- Case report
- Open access
- Published: 21 February 2018

Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
- Virginia Mirra 1 ,
- Silvia Montella 1 &
- Francesca Santamaria 1
BMC Pediatrics volume 18 , Article number: 73 ( 2018 ) Cite this article
11k Accesses
11 Citations
12 Altmetric
Metrics details
The primary goal of asthma management is to achieve disease control for reducing the risk of future exacerbations and progressive loss of lung function. Asthma not responding to treatment may result in significant morbidity. In many children with uncontrolled symptoms, the diagnosis of asthma may be wrong or adherence to treatment may be poor. It is then crucial to distinguish these cases from the truly “severe therapy-resistant” asthmatics by a proper filtering process. Herein we report on four cases diagnosed as difficult asthma, detail the workup that resulted in the ultimate diagnosis, and provide the process that led to the prescription of omalizumab.
Case presentation
All children had been initially referred because of asthma not responding to long-term treatment with high-dose inhaled steroids, long-acting β 2 -agonists and leukotriene receptor antagonists. Definitive diagnosis was severe asthma. Three out four patients were treated with omalizumab, which improved asthma control and patients’ quality of life. We reviewed the current literature on the diagnostic approach to the disease and on the comorbidities associated with difficult asthma and presented the perspectives on omalizumab treatment in children and adolescents. Based on the evidence from the literature review, we also proposed an algorithm for the diagnosis of pediatric difficult-to-treat and severe asthma.
Conclusions
The management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma. The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism in the management of children and adolescents with atopic severe asthma.
Peer Review reports
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [ 1 ]. Therefore, the primary goal of asthma management at all ages is to achieve disease control [ 2 , 3 , 4 ].
According to recent international guidelines, patients with uncontrolled asthma require a prolonged maintenance treatment with high-dose inhaled corticosteroids (ICS) in association with a long-acting β 2 -agonist (LABA) plus oral leukotriene receptor antagonist (LTRA) (Table 1 ) [ 5 ].
Nevertheless, in the presence of persistent lack of control, reversible factors such as adherence to treatment or inhalation technique should be first checked for, and diseases that can masquerade as asthma should be promptly excluded. Finally, additional strategies, in particular anti-immunoglobulin E (anti-IgE) treatment (omalizumab), are suggested for patients with moderate or severe allergic asthma that remains uncontrolled in Step 4 [ 5 ].
Herein, we reviewed the demographics, clinical presentation and treatment of four patients with uncontrolled severe asthma from our institution in order to explain why we decided to prescribe omalizumab. We also provided a review of the current literature that focuses on recent advances in the diagnosis of pediatric difficult asthma and the associated comorbidities, and summarizes the perspectives on anti-IgE treatment in children and adolescents.
Case presentations
Table 2 summarizes the clinical characteristics and the triggers/comorbidities of the cases at referral to our Institution. Unfortunately, data on psychological factors, sleep apnea, and hyperventilation syndrome were not available in any case. Clinical, lung function and airway inflammation findings at baseline and after 12 months of follow-up are reported in Table 3 . In the description of our cases, we used the terminology recommended by the ERS/ATS guidelines on severe asthma [ 6 ].
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 11, severe asthma was diagnosed. Sensitization to multiple inhalant allergens (i.e., house dust mites, dog dander, Graminaceae pollen mix, and Parietaria judaica ) and high serum IgE levels (1548 KU/l) were found. Body mass index (BMI) was within normal range. Combined treatment with increasing doses of ICS (fluticasone, up to 1000 μg/day) in association with LABA (salmeterol, 100 μg/day) plus LTRA (montelukast, 5 mg/day) has been administered over 2 years. Nevertheless, persistent symptoms and monthly hospital admissions due to asthma exacerbations despite correct inhaler technique and good adherence were reported. Parents refused to perform any test to exclude gastroesophageal reflux (GER) as comorbidity [ 6 ]. However, an ex-juvantibus 2-month-course with omeprazole was added to asthma treatment [ 7 ], but poor control persisted. Anterior rhinoscopy revealed rhinosinusitis that was treated with nasal steroids for six months [ 8 ], but asthma symptoms were unmodified. Treatment with omalizumab was added at age 12. Reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 2.0 up to 6.7 out of a maximum of 7 points) were documented over the following months. Unfortunately, after one year of treatment, adherence to omalizumab decreased because of family complaints, and eventually parents withdrew their informed consent and discontinued omalizumab. Currently, by age 17, treatment includes inhaled salmeterol/fluticasone (100 μg/500 μg∙day -1 , respectively) plus oral montelukast (10 mg/day). Satisfactory symptom control is reported, with no asthma exacerbations.
A full-term male, who had a recurrent severe preschool wheezing, at 6 years of age developed exercise-induced asthma. At age 10, severe asthma was diagnosed. High serum IgE levels (1300 KU/l) and skin prick tests positive to house dust mites were found. Despite a 3-year treatment with progressively increasing doses of inhaled fluticasone (up to 1000 μg/day) combined with salmeterol (100 μg/day) and oral montelukast (5 mg/day), monthly hospital admissions with systemic steroids use were reported. At age 13, a 24-h esophageal impedance/pH study demonstrated the presence of acid and non-acid GER [ 7 ]. Esomeprazole was added to asthma medications, but with an incomplete clinical benefit for respiratory symptoms. Esomeprazole was withdrawn after 3 months, and parents refused to re-test for GER. As respiratory symptoms persisted uncontrolled despite treatment, severe asthma was definitively diagnosed [ 6 ]. BMI was within the normal range and anterior rhinoscopy excluded rhinosinusitis. Inhaler technique and adherence were good; thus we considered the anti-IgE treatment option [ 9 ]. Subcutaneous omalizumab was started, with fast improvement of both symptoms and QoL score (from 3.9 up to 6.5). Seventeen months later, the dose of ICS had been gradually tapered and oral montelukast definitely discontinued. Currently, at age 14, treatment includes the combined administration of bimonthly subcutaneous omalizumab and of daily inhaled salmeterol/fluticasone (50 μg/100 μg∙day - 1 , respectively). Asthma control is satisfactory and no side effects are reported. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with acute respiratory failure that frequently required intensive care unit (ICU) admission. At age 6, sensitization to multiple perennial inhalant (i.e., house dust mites, dog and cat danders, Alternaria alternata , Graminaceae pollen mix, Artemisia vulgaris , Parietaria judaica , and Olea europaea pollen) and food allergens (i.e., egg, milk, and peanut) was diagnosed. Serum IgE levels were 2219 KU/l. Weight and height were appropriate for age and sex. The patient has been treated over 3 years with a combined scheme of high-dose inhaled fluticasone (up to 1000 μg/day) plus salmeterol (100 μg/day) and oral montelukast (5 mg/day), with correct inhaler technique and good adherence. Despite this, monthly hospital admissions with systemic steroids use were recorded. Rhinosinusitis and GER were excluded on the basis of appropriate testing; thus treatment with omalizumab was started when the patient was 9 years old. At age 11, adherence to treatment is satisfactory, with no side effects. More importantly, reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 6.4 to 6.8) were reported. Finally, progressive step-down of anti-asthma treatment was started, and at present (by 11.5 years) inhaled fluticasone (200 μg/day) plus bimonthly subcutaneous omalizumab provide good control of symptoms. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 4, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 8, multiple perennial inhalants and food sensitization (i.e., house dust mites, dog dander, Graminaceae pollen mix, Olea europaea pollen, tomatoes, beans, shrimps, and peas) and high serum IgE levels (1166 KU/l) were found. The patient has been treated over 5 years with inhaled fluticasone (up to 1000 μg/day) in association with salmeterol (100 μg/day) and oral montelukast (5 mg/day). Despite this, monthly hospital admissions with systemic steroids need were recorded. After checking the inhaler technique and adherence to treatment, comorbidities including obesity, rhinosinusitis and GER were excluded. Omalizumab was proposed, but parents refused it. By 13.6 years, despite a treatment including the association of inhaled salmeterol/fluticasone (100 μg/1000 μg∙day − 1 , respectively) plus oral montelukast (10 mg/day), monthly exacerbations requiring systemic steroids are reported.
Discussion and conclusions
Most children and adolescents with asthma respond well to inhaled short-acting beta 2 -agonists (SABA) on demand if symptoms are intermittent, or to low dose controller drugs plus as-needed SABA if the risk of exacerbations increases [ 1 ]. Nevertheless, a proportion of patients is referred to specialists because this strategy is not working and asthma is persistently uncontrolled [ 4 ]. For these children, assessment is primarily aimed at investigating the reasons for poor control. Indeed, when the child is initially referred, before the label of “severe, therapy-resistant asthma” (i.e., not responding to treatment even when factors as exposure to allergens and tobacco smoke have been considered) is assigned, three main categories need to be identified: 1) “not asthma at all”, in which response to treatment is suboptimal because the diagnosis is wrong; 2) “asthma plus ”, when asthma is mild but exacerbated by one or more comorbidities; and 3) “difficult-to-treat asthma”, when asthma is uncontrolled because of potentially reversible factors [ 10 ].
The reported cases highlight some aspects of the disease process that may expand the diagnosis and improve patients’ care. At our institution, the severe asthma program includes a multidisciplinary approach with consultations by gastroenterologists as well as ear, nose and throat experts. Recently, sleep medicine experts joined this multidisciplinary team; thus, unfortunately, sleep-disordered breathing (SDB) could not be excluded at the time of our patients’ assessment. Inhalation technique is periodically evaluated by nurses or doctors in each patient. Unfortunately, in Italy an individual prescription database is not available and thus we cannot assess patients’ use of medication. In two cases, the filtering process eventually identified GER and rhinosinusitis, but poor control of asthma persisted even after comorbidities were treated. In all subjects, inhaler skills, treatment adherence, and environmental exposure to indoor/outdoor allergens as well as to second- and third-hand smoke were excluded as cause of lack of control. Eventually, three out of four patients started anti-IgE treatment; asthma control was obtained and maintenance drugs were progressively reduced. In the case that refused omalizumab therapy, pulmonary function, clinical features and controller treatment including high-dose ICS were unchanged.
Previous studies have highlighted an association between increasing asthma severity in children and reduced QoL [ 11 , 12 , 13 ]. Uncontrolled asthma symptoms not only affect children physically, but can impair them socially, emotionally, and educationally [ 13 ]. In line with previous observations, 3 out 4 of our cases had poor QoL, assessed by a standardized questionnaire [ 14 ]. It is well known that improving QoL in difficult asthma is not an easy task, despite a variety of treatments aimed at achieving control [ 12 ], and much more remains to be done to address the problem. Nevertheless, 2 of our 3 cases showed a remarkable improvement of QoL after one year of treatment with omalizumab.
Reduction in forced expiratory volume in the first second (FEV 1 ) is often used to define childhood asthma severity in treatment guidelines and clinical studies [ 5 , 11 , 15 ]. Nevertheless, children with severe asthma often have a normal FEV 1 that does not improve after bronchodilators, indicating that spirometry may be a poor predictor of asthma severity in childhood [ 6 , 16 , 17 ]. Actually, children with a normal FEV 1 , both before and after β 2 -agonist, may show a bronchodilator response in terms of forced expiratory flow between 25% and 75% (FEF 25–75 ) [ 18 ]. However, the utility of FEF 25–75 in the assessment or treatment of severe asthma is currently unknown. Interestingly, all the reported cases showed normal or slightly reduced values of FEV 1 but severe impairment of FEF 25–75 . Two cases showed a bronchodilator response in terms of FEV 1 (subjects 3 and 4), while 3 patients had a significant increase of FEF 25–75 (cases 1, 3 and 4). Unfortunately, we could not provide the results of bronchodilator response during or after the treatment with omalizumab in any case.
Available literature on the diagnostic approach to difficult asthma in children offers a number of reviews which basically summarize the steps needed to fill the gap between a generic diagnosis of “difficult asthma” and more specific labels (i.e., “severe” asthma, “difficult-to-treat” asthma, or even different diagnoses) [ 3 , 5 , 6 , 8 , 10 , 19 , 20 , 21 ]. So far, few original articles and case reports have been published, probably due to the peculiarity of the issue, which makes retrospective discussion of cases easier than the design of a prospective clinical study [ 4 , 22 , 23 , 24 , 25 , 26 ]. Available knowledge mainly derives from the experience of specialized centers.
The evaluation of a child referred for uncontrolled asthma should start with a careful history focused on typical respiratory symptoms and on the definition of possible triggers. In the “severe asthma” process, it is crucial for clinicians to maintain a high degree of skepticism about the ultimate diagnosis, particularly in the presence of relevant discrepancies between history, physical features and lung function, as many conditions may be misdiagnosed as asthma. In order to simplify this process, herein we propose an algorithm for the diagnosis of difficult-to-treat and severe asthma (Fig. 1 ). Confirmation of the diagnosis through a detailed clinical and laboratory re-evaluation is important because in 12–50% of cases assumed to have severe asthma this might not be the correct diagnosis [ 10 ]. Several documents have indicated the main steps of the process that should be followed in children with uncontrolled asthma [ 3 , 8 , 10 ]. The translation of these procedures into real life practice may deeply change from one subject to another due to the variability of individual patients’ history and clinical features, which will often lead the diagnostic investigations towards the most likely reason for uncontrolled asthma. For children with apparently severe asthma, the first step is to confirm the diagnosis and, before proceeding to broader investigations, to verify that the poor control is not simply determined by poor adherence to treatment, inadequate inhaler skills and/or environmental exposure to triggers. A nurse-led assessment, including a home visit, despite not being applicable in all settings, may be useful for identifying potentially modifiable factors in uncontrolled pediatric asthma [ 27 ].
A practical algorithm for the diagnosis of difficult-to-treat and severe asthma. ICS, inhaled corticosteroids; OCS, oral corticosteroids
A number of comorbidities have been increasingly recognized as factors that may impact asthma clinical expression and control in childhood [ 10 , 28 ]. Children with uncontrolled disease should be investigated for GER, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, obesity, psychological factors, smoke exposure, hormonal influences, and ongoing drugs [ 3 , 6 , 8 , 20 ]. Indeed, the exact role played by comorbidities in pediatric asthma control is still debated [ 28 ]. The most impressive example is GER. Several pediatric documents recommend assessing for GER because reflux may be a contributing factor to problematic or difficult asthma [ 7 , 29 ]. Nevertheless, GER treatment might not be effective for severe asthma [ 30 , 31 ], as confirmed by current cases 1 and 2. There is an established evidence that chronic rhinosinusitis is associated with more severe asthma in children [ 32 , 33 , 34 ]. Therefore, examination of upper airways and ad hoc treatment if rhinosinusitis is evident are recommended in children with severe asthma [ 3 , 8 , 35 ]. However, intranasal steroids for rhinitis resulted in a small reduction of asthma risk in school-aged children [ 36 ], and actual placebo-controlled studies on the effect of treatment of rhinosinusitis on asthma control in children are lacking [ 10 , 37 ].
Dysfunctional breathing, including hyperventilation and vocal cord dysfunction, is associated with poorer asthma control in children [ 8 , 10 , 38 , 39 ]. Unfortunately, there is scarce literature on the effect of its treatment on the control of severe asthma in children [ 40 ]. SDB ranging from primary snoring to obstructive sleep apnea syndrome is very common in children [ 41 ], and an increased prevalence of SDB together with increasing asthma severity has been reported [ 42 ]. Interestingly, GER may also be worsened by recurrent episodes of upper airway obstruction associated with SDB, and this may further trigger bronchial obstruction. Asthma guidelines recommend the assessment of SDB through nocturnal polysomnography in poorly controlled asthmatics, particularly if they are also obese [ 5 ]. There are no studies examining whether pediatric asthma improves after SDB has been treated, for example, with nasal steroids, adenotonsillectomy, continuous positive airway pressure or weight reduction if the child is also obese [ 43 ]. The parallel increase in obesity and asthma suggests that the two conditions are linked and that they can aggravate each other [ 44 , 45 ], even though the exact mechanisms that underlie this association remain unclear [ 46 ]. Indeed, other coexisting comorbidities such as SDB or GER may play a confounding role in the development of the interactions between obesity and the airways [ 47 , 48 ]. Obesity is associated with increased markers of inflammation in serum and adipose tissue and yet decreased airway inflammation in obese people with asthma [ 49 ]. Several interventions, including behavioral and weight reduction programs or bariatric surgery, may result in improved asthma control, quality of life and lung function in adult obese asthmatics [ 50 ]. Although reports of adolescent bariatric surgery demonstrate a significant body weight decrease, this approach is not widely available and there are no published reports on its effect on pediatric severe asthma control [ 51 ]. Finally, although it is still unclear whether food allergy is causative or shares a common pathway with difficult asthma, it might explain the loss of asthma control at least in some children and thus be considered as a comorbid condition [ 10 , 16 , 52 ].
In conclusion, establishing the impact of comorbidities on asthma control may be cumbersome, and an ex-juvantibus treatment is sometimes necessary to assess their role. Comorbid conditions can also worsen each other, and symptoms arising from some of them may mimic asthma [ 6 ]. Although the ability to improve pediatric severe asthma by treating comorbidities remains unconfirmed, they should be treated appropriately [ 9 ].
The vast majority of asthmatic children exhibit a mild or at most a moderate disease that can be fully controlled with low-to-medium dose ICS associated or not with other controllers [ 5 , 6 ]. However, a subset of asthmatics remains difficult-to-treat [ 5 , 6 ]. With the advent of biologics, these severe steroid-dependent asthmatics have alternative options for treatment, as steroid-related adverse events are common in severe asthma [ 53 ]. Omalizumab, an anti-IgE monoclonal antibody, is the only biologic therapy recommended in children with moderate-to-severe asthma by the recent guidelines [ 5 , 6 ]. In Italy, this treatment is fully covered by the National Health System. Therefore, there is no influence by any funding on treatment decisions. It was approved by the US (Food and Drug Administration) in 2003 and by the European Union (European Medicines Agency) in 2005 as an add-on treatment for patients aged > 12 years with severe persistent allergic asthma and who have a positive skin test or in-vitro reactivity to a perennial aeroallergen, FEV 1 < 80% predicted, frequent daytime symptoms or nighttime awakenings, and multiple documented severe asthma exacerbations despite daily ICS plus a LABA [ 54 , 55 ]. In 2009, it also received approval in Europe for treating patients aged 6–12 years. Figure 2 illustrates current indications for treatment with omalizumab in children and adolescents with severe asthma.
Indications for omalizumab in children and adolescents with severe asthma
IgE antibodies, Th 2 -derived cytokines and eosinophils play a major role in the development of chronic airway inflammation in asthmatic subjects [ 56 ]. Once released from plasma cells, IgE binds principally to the high-affinity IgE receptor (FcεRI) on mast cells, triggering different effector responses, including the release of mediators leading to allergic inflammatory reactions [ 56 ]. The activation of the allergic cascade by IgE, under constant allergen stimulation, leads to the establishment of chronic allergic inflammation in the airways of asthmatic patients, with IgE being a key element of the vicious circle that maintains it. Cytokines produced during the late phase and subsequent chronic inflammation stage have been directly associated with the induction of airway remodelling, indirectly implicating IgE in the process [ 56 ]. At present, omalizumab is the only commercially available recombinant humanized anti-IgE monoclonal antibody that specifically binds serum free IgE at its CH 3 domain, in the proximity of the binding site for FcεRI, thus preventing IgE from interacting with its receptor on mast cells, basophils, antigen-presenting cells and other inflammatory cells [ 57 ]. The rapid reduction of free IgE levels leads to a downregulation of the FcεRI expression on inflammatory cells and an interruption of the allergic cascade, which results in the reduction of peripheral and bronchial tissue eosinophilia and of levels of granulocyte macrophage colony stimulating factor, interleukin (IL)-2, IL-4, IL-5, and IL-13 [ 58 ]. Moreover, basophils have a relevant role in the initiation and progression of allergic inflammation, suggesting that they may represent a viable therapeutic target. Indeed, in children with severe asthma, it has been reported that omalizumab therapy is associated with a significant reduction in circulating basophil numbers, a finding that is concurrent with improved clinical outcomes [ 59 ]. This finding supports a mechanistic link between IgE levels and circulating basophil populations, and may provide new insights into one mechanism by which omalizumab improves asthma symptoms.
Several clinical controlled and real-life studies of adults with severe, inadequately controlled allergic asthma have demonstrated the efficacy and safety of omalizumab in reducing asthma-related symptoms, corticosteroid use, exacerbation rates, and healthcare resource utilization, and in improving QoL and lung function [ 60 , 61 , 62 , 63 ]. Fewer studies have been published in children. In two double-blind, randomized, placebo-controlled trials (RCTs) of children aged 6 to 12 years with moderate-to-severe allergic asthma, treatment with omalizumab reduced the requirement for ICS and protected against disease exacerbations, but there was little change in asthma symptom scores or spirometry [ 9 , 64 ]. These findings were confirmed and extended in older children [ 65 , 66 , 67 ].
The results of the ICATA study, a multicenter RCT of 419 inner-city children, adolescents and young adults with persistent allergic asthma, showed that, compared to placebo, omalizumab reduces the number of days with asthma symptoms and the proportion of participants with at least one exacerbation by approximately 25% and 19%, respectively ( p < 0.001), thus reducing the need for asthmatic symptom controllers [ 68 ]. Another multicenter RCT of inner-city children and adolescents showed that the addition of omalizumab to ongoing guidelines-based care before patients return to school reduces fall asthma exacerbations (odds ratio, 0.48), particularly in subjects with a recent exacerbation [ 69 ]. Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases ( p < 0.001), while FEV 1 improved by 4.9% ( p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% ( p < 0.001) [ 70 ]. The same authors also showed that, after two years of treatment, exacerbation rate and healthcare utilisation were further decreased by 83% and 100%, respectively, while level of asthma control, steroid use and lung function remained unchanged [ 71 ].
A systematic review of pediatric RCTs pooled the data of 1381 children and adolescents with moderate-to-severe allergic asthma in order to establish the efficacy of omalizumab as an add-on therapy [ 72 ]. During the stable-steroid phase, omalizumab decreased the number of patients with at least one exacerbation (risk ratio, 0.69; p < 0.001), the mean number of asthma exacerbations per patient (risk ratio, 0.35; p < 0.001), and the asthma symptom score (mean difference, 0.12; p = 0.005) when compared to placebo. During the steroid reduction phase, omalizumab further reduced the number of patients with at least one exacerbation (risk ratio, 0.48; p < 0.001) and the mean number of asthma exacerbations per patient (mean difference, 0.12; p < 0.05).
Given the cost of omalizumab, many authors have argued for the importance of identifying specific asthma populations who will have significant benefit from it [ 68 , 73 , 74 ]. In the ICATA study, baseline predictors of good response to treatment were sensitization and exposure to cockroach allergen, sensitization to house dust mite allergens, a serum IgE level of more than 100 IU per milliliter, a BMI of 25 or more, and a history of at least one unscheduled medical visit in the previous year [ 68 ].
Several studies have assessed the long-term safety of omalizumab in children and adults. A pooled analysis of 67 RCTs conducted over 2 decades on 4254 children and adults treated with omalizumab showed no association between omalizumab treatment and risk of malignancy [ 75 ]. In an RCT evaluating 225 school-aged children, omalizumab was well tolerated, there were no serious adverse events, and the frequency and types of all adverse events were similar to the placebo group [ 9 ]. These results have been further confirmed by a recent systematic review of RCTs that concluded that treatment with omalizumab does not result in increased risk of malignancy or hypersensitivity reactions [ 72 ].
While the rationale for long-term treatment with omalizumab is supported by pharmacokinetic-pharmacodynamic models [ 76 ], the duration of treatment is still under discussion. Results from published studies suggest that omalizumab should be continued for > 1 year [ 77 , 78 ]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was ‘excellent’ in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years [ 77 ]. After the discontinuation of treatment, loss of asthma control was documented in 69.2% of the patients who had received omalizumab for < 1 year, 59.1% of the subjects treated for 1–2 years, and 46.1% of the cases treated for > 2 years. Time to loss of control was shorter in younger children and longer in patients with an ‘excellent’ response compared with patients with a ‘good’ response. No early loss of control (within 6 months) was observed among patients with > 3.5 years of continuous treatment with omalizumab. Finally, 20% of patients in whom omalizumab was re-prescribed because of loss of control did not respond to the treatment anymore [ 77 ]. Despite these encouraging findings, the impact of omalizumab on the natural history of severe asthma in children deserves to be further investigated by long-term studies that will also define the criteria and timing for discontinuing the treatment.
It is well known that asthma pharmacotherapy is effective in controlling symptoms and bronchial inflammation, but cannot affect the underlying immune response, thus leading to the possibility of symptom reappearance after its discontinuation [ 79 ]. In this scenario, allergen-specific immunotherapy (AIT) has been proposed as the only therapeutic method that can modulate the underlying immune pathophysiology in allergic asthma [ 80 ].
AIT is currently indicated in children and adults with mild-moderate allergic asthma that is completely or partially controlled by pharmacotherapy and with the evidence of a clear relationship between symptoms and exposure to a specific allergen [ 81 , 82 , 83 , 84 ]. However, according to recent guidelines, the efficacy of AIT in asthmatic subjects is limited, and its potential benefits must be weighed against the risk of side effects and the inconvenience and costs of the prolonged therapy [ 5 ]. Moreover, severe or uncontrolled asthma (regardless of its severity) is a major independent risk factor for non-fatal or even fatal adverse reactions, thus representing a contraindication for AIT [ 85 , 86 , 87 ]. Finally, children with severe asthma are often sensitized to multiple allergens, thus making AIT prescription even more complicated [ 88 ].
In subjects with uncontrolled and/or severe allergic asthma, a combination of omalizumab and AIT has been proposed [ 88 ]. Surprisingly, only a few studies have addressed this issue [ 89 , 90 , 91 , 92 ]. However, pre-treatment with omalizumab seems to improve the efficacy and tolerability of subcutaneous AIT in children and adults with severe allergic asthma both during omalizumab treatment and after its discontinuation [ 89 , 91 , 92 ]. Omalizumab has also been successfully used as a supplementary treatment to AIT in order to improve asthma control in children ≥6 years with severe persistent allergic asthma [ 90 ]. Given the scarcity of studies on AIT plus omalizumab in children with severe allergic asthma, further research is warranted to assess risks and benefits of the combined treatment.
Children with severe asthma require a detailed and individualized approach including re-assessment for differential diagnoses, comorbidities and contributory factors, environmental triggers, lung function and inflammation, adherence and response to therapy, and QoL. Treatment of pediatric severe asthma still relies on the maximal optimal use of corticosteroids, bronchodilators and other controllers recommended for moderate-to-severe disease. However, the management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma.
In the current paper, we described the characteristics of four children with severe asthma in whom omalizumab was prescribed. A review of the relevant literature on the topic was also performed. Finally, we provided an algorithm for the diagnosis of difficult-to-treat and severe asthma in children and adolescents, based on the evidence from the literature review. As all algorithms, it is not meant to replace clinical judgment, but it should drive physicians to adopt a systematic approach towards difficult and severe asthma and provide a useful guide to the clinician.
The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism of outcome improvements in patients with allergic severe asthma. As severe asthma is a heterogeneous condition consisting of different phenotypes, the future of asthma management will likely involve phenotypic and potentially even genotypic characterization in selected cases in order to determine appropriate therapy and thus to provide the highest possible benefit, especially if specific responder phenotypes can be identified and selected for this highly specific treatment.
Abbreviations
Anti-immunoglobulin E
Body mass index
IgE receptor
Forced expiratory flow between 25% and 75%
Forced expiratory volume in the first second
Gastroesophageal reflux
Inhaled corticosteroids
Intensive care unit
Interleukin
Long-acting β 2 -agonist
Oral leukotriene receptor antagonist
Quality of life
Randomized controlled trials
Short-acting β 2 -agonists
Sleep-disordered breathing
O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143:511–3.
Article PubMed Google Scholar
National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–8.
Article Google Scholar
Hedlin G. Management of severe asthma in childhood-state of the art and novel perspectives. Pediatr Allergy Immunol. 2014;25:111–21.
Konradsen JR, Nordlund B, Lidegran M, Pedroletti C, Grönlund H, van Hage M, et al. Problematic severe asthma: a proposed approach to identifying children who are severely resistant to therapy. Pediatr Allergy Immunol. 2011;22:9–18.
Global Initiative for Asthma Report. Global strategy for asthma management and prevention (updated 2016). https://www.ginasthma.org . Accessed 07 June 2017.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–53.
Article CAS PubMed Google Scholar
Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the north American Society for Pediatric Gastroenterology, Hepatology, and nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–507.
Lødrup Carlsen KC, Hedlin G, Bush A, Wennergren G, de Benedictis FM, De Jongste JC, et al. Assessment of problematic severe asthma in children. Eur Respir J. 2011;37:432–40.
Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–5.
Article CAS PubMed PubMed Central Google Scholar
Lang A, Mowinckel P, Sachs-Olsen C, Riiser A, Lunde J, Carlsen KH, et al. Asthma severity in childhood, untangling clinical phenotypes. Pediatr Allergy Immunol. 2010;21:945–53.
Nordlund B, Konradsen JR, Pedroletti C, Kull I, Hedlin G. The clinical benefit of evaluating health-related quality-of-life in children with problematic severe asthma. Acta Paediatr. 2011;100:1454–60.
Dean BB, Calimlim BC, Sacco P, Aguilar D, Maykut R, Tinkelman D. Uncontrolled asthma: assessing quality of life and productivity of children and their caregivers using a cross-sectional internet-based survey. Health Qual Life Outcomes. 2010;8:6.
Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46.
British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, 2014. https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline . Accessed 13 Apr 2016.
Montella S, Baraldi E, Cazzato S, Aralla R, Berardi M, Brunetti LM, et al. Severe asthma features in children: a case-control online survey. Ital J Pediatr. 2016;42:9.
Article PubMed PubMed Central Google Scholar
Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25.
Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34.
Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36:196–201.
Fitzpatrick AM, Teague WG. Severe asthma in children: insights from the National Heart, Lung, and Blood Institute's severe asthma research program. Pediatr Allergy Immunol Pulmonol. 2010;23:131–8.
Konradsen JR, Caffrey Osvald E, Hedlin G. Update on the current methods for the diagnosis and treatment of severe childhood asthma. Expert Rev Respir Med. 2015;9:769–77.
Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, et al. Identifying problematic severe asthma in the individual child—does lung function matter? Acta Paediatr. 2010;99:404–10.
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul WJ. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. Asthma. 2012;49:586–92.
Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–8.
Cicutto LC, Chapman KR, Chamberlain D, Downey GP. Difficult asthma: consider all of the possibilities. Can Respir J. 2000;7:415–8.
Wener RR, Bel EH. Severe refractory asthma: an update. Eur Respir Rev. 2013;22:227–35.
Bracken M, Fleming L, Hall P, et al. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94:780–4.
De Groot EP, Kreggemeijer WJ, Brand PL. Getting the basics right resolves most cases of uncontrolled and problematic asthma. Acta Paediatr. 2015;104:916–21.
Grimaldi-Bensouda L, Zureik M, Aubier M, Humbert M, Levy J, Benichou J, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–9.
Article PubMed Central Google Scholar
Writing Committee for the American Lung Association Asthma Clinical Research Centers, Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012;307:373-381.
Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94:895–901.
CAS PubMed Google Scholar
De Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–7.
Rotiroti G, Roberts G, Scadding GK. Rhinitis in children: common clinical presentations and differential diagnoses. Pediatr Allergy Immunol. 2015;26:103–10.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA). 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:S8–160.
Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy. 2014;69:1515–21.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120:855–64.
De Groot EP, Duiverman EJ, Brand PL. Dysfunctional breathing in children with asthma: a rare but relevant comorbidity. Eur Respir J. 2013;41:1068–73.
Barker NJ, Jones M, O'Connell NE, Everard ML. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in children. Cochrane Database Syst Rev. 2013;12:CD010376.
Google Scholar
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12.
Goldstein NA, Aronin C, Kantrowitz B, Hershcopf R, Fishkin S, Lee H, Weaver DE, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 2015;50:1128–36.
Ross KR, Storfer-Isser A, Hart MA, Kibler AM, Rueschman M, Rosen CL, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42.
Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, et al. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring). 2011;19:1623–8.
Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. 2015;21:80–5.
Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14:35–43.
Santamaria F, Montella S, Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes Rev. 2012;13:822–33.
Lang JE, Hossain J, Holbrook JT, Teague WG, Gold BD, Wise RA, et al. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71:238–46.
Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005;116:1163–4.
Van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67.
Katzmarzyk PT, Bouchard C. Where is the beef? Waist circumference is more highly correlated with BMI and total body fat than with abdominal visceral fat in children. Int J Obes. 2014;38:753–4.
Article CAS Google Scholar
De Groot EP, Duiverman EJ, Brand PL. Comorbidities of asthma during childhood: possibly important, yet poorly studied. Eur Respir J. 2010;36:671–8.
Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016; https://doi.org/10.1136/thoraxjnl-2015-207630 .
Federal Drug Administration Advisory for Omalizumab. Available at: https://wayback.archive-it.org/7993/20170111075347/ . http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm . Accessed 4 Feb 2018.
European Medicines Agency: assessment report for Xolair. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124 . Accessed 7 June 2017.
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–96.
Jensen RK, Plum M, Tjerrild L, Jakob T, Spillner E, Andersen GR. Structure of the omalizumab Fab. Acta Crystallogr F Struct Biol Commun. 2015;71:419–26.
Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36.
Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy. 2014;69:674–7.
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, Cao C, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191.
Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. “real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2015; https://doi.org/10.1111/all.12815 .
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6.
Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61.
Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004;28:152–7.
Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, et al. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. 2015;64:364–70.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–33.
Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–9.
Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26:551–6.
Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9.
Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65:1141–8.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129:983–9.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–10.
Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–6.
Busse WW, Trzaskoma B, Omachi TA, Canvin J, Rosen K, Chipps BE, et al. Evaluating Xolair persistency of response after long-term therapy (XPORT). Am J Respir Crit Care Med. 2014;189:A6576.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97.
Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–49.
National Heart, Lung, and Blood Institute. Expert panel report 3: Guidelines for the diagnosis and management of asthma—full report 2007. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf . Accessed 4 Feb 2018.
Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunolgy. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85.
Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30.
Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. 2017;43:13.
Pitsios C, Demoly P, Bilo MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909.
Tsabouri S, Mavroudi A, Feketea G, Guibas GV. Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pediatr. 2017;5:82.
Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68.
Hedlin G, van Hage M. The role of immunotherapy in the management of childhood asthma. Ther Adv Respir Dis. 2012;6:137–46.
Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25:829–32.
Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann K-C, Sieder C. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9.
Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–9.
Stelmach I, Kaczmarek-Woźniak J, Majak P, Olszowiec-Chlebna M, Jerzynska J. Efficacy and safety of high-doses sublingual immunotherapy in ultra-rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39:401–8.
Download references
Acknowledgements
The authors gratefully thank Dr. Marco Maglione for his contribution in the clinical assessment of the described cases. Medical writing assistance was provided by Stephen Walters on behalf of City Hills Proofreading.
No funding was secured for this study.
Availability of data and materials
All relevant data and materials are published in the manuscript.
Author information
Authors and affiliations.
Department of Translational Medical Sciences, Federico II University, Via Sergio Pansini 5, 80131, Naples, Italy
Virginia Mirra, Silvia Montella & Francesca Santamaria
You can also search for this author in PubMed Google Scholar
Contributions
VM, SM and FS, authors of the current manuscript, declare that they have participated sufficiently in the work to take public responsibility for appropriate portions of the content. VM and SM carried out the initial investigations, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. FS conceptualized and designed the study, and critically reviewed and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Francesca Santamaria .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee “Carlo Romano”, Federico II University, Naples, Italy. Children’s parents/legal guardians gave informed written consent to participate. The description of our cases adheres to the CARE standards of reporting checklist.
Consent for publication
Children’s parents/legal guardians provided informed written consent for the case report to be published.
Competing interests
The authors declare that they have no competing interests to disclose. Authors have no financial relationships relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Mirra, V., Montella, S. & Santamaria, F. Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr 18 , 73 (2018). https://doi.org/10.1186/s12887-018-1019-9
Download citation
Received : 24 May 2016
Accepted : 29 January 2018
Published : 21 February 2018
DOI : https://doi.org/10.1186/s12887-018-1019-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Adolescents
- Asthma exacerbations
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search Menu
- Advance Articles
- Supplements
- Author Guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Advertising & Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Paediatrics & Child Health
- About The Canadian Paediatric Society
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Case 1 diagnosis: allergy bullying, clinical pearls.
- < Previous
Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation
- Article contents
- Figures & tables
- Supplementary Data
Lopamudra Das, Michelle GK Ward, Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation, Paediatrics & Child Health , Volume 19, Issue 2, February 2014, Pages 69–70, https://doi.org/10.1093/pch/19.2.69
- Permissions Icon Permissions
A 12-year-old girl with a history of asthma presented to the emergency department with a three-day history of increased work of breathing, cough and wheezing. She reported no clear trigger for her respiratory symptoms, although she had noted some symptoms of a mild upper respiratory tract infection. With this episode, the patient had been using a short-acting bronchodilator more frequently than she had in the past, without the expected resolution of symptoms.
On the day of presentation, the patient awoke feeling ‘suffocated’ and her mother noted her lips to be blue. In the emergency department, her oxygen saturation was 85% and her respiratory rate was 40 breaths/min. She had significantly increased work of breathing and poor air entry bilaterally to both lung bases, with wheezing in the upper lung zones. She was treated with salbutamol/ipratropium and received intravenous steroids and magnesium sulfate. Her chest x-ray showed hyperinflation and no focal findings.
Her medical history revealed that she was followed by a respirologist for her asthma, had good medication adherence and had not experienced a significant exacerbation for six months. She also had a history of wheezing, dyspnea and pruritis with exposure to peanuts, chickpeas and lentils; she had been prescribed an injectible epinephrine device for this. However, her device had expired at the time of presentation. In the past, her wheezing episodes had been seasonal and related to exposure to grass and pollens; this presentation occurred during the winter. Further history revealed the probable cause of her presentation.
Although reluctant to disclose the information, our patient later revealed that she had been experiencing significant bullying at school, which was primarily related to her food allergies. Three days before her admission, classmates had smeared peanut butter on one of her schoolbooks. She developed pruritis immediately after opening the book and she started wheezing and coughing later that day. This event followed several months of being taunted with peanut products at school. The patient was experiencing low mood and reported new symptoms of anxiety related to school. The review of systems was otherwise negative, with no substance use.
The patient's asthma exacerbation resolved with conventional asthma treatment. Her pulmonary function tests were nonconcerning (forced expiratory volume in 1 s 94% and 99% of predicted) after her recovery. The trigger for her asthma exacerbation was likely multifactorial, related to exposure to the food allergen as well as the upper respiratory infection. A psychologist was consulted to assess the symptoms of anxiety and depression that had occurred as a result of the bullying. During the hospitalization, the medical team contacted the patient's school to provide education on allergy bullying, treatment of severe allergic reactions and its potential for life-threatening reactions with exposure to allergens. The medical team also recommended community resources for further education of students and staff about allergy bullying and its prevention.
Allergy bullying is a form of bullying with potentially severe medical outcomes. In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study [ 1 ]), this bullying is related directly to the food allergy. From a medical perspective, there are little published data regarding allergy bullying, and many health care providers may not be aware of the issue.
Allergy bullying can include teasing a child about their allergy, throwing food at a child, or even forcing them to touch or eat allergenic foods. Most episodes of allergy bullying occur at school, and can include episodes perpetrated by teachers and/or staff ( 2 ).
Allergy bullying can lead to allergic reactions, which may be mild or severe (eg, urticaria, wheezing, anaphylaxis), but may also lead to negative emotional consequences (sadness, depression) ( 2 ) and an overall decrease in quality of life measures ( 1 ). Adolescents commonly resist using medical devices, such as injectible epinephrine devices, and bullying may be a contributing factor for this ( 3 ). Attempting to conceal symptoms in a bullying situation may place children at risk for a worse outcome.
Physicians can play a key role in detecting allergy bullying and its health consequences. In many cases, children have not discussed this issue with their parents ( 1 ). Given the prevalence of bullying, its potential to lead to severe harm, including death, and the lack of awareness of this issue, clinicians should specifically ask about bullying in all children and teens with allergies. Physicians can also work with families and schools to support these children, educate their peers and school staff, and help prevent negative health outcomes from allergy bullying.
Online resources
www.anaphylaxis.ca − A national charity that aims to inform, support, educate and advocate for the needs of individuals and families living with anaphylaxis, and to support and participate in research. This website includes education modules for schools and links to local support groups throughout Canada.
www.whyriskit.ca/pages/en/live/bullying.php − A website for teenagers with food allergies; includes a segment that addresses food bullying.
www.foodallergy.org − Contains numerous resources for children and their families, including a significant discussion on bullying and ways to prevent it.
Allergy bullying is common but is often unrecognized as a factor in clinical presentations of allergic reactions.
Physicians should make a point of asking about bullying in patients with allergies and become familiar with resources for dealing with allergy bullying.
Physicians can play roles as advocates, educators and collaborators with the school system to help make the school environment safer for children with allergies who may be at risk for allergy bullying.
Google Scholar
Email alerts
Citing articles via, looking for your next opportunity.
- About Paediatrics & Child Health
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1918-1485
- Print ISSN 1205-7088
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- News and Events
- < Back To All News
Protecting Navajo children with asthma: A case study

Joncita Todechine, a mother of four who lives on the Navajo Nation, knows all too well what can trigger asthma symptoms in her daughter Ashley. But she didn’t always. She recalls a time in 2013, living in Phoenix and attending medical assistant school, when she rushed her then-three-year-old to the Indian Medical Center.
“She was really sick,” Todechine said. “She was fevering, coughing, and had shortness of breath. We had no idea what was wrong.”
Ashley was admitted to the hospital and stayed for an entire week before the doctors could make a diagnosis of asthma. Now a thriving 13-year-old, Ashley loves gaming, social media, and riding on her hoverboard. These days she lives on the Navajo reservation with her family, who moved there shortly after her mother finished school. For the most part, she keeps her asthma under control by taking medication and doing her best to avoid her asthma triggers.
But that can be challenging.
On the Navajo Nation, there are many asthma triggers. The semi-arid environment is plagued by drought, so on windy days, the gusts kick up ever-present dust and sand into the air. Shuttered coal-fired powerplants dot the landscape and, though they are closed, residual soot still dirties the air. Uranium and other heavy metals contaminate the landscape, and people breathe diesel fumes from the buses that take children to and from school every day. The many dogs and livestock roaming the reservation carry other allergens.
“And that’s just the outdoor pollution,” said Bruce Bender, Ph.D., professor in the pediatrics department at National Jewish Health in Denver, Colorado. “Seventy percent of households heat with indoor stoves that burn wood or charcoal and can leak a lot of smoke into the air.”
Bender would know. He’s co-project leader of an NHLBI-funded project focused on reducing health disparities in children living on the Navajo Nation, and he’s studied some of the factors that make those disparities worse. He’s also looked at the health data overall and found that while Native adults suffer from higher rates of chronic conditions like cardiovascular diseases and diabetes, it’s asthma that remains one of the most common chronic diseases in children. Some 18% of children on the Navajo reservation have it, compared to 10.2% of children nationwide.
“Asthma can be incredibly scary for children and their families, especially those who cannot get emergency care easily or quickly,” said Michelle Freemer, M.D., M.P.H., director of the asthma program in NHLBI’s Division of Lung Diseases.
The Navajo Nation extends across more than 27,000 square miles, making it the largest Native land area in the U.S. "For families of children with asthma, the distances and travel conditions on the reservation may add challenges,” said Freemer. “The investigators partnered with the community to find solutions that work where they live, not simply provide asthma care that has been shown to work in other places."
A local solution
Bender and his colleague, Lynn B. Gerald, Ph.D., M.S.P.H., assistant vice chancellor for population health at the University of Illinois-Chicago, started a large-scale effort to teach educators, children, their families, and local medical providers on the Navajo Nation how to identify an asthma attack and what to do in an emergency. (Gerald had worked previously at the University of Arizona and had gained a wealth of knowledge from the university's Native collaborators.) The program rolled out in three Arizona communities on the reservation: Tuba City, Chinle, and Fort Defiance. Combined, these towns represent 43% of the Navajo Nation population and are home to more than 8,000 children with asthma.
But before they began, the investigators knew they needed to build relationships with the Navajo people – who refer to themselves as Diné – as the community’s prior experiences with non-Native researchers had left them skeptical. The research team began by ensuring the program was tailored to the needs and wishes of the community itself.
“The Navajo Nation human research review board is very careful and thorough,” Bender said. “They’re protecting their population. We had to earn their trust.”
Once the investigators got approval, they hit the ground running, starting in Tuba City. In the hospitals, the research team provided tools for medical professionals, using self-directed online learning and in-person workshops, to increase their use of practices that have been shown to be important in asthma care.
In the schools, the investigators provided education using the American Lung Association’s Asthma Basics and Open Airways for Schools® training, to teach school staff about asthma, its triggers, and what to do when a child is having an attack and to teach Diné children how to manage their asthma.
Using a “train-the-trainer” model, school staff, community health workers, respiratory therapists, and pharmacists became students and then instructors. This made it possible for the Diné participants to teach additional staff, ensuring the community can sustain the program after the research funding ends.
Still, there was another urgent need that Bender and Gerald realized had not been addressed. “Less than 15% of children with asthma actually have an inhaler at school when they need it,” Gerald said. In response, the team helped start a program in two of the three communities that provided stock inhalers to schools for children who need them.
A global threat
After starting the program in Tuba City as planned and spending a year there, the research team moved their focus to Chinle. The goal was to be able to compare how well the program worked in each of the three communities. But a global pandemic had other plans.
“The COVID-19 pandemic hit right in the middle of our time in Chinle,” Bender said. “After that, we weren’t allowed on the reservation for two years.”
While the pandemic changed life for all Americans, it devastated many Native communities. Schools closed and medical clinics focused on emergencies. The research team pivoted: they continued some training virtually and were able to keep learning from families about their needs, especially using the Diné members of the research team who were on the reservation.
Taking stock
Today, despite the challenges of the pandemic, all three communities have completed the original program, and 439 Diné members have been trained to identify asthma and its triggers. Yet the work is far from over. The investigators are analyzing the data they collected. “Particularly important is returning the results to the community,” Gerald said. As soon as they are ready, she said, they will be meeting with the school boards and health boards and joining community meetings to share them.
Freemer said that all the materials the researchers developed through their NHLBI funding are available to the community and have also been shared with those at the Indian Health Service leading the Asthma Control in Tribal Communities program.
“The researchers also took the opportunity to build research capacity,” she said. They developed an agreement with Diné College, the only four-year college on the reservation, to provide training through their Summer Research Experience Program. “In that program, students learned about research and were able to readily reach the families who appreciated the interactions with Diné research team members.”
Todechine said knowing that her child will be cared for if the worst happens has given her peace of mind. “Now the school systems have their own asthma alert systems that the employees and even the bus drivers take part in,” she said. “For me, I feel safer for her to be at school without me.”
Resources:
Learn more about Asthma in Our Communities with specific resources for American Indians.
Related Health Topics
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Patient Presentation
A 12 year old girl, Annie, enters the Pediatrician’s office with complaints of dyspnea, wheezing, and chest tightness.
The history of the illness from the family includes:
A recent upper respiratory infection with cough, congestion and runny nose with a low grade fever of 100.8 degrees Fahrenheit.
Mother also states this has happened the last few times Annie had cold symptoms and does seem to get relief from Albuterol that was prescribed previously. Annie is behind on her well checks and usually only comes in for sick visits.
Family/Social History
Family history revealed that mother has asthma and because of this uses a lot of hand sanitizer for herself and family to keep them from “catching colds”.
In the home lives Annie, her mother, father and little brother age 6. Annie’s father is a smoker but mother states he smokes outside.
The family is living in a home currently under renovations.
Upon assessment Annie has the following:

(Parakh, 2019)
- intermittent coughing
- expiratory wheezing
- subcostal retractions
- temperature of 100.8 PO
- heart rate of 100 bpm
- respiratory rate of 40
- pulse oximetry 92 %
- purulent mucous from bilateral nares
- catching her breath often while speaking
- Introduction
- Conclusions
- Article Information
The figures shows cohort-specific boxplots, where the box is used to represent the IQR, or the data between the first and third quartile, the line within the box represents the median, the whiskers extend from each quartile to the minimum and maximum values, and the points beyond the whiskers represents the outliers. Bal indicates Baltimore; Bos, Boston; CAS, Children’s Asthma Study; CCAAPS, Cincinnati Childhood Allergy and Air Pollution Study; CCCEH, Columbia Center for Children’s Environmental Health Cohort; COAST, Childhood Origins of Asthma Study; COI, Child Opportunity Index; EHAAS, Epidemiology of Home Allergens and Asthma Study; IIS, Infant Immune Study; NO 2 , nitrogen dioxide air pollution; NY, New York; PM 2.5 , fine particulate matter air pollution; StL, St Louis; SVI, Social Vulnerability Index; URECA, Urban Environment and Childhood Asthma Study; WHEALS, Wayne County Health Environment Allergy and Asthma Longitudinal Study.
eMethods 1. Description of the Participating CREW Cohorts
eMethods 2. Supplementary Material and Methods
eTable 1. Overview of Participating CREW Cohorts
eTable 2. Child’s Demographic Characteristics and Caregiver Demographic Characteristics Among CREW Participants When Asthma Incidence Is Missing
eTable 3. Child’s Demographic Characteristics, Caregiver Demographic Characteristics and Distribution of Respiratory Health Outcomes Among CREW Participants When PM 2.5 Averaged Over Years 1-3 Is Missing
eTable 4. Child’s Demographic Characteristics, Caregiver Demographic Characteristics and Distribution of Respiratory Health Outcomes Among CREW Participants When NO 2 Averaged Over Years 1-3 Is Missing
eFigure 1. Social Vulnerability Index (SVI) and Its Domains
eFigure 2. Child Opportunity Index (COI) and Its Domains
eFigure 3. Strobe Diagram of Analytical Cohort
eFigure 4. Correlations Among PM 2.5 , NO 2 , U.S. Census Variables and COI and SVI
eFigure 5. Odds Ratios (OR) of Asthma by Age 4 and 11 (A) and Hazard Ratios (HR) of Asthma Incidence (B) for PM 2.5 and NO 2 for the First Year of Life and Up to 4 Years and for the Averages of Years 1 and 2 and 1 Through 4
eFigure 6. Effect Modification of PM 2.5 by Neighborhood Characteristics
eFigure 7. Effect Modification of NO 2.5 by Neighborhood Characteristics
eFigure 8. Sensitivity Analysis: Odds Ratio of Asthma by Age 4 With Persistent Wheeze for One IQR Increase in Each Exposure Average
eFigure 9. Odds Ratios (OR) of Asthma by Age 4 and Ages 5-11 for an IQR Increase in PM 2.5 and NO 2 for the First Year of Life and for the Averages of Year 1 and 2 and 1 Through 4 Using a Multinomial Regression
eReferences
Nonauthor Collaborators
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zanobetti A , Ryan PH , Coull BA, et al. Early-Life Exposure to Air Pollution and Childhood Asthma Cumulative Incidence in the ECHO CREW Consortium. JAMA Netw Open. 2024;7(2):e240535. doi:10.1001/jamanetworkopen.2024.0535
Manage citations:
© 2024
- Permissions
Early-Life Exposure to Air Pollution and Childhood Asthma Cumulative Incidence in the ECHO CREW Consortium
- 1 Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts
- 2 Department of Pediatrics, University of Cincinnati, College of Medicine, Cincinnati, Ohio
- 3 Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio
- 4 Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts
- 5 Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts
- 6 Center for Geographic Analysis, Harvard University, Cambridge, Massachusetts
- 7 Asthma and Airways Disease Research Center, University of Arizona, Tucson
- 8 Department of Community, Environment, and Policy, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson
- 9 Division of Clinical Immunology, Icahn School of Medicine at Mount Sinai, New York, New York
- 10 Rho Inc, Federal Research Operations, Durham, North Carolina
- 11 Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, New York
- 12 Monroe Carell Jr Children’s Hospital at Vanderbilt, Division of Pediatric Allergy, Immunology, and Pulmonary Medicine, Nashville, Tennessee
- 13 Vanderbilt University School of Medicine, Division of Allergy, Pulmonary, and Critical Care Medicine, Nashville, Tennessee
- 14 Department of Public Health Sciences, Henry Ford Health, Detroit, Michigan
- 15 Division of Allergy and Immunology, Augusta University, Augusta, Georgia
- 16 Cincinnati Children’s Hospital, Division of Asthma Research, Cincinnati, Ohio
- 17 Department of Pediatrics, Indiana University, Indianapolis
- 18 Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison
- 19 Department of Medicine, Henry Ford Health, Detroit, Michigan
- 20 Division of Pulmonary and Sleep Medicine, Department of Pediatrics, College of Medicine, University of Arizona, Tucson
Question Is there an association between early life exposure to air pollution and the risk of asthma by early and middle childhood, and is this association modified by individual and community-level characteristics?
Findings In this cohort study of 5279 children, mean fine particulate matter (PM 2.5 ) and mean nitrogen dioxide (NO 2 ) air pollution during the first 3 years of life were associated both with asthma incidence by early and by middle childhood, after adjusting for individual-level characteristics. The association of ambient pollution (PM 2.5 or NO 2 ) with incident asthma was modified by community-level and individual-level socioeconomic circumstances, including maternal education and race.
Meaning These findings suggest that exposure to PM 2.5 or NO 2 air pollution during early childhood may play a role in the development of childhood asthma, with higher risk among minoritized families living in densely populated communities characterized by fewer opportunities and resources and multiple environmental coexposures.
Importance Exposure to outdoor air pollution contributes to childhood asthma development, but many studies lack the geographic, racial and ethnic, and socioeconomic diversity to evaluate susceptibility by individual-level and community-level contextual factors.
Objective To examine early life exposure to fine particulate matter (PM 2.5 ) and nitrogen oxide (NO 2 ) air pollution and asthma risk by early and middle childhood, and whether individual and community-level characteristics modify associations between air pollution exposure and asthma.
Design, Setting, and Participants This cohort study included children enrolled in cohorts participating in the Children’s Respiratory and Environmental Workgroup consortium. The birth cohorts were located throughout the US, recruited between 1987 and 2007, and followed up through age 11 years. The survival analysis was adjusted for mother’s education, parental asthma, smoking during pregnancy, child’s race and ethnicity, sex, neighborhood characteristics, and cohort. Statistical analysis was performed from February 2022 to December 2023.
Exposure Early-life exposures to PM 2.5 and NO 2 according to participants’ birth address.
Main Outcomes and Measures Caregiver report of physician-diagnosed asthma through early (age 4 years) and middle (age 11 years) childhood.
Results Among 5279 children included, 1659 (31.4%) were Black, 835 (15.8%) were Hispanic, 2555 (48.4%) where White, and 229 (4.3%) were other race or ethnicity; 2721 (51.5%) were male and 2596 (49.2%) were female; 1305 children (24.7%) had asthma by 11 years of age and 954 (18.1%) had asthma by 4 years of age. Mean values of pollutants over the first 3 years of life were associated with asthma incidence. A 1 IQR increase in NO 2 (6.1 μg/m 3 ) was associated with increased asthma incidence among children younger than 5 years (HR, 1.25 [95% CI, 1.03-1.52]) and children younger than 11 years (HR, 1.22 [95% CI, 1.04-1.44]). A 1 IQR increase in PM 2.5 (3.4 μg/m 3 ) was associated with increased asthma incidence among children younger than 5 years (HR, 1.31 [95% CI, 1.04-1.66]) and children younger than 11 years (OR, 1.23 [95% CI, 1.01-1.50]). Associations of PM 2.5 or NO 2 with asthma were increased when mothers had less than a high school diploma, among Black children, in communities with fewer child opportunities, and in census tracts with higher percentage Black population and population density; for example, there was a significantly higher association between PM 2.5 and asthma incidence by younger than 5 years of age in Black children (HR, 1.60 [95% CI, 1.15-2.22]) compared with White children (HR, 1.17 [95% CI, 0.90-1.52]).
Conclusions and Relevance In this cohort study, early life air pollution was associated with increased asthma incidence by early and middle childhood, with higher risk among minoritized families living in urban communities characterized by fewer opportunities and resources and multiple environmental coexposures. Reducing asthma risk in the US requires air pollution regulation and reduction combined with greater environmental, educational, and health equity at the community level.
Air pollution is a near ubiquitous exposure and the largest environmental contributor to disease and premature death in the world, including for children. 1 , 2 Exposure to air pollution has been consistently associated with respiratory morbidity, including wheeze and exacerbation of asthma in children. Several reviews 3 - 6 on air pollution, asthma, and respiratory symptoms in children concluded that outdoor traffic pollution contributes to the development of childhood asthma.
However, community-level and individual-level contextual factors that increase not only exposure, but also susceptibility to air pollution–related childhood asthma effects, including the developmental stage and age of onset of asthma, remain poorly understood. Most prior cohort studies with individual-level information lacked the geographic, racial and ethnic, and socioeconomic diversity to explore the modifying role of community-level contextual factors and the association between air pollution exposure and asthma development.
The Environmental Influences on Child Health Outcomes (ECHO) Children’s Respiratory and Environmental Workgroup (CREW) 7 consortium, a US nationwide birth cohorts network, is well-positioned to address these questions given the multiple decades of recruitment and follow-up of birth cohorts, the geographic and demographic diversity of study participants, 8 the wide distribution of state-of-the-art spatio-temporally-resolved fine particulate matter (PM 2.5 ) and nitrogen dioxide (NO 2 ) estimates, and the diverse individual and area-level socioeconomic and built environment factors that vary across and within cohorts.
Previously, we found in the CREW consortium that Black and Hispanic children and children who resided in census tracts with higher rates of household poverty and population density were at increased risk for developing childhood asthma. 9 Building upon these findings, we hypothesized that (1) early life exposure to PM 2.5 and NO 2 is associated with increased asthma risk by early and middle childhood, and (2) not only individual-level, but also community-level factors (eg, living in areas with higher level of poverty, and less opportunity) are associated with increased child susceptibility to pollution effects on asthma risk.
Our study population included 8 of the 12 longitudinal birth cohorts participating in the Children’s Respiratory and Environmental Workgroup (CREW), representing a diverse sample of children and their families residing throughout the US in urban, suburban, and rural environments (eMethods 1 and eTable 1 in Supplement 1 ). We excluded cohorts with young children without harmonized asthma outcomes, and we excluded our oldest cohort for which early-life air pollution estimates were not available.
Eligibility criteria, study recruitment, and other methods, have been previously described. 7 Birth years of cohort participants spanned from 1987 to 2007, and participants were followed up to age 11. All cohorts had institutional review board approvals from each participating cohort’s institution and all participants provided written informed consent. This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
We defined asthma as caregiver report of physician-diagnosed asthma. We ascertained the outcomes through survival analysis as asthma incidence by less than 5 years of age (early childhood) and by less than 12 years of age (middle childhood); and, secondarily, through logistic regression as ever asthma through age 4 years and through age 11 years. As a sensitivity analysis, we also considered ever asthma before age 4 years with any wheeze reported after age 3 years, to ascertain whether associations were consistent for those children diagnosed before age 4 years who had an indication of persistent (rather than transient) asthma.
Cohorts provided individual characteristics, including child’s sex, caregiver-reported child race and ethnicity (Hispanic, non-Hispanic Black [Black], non-Hispanic White [White], and other [for any race or ethnicity not in the preceding categories]), mother’s education (no high school diploma, high school diploma, and college and graduate school), maternal-reported smoking during pregnancy, and parental history of asthma defined as history in the mother, father, or both vs no parental history. We acknowledge that race is a social construct and correlates with poverty, adverse physical environments, unequal access to health care, and a multitude of structural, systemic, and institutional determinants. The CREW Biostatistics/Bioinformatics Core collected and harmonized data from each cohort.
We obtained daily estimates of PM 2.5 and NO 2 from 2000 to 2016 at a 1 km 2 resolution from previously validated prediction models 10 - 12 and monthly PM 2.5 predictions at the 6 km 2 grid for the years 1988-2007 from a previously published model 12 to estimate PM 2.5 and NO 2 exposures (eMethods 2 in Supplement 1 ).
The 1 km 2 estimates were linked to the home addresses for each CREW participant using our Decentralized Geomarker Assessment for Multi-site Studies (DeGAUSS) software 13 , 14 approach (eMethods 2 in Supplement 1 ). For the cohorts with person-time before 2000 when the 1 km 2 estimates were not available, we created cohort-specific calibration factors based on monthly mean values, using all available overlap data to obtain calibrated annual, monthly, and prenatal 6 km 2 exposure estimates for periods before 2000 (eMethods 2 in Supplement 1 ).
Annual mean values for each year of life beginning at the birth date through age 1 year and up to age 5 years were calculated. In addition, we calculated mean values from birth through age 1 year, 1 to 2 years, 1 to 3 years, and 1 to 4 years. As NO 2 estimates were only available from 2000 and later, we were not able to assign early life NO 2 exposures to 2 cohorts (Children’s Asthma Study, Epidemiology of Home Allergens and Asthma Study), whose children enrolled at birth in the 1990s.
We obtained US census data for percentage population with low income, percentage Black population, population density, median household income, and percentage low-income families. We also obtained the Child Opportunity Index (COI), 15 , 16 a measure of the quality of neighborhoods in which children live, and the social vulnerability index (SVI), 17 a measure used to identify high-risk populations that are especially at risk during public health emergencies. COI is a score based on neighborhood-level indicators, grouped into 3 domains, with higher scores reflecting more favorable neighborhood opportunities. SVI is computed from census variables as percentile ranging from 0 (lowest risk) to 1 (highest risk), grouped into 4 domains (see eMethods 2, eFigure, 1, and eFigure 2 in Supplement 1 ).
We analyzed the association between exposures and asthma incidence with a Cox proportional hazard model, adjusting for potential confounders including mother’s education, child’s race and ethnicity, sex, smoking during pregnancy, parental history of asthma and an indicator variable for cohort. In secondary analysis, we examined the association between exposure and ever asthma using logistic regression models, adjusting for the same confounders as in the survival analysis.
We examined effect modification by child’s race and ethnicity and sex, mother’s education, and neighborhood socioeconomic factors, including an interaction term between each pollutant and each modifier in separate models. We then computed the association of pollution in each category of the modifier. For continuous modifiers (neighborhood socioeconomic factors), we computed the association of pollution at the 10th (Low) and 90th (High) percentiles of the modifier. We reported the results as hazard ratios (HRs) for the survival analysis and odds ratios (ORs) for the logistic regression with 95% CI for a 1 IQR increase in each pollutant. P < .05 was considered as the significance level. Statistical analysis was performed using R version 4.4.1 (R Project for Statistical Computing) and SAS version 9.4 (SAS Institute) from February 2022 to December 2023.
We repeated the analysis using asthma incidence by less than 5 years of age with any wheeze reported after age 3 years. To account for potential residual spatial correlation, we repeated the analysis using mixed-effect models adding random intercepts for census tract, in addition to the indicator variable for cohort. We then applied a multinomial regression, where the outcome was a categorical variable defined as asthma by age 4 years and first asthma diagnosis between ages 5 and 11 years.
Among a total of 5279 children included, 1659 (31.4%) were Black, 835 (15.8%) were Hispanic, 2555 (48.4%) where White, and 229 (4.3%) were other race or ethnicity; 2721 (51.5%) were male and 2596 (49.2%) were female; 1305 children (24.7%) had asthma by 11 years of age and 954 (18.1%) had asthma by 4 years of age; 3315 mothers (62.8%) had some college or higher education, 565 mothers (10.7%) smoked during pregnancy, and 1893 children (35.9%) had parents with history of asthma. Table 1 presents these characteristics for each CREW cohort. eFigure 3 in Supplement 1 shows the flowchart of the analytic data set. eTables 2, 3, 4, and 5 in Supplement 1 present the characteristics when asthma, PM 2.5 , and NO 2 are missing. The distribution of the outcomes and the individual characteristics varied widely across cohorts, demonstrating racial and socioeconomic diversity among participants. The Figure shows the distributions of PM 2.5 and NO 2 as well as the neighborhood-level variables; all of the variables present substantial variability across and within cohorts. eFigure 4 in Supplement 1 shows the correlation among the neighborhood-level variables and the pollutants. PM 2.5 was not correlated with any variables, whereas NO 2 correlated with population density ( r = 0.63). The census variables were positively correlated with SVI (eg, correlation between total SVI and percentage of Black population: r = 0.63) and negatively correlated with COI (eg, correlation between total COI and percentage of Black population: r = −0.64).
Table 2 and eFigure 5 in Supplement 1 present the results of the association between early life air pollution exposure and asthma incidence by younger than 5 and younger than 12 years of age using survival analysis and logistic regression. We found stronger associations with both outcomes and pollutants averaged over the first 2 and 3 years of life compared with other pollution averages (eFigure 5 in Supplement 1 ). Specifically, we found that 1 IQR increase in mean NO 2 (6.1 μg/m 3 ) over the first 3 years of life was associated with increased asthma incidence through the first 4 years of life (HR, 1.25 [95% CI, 1.03-1.52]) and through the first 11 years of life (HR, 1.22 [95% CI, 1.04-1.44]). For the same 3-year average, a 1 IQR increase in mean PM 2.5 (3.4 μg/m 3 ) was associated with increased asthma incidence among children younger than 5 years (HR, 1.31 [95% CI, 1.04-1.66]) and children younger than 12 years (HR, 1.23 [95% CI, 1.01-1.507]). NO 2 and PM 2.5 were also significantly associated with increased odds of asthma through age 4 years and through age 11 years.
Table 3 presents results of air pollution modified by individual characteristics and by a selection of neighborhood-level variables separately for asthma incidence through the first 4 and 11 years of life. We found that the associations were stronger when mothers had less than a high school diploma and among Black children; for example, there was a significantly higher association between PM 2.5 and asthma incidence by less than 5 years of age in Black children (HR, 1.60 [95% CI, 1.15-2.22]) compared with White children (HR, 1.17 [95% CI, 0.90-1.52]) ( Table 3 ).
When we examined effect modification of air pollution averaged over the first 3 years of life by neighborhood factors ( Table 3 ; eFigures 6 and 7 in Supplement 1 ), we found that for an IQR increase in PM 2.5 , children who resided in areas with lower education and health and environment opportunity had higher asthma incidence in the first 4 and 11 years of life. For an IQR increase in NO 2 , children who resided in areas with higher proportion of Black population and more urban areas had higher asthma incidence through the first 4 and 11 years of life. ( Table 3 )
In sensitivity analyses, the results were similar when we restricted analyses to those with documented wheeze symptoms during middle childhood following an asthma diagnosis by age 4 years (eFigure 8 in Supplement 1 ). In mixed-effect models with a random intercept for census tract in addition to cohort, the results did not change (eFigure 9 in Supplement 1 ). The multinomial regression results (eFigure 10 in Supplement 1 ) showed similar associations between PM 2.5 and NO 2 and asthma by age 4 years. The associations were weaker with asthma between ages 5 and 11, mostly for PM 2.5 .
In this multicohort study, we found that exposure to PM 2.5 and NO 2 during the first 3 years of life were associated with increased asthma incidence by early (<5 years) and middle (<12 years) childhood. Individual-level characteristics, including Black race and lower maternal educational attainment, and community-level factors, including lower health and environment child opportunity indices, population density, and neighborhoods with higher proportion of Black population, were associated with increased magnitude of the association between air pollution exposure and risk of childhood asthma.
Early childhood is a period of heightened concern, as higher exposures may lead to altered trajectories of airway and immune system development, with decreased lung function and asthma pathogenesis. 18 , 19 The Tucson Children’s Respiratory Study found, with suggestion of a larger response in Black children, that higher childhood NO 2 was associated with lower CC16, a biomarker in which its decrease has been associated with oxidative stress and reduced lung function. PM 2.5 and NO 2 may influence asthma development not only through oxidative stress leading to inflammation (eg, IL-6), but also through altered immune development, increased IgE-mediated allergic sensitization, and Th17-associated responses. 20 , 21
By examining multiple periods of exposure and health outcomes, 5 this analysis adds to the growing epidemiologic evidence that early-life air pollution exposures are associated with the onset of childhood asthma. In the Southern California Children’s Health Study (CHS) 22 traffic-related pollution exposures during childhood, including NO 2 at school 23 and homes, 22 were associated with increased asthma incidence. A follow-up study using 3 waves of the CHS Study 24 found that decreases in NO 2 and PM 2.5 were significantly associated with lower asthma incidence. In Boston, 25 first year of life and lifetime exposure to PM 2.5 were associated with increased risk of pediatric asthma during early childhood (3-5 years of age). A previous analysis in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), 26 found that exposure during the first 2 years of life, but not exposure during later childhood, was associated with asthma development by age 7 years. Another birth cohort study 27 found increased risk of having an asthma diagnosis at age 13 years with NO 2 postnatal exposure in the first year of life. Our study is consistent with these prior studies and suggests that the first 1 to 3 years of life are the most susceptible period for air pollution exposure to promote asthma development.
Our previous analysis 9 of asthma incidence in the CREW consortium found that adverse neighborhood characteristics and Black race were associated with increased childhood asthma incidence. We interpreted race to signify measured and unmeasured exposures resulting from racism. Here, we leveraged the geographic, racial, and socioeconomic diversity of participant families and their communities to examine factors modifying associations between air pollution and asthma. We found that associations of air pollution exposure with asthma were elevated among Black children and children born to mothers without a high school diploma. These results are consistent with prior studies showing that socioeconomic position (SEP) and race are key drivers of elevated environmental exposures, including air pollution, and race and SEP, each of which can be independently or synergistically associated with elevated physiologic stress leading to inflammation that increases susceptibility to disease, including asthma. 28 , 29 In addition, Black children are more likely to be exposed to adverse childhood experiences, poor housing quality and indoor environments, and have less access to healthy food and greenspace. 30 These factors, likely in combination, may inequitably affect Black children such that the negative health effects due to exposure to air pollution are heightened.
Similar to individual-level race and SEP, we also found that neighborhood measures, including the percentage of Black population and lower health and environment COI, also were significant modifiers. The COI domain of health and environment represents the quality of neighborhoods in terms of housing, access to food, built environment, and exposure to pollution and heat. 15 Previous studies have shown disparities in asthma prevalence and morbidity across communities related to violence, poor housing, elevated environmental exposures, lack of access to health care. 30 The COI has been previously associated with population-level asthma morbidity in the US. 31 A Swedish study using administrative data 32 found that PM 2.5 during the first 3 years of life increased asthma risk and that pollution-asthma associations were stronger in areas with lower education.
Our study has several strengths. To our knowledge, this is the first multicohort study of air pollution and asthma in the US that focused on the independent and interacting environmental and social influences on the age and child life-stage of asthma onset, demonstrating heightening of early-life pollution effects by adverse community-level exposures. We report findings that have a consistent pattern and are robust to different methods of statistically testing associations. 33
The added value of this study includes cohorts that were specifically focused on measuring outcomes related to childhood asthma, with multiple decades of recruitment, varying in terms of the population selection criteria (general and high risk), and with socioeconomic, racial and ethnic, geographic and temporal diversity, and with a wide distribution of state-of-the-art modeling for spatio-temporally-resolved estimation of pollutants.
In Europe, a multicity study 34 did not find significant association between air pollution exposure and childhood asthma. These cohorts were recruited in the late 1990s, and air pollution exposure was determined with land use regression models. In CREW, collecting and harmonizing data from each cohort enabled us to examine early and middle childhood onset of asthma as well as examine whether the associations differed by both individual-level and area-level factors in urban US settings. Given the longitudinal nature of the study, we were able to examine incident cases instead of a prevalence. The use of the DeGAUSS approach, 13 , 14 a software containerization platform, enabled individual sites to geocode their birth addresses and then assign tract-level and exposure variables to those geocodes, so that all individual study sites had identically constructed data sets. Examining modification of air pollution on asthma by COI and SVI (indexes used in previous studies 35 , 36 on body mass index and cardiometabolic risk) enabled us to characterize how composite, as well as individual metrics of community resources and risk were associated with worse pollution effects on childhood asthma.
This study had limitations. While the census variables are available every 10 years and have been merged to the nearest year of birth, SVI was available for the years 2000 and 2010 and COI only for 2010; therefore, some misclassification is possible. In these analyses, we examined NO 2 and PM 2.5 , although other pollutants common in urban environments like ozone and other traffic-related air pollution may also contribute to asthma and interact with NO 2 and PM 2.5 . Similarly, we do not have information about the contribution of pollution from indoor environments. While our analysis focused on early life exposures, future studies could test whether exposures in later life add cumulatively to lifetime risk of asthma. 25
In this cohort study of children from a highly diverse US population, PM 2.5 and NO 2 averaged over the first 3 years of life were associated with increased asthma incidence by early and middle childhood. The associations of PM 2.5 and NO 2 were greater among families living in urban US communities with fewer opportunities and resources with multiple environmental coexposures. Air pollution continues to be a global burden with serious consequences on childhood health. Reducing asthma risk in the US requires regulation and reduction of air pollution combined with creation of greater environmental, educational, and health equity at a community level.
Accepted for Publication: December 31, 2023.
Published: February 28, 2024. doi:10.1001/jamanetworkopen.2024.0535
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Zanobetti A et al. JAMA Network Open .
Corresponding Author: Antonella Zanobetti, PhD, Department of Environmental Health, Harvard T.H. Chan School of Public Health, 401 Park Dr, Boston, MA 02215 ( [email protected] ).
Author Contributions: Dr Zanobetti had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zanobetti, Ryan, Coull, Lothrop, Miller, Ownby, Joseph, Martinez, Gold.
Acquisition, analysis, or interpretation of data: Zanobetti, Ryan, Coull, Luttmann-Gibson, Datta, Blossom, Brokamp, Lothrop, Miller, Beamer, Visness, Andrews, Bacharier, Hartert, Cole Johnson, Ownby, Khurana Hershey, Mendonça, Jackson, Zoratti, Wright, Seroogy, Ramratnam, Calatroni, Gern, Gold.
Drafting of the manuscript: Zanobetti, Ryan, Lothrop, Martinez, Calatroni, Gold.
Critical review of the manuscript for important intellectual content: Zanobetti, Ryan, Coull, Luttmann-Gibson, Datta, Blossom, Brokamp, Miller, Beamer, Visness, Andrews, Bacharier, Hartert, Cole Johnson, Ownby, Khurana Hershey, Joseph, Mendonça, Jackson, Zoratti, Wright, Seroogy, Ramratnam, Calatroni, Gern.
Statistical analysis: Zanobetti, Coull, Datta, Lothrop, Mendonça, Calatroni.
Obtained funding: Coull, Miller, Hartert, Cole Johnson, Ownby, Jackson, Zoratti, Seroogy, Gern, Gold.
Administrative, technical, or material support: Ryan, Luttmann-Gibson, Datta, Blossom, Brokamp, Lothrop, Beamer, Visness, Andrews, Cole Johnson, Ownby, Khurana Hershey, Mendonça, Zoratti.
Supervision: Miller, Visness, Cole Johnson, Zoratti, Martinez, Seroogy.
Conflict of Interest Disclosures: Dr Beamer reported grants from the Environmental Protection Agency, the Department of Education, and the National Institutes of Health (NIH) outside the submitted work. Dr Bacharier reported personal fees from AstraZeneca, Sanofi/Regeneron, GlaxoSmithKline, Novartis/Genentech, DBV Technologies, Vertex, Aravax, Recludix, Teva, OM Pharma, and Kinaset outside the submitted work; and royalties from Elsevier. Dr Hartert reported personal fees from the American Thoracic Society (co-chair of Vaccine and Immunization Initiative), Parker B Francis Council of Scientific Advisors, NIH/National Heart, Lung, and Blood Institute (council member), Sanofi (Infectious Diseases Forum), and Pfizer (Data Safety Monitoring Board member) outside the submitted work. Dr Jackson reported grants from GlaxoSmithKline and Regeneron; and personal fees from AstraZeneca, Avillion, Genentech, Sanofi-Regeneron, GlaxoSmithKline, and Areteia outside the submitted work. Dr Zoratti reported grants from the National Institute of Allergy and Infectious Diseases (NIAID) outside the submitted work. Dr Martinez reported personal fees from OM Pharma outside the submitted work. Dr Ramratnam reported personal fees from Sanofi outside the submitted work. Dr Gern reported personal fees and stock options from Meissa Vaccines Inc and personal fees from AstraZeneca outside the submitted work. No other disclosures were reported.
Funding/Support: The Harvard NIEHS Center Grant P30-ES000002 is a source of support for Drs Zanobetti, Coull, and Gold. Apple Inc is a source of support for Dr Coull. This study was funded by NIH grants UH3OD023282 (Drs Miller, Hartert, Johnson, Ryan, Mendonça, Seroogy, Gern, Gold); UM2 AI117870, HHSN272201000052I (Dr Visness); 5P01AI089473-07 (Dr Ownby); UM1 AI114271, UM1 AI160040 (Dr Jackson); U19 AI104317 (Dr Seroogy). The Children’s Respiratory and Environmental Workgroup (CREW) is funded by the Environmental Influences on Child Health Outcomes (ECHO) program.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The members of the ECHO Children’s Respiratory and Environmental Workgroup are listed in Supplement 2 .
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data Sharing Statement: See Supplement 3 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Topic collections
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 6, Issue 1
- Diagnosis and management of asthma in children
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Joanne Martin 1 , 2 , 3 ,
- Jennifer Townshend 4 ,
- http://orcid.org/0000-0003-4591-8299 Malcolm Brodlie 1 , 4
- 1 Translational and Clinical Research Institute , Newcastle University , Newcastle upon Tyne , UK
- 2 Northern Foundation School , Health Education England North East , Newcastle upon Tyne , UK
- 3 James Cook University Hospital , South Tees NHS Foundation Trust , Middlesbrough , UK
- 4 Paediatric Respiratory Medicine , Great North Children's Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust , Newcastle upon Tyne , UK
- Correspondence to Dr Malcolm Brodlie; malcolm.brodlie{at}newcastle.ac.uk
Asthma is the the most common chronic respiratory condition of childhood worldwide, with around 14% of children and young people affected. Despite the high prevalence, paediatric asthma outcomes are inadequate, and there are several avoidable deaths each year. Characteristic asthma features include wheeze, shortness of breath and cough, which are typically triggered by a number of possible stimuli. There are several diagnostic challenges, and as a result, both overdiagnosis and underdiagnosis of paediatric asthma remain problematic.
Effective asthma management involves a holistic approach addressing both pharmacological and non-pharmacological management, as well as education and self-management aspects. Working in partnership with children and families is key in promoting good outcomes. Education on how to take treatment effectively, trigger avoidance, modifiable risk factors and actions to take during acute attacks via personalised asthma action plans is essential.
This review aimed to provide an overview of good clinical practice in the diagnosis and management of paediatric asthma. We discuss the current diagnostic challenges and predictors of life-threatening attacks. Additionally, we outline the similarities and differences in global paediatric asthma guidelines and highlight potential future developments in care. It is hoped that this review will be useful for healthcare providers working in a range of child health settings.
- therapeutics
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjpo-2021-001277
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Key messages
Paediatric asthma outcomes are poor and many deaths are preventable.
Diagnosing asthma in childhood can be challenging, and the diagnosis should be reviewed during follow-up to ensure it is correct.
Asthma attacks should be viewed as never events. Postattack reviews are essential to optimise maintenance therapy and prevent future attacks.
Education is key to improving asthma outcomes.
Personalised asthma action plans are essential, and a significant number of children with asthma do not have one.
Introduction
Asthma is a chronic respiratory disease characterised by episodes of wheeze, cough, and shortness of breath. Around 14% of children worldwide have a diagnosis of asthma, making it the most common chronic respiratory disease of childhood. 1
Poor asthma control is associated with a number of negative effects on children and families. For example, they are more likely to be absent from school, have additional educational needs and have lower educational attainment. 2 Caregivers also experience missed work days and financial challenges as a result. 3 Some children will experience severe symptoms and life-threatening attacks. 4
Taking the UK as an example, paediatric asthma outcomes are poor overall with considerable associated morbidity and high rates of emergency hospital admissions, and most pertinently, there are several preventable deaths each year. 5 Alarmingly, the National Review of Asthma Deaths (NRAD) found that in almost all paediatric cases, there were a number of significant avoidable contributing factors and that these deaths may have been preventable. 6
There are several factors that make the diagnosis and management of asthma in children challenging. The aim of this review was to explore these issues and highlight good clinical practice in the diagnosis and management of paediatric asthma.
Presentation of asthma
Children with asthma typically present with a symptom triad of wheeze, shortness of breath and cough. However, ‘asthma’ is an umbrella term used to describe this collection of symptoms and, when present, should prompt practitioners to ask, ‘What type of asthma is this?’ There are a number of asthma subtypes that present and respond to treatment differently. Identification of the features of asthma and modifiable or treatable traits should only be the start of the diagnostic journey. 7 Asthma symptoms are normally intermittent in nature and may not be present at the time of clinical review, making the diagnosis challenging in some cases. 8 Additionally, disease phenotypes are not fixed and may evolve over time, necessitating ongoing review of symptoms and treatment. 9
Wheeze is a key feature of asthma and, if not present, a diagnosis of asthma in a child is unlikely. Wheeze is an expiratory high-pitched whistle that occurs as a result of inflammation and narrowing of the small airways. Parental understanding of wheeze varies, and clarifying what is meant when it is reported is key in making an accurate diagnosis. 10
The prevalence of ‘preschool wheeze’ is an additional challenge when diagnosing asthma in young children. In the first few years of life, many children will experience wheeze, but not all will go on to develop true asthma. The diagnosis of asthma should therefore be reviewed routinely to identify true asthma and alter treatment where necessary. 11 Favourable response to an appropriate trial of asthma treatment is an important confirmatory piece of diagnostic evidence.
Clinical examination may be normal in children and adolescents with asthma if they present during asymptomatic periods. During acute attacks, use of accessory muscles of respiration and widespread wheeze may be present. 12 Chest hyperinflation may be identified in acute and chronic disease settings.
Asthma triggers
Asthma attacks commonly occur following exposure to one or several triggers. Viral respiratory infections remain the leading cause, 13 but there are a number of other known triggers ( box 1 ), including aeroallergens, secondhand smoke exposure, or changes in ambient air temperature or humidity. Identification and documentation of specific asthma triggers should be part of routine care. Education on trigger recognition and avoidance is essential.
Common asthma triggers
Viral respiratory tract infections 6
Exercise 6 59
Weather changes in temperature and humidity 6 59
Domestic pollutants (eg, pests, mould and dust mites) 6
Environmental pollutants (eg, air pollution) 6
Secondhand smoke exposure 13 59
Pets and animals 13
Strong odours 13
Anxiety or strong emotions 59
Drugs (eg, non-steroidal anti-inflammatory drugs and beta blockers) 59
Gastro-oesophageal reflux 59
Risk factors for asthma
There are a number of risk factors that should be explored in the history of children who present with features of asthma. In symptomatic children, a personal or family history of atopic features, including asthma, eczema or rhinitis, supports a diagnosis of asthma. Some additional risk factors are outlined in box 2 . Education on modifiable risk factors, for example, exposure to secondhand smoke or air pollution and obesity, should be delivered routinely during consultations and asthma reviews. A range of social determinants that are linked to poverty impact on outcomes and the health of children with asthma. 14
Asthma risk factors
Personal or family history of atopy: eczema, allergic rhinitis or nasal polyposis 60
Family history of asthma 60
Exposure to secondhand smoke 60
Preterm birth 21
Low birth weight 61
Poor housing quality/mould and dampness 6
Air pollution 63
Paediatric asthma phenotypes
Asthma is a heterogeneous disease in which there are several phenotypes and underlying endotypes. Phenotypes are subtypes of asthma that share clinical characteristics such as symptom triggers, atopic features, disease severity and response to treatment. Endotypes are subtypes of asthma that are characterised by similar underlying biological mechanisms. 15
Key endotypes include ‘type 2-high’ and ‘type 2-low’ asthma. 16 Identifying asthma phenotypes and endotypes can facilitate targeted treatment based on the pathophysiology occurring in a specific individual. 17 For example, allergic or eosinophilic asthma that frequently starts in childhood is type 2-high and is characterised by eosinophilic airway inflammation, raised IgE and fractional exhaled nitric oxide (FeNO) levels. 15 Typically, type 2-high asthma responds well to inhaled corticosteroid (ICS) treatment. 7 A number of biologic agents can be used in the management of asthma, under specialist supervision, and their use varies on asthma endotypes (table 8). 18
Differential diagnoses and diagnostic uncertainty
Misdiagnosis of asthma remains a major problem with rates of both underdiagnosis and overdiagnosis being high. 19 Overdiagnosis is problematic as it exposes children to unnecessary side effects of medications and runs the risk of trivialising asthma. 7
There are several conditions that may be associated with chronic cough, wheeze and/or shortness of breath in children and therefore present similarly to asthma ( table 1 ). Due to the difficulties with diagnosis, especially in young children where objective testing is not possible, the diagnosis of asthma should be reviewed at each clinical presentation and interaction.
- View inline
Asthma differentials and clues in medical history
Diagnosing asthma in children
There is no single ‘gold-standard’ test that can be used to accurately diagnose asthma. In practice, a diagnosis should be made based on characteristic symptom patterns, evidence of variability in airflow limitation in the presence of airway inflammation, likelihood of alternative diagnoses and response to treatment. Getting the diagnosis correct is key for optimal management of paediatric asthma.
Lung function tests can be used to aid the diagnosis of asthma in children over the age of 5 years. Peak expiratory flow (PEF) and spirometry are commonly used to assess airflow obstruction and reversibility. PEF can be used to detect diurnal variation, which is a typical feature of asthma. The Global Initiative for Asthma (GINA) specifically recommends the use of either PEF or spirometry in the diagnosis of asthma in children over 5 years. 20 Once a child is old enough to reliably perform lung function testing, it is recommended that this be undertaken if the diagnosis of asthma has not been previously confirmed. In children under 5, lung function testing is rarely practical outside a research setting. This makes diagnosis in this age group additionally challenging. 21 Guidelines vary between countries and regions with regard to diagnostic criteria. An overview of the similarities and differences between these guidelines is displayed in table 2 . Lung function testing is frequently used to monitor progress of children with asthma as part of their care. Objective testing should be repeated if there is poor response to treatment or diagnostic uncertainty.
Summary of paediatric asthma national guidelines: focusing on diagnosis
FeNO is used to detect and quantify eosinophilic airway inflammation with levels elevated in those with eosinophilic asthma. 22 Once staff are trained, and provided equipment is available, FeNO is a practically useful test that is quick to perform in school-aged children. The exact positioning of FeNO testing varies between guidelines worldwide ( table 2 ). FeNO monitoring may also be useful in titrating dosage of ICS in those with an established diagnosis of asthma. 23
Allergy testing (skin prick testing or measurement of specific IgE levels) is not routinely carried out in the diagnostic process; however, it is recommended in a number of clinical guidelines and may identify individual triggers. 24–27
There are several aspects that make paediatric asthma diagnosis challenging. Most diagnoses are made in primary care where there is often limited access to objective testing at present. Despite guideline recommendations, objective testing is frequently only available in secondary or tertiary care settings where equipment and trained staff are available. The COVID-19 pandemic has served to exacerbate these issues and increase backlogs. Various solutions have been proposed, including community diagnostic hubs. 28 In some healthcare systems, the cost of undergoing objective testing is a cause of health inequalities.
Additionally, the symptom onset for most cases of paediatric asthma occurs before the age of 3 years 29 when lung function testing cannot be used to aid diagnosis. In this age group, response to an asthma treatment trial is useful to aid diagnostic decision making and is recommended in a number of national guidelines. 27 30–32

Management of asthma in children
The management of asthma is multifactorial, and to optimise disease control, a number of pharmacological, non-pharmacological and self-management aspects need to be considered.
Pharmacological management
The pharmacological management of asthma involves two key components: maintenance and reliever therapies. Maintenance therapies are the mainstay of asthma management, and the treatment aim is that no reliever therapies are required. Use of reliever therapy suggests asthma control is poor.
An overview of maintenance and reliever therapies is outlined in tables 3 and 4 , respectively. A stepwise approach to asthma management is encouraged, and pharmacological management varies on age, symptom control and the national guideline used. An overview of management approach in a number of national guidelines is summarised in table 5 .
Maintenance therapies
Reliever therapies
Summary of paediatric asthma national guidelines: focusing on management
Biologic agents used in the management of asthma
GINA guidelines recommend dual ICS and short-acting beta-2 agonist (SABA) therapy to children over the age of 5. 20 SABA monotherapy was previously the main management starting point; however, compared with combined treatment, SABA monotherapy has been shown to be associated with asthma mortality. 33 SABA monotherapy is now only recommended by GINA for use in children aged 5 or less. 20 As seen in table 5 , GINA recommends symptom-driven ICS use, compared with daily ICS use, as initial therapy in children over 6 years of age. In comparison to daily ICS use, symptom-driven use has demonstrated a similar exacerbation risk and reduces the risk of ICS adverse effects. 34
Single maintenance and reliever therapy (SMART) inhalers are combined inhalers offering both maintenance and reliever therapy in those with asthma. These inhalers contain a number of maintenance and reliever therapies in different combinations. The use of these inhalers have been shown to reduce the risk of asthma attacks and emergency department (ED) admissions, 35 improve lung function and decrease the need for reliever therapy. 36 There is limited evidence in the effectiveness of SMART inhalers in children, but children over 12 years may be prescribed a SMART inhaler, which acts as both a maintenance and reliever therapy, if symptoms are not well controlled. 37
There are a number of biologic agents ( table 6 ) that may be used in the management of paediatric asthma. These are endotype-specific, targeted therapies that should be used only under the supervision of specialists. Their availability and cost vary between countries and different healthcare systems. Detailed appraisal of the evidence base for their use is provided in the individual management guidelines and has been recently reviewed. 17
Non-pharmacological management
Non-pharmacological aspects of asthma management include providing education on modifiable risk factors and comorbidities to caregivers and conducting annual asthma reviews to assess control and future risk.
Education is key to improving caregiver and child understanding of asthma and its management. Clear information regarding modifiable risk factors, such as smoke exposure, domestic pollutants and obesity, should be given. Short-term educational interventions aimed to improve self-management have been shown to increase medication adherence, 38 improve symptom control and reduce mortality. 39
All young people with asthma should have asthma reviews at least annually. These reviews should focus on current symptom control and management, previous attacks, triggers, modifiable risk factors and personal asthma action plans (PAAPs). Asthma reviews are opportunities to assess child and caregiver understanding of asthma and provide education, if necessary. Annual asthma reviews are also opportunities to assess inhaler technique (including spacer use) and provide education on this if necessary. Poor inhaler technique is common in young people with asthma 40 and associated with poor disease control. 41
Taking time to understand the perceptions of young people and their caregivers in relation to their asthma diagnosis and management is important, and exploring such perceptions may enhance engagement during consultations, subsequently improving outcomes for young people. 42
Self-management
Self-management aspects of paediatric asthma management include asthma education and PAAPs. PAAPs are written documents that are given to young people and/or caregivers that advise them on day-to-day asthma management and what to do in the event of an attack. 43 Action plans should be created with patient/caregiver input, shared with relevant individuals (eg, school teachers) and should be reviewed and updated regularly. PAAPs have been shown to reduce ED attendance and missed school days and to increase caregiver confidence when managing attacks. 44 The 2018 Annual Asthma Survey found that over 50% of children with asthma in the UK had no PAAP, and around 20% of caregivers did not seek medical advice during acute asthma attacks, highlighting large gaps in education. 45
Diet and exercise are additional important self-management aspects within paediatric asthma care. A number of short-term exercise interventions have demonstrated improvements in lung function and symptom control. 46 Healthy eating interventions can help reduce body mass index and improve the quality of life of both young people and their caregivers. 47
Withdrawing management/stepping down
Asthma control should be reviewed at every medical contact. When asthma symptoms are well controlled on pharmacological therapy, stopping or stepping down medication should be considered to protect young people from unnecessary adverse effects.
The GINA 2021 guidelines advise that clinicians should consider stepping down asthma management to the lowest effective treatment regimen when good symptom control has been achieved for at least 3 months. 20 When stepping down treatment, an individualised risk–benefit approach should be taken with focus on the child’s medical history, including frequency of oral corticosteroid use, frequency of asthma attacks, and previous intensive or high-dependency care admissions. 48
When to refer to a specialist
Most paediatric asthma cases are diagnosed in primary care without the input of general paediatricians or paediatric respiratory physicians. 6 However, a number of children with asthma may need to be referred to specialists for diagnostic or management input. Common indications for specialist referral include no or poor response to asthma treatments, inconclusive objective testing, poor symptom control with appropriate treatment, frequent oral corticosteroid use or the occurrence of a severe asthma attack. 20 27 30 31 49 50 A key element of specialist care is a multidisciplinary team consisting of a number of professionals, including specialist nurses, psychologists, physiologists and pharmacists.
Healthcare professionals must consider any safeguarding implications at all paediatric asthma reviews as part of delivering holistic care. Unexplained or frequent ‘do not attend’ appointments or suspicion of poor medical management at home should be flagged and acted on locally.
Predictors of life-threatening attacks
The following features have been shown to increase the likelihood of future severe attacks, and particular attention should be given to these factors during asthma reviews:
Previous attack. The strongest risk factor for a future asthma attack is a personal history of a previous attack. One large systematic review and meta-analysis found that children with a recent history of ED attendance with an asthma attack were up to 5.8 times more likely to have another ED attendance and up to three times more likely to be admitted to the hospital with a future asthma attack. 51
Frequent SABA use and prescription requests. Frequent use of SABA reliever therapy suggests poor control of asthma symptoms. If asthma symptoms are well controlled, no more than two SABA inhalers should be required annually. 52 The UK NRAD found that excess SABA prescription and use were prominent in individuals who died of asthma attacks. For those with data available, around 40% had been prescribed 12 or more SABA inhalers in the 12 months before death. 6
Postattack review
Asthma attacks should be viewed as never events. It is essential that a postattack review is conducted to review asthma maintenance treatment, as this is likely to be suboptimal. Failure to review patients post attack, and to alter treatment where appropriate, is likely to predispose to future attacks, which could be life-threatening. Management of the current attack should be reviewed to ensure treatment is appropriate and symptoms are resolving. Some individuals may require additional courses of oral corticosteroids to settle symptoms. 7
Current NICE quality standards (UK) state that all individuals hospitalised with an asthma attack should receive a follow-up review in primary care within two working days of discharge, 49 to review maintenance management and ensure resolution of symptoms. However, the 2018 National Asthma Survey completed in the UK found that 64% of respondents had no primary care follow-up post attack, and most patients were not aware that this was required. 45
Salbutamol weaning
Salbutamol weaning plans are commonly used by a number of healthcare organisations following discharge after an asthma attack. These plans direct caregivers to provide regular SABA therapy, often in a reducing regime, in the days following discharge. There have been a number of concerns raised with regard to these plans with some believing that providing regular SABA therapy may potentially mask deterioration and could delay care givers seeking medical advice. 53 Healthcare professionals should enquire about salbutamol weaning plans during postattack reviews and urge caregivers to seek medical advice if they have concerns or the effects of SABA are not lasting the 4 hours of duration.
Future developments in care
The management of paediatric asthma is changing over time with, just as two examples, developments in technology and service structure:
Technology. The growing use of technology in asthma care has huge potential to improve clinical outcomes. Smartphone applications can be used to provide medication reminders to users, and this has been shown to increase ICS adherence. 54 Applications can also be used to provide educational content to young people and caregivers, 55 as well as store PAAPs. 56 ‘Smart’ inhalers, not to be confused with SMART inhalers, are devices that can provide audio reminders to users and record when they are used. One paediatric study found that the use of smart inhalers increased treatment adherence to 84%, compared with 30% in the control group. 57
Diagnostic hubs. In the UK, regional diagnostic hubs for asthma care have been recommended in NHS England’s Long Term Plan. 58 Implementation of diagnostic hubs is hoped to result in earlier and more accurate asthma diagnoses by improving access to objective testing and specialised interpretation. Hubs are designed to improve asthma outcomes by enabling most appropriate treatment initiation and monitoring. There is currently no evidence in the literature of the clinical effects of diagnostic hubs being used in the management of paediatric asthma.
Conclusions
Paediatric asthma outcomes are currently poor and many deaths are preventable. The aim should be to avoid asthma attacks occurring with appropriate maintenance therapy, and they should be viewed as never events. In order to improve outcomes, accurate diagnosis and management are essential. Good asthma care extends beyond providing medication and should include education, as well as supported self-management advice. The use of PAAPs remains limited and a significant number of young people with asthma do not have one. Postattack asthma reviews are a key opportunity to review maintenance medication and current symptom control.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Fleming M ,
- Fitton CA ,
- Steiner MFC , et al
- Nichols M ,
- Treiber F , et al
- FitzGerald JM ,
- Barnes PJ ,
- Chipps BE , et al
- Fleming L ,
- Warner JO , et al
- Physicians RCo
- McCormack MC ,
- Kuruvilla ME ,
- Silverman M ,
- Strippoli M-PF , et al
- Fainardi V ,
- Santoro A ,
- Caffarelli C
- Townshend J ,
- Gautier C ,
- Matsui EC ,
- McCormack MC , et al
- Castagnoli R ,
- Brambilla I , et al
- Brusselle GG ,
- Koppelman GH
- McGregor MC ,
- Krings JG ,
- Nair P , et al
- Kavanagh J ,
- Jackson DJ ,
- Lugtenberg MJ ,
- Smets E , et al
- Shaw DE , et al
- Petsky HL ,
- Plaza Moral V ,
- Alonso Mostaza S ,
- Alvarez Rodríguez C , et al
- Papadopoulos NG ,
- Arakawa H ,
- Carlsen K-H , et al
- Ebisawa M , et al
- Sylvester KP ,
- Rutter MA , et al
- Devonshire AL ,
- Australia TNAC
- Zealand AaRFN
- Mitchell P , et al
- Benayoun S , et al
- Muneswarao J ,
- Hassali MA ,
- Ibrahim B , et al
- Scicchitano R ,
- Aalbers R ,
- Ukena D , et al
- Sobieraj DM ,
- Nguyen E , et al
- Boutopoulou B ,
- Koumpagioti D ,
- Matziou V , et al
- Guevara JP ,
- Grum CM , et al
- Gillette C ,
- Rockich-Winston N ,
- Kuhn JA , et al
- Capanoglu M ,
- Dibek Misirlioglu E ,
- Toyran M , et al
- Henderson J , et al
- Ducharme FM ,
- Lakupoch K ,
- Manuyakorn W ,
- Preutthipan A , et al
- Wanrooij VHM ,
- Willeboordse M ,
- Dompeling E , et al
- Kallenbach JM ,
- Frankel AH ,
- Lapinsky SE , et al
- (NICE) NIfHaCE
- Dermot Nolan DM
- Ardura-Garcia C ,
- Stolbrink M ,
- Zaidi S , et al
- Ekström M ,
- Hasvold P , et al
- Mosnaim G ,
- Martin M , et al
- Burbank AJ ,
- Hewes M , et al
- Stewart AW ,
- Harrison J , et al
- Tarasidis GS ,
- Bai M-J , et al
- Ettinger AS ,
- Pedersen CB ,
- Thygesen M , et al
- Ullmann N ,
- Di Marco A , et al
- Rugg-Gunn CE ,
- Sellick V , et al
- Society SAT
- Doroudchi A ,
- Pathria M ,
- Morgan WJ ,
- Gergen PJ , et al
- Garcia E , et al
- Ortiz B , et al
- Korenblat PE
- Chanez P , et al
- Pavord ID , et al
- Godor D , et al
- Ortega HG ,
- Pavord ID ,
- Howarth P , et al
- Gibson PG , et al
- Geng B , et al
- Wechsler M ,
- Virchow JC ,
- Brusselle GG , et al
- Kolbeck R ,
- Kozhich A ,
- Koike M , et al
- Harrison TW ,
- Menzella F , et al
JT and MB contributed equally.
Contributors All authors conceived the ideas for the article. JM wrote the first draft that was then commented on by JT and MB.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests MB received investigator-led research grants from Pfizer and Roche Diagnostics; speaker fees paid to Newcastle University from Novartis, Roche Diagnostics and TEVA; and travel expenses to educational meetings Boehringer Ingelheim and Vertex Pharmaceuticals.
Provenance and peer review Commissioned; externally peer reviewed.
Read the full text or download the PDF:
YES WE CAN Children’s Asthma Program
This case study was prepared for CDC by Dr. LaMar Palmer of MAS Consultants. The purpose of the case study is to share the experience of one community as they attempt to address the problem of asthma. It does not represent an endorsement of this approach by CDC.
- YES WE CAN Children’s Asthma Program: Background
- Design and Development
- Program Components
- Operation of the Program
- Patient and Family Education
- Intervention Research Outcomes
- Strengths and Challenges of the Program
- Lessons Learned
- Success Stories
- The YES WE CAN Toolkit
- Ordering and Contact Information
Follow @CDCasthma on Twitter to learn more about helping people with asthma live healthier lives by gaining control over their asthma.
- Asthma Action Plan
- America Breathing Easier pdf [PDF – 1.1 MB]
- ASL Asthma Film
- Asthma Clinical Guidelines
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Pediatr

Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
Virginia mirra.
Department of Translational Medical Sciences, Federico II University, Via Sergio Pansini 5, 80131 Naples, Italy
Silvia Montella
Francesca santamaria, associated data.
All relevant data and materials are published in the manuscript.
The primary goal of asthma management is to achieve disease control for reducing the risk of future exacerbations and progressive loss of lung function. Asthma not responding to treatment may result in significant morbidity. In many children with uncontrolled symptoms, the diagnosis of asthma may be wrong or adherence to treatment may be poor. It is then crucial to distinguish these cases from the truly “severe therapy-resistant” asthmatics by a proper filtering process. Herein we report on four cases diagnosed as difficult asthma, detail the workup that resulted in the ultimate diagnosis, and provide the process that led to the prescription of omalizumab.
Case presentation
All children had been initially referred because of asthma not responding to long-term treatment with high-dose inhaled steroids, long-acting β 2 -agonists and leukotriene receptor antagonists. Definitive diagnosis was severe asthma. Three out four patients were treated with omalizumab, which improved asthma control and patients’ quality of life. We reviewed the current literature on the diagnostic approach to the disease and on the comorbidities associated with difficult asthma and presented the perspectives on omalizumab treatment in children and adolescents. Based on the evidence from the literature review, we also proposed an algorithm for the diagnosis of pediatric difficult-to-treat and severe asthma.
Conclusions
The management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma. The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism in the management of children and adolescents with atopic severe asthma.
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [ 1 ]. Therefore, the primary goal of asthma management at all ages is to achieve disease control [ 2 – 4 ].
According to recent international guidelines, patients with uncontrolled asthma require a prolonged maintenance treatment with high-dose inhaled corticosteroids (ICS) in association with a long-acting β 2 -agonist (LABA) plus oral leukotriene receptor antagonist (LTRA) (Table 1 ) [ 5 ].
Recommended options for initial controller treatment in children and adults according to GINA Guidelines [ 5 ]
Theophylline is not recommended for children 6–11 years, while tiotropium is not indicated in patients < 18 years
ICS inhaled corticosteroids, LTRA leukotriene receptor antagonist, LABA long-acting β 2 -agonist, anti-IgE anti-immunoglobulin E therapy, OCS oral corticosteroids
Nevertheless, in the presence of persistent lack of control, reversible factors such as adherence to treatment or inhalation technique should be first checked for, and diseases that can masquerade as asthma should be promptly excluded. Finally, additional strategies, in particular anti-immunoglobulin E (anti-IgE) treatment (omalizumab), are suggested for patients with moderate or severe allergic asthma that remains uncontrolled in Step 4 [ 5 ].
Herein, we reviewed the demographics, clinical presentation and treatment of four patients with uncontrolled severe asthma from our institution in order to explain why we decided to prescribe omalizumab. We also provided a review of the current literature that focuses on recent advances in the diagnosis of pediatric difficult asthma and the associated comorbidities, and summarizes the perspectives on anti-IgE treatment in children and adolescents.
Case presentations
Table 2 summarizes the clinical characteristics and the triggers/comorbidities of the cases at referral to our Institution. Unfortunately, data on psychological factors, sleep apnea, and hyperventilation syndrome were not available in any case. Clinical, lung function and airway inflammation findings at baseline and after 12 months of follow-up are reported in Table 3 . In the description of our cases, we used the terminology recommended by the ERS/ATS guidelines on severe asthma [ 6 ].
Clinical characteristics of described patients with difficult asthma
GER Gastroesophageal reflux, ICU Intensive care unit
Clinical findings at baseline and after 12 months of follow-up in patients with difficult asthma
BD bronchodilator, Δ % predicted changes from the pre-bronchodilator values, FeNO fractional exhaled nitric oxide, Ppb part per billion, QoL Quality of Life defined according to references [ 14 ], c-ACT Children Asthma Control Test evaluated according to references [ 79 , 80 ], NA Not Available
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 11, severe asthma was diagnosed. Sensitization to multiple inhalant allergens (i.e., house dust mites, dog dander, Graminaceae pollen mix, and Parietaria judaica ) and high serum IgE levels (1548 KU/l) were found. Body mass index (BMI) was within normal range. Combined treatment with increasing doses of ICS (fluticasone, up to 1000 μg/day) in association with LABA (salmeterol, 100 μg/day) plus LTRA (montelukast, 5 mg/day) has been administered over 2 years. Nevertheless, persistent symptoms and monthly hospital admissions due to asthma exacerbations despite correct inhaler technique and good adherence were reported. Parents refused to perform any test to exclude gastroesophageal reflux (GER) as comorbidity [ 6 ]. However, an ex-juvantibus 2-month-course with omeprazole was added to asthma treatment [ 7 ], but poor control persisted. Anterior rhinoscopy revealed rhinosinusitis that was treated with nasal steroids for six months [ 8 ], but asthma symptoms were unmodified. Treatment with omalizumab was added at age 12. Reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 2.0 up to 6.7 out of a maximum of 7 points) were documented over the following months. Unfortunately, after one year of treatment, adherence to omalizumab decreased because of family complaints, and eventually parents withdrew their informed consent and discontinued omalizumab. Currently, by age 17, treatment includes inhaled salmeterol/fluticasone (100 μg/500 μg∙day -1 , respectively) plus oral montelukast (10 mg/day). Satisfactory symptom control is reported, with no asthma exacerbations.
A full-term male, who had a recurrent severe preschool wheezing, at 6 years of age developed exercise-induced asthma. At age 10, severe asthma was diagnosed. High serum IgE levels (1300 KU/l) and skin prick tests positive to house dust mites were found. Despite a 3-year treatment with progressively increasing doses of inhaled fluticasone (up to 1000 μg/day) combined with salmeterol (100 μg/day) and oral montelukast (5 mg/day), monthly hospital admissions with systemic steroids use were reported. At age 13, a 24-h esophageal impedance/pH study demonstrated the presence of acid and non-acid GER [ 7 ]. Esomeprazole was added to asthma medications, but with an incomplete clinical benefit for respiratory symptoms. Esomeprazole was withdrawn after 3 months, and parents refused to re-test for GER. As respiratory symptoms persisted uncontrolled despite treatment, severe asthma was definitively diagnosed [ 6 ]. BMI was within the normal range and anterior rhinoscopy excluded rhinosinusitis. Inhaler technique and adherence were good; thus we considered the anti-IgE treatment option [ 9 ]. Subcutaneous omalizumab was started, with fast improvement of both symptoms and QoL score (from 3.9 up to 6.5). Seventeen months later, the dose of ICS had been gradually tapered and oral montelukast definitely discontinued. Currently, at age 14, treatment includes the combined administration of bimonthly subcutaneous omalizumab and of daily inhaled salmeterol/fluticasone (50 μg/100 μg∙day - 1 , respectively). Asthma control is satisfactory and no side effects are reported. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with acute respiratory failure that frequently required intensive care unit (ICU) admission. At age 6, sensitization to multiple perennial inhalant (i.e., house dust mites, dog and cat danders, Alternaria alternata , Graminaceae pollen mix, Artemisia vulgaris , Parietaria judaica , and Olea europaea pollen) and food allergens (i.e., egg, milk, and peanut) was diagnosed. Serum IgE levels were 2219 KU/l. Weight and height were appropriate for age and sex. The patient has been treated over 3 years with a combined scheme of high-dose inhaled fluticasone (up to 1000 μg/day) plus salmeterol (100 μg/day) and oral montelukast (5 mg/day), with correct inhaler technique and good adherence. Despite this, monthly hospital admissions with systemic steroids use were recorded. Rhinosinusitis and GER were excluded on the basis of appropriate testing; thus treatment with omalizumab was started when the patient was 9 years old. At age 11, adherence to treatment is satisfactory, with no side effects. More importantly, reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 6.4 to 6.8) were reported. Finally, progressive step-down of anti-asthma treatment was started, and at present (by 11.5 years) inhaled fluticasone (200 μg/day) plus bimonthly subcutaneous omalizumab provide good control of symptoms. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 4, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 8, multiple perennial inhalants and food sensitization (i.e., house dust mites, dog dander, Graminaceae pollen mix, Olea europaea pollen, tomatoes, beans, shrimps, and peas) and high serum IgE levels (1166 KU/l) were found. The patient has been treated over 5 years with inhaled fluticasone (up to 1000 μg/day) in association with salmeterol (100 μg/day) and oral montelukast (5 mg/day). Despite this, monthly hospital admissions with systemic steroids need were recorded. After checking the inhaler technique and adherence to treatment, comorbidities including obesity, rhinosinusitis and GER were excluded. Omalizumab was proposed, but parents refused it. By 13.6 years, despite a treatment including the association of inhaled salmeterol/fluticasone (100 μg/1000 μg∙day − 1 , respectively) plus oral montelukast (10 mg/day), monthly exacerbations requiring systemic steroids are reported.
Discussion and conclusions
Most children and adolescents with asthma respond well to inhaled short-acting beta 2 -agonists (SABA) on demand if symptoms are intermittent, or to low dose controller drugs plus as-needed SABA if the risk of exacerbations increases [ 1 ]. Nevertheless, a proportion of patients is referred to specialists because this strategy is not working and asthma is persistently uncontrolled [ 4 ]. For these children, assessment is primarily aimed at investigating the reasons for poor control. Indeed, when the child is initially referred, before the label of “severe, therapy-resistant asthma” (i.e., not responding to treatment even when factors as exposure to allergens and tobacco smoke have been considered) is assigned, three main categories need to be identified: 1) “not asthma at all”, in which response to treatment is suboptimal because the diagnosis is wrong; 2) “asthma plus ”, when asthma is mild but exacerbated by one or more comorbidities; and 3) “difficult-to-treat asthma”, when asthma is uncontrolled because of potentially reversible factors [ 10 ].
The reported cases highlight some aspects of the disease process that may expand the diagnosis and improve patients’ care. At our institution, the severe asthma program includes a multidisciplinary approach with consultations by gastroenterologists as well as ear, nose and throat experts. Recently, sleep medicine experts joined this multidisciplinary team; thus, unfortunately, sleep-disordered breathing (SDB) could not be excluded at the time of our patients’ assessment. Inhalation technique is periodically evaluated by nurses or doctors in each patient. Unfortunately, in Italy an individual prescription database is not available and thus we cannot assess patients’ use of medication. In two cases, the filtering process eventually identified GER and rhinosinusitis, but poor control of asthma persisted even after comorbidities were treated. In all subjects, inhaler skills, treatment adherence, and environmental exposure to indoor/outdoor allergens as well as to second- and third-hand smoke were excluded as cause of lack of control. Eventually, three out of four patients started anti-IgE treatment; asthma control was obtained and maintenance drugs were progressively reduced. In the case that refused omalizumab therapy, pulmonary function, clinical features and controller treatment including high-dose ICS were unchanged.
Previous studies have highlighted an association between increasing asthma severity in children and reduced QoL [ 11 – 13 ]. Uncontrolled asthma symptoms not only affect children physically, but can impair them socially, emotionally, and educationally [ 13 ]. In line with previous observations, 3 out 4 of our cases had poor QoL, assessed by a standardized questionnaire [ 14 ]. It is well known that improving QoL in difficult asthma is not an easy task, despite a variety of treatments aimed at achieving control [ 12 ], and much more remains to be done to address the problem. Nevertheless, 2 of our 3 cases showed a remarkable improvement of QoL after one year of treatment with omalizumab.
Reduction in forced expiratory volume in the first second (FEV 1 ) is often used to define childhood asthma severity in treatment guidelines and clinical studies [ 5 , 11 , 15 ]. Nevertheless, children with severe asthma often have a normal FEV 1 that does not improve after bronchodilators, indicating that spirometry may be a poor predictor of asthma severity in childhood [ 6 , 16 , 17 ]. Actually, children with a normal FEV 1 , both before and after β 2 -agonist, may show a bronchodilator response in terms of forced expiratory flow between 25% and 75% (FEF 25–75 ) [ 18 ]. However, the utility of FEF 25–75 in the assessment or treatment of severe asthma is currently unknown. Interestingly, all the reported cases showed normal or slightly reduced values of FEV 1 but severe impairment of FEF 25–75 . Two cases showed a bronchodilator response in terms of FEV 1 (subjects 3 and 4), while 3 patients had a significant increase of FEF 25–75 (cases 1, 3 and 4). Unfortunately, we could not provide the results of bronchodilator response during or after the treatment with omalizumab in any case.
Available literature on the diagnostic approach to difficult asthma in children offers a number of reviews which basically summarize the steps needed to fill the gap between a generic diagnosis of “difficult asthma” and more specific labels (i.e., “severe” asthma, “difficult-to-treat” asthma, or even different diagnoses) [ 3 , 5 , 6 , 8 , 10 , 19 – 21 ]. So far, few original articles and case reports have been published, probably due to the peculiarity of the issue, which makes retrospective discussion of cases easier than the design of a prospective clinical study [ 4 , 22 – 26 ]. Available knowledge mainly derives from the experience of specialized centers.
The evaluation of a child referred for uncontrolled asthma should start with a careful history focused on typical respiratory symptoms and on the definition of possible triggers. In the “severe asthma” process, it is crucial for clinicians to maintain a high degree of skepticism about the ultimate diagnosis, particularly in the presence of relevant discrepancies between history, physical features and lung function, as many conditions may be misdiagnosed as asthma. In order to simplify this process, herein we propose an algorithm for the diagnosis of difficult-to-treat and severe asthma (Fig. 1 ). Confirmation of the diagnosis through a detailed clinical and laboratory re-evaluation is important because in 12–50% of cases assumed to have severe asthma this might not be the correct diagnosis [ 10 ]. Several documents have indicated the main steps of the process that should be followed in children with uncontrolled asthma [ 3 , 8 , 10 ]. The translation of these procedures into real life practice may deeply change from one subject to another due to the variability of individual patients’ history and clinical features, which will often lead the diagnostic investigations towards the most likely reason for uncontrolled asthma. For children with apparently severe asthma, the first step is to confirm the diagnosis and, before proceeding to broader investigations, to verify that the poor control is not simply determined by poor adherence to treatment, inadequate inhaler skills and/or environmental exposure to triggers. A nurse-led assessment, including a home visit, despite not being applicable in all settings, may be useful for identifying potentially modifiable factors in uncontrolled pediatric asthma [ 27 ].

A practical algorithm for the diagnosis of difficult-to-treat and severe asthma. ICS, inhaled corticosteroids; OCS, oral corticosteroids
A number of comorbidities have been increasingly recognized as factors that may impact asthma clinical expression and control in childhood [ 10 , 28 ]. Children with uncontrolled disease should be investigated for GER, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, obesity, psychological factors, smoke exposure, hormonal influences, and ongoing drugs [ 3 , 6 , 8 , 20 ]. Indeed, the exact role played by comorbidities in pediatric asthma control is still debated [ 28 ]. The most impressive example is GER. Several pediatric documents recommend assessing for GER because reflux may be a contributing factor to problematic or difficult asthma [ 7 , 29 ]. Nevertheless, GER treatment might not be effective for severe asthma [ 30 , 31 ], as confirmed by current cases 1 and 2. There is an established evidence that chronic rhinosinusitis is associated with more severe asthma in children [ 32 – 34 ]. Therefore, examination of upper airways and ad hoc treatment if rhinosinusitis is evident are recommended in children with severe asthma [ 3 , 8 , 35 ]. However, intranasal steroids for rhinitis resulted in a small reduction of asthma risk in school-aged children [ 36 ], and actual placebo-controlled studies on the effect of treatment of rhinosinusitis on asthma control in children are lacking [ 10 , 37 ].
Dysfunctional breathing, including hyperventilation and vocal cord dysfunction, is associated with poorer asthma control in children [ 8 , 10 , 38 , 39 ]. Unfortunately, there is scarce literature on the effect of its treatment on the control of severe asthma in children [ 40 ]. SDB ranging from primary snoring to obstructive sleep apnea syndrome is very common in children [ 41 ], and an increased prevalence of SDB together with increasing asthma severity has been reported [ 42 ]. Interestingly, GER may also be worsened by recurrent episodes of upper airway obstruction associated with SDB, and this may further trigger bronchial obstruction. Asthma guidelines recommend the assessment of SDB through nocturnal polysomnography in poorly controlled asthmatics, particularly if they are also obese [ 5 ]. There are no studies examining whether pediatric asthma improves after SDB has been treated, for example, with nasal steroids, adenotonsillectomy, continuous positive airway pressure or weight reduction if the child is also obese [ 43 ]. The parallel increase in obesity and asthma suggests that the two conditions are linked and that they can aggravate each other [ 44 , 45 ], even though the exact mechanisms that underlie this association remain unclear [ 46 ]. Indeed, other coexisting comorbidities such as SDB or GER may play a confounding role in the development of the interactions between obesity and the airways [ 47 , 48 ]. Obesity is associated with increased markers of inflammation in serum and adipose tissue and yet decreased airway inflammation in obese people with asthma [ 49 ]. Several interventions, including behavioral and weight reduction programs or bariatric surgery, may result in improved asthma control, quality of life and lung function in adult obese asthmatics [ 50 ]. Although reports of adolescent bariatric surgery demonstrate a significant body weight decrease, this approach is not widely available and there are no published reports on its effect on pediatric severe asthma control [ 51 ]. Finally, although it is still unclear whether food allergy is causative or shares a common pathway with difficult asthma, it might explain the loss of asthma control at least in some children and thus be considered as a comorbid condition [ 10 , 16 , 52 ].
In conclusion, establishing the impact of comorbidities on asthma control may be cumbersome, and an ex-juvantibus treatment is sometimes necessary to assess their role. Comorbid conditions can also worsen each other, and symptoms arising from some of them may mimic asthma [ 6 ]. Although the ability to improve pediatric severe asthma by treating comorbidities remains unconfirmed, they should be treated appropriately [ 9 ].
The vast majority of asthmatic children exhibit a mild or at most a moderate disease that can be fully controlled with low-to-medium dose ICS associated or not with other controllers [ 5 , 6 ]. However, a subset of asthmatics remains difficult-to-treat [ 5 , 6 ]. With the advent of biologics, these severe steroid-dependent asthmatics have alternative options for treatment, as steroid-related adverse events are common in severe asthma [ 53 ]. Omalizumab, an anti-IgE monoclonal antibody, is the only biologic therapy recommended in children with moderate-to-severe asthma by the recent guidelines [ 5 , 6 ]. In Italy, this treatment is fully covered by the National Health System. Therefore, there is no influence by any funding on treatment decisions. It was approved by the US (Food and Drug Administration) in 2003 and by the European Union (European Medicines Agency) in 2005 as an add-on treatment for patients aged > 12 years with severe persistent allergic asthma and who have a positive skin test or in-vitro reactivity to a perennial aeroallergen, FEV 1 < 80% predicted, frequent daytime symptoms or nighttime awakenings, and multiple documented severe asthma exacerbations despite daily ICS plus a LABA [ 54 , 55 ]. In 2009, it also received approval in Europe for treating patients aged 6–12 years. Figure 2 illustrates current indications for treatment with omalizumab in children and adolescents with severe asthma.

Indications for omalizumab in children and adolescents with severe asthma
IgE antibodies, Th 2 -derived cytokines and eosinophils play a major role in the development of chronic airway inflammation in asthmatic subjects [ 56 ]. Once released from plasma cells, IgE binds principally to the high-affinity IgE receptor (FcεRI) on mast cells, triggering different effector responses, including the release of mediators leading to allergic inflammatory reactions [ 56 ]. The activation of the allergic cascade by IgE, under constant allergen stimulation, leads to the establishment of chronic allergic inflammation in the airways of asthmatic patients, with IgE being a key element of the vicious circle that maintains it. Cytokines produced during the late phase and subsequent chronic inflammation stage have been directly associated with the induction of airway remodelling, indirectly implicating IgE in the process [ 56 ]. At present, omalizumab is the only commercially available recombinant humanized anti-IgE monoclonal antibody that specifically binds serum free IgE at its CH 3 domain, in the proximity of the binding site for FcεRI, thus preventing IgE from interacting with its receptor on mast cells, basophils, antigen-presenting cells and other inflammatory cells [ 57 ]. The rapid reduction of free IgE levels leads to a downregulation of the FcεRI expression on inflammatory cells and an interruption of the allergic cascade, which results in the reduction of peripheral and bronchial tissue eosinophilia and of levels of granulocyte macrophage colony stimulating factor, interleukin (IL)-2, IL-4, IL-5, and IL-13 [ 58 ]. Moreover, basophils have a relevant role in the initiation and progression of allergic inflammation, suggesting that they may represent a viable therapeutic target. Indeed, in children with severe asthma, it has been reported that omalizumab therapy is associated with a significant reduction in circulating basophil numbers, a finding that is concurrent with improved clinical outcomes [ 59 ]. This finding supports a mechanistic link between IgE levels and circulating basophil populations, and may provide new insights into one mechanism by which omalizumab improves asthma symptoms.
Several clinical controlled and real-life studies of adults with severe, inadequately controlled allergic asthma have demonstrated the efficacy and safety of omalizumab in reducing asthma-related symptoms, corticosteroid use, exacerbation rates, and healthcare resource utilization, and in improving QoL and lung function [ 60 – 63 ]. Fewer studies have been published in children. In two double-blind, randomized, placebo-controlled trials (RCTs) of children aged 6 to 12 years with moderate-to-severe allergic asthma, treatment with omalizumab reduced the requirement for ICS and protected against disease exacerbations, but there was little change in asthma symptom scores or spirometry [ 9 , 64 ]. These findings were confirmed and extended in older children [ 65 – 67 ].
The results of the ICATA study, a multicenter RCT of 419 inner-city children, adolescents and young adults with persistent allergic asthma, showed that, compared to placebo, omalizumab reduces the number of days with asthma symptoms and the proportion of participants with at least one exacerbation by approximately 25% and 19%, respectively ( p < 0.001), thus reducing the need for asthmatic symptom controllers [ 68 ]. Another multicenter RCT of inner-city children and adolescents showed that the addition of omalizumab to ongoing guidelines-based care before patients return to school reduces fall asthma exacerbations (odds ratio, 0.48), particularly in subjects with a recent exacerbation [ 69 ]. Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases ( p < 0.001), while FEV 1 improved by 4.9% ( p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% ( p < 0.001) [ 70 ]. The same authors also showed that, after two years of treatment, exacerbation rate and healthcare utilisation were further decreased by 83% and 100%, respectively, while level of asthma control, steroid use and lung function remained unchanged [ 71 ].
A systematic review of pediatric RCTs pooled the data of 1381 children and adolescents with moderate-to-severe allergic asthma in order to establish the efficacy of omalizumab as an add-on therapy [ 72 ]. During the stable-steroid phase, omalizumab decreased the number of patients with at least one exacerbation (risk ratio, 0.69; p < 0.001), the mean number of asthma exacerbations per patient (risk ratio, 0.35; p < 0.001), and the asthma symptom score (mean difference, 0.12; p = 0.005) when compared to placebo. During the steroid reduction phase, omalizumab further reduced the number of patients with at least one exacerbation (risk ratio, 0.48; p < 0.001) and the mean number of asthma exacerbations per patient (mean difference, 0.12; p < 0.05).
Given the cost of omalizumab, many authors have argued for the importance of identifying specific asthma populations who will have significant benefit from it [ 68 , 73 , 74 ]. In the ICATA study, baseline predictors of good response to treatment were sensitization and exposure to cockroach allergen, sensitization to house dust mite allergens, a serum IgE level of more than 100 IU per milliliter, a BMI of 25 or more, and a history of at least one unscheduled medical visit in the previous year [ 68 ].
Several studies have assessed the long-term safety of omalizumab in children and adults. A pooled analysis of 67 RCTs conducted over 2 decades on 4254 children and adults treated with omalizumab showed no association between omalizumab treatment and risk of malignancy [ 75 ]. In an RCT evaluating 225 school-aged children, omalizumab was well tolerated, there were no serious adverse events, and the frequency and types of all adverse events were similar to the placebo group [ 9 ]. These results have been further confirmed by a recent systematic review of RCTs that concluded that treatment with omalizumab does not result in increased risk of malignancy or hypersensitivity reactions [ 72 ].
While the rationale for long-term treatment with omalizumab is supported by pharmacokinetic-pharmacodynamic models [ 76 ], the duration of treatment is still under discussion. Results from published studies suggest that omalizumab should be continued for > 1 year [ 77 , 78 ]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was ‘excellent’ in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years [ 77 ]. After the discontinuation of treatment, loss of asthma control was documented in 69.2% of the patients who had received omalizumab for < 1 year, 59.1% of the subjects treated for 1–2 years, and 46.1% of the cases treated for > 2 years. Time to loss of control was shorter in younger children and longer in patients with an ‘excellent’ response compared with patients with a ‘good’ response. No early loss of control (within 6 months) was observed among patients with > 3.5 years of continuous treatment with omalizumab. Finally, 20% of patients in whom omalizumab was re-prescribed because of loss of control did not respond to the treatment anymore [ 77 ]. Despite these encouraging findings, the impact of omalizumab on the natural history of severe asthma in children deserves to be further investigated by long-term studies that will also define the criteria and timing for discontinuing the treatment.
It is well known that asthma pharmacotherapy is effective in controlling symptoms and bronchial inflammation, but cannot affect the underlying immune response, thus leading to the possibility of symptom reappearance after its discontinuation [ 79 ]. In this scenario, allergen-specific immunotherapy (AIT) has been proposed as the only therapeutic method that can modulate the underlying immune pathophysiology in allergic asthma [ 80 ].
AIT is currently indicated in children and adults with mild-moderate allergic asthma that is completely or partially controlled by pharmacotherapy and with the evidence of a clear relationship between symptoms and exposure to a specific allergen [ 81 – 84 ]. However, according to recent guidelines, the efficacy of AIT in asthmatic subjects is limited, and its potential benefits must be weighed against the risk of side effects and the inconvenience and costs of the prolonged therapy [ 5 ]. Moreover, severe or uncontrolled asthma (regardless of its severity) is a major independent risk factor for non-fatal or even fatal adverse reactions, thus representing a contraindication for AIT [ 85 – 87 ]. Finally, children with severe asthma are often sensitized to multiple allergens, thus making AIT prescription even more complicated [ 88 ].
In subjects with uncontrolled and/or severe allergic asthma, a combination of omalizumab and AIT has been proposed [ 88 ]. Surprisingly, only a few studies have addressed this issue [ 89 – 92 ]. However, pre-treatment with omalizumab seems to improve the efficacy and tolerability of subcutaneous AIT in children and adults with severe allergic asthma both during omalizumab treatment and after its discontinuation [ 89 , 91 , 92 ]. Omalizumab has also been successfully used as a supplementary treatment to AIT in order to improve asthma control in children ≥6 years with severe persistent allergic asthma [ 90 ]. Given the scarcity of studies on AIT plus omalizumab in children with severe allergic asthma, further research is warranted to assess risks and benefits of the combined treatment.
Children with severe asthma require a detailed and individualized approach including re-assessment for differential diagnoses, comorbidities and contributory factors, environmental triggers, lung function and inflammation, adherence and response to therapy, and QoL. Treatment of pediatric severe asthma still relies on the maximal optimal use of corticosteroids, bronchodilators and other controllers recommended for moderate-to-severe disease. However, the management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma.
In the current paper, we described the characteristics of four children with severe asthma in whom omalizumab was prescribed. A review of the relevant literature on the topic was also performed. Finally, we provided an algorithm for the diagnosis of difficult-to-treat and severe asthma in children and adolescents, based on the evidence from the literature review. As all algorithms, it is not meant to replace clinical judgment, but it should drive physicians to adopt a systematic approach towards difficult and severe asthma and provide a useful guide to the clinician.
The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism of outcome improvements in patients with allergic severe asthma. As severe asthma is a heterogeneous condition consisting of different phenotypes, the future of asthma management will likely involve phenotypic and potentially even genotypic characterization in selected cases in order to determine appropriate therapy and thus to provide the highest possible benefit, especially if specific responder phenotypes can be identified and selected for this highly specific treatment.
Acknowledgements
The authors gratefully thank Dr. Marco Maglione for his contribution in the clinical assessment of the described cases. Medical writing assistance was provided by Stephen Walters on behalf of City Hills Proofreading.
No funding was secured for this study.
Availability of data and materials
Abbreviations, authors’ contributions.
VM, SM and FS, authors of the current manuscript, declare that they have participated sufficiently in the work to take public responsibility for appropriate portions of the content. VM and SM carried out the initial investigations, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. FS conceptualized and designed the study, and critically reviewed and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the ethics committee “Carlo Romano”, Federico II University, Naples, Italy. Children’s parents/legal guardians gave informed written consent to participate. The description of our cases adheres to the CARE standards of reporting checklist.
Consent for publication
Children’s parents/legal guardians provided informed written consent for the case report to be published.
Competing interests
The authors declare that they have no competing interests to disclose. Authors have no financial relationships relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Virginia Mirra, Email: [email protected] .
Silvia Montella, Email: ti.oiligriv@4002anima .
Francesca Santamaria, Phone: +39 0817463495, Email: ti.aninu@ramatnas .
Determinants of Childhood Asthma: A Case Control Study from a Tertiary Care Hospital in Bengaluru, South India
Affiliations.
- 1 St. John's Medical College, Bangalore, Karnataka, India.
- 2 Department of Paediatrics, St. John's Medical College, Bangalore, Karnataka, India.
- 3 Department of Community Health, St. John's Medical College, Bangalore, Karnataka, India.
- PMID: 37668442
- PMCID: PMC10478677
- DOI: 10.34763/jmotherandchild.20232701.d-22-00059
Background: Childhood asthma is a common, and often serious, chronic disease with episodic exacerbations in infants and children. There is an increasing trend in the prevalence of childhood asthma in developing countries. Objectives: To identify the determinants of childhood asthma.
Methods: A case control study with 30 cases of childhood asthma and 30 gender- and aged-matched controls selected from the paediatric outpatient department and paediatric ward of a tertiary hospital. The primary caregiver was interviewed to capture sociodemographic details, prenatal and birth history, and history of exposure to environmental risk factors. Odds ratios with 95% confidence intervals were calculated to determine the strength of association between childhood asthma and independent co-variates, followed by subgroup multiple logistic regression analysis.
Results: We found that children with a parental history of allergy/atopy [OR=2.88 (1.94-4.27), P<0.001], residence in houses located in industrial areas [AOR=2.72 (2.6-323.1), P<0.001], exposure to incense at home [AOR=2.03 (1.14-29.42), P<0.001], or a history of allergic rhinitis [AOR=3.09 (2.22-243.25), P<0.001] had significantly higher odds of developing childhood asthma.
Conclusion: Our study found that having homes located in industrial areas, burning incense at home, parental history of allergy, and history of allergic rhinitis in the child are determinants of childhood asthma. The findings from our study can be used to generate awareness regarding risk factors that are linked to childhood asthma.
Keywords: asthma; childhood; determinants; risk factors.
© 2023 Andrea Daniella Johnson et al., published by Sciendo.
- Asthma* / epidemiology
- Asthma* / etiology
- Case-Control Studies
- India / epidemiology
- Rhinitis, Allergic* / epidemiology
- Tertiary Care Centers
Website cookies
This website uses cookies to help us understand the way visitors use our website. We can't identify you with them and we don't share the data with anyone else. If you click Reject we will set a single cookie to remember your preference. Find out more in our privacy policy .
Accept cookies Reject cookies
Case study 1: Improving the experience of children with asthma
The PFCC team at Walsall Manor Hospital chose to work to improve the experience of children with asthma, following a patient safety incident some years earlier. The team felt that nationally, management of asthma could be better, and they wanted to work on this as a local priority.
Specifically, the team wanted to work with patients to co-design the new pathway and make it more clinically consistent as well as improving the experience for children and families.
Initially, the team wanted to transform the whole experience across the health economy. But as time went on, they became aware of the enormity of the task they had set themselves, and they decided to concentrate on arrangements for discharge as their first project, with other aspects of care to follow later.
The team embraced the PFCC method, establishing a guiding council and working groups , and meeting regularly. They also made sure that shadowing was carried out by a number of team members. Many of the clinical team were involved in the work, as well as service users, and it was led by two consultant paediatricians. The organisation also put in place some project support to help the team.
Eventually, the team focused on three specific projects. The first focused on clarifying the process of care from admission to discharge, and producing a patient journey map. The second involved developing resources for families to increase their knowledge about asthma and their confidence in managing it. The third involved implementing follow-up phone calls for families after their children came home from hospital.
The team made some improvements in the process of care, and worked with colleagues to carry out small tests of change, to improve the care bundle until they felt confident with it. Patients and families reported finding the new resources useful.
The team’s work to shadow patients had unintended consequences, too. It helped them to think about new ways to make the environment more user-friendly. For example, one of the first signs visible on entering the ward points to the ‘resuscitation room’. They considered how, from the families’ point of view, it might feel to read this on first arriving.
The team’s successes have fuelled their enthusiasm to spread the PFCC method to other care experiences and, as some members of the team have moved to other organisations, to spread it to other parts of the NHS.
Case study 2: Improving the care of children with acute abdominal pain
Share this content
ORIGINAL RESEARCH article
Preschool children’s asthma medication: parental knowledge, attitudes, practices, and adherence.

- Department of Respiratory, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Engineering Research Center of Stem Cell Therapy, Chongqing, China
Introduction: As parents or legal guardians primarily care for children with asthma, understanding their knowledge, attitudes, and practices (KAP) barriers to treatment and medication adherence is of essential importance. This study aimed to analyze the KAP toward asthma medication and adherence among preschool-aged asthmatic children’s parents and explore the factors influencing adherence.
Methods: This cross-sectional study was conducted between February 2023 and April 2023. Parents of preschool children with asthma were asked to complete the questionnaire containing knowledge, attitude, practice dimensions, and demographic characteristics. The Morisky Medication Adherence Scale (MMAS) was used to investigate adherence.
Results: A total of 632 valid questionnaires (154 male and 478 female) were included. Parents showed moderate knowledge (9.49 ± 2.86, 63.27%, possible range: 0-15) and moderate attitudes (26.18 ± 2.51, 74.80%, possible range: 7-35) towards asthma medication, while their practices (27.46 ± 5.26, 91.53%, possible range: 6-30) were proactive; however, medication adherence was low (4.84 ± 1.78, total score: 8). The attitude scores (OR = 1.10, 95% CI: 1.01-1.19, P=0.020), practice scores (OR = 1.16, 95%CI: 1.12-1.21, p < 0.001), and smoking (OR = 1.64, 95%CI: 1.14-2.37, p = 0.008) were associated with medication adherence.
Discussion: Preschool-aged asthmatic children’s parents showed moderate knowledge, attitudes, and proactive practice toward asthma medication. Continuous training and education programs should be provided for parents to improve asthma medication management in preschool children.
Introduction
Asthma is a long-term condition characterized by coughing, wheezing, shortness of breath, and chest tightness, varying in intensity and frequency. These symptoms are accompanied by chronic airway inflammation and variable airflow obstruction ( National Asthma and Prevention, 2007 ; Healthcare Improvement Scotland, 2019 ; Global Initiative for Asthma, 2023 ). According to recent statistics, more than 300 million people worldwide are affected by asthma ( Papi et al., 2018 ), and the incidence has been increasing in children and adolescents ( Papi et al., 2018 ; Zahran et al., 2018 ). In the United States, the prevalence rate of asthma has been over 8% in the last two decades ( Zahran et al., 2018 ), while in China, the prevalence in children increased from 0.91% in 1990 to 3.02% in 2010 ( National Collaborative Group on Pediatric Asthma, 2004 ; Asthma, 2013 ). Viral respiratory infections, allergens, and tobacco smoke are common triggers for asthma attacks ( National Asthma and Prevention, 2007 ; Healthcare Improvement Scotland, 2019 ; Global Initiative for Asthma, 2023 ). Although asthma-related mortality is generally low, patients with multiple psychosocial risk factors and severe asthma may have a higher mortality risk.
The pharmacological management of asthma involves a stepwise approach to therapy, in which pharmacotherapy is adjusted as necessary, and inhaled short-acting beta-2 agonists (SABAs) are prescribed as reliever medication to all children with symptomatic asthma unless they are on maintenance and reliever therapy with inhaled corticosteroids (ICS)-formoterol ( National Asthma and Prevention, 2007 ; Healthcare Improvement Scotland, 2019 ; Global Initiative for Asthma, 2023 ). The current Global Asthma Management and Prevention Strategy (GINA) guidelines also suggest anti-inflammatory reliever therapy for asthma exacerbations ( Global Initiative for Asthma, 2023 ). Treatment adherence is crucial for achieving optimal respiratory outcomes, preventing attacks, and reducing healthcare costs ( Pearce and Fleming, 2018 ; Kaplan and Price, 2020 ; Wong and Mortin, 2021 ).
Preschoolers represent a special group of patients with asthma. Indeed, accurately assessing symptoms in younger children is difficult because children lack clarity when asked to describe their feelings. In addition, the immature respiratory system can influence the detection of signs and symptoms. Also, lung function tests cannot be performed, and several childhood diseases (e.g., bronchiolitis) can complicate the diagnosis. Therefore, diagnostic methods require the objective documentation of signs or convincing reports from the parents without suspicion of an alternate diagnosis ( Cave and Atkinson, 2014 ; Ducharme et al., 2015 ; Yang et al., 2021 ). Furthermore, patients developing asthma at a very young age are at high risk of decreased respiratory function and lung diseases (early loss of lung function) in adulthood; thus, accurate asthma management is crucial for prognosis ( Montuschi and Barnes, 2011 ; Belgrave et al., 2014 ; Trivedi and Denton, 2019 ).
The knowledge, attitudes, and practices (KAP) methodology can help identify and assess the barriers and misconceptions of a particular population regarding a specific subject ( World Health Organization, 2008 ; Andrade et al., 2020 ). It provides quantitative and qualitative data that can be used to design and implement effective training or educational interventions. Previous studies have shown that although parents’ or legal guardians’ KAP regarding childhood asthma was moderate, their understanding of asthma medication was inadequate ( Zhao et al., 2002 ; Ho et al., 2003 ; Zhao et al., 2013 ; AlOtaibi and AlAteeq, 2018 ; Noureddin et al., 2019 ; Alhammad et al., 2020 ; Alruwaili et al., 2021 ). Moreover, poor KAP regarding asthma medication was linked to inadequate asthma control in children ( Ho et al., 2003 ; Alhammad et al., 2020 ) and poor medication compliance ( Zhao et al., 2002 ). As parents or legal guardians primarily care for children with asthma, understanding their KAP barriers to treatment and medication adherence is of essential importance.
This study aimed to analyze the KAP toward asthma medication and adherence among preschool-aged asthmatic children’s parents and explore the factors influencing adherence.
Materials and methods
Participants.
This study was conducted between February 2023 and April 2023 at our Hospital (respiratory department). Parents of preschool children with asthma were asked to complete the questionnaire containing knowledge, attitude, practice dimensions, and demographic characteristics. The study was advertised in the waiting rooms. The parents could approach the investigator or the research team if interested. The Ethics Committee approved the study [approval # (2023) Lunshen (Research) No. ( Lajunen et al., 2019 )]. Written informed consent was obtained from the study subjects before completing the survey. All eligible and willing-to-participate participants during the time frame were enrolled.
The inclusion criteria were the following: ( Global Initiative for Asthma, 2023 ): parents of preschool children (1–6 years old) diagnosed with asthma according to the GINA ( Global Initiative for Asthma, 2021 ; Healthcare Improvement Scotland, 2019 ) voluntarily participated in this study; ( Papi et al., 2018 ); proficient in using WeChat to answer the questionnaire. The patients with serious physical or mental illnesses prevented from cooperating were excluded. The diagnostic criteria in young children are 1) symptom patterns including wheezing, cough, breathlessness (seen as activity limitation), nocturnal symptoms or awakenings, 2) risk factors for asthma including a family history of atopy, allergic sensitization, allergy or asthma, or a personal history of food allergy or atopic dermatitis, and 3) exclusion of alternate diagnoses ( Global Initiative for Asthma, 2021 ) (the 2021 version was the current one when the study was designed).
Questionnaire design
The questionnaire was designed by the authors for the purpose of the present study with reference to previous literature exploring parental attitudes toward asthma and factors related to children’s medication adherence ( van Dellen et al., 2008 ; Ponieman et al., 2009 ; Klok et al., 2015 ; Koste et al., 2015 ). A random sample of 66 parents was tested for reliability; Cronbach’s α was 0.805, suggesting high internal consistency.
The final questionnaire was in Chinese, and it contained four dimensions: demographic characteristics (age, gender, ethnicity, education, work type, residence, marital status, monthly per capita income, whether anyone in the household smoking, family history of asthma, the severity and duration of asthma, and asthma medication) and knowledge, attitude, and practice dimensions ( Supplementary Material ). The knowledge dimension consisted of 15 questions: 0 points for wrong or unclear answers, 1 point for correct answers, and a total score ranging from 0 to 15 points. The attitude dimension had seven questions: positive attitudes (items A1, A2, A4, A5, and A6) were normally scored with scores ranging from 5–1, indicating a strong agreement to strong disagreement, and negative attitudes (items A3 and A7) were scored reversely with scores ranging from 1 to 5 indicating a strong agreement to strong disagreement; the total score range of the attitude dimension was 7–35. The practice dimension contained 14 items, and items P1-P6 were scored using a five-point Likert scale, with always to never being assigned a score of 5 to 1, with a score range of 6–30. The Morisky Medication Adherence Scale (MMAS) ( Morisky et al., 2008 ) was used to investigate adherence ( Yan et al., 2014 ). The maximum score was 8, where <6 was considered poor adherence, a score between 6 and 7 was considered moderate adherence, and a score of 8 was considered good adherence.
Questionnaire distribution and quality control
The Sojump online platform was used, and the questionnaire was verified after data were imported into the platform. The questionnaires were distributed to the study participants by scanning the QR code onsite via WeChat. A specific IP address could be used to submit a questionnaire only once. Instructions and clarifications were given on the requirements for completing the questionnaire before it was distributed, and any doubts about the questions were answered by trained staff at any time during the questionnaire process.
The questionnaires were automatically anonymized and given serial numbers. Answers to all items were mandatory for submission. The questionnaires with obvious filling patterns (e.g., all first choices), logical errors, and those that took <2 min to complete were excluded.
Statistical analysis
Statistical analysis was performed using STATA 17.0 (Stata Corporation, College Station, TX, United States). The continuous variables were first tested for normality using the Kolmogorov-Smirnov test; the continuous variables conforming to the normal distribution were analyzed using ANOVA (three or more groups) and Student’s t-test (two groups) and presented as means ± standard deviation. Those variables not conforming to the normal distribution were analyzed by Kruskal–Wallis analysis of variance (three or more groups) or Wilcoxon-Mann-Whitney U -test (two groups) and presented as medians (ranges). The categorical variables were presented as n (%) and analyzed using Fisher’s exact or Chi-square tests. Pearson correlation was used to analyze the correlation among the KAP dimensions. Logistic multivariable regression was performed with medication adherence as the dependent variable to analyze the factors associated with medication adherence. Medication adherence was divided into ≥6 points (moderate to good adherence) and <6 (poor adherence), after which a forest plot was made. Two-sided p -values <0.05 were considered statistically significant.
General characteristics
Among a total of 640 collected questionnaires, 8 were excluded as the parents of those children were <18 years old, resulting in 632 valid questionnaires. Most participants were <35 years of age (64.87%), female (75.63%), Chinese Han (93.35%), employed with stable jobs (55.22%), living in urban areas (84.97%), married (98.42%), with a junior college/bachelor’s degree (62.03%), with a monthly income of 5,000–10,000 CNY (45.73%), smoking (62.18%), and without a family history of asthma (68.51%). Most of their children had a history of asthma of 1–3 years (48.73%), had intermittent asthma (48.26%), and were prescribed one drug for asthma (84.65%) ( Table 1 ). The most common drugs were fluticasone propionate (inhalation powder) (62.34%) and fluticasone propionate inhaled aerosol and salmeterol xinafoate (26.42%) ( Table 2 ).
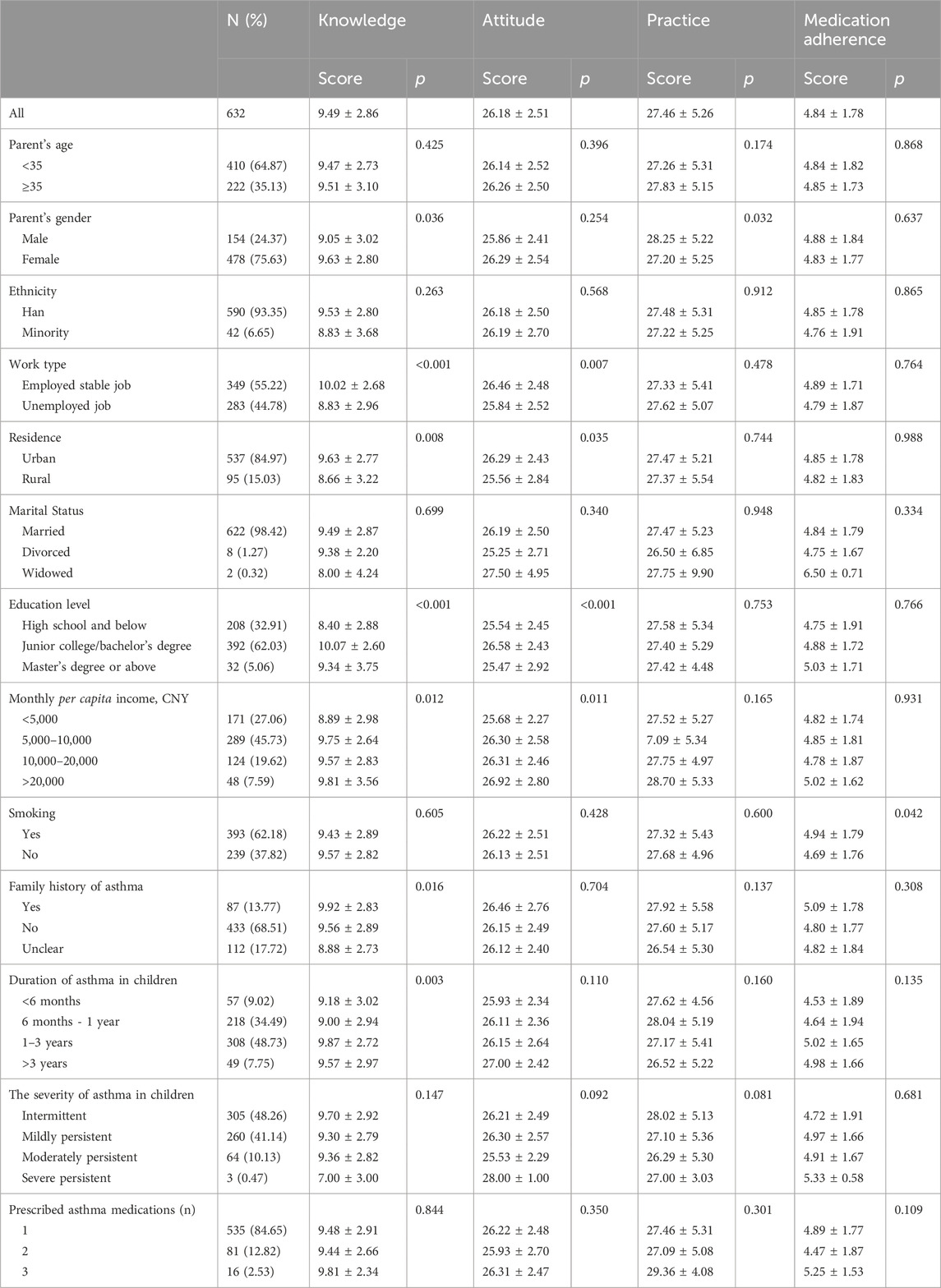
Table 1 . Characteristics of the participants.
Table 2 . Medication status of children with asthma (multiple choice, number).
The knowledge score was 9.49 ± 2.86 (63.27%, possible score range: 0–15), indicating moderate knowledge. Higher knowledge scores were observed in females, participants who were employed and with a stable job, living in urban areas, with a higher education level and higher income, with a family history of asthma, and with longer child’s asthma history (all p < 0.05) ( Table 1 ). As shown in Supplementary Table S1 , the knowledge items with the lowest rates of correct answers were K8 (19.30%, “When a child is exposed to asthma triggers, he or she should wait before taking the medication until symptoms appear”), K12 (32.92%, “Oral medication works as quickly as inhaled medication”), K10 (37.97%, “Long-term inhalation of glucocorticoids is the most effective way to prevent asthma attacks in children”), K13 (40.66, “Inhaled medication has fewer side effects than oral medication”), K11 (42.25%, “Inhaled glucocorticosteroids should be used even when your child is not having an asthma attack”), K3 (54.59%, “3 or more repeated wheezing episodes indicate asthma”), and K2 (59.49%, “Asthma is a neurological or psychological disorder”).
The attitude score was 26.18 ± 2.51 (74.80%, possible score range: 7–35), indicating moderate attitudes. Higher attitude scores were observed in participants with employed stable jobs, junior college/bachelor’s degrees, living in urban areas, and higher income (all p < 0.05) ( Table 1 ). The practice score was 27.46 ± 5.26 (91.53%, possible score range: 6–30), indicating proactive practice. Higher practice scores were also observed in males ( p = 0.032) ( Table 1 ).
The knowledge scores were correlated to practice and attitude scores (practice scores: r = 0.145, p < 0.001; attitude scores: r = 0.359, p < 0.001). The attitude scores were correlated to the practice scores (r = 0.251, p < 0.001) ( Table 3 ).
Table 3 . Correlation analysis between KAP dimensions.
Factors associated with children’s medication adherence
As shown in Figure 1 , the attitude scores (OR = 1.10, 95%CI: 1.01–1.19, p = 0.020), practice scores (OR = 1.16, 95%CI: 1.12–1.21, p < 0.001), and smoking (OR = 1.64, 95%CI: 1.14–2.37, p = 0.008) were associated with medication adherence.
Figure 1 . Multivariable analysis of medication adherence. References: <35 years old, female, minority, non-fixed job, rural, high school or below, <5000 CNY, no smoking, no family history of asthma, duration of asthma in children <6 months, severity of asthma in children (intermittent), and a number of asthma medications ( Global Initiative for Asthma, 2023 ).
Our data showed that the parents of preschoolers in the Southwest region of Chongqing (China) with asthma showed moderate knowledge, attitudes, and active practice toward asthma medication. The attitude and practice scores, as well as smoking, were associated with treatment adherence.
Most participants were <35 years old, which is consistent with the requirement for their children to be <6 years old, as indicated by a previous study ( Al-Binali et al., 2010 ). Also, previous studies showed that a family history of asthma is a crucial risk factor for developing the condition ( Hijazi et al., 2000 ); however, most participants in this study reported no such history. Additionally, the socioeconomic status of the participants was relatively high.
Previous analysis has shown that parents of children with asthma generally have poor KAP toward asthma medication. For instance, a study conducted in Saudi Arabia found that while knowledge about asthma was moderate, knowledge about asthma medication was poor, with several misconceptions prevailing ( AlOtaibi and AlAteeq, 2018 ; Alhammad et al., 2020 ). Similarly, a Khartoum (Sudan) study found that mothers admitted to asthma or emergency departments had inadequate knowledge about asthma ( Noureddin et al., 2019 ). Surprisingly, even in developed countries like the United States, a lack of knowledge about asthma and its treatments has been reported ( Ho et al., 2003 ). A systematic review of eight studies also revealed that poor parental KAP on asthma care was prevalent in both Eastern and Western countries, where being a mother, of young age, and with low socioeconomic status were associated with inadequate KAP ( Alruwaili et al., 2021 ). In Nanjing (China), 55% of parents had poor knowledge of asthma and its management ( Zhao et al., 2002 ), while 29 studies across China showed similar results ( Zhao et al., 2013 ). In contrast to previous studies, we found that parents in the Southwest region of Chongqing had moderate knowledge, attitudes, and active practices toward asthma medication. This could be attributed to the relatively higher socioeconomic status of the participants ( DeWalt et al., 2007 ; Gong et al., 2014 ) and the fact that most of the children in the study were <6 years old and had a history of asthma for 1–3 years. Previous studies presented above-included preschoolers but were not focused on preschoolers, which could influence the results. Nevertheless, the study identified some areas that require education among parents of children with asthma, such as the nature of asthma, triggers, and treatments. The women had higher knowledge scores, while the men had higher practice scores. There are gender differences in access to healthcare, use of healthcare, and help-seeking behaviors between genders ( Mauvais-Jarvis et al., 2020 ). The difference in the knowledge score is consistent with the literature showing a higher health literacy in women compared with men ( Clouston et al., 2017 ; Lee et al., 2015 ; K et al., 2019 ). A study showed that men were using more healthcare services than women ( Cameron et al., 2010 ; Azad et al., 2020 ). Still, the present study was not designed to examine the parents’ gender differences in the management of childhood asthma, but it will be worth studying in the future.
Treatment adherence is crucial for children with asthma ( Pearce and Fleming, 2018 ; Kaplan and Price, 2020 ; Wong and Mortin, 2021 ), particularly in preschoolers ( Belgrave et al., 2014 ; Yang et al., 2021 ). To the best of our knowledge, there are no reports on data assessing the relationship between parental KAP and asthma control, specifically in preschoolers. A study conducted in Saudi Arabia by Alhammad et al. (2020) reported a correlation between poor asthma control and poor parental KAP. However, Ho et al. (2003) reported no association between KAP and asthma outcomes or treatment adherence in United States patients in general. In China, Zhao et al. (2002) found a correlation between poor KAP and poor medication compliance in children. Many parents are concerned about the long-term effects of corticosteroids on their children, including side effects, impacts on development, and possible dependence ( Orrell-Valente et al., 2007 ; Zhao et al., 2013 ).
In the present study, higher practice and attitude scores, as well as smoking, were associated with greater adherence among parents to asthma medication for their preschoolers. As previously reported, the association between attitude and practice scores and adherence is not surprising ( Adams, 2010 ; Carrillo Zuniga et al., 2012 ). Yet, the association with smoking is somewhat surprising. Some studies suggested that exposure to secondary smoke is a significant risk factor for asthma development in children ( National Asthma and Prevention, 2007 ; Healthcare Improvement Scotland, 2019 ; Global Initiative for Asthma, 2023 ) and has a negative effect on asthma control ( Soussan et al., 2003 ; Gerald et al., 2009 ). Tobacco exposure can affect lung function in preschoolers with asthma for as long as 10 years after exposure ( Lajunen et al., 2019 ). Nonetheless, parents may be aware of the harmful effects of smoking on preschool children, prompting them to adhere more to treatment to prevent asthma attacks or make them smoke outdoors to protect their children. Ensuring high adherence may be easier for them than quitting smoking.
The present study has a few limitations. First, only a small proportion of Chinese children were enrolled ( Xiaoyan and Shuxia, 2020 ). Also, only participants from the Southwest region of Chongqing, China, were enrolled. Only the number of prescribed drugs was collected, not their formulation nor their frequency of use. Furthermore, our results reflect a single point in time ( World Health Organization, 2008 ; Andrade et al., 2020 ), but they can serve as a baseline to examine the impact of future educational interventions. As with any KAP survey, there is a possibility of social desirability bias, in which participants may provide socially acceptable answers instead of their actual behaviors ( Bergen and Labonte, 2020 ). Finally, lung function data of children were not collected, which could have provided valuable insights into the impact of treatment adherence on asthma control.
Parents of preschool asthmatic children from the Southwest region of Chongqing, China, showed moderate knowledge, attitudes, and proactive practices toward asthma medication. The attitude and practice scores and smoking were associated with treatment adherence. It is recommended that continuous training and education programs be provided for parents to improve the management of asthma medication among preschool children.
Scope statement
This study aimed to investigate the knowledge, attitude, and practice of parents of preschool children with asthma towards their children’s medication adherence and to explore the factors associated with medication adherence. A cross-sectional study was conducted between February 2023 and April 2023 at the respiratory department of the Children’s Hospital Affiliated to Chongqing Medical University, China. A final questionnaire was developed, containing four dimensions: demographic characteristics, knowledge dimension, attitude dimension, and practice dimension. The Morisky Medication Adherence Scale (MMAS) was used to investigate adherence. The study enrolled 632 parents of preschool children with asthma, and the majority of the participants were female, Chinese Han, living in urban areas, with a junior college/bachelor’s degree, and a monthly income of 5,000–10,000. The results showed that the knowledge and practice scores were low, while the attitude scores were relatively high. Furthermore, logistic multivariable regression showed that low monthly per capita income, poor knowledge, negative attitude, and poor practice were independent risk factors for poor adherence to asthma medication. The findings suggest that interventions should be implemented to improve parental knowledge, attitude, and practice toward asthma medication adherence, particularly among those with low monthly per capita income.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (2023-48). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JT: Writing–review and editing, Investigation. ZZ: Writing–review and editing, Investigation, Supervision, Project administration. RG: Writing–original draft, Methodology, Supervision, Formal analysis, Project administration, Investigation. CN: Writing–review and editing, Methodology, Software. RZ: Writing–review and editing, Investigation. LW: Writing–review and editing, Methodology, Software. NL: Writing–review and editing, Investigation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2020FYYX064); Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2023MSXM047); Research Project of the Children’s Hospital Affiliated to Chongqing Medical University in 2019 (No. CHCQMU 2019.16); Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1292308/full#supplementary-material
Adams, R. J. (2010). Improving health outcomes with better patient understanding and education. Risk Manag. Healthc. Policy 3, 61–72. doi:10.2147/RMHP.S7500
PubMed Abstract | CrossRef Full Text | Google Scholar
Al-Binali, A. M., Mahfouz, A. A., Al-Fifi, S., Naser, S. M., and Al-Gelban, K. S. (2010). Asthma knowledge and behaviours among mothers of asthmatic children in Aseer, southwest Saudi Arabia. East Mediterr. Health J. 16 (11), 1153–1158. doi:10.26719/2010.16.11.1153
Alhammad, A. M., Alajmi, G., Alenizi, A., Alrashidi, E., Alghannam, G., Alaki, E., et al. (2020). Parental attitude and knowledge towards asthma care measures for their children in Saudi Arabia. Pediatr. Pulmonol. 55 (11), 2901–2907. doi:10.1002/ppul.25060
AlOtaibi, E., and AlAteeq, M. (2018). Knowledge and practice of parents and guardians about childhood asthma at king abdulaziz medical city for national guard, riyadh, Saudi Arabia. Risk Manag. Healthc. Policy 11, 67–75. doi:10.2147/RMHP.S143829
Alruwaili, O. M. B., Alaql, M. K. H., Alrubayyi, S. F. W., Alsharatin, A. M. R., Alnour, M. K. A., and Ahmad, A. R. (2021). Awareness and attitude of parents toward asthma care measures in children, a systematic review. Intl J. Med. Dev. Ctries. 5 (1), 347–352. doi:10.24911/IJMDC51-1603214825
CrossRef Full Text | Google Scholar
Andrade, C., Menon, V., Ameen, S., and Kumar Praharaj, S. (2020). Designing and conducting knowledge, attitude, and practice surveys in psychiatry: practical guidance. Indian J. Psychol. Med. 42 (5), 478–481. doi:10.1177/0253717620946111
Asthma, N. C. G. C. (2013). C. C. f. D. C. a. P. Institute of Environmental Health and Related Product Safety and C. C. f. D. C. a. Prevention: third nationwide survey of childhood asthma in urban areas of China [in Chinese with English abstract]. Zhonghua Er Ke Za Zhi 51, 729–735.
PubMed Abstract | Google Scholar
Azad, A. D., Charles, A. G., Ding, Q., Trickey, A. W., and Wren, S. M. (2020). The gender gap and healthcare: associations between gender roles and factors affecting healthcare access in Central Malawi, June-August 2017. Arch. Public Health 78 (1), 119. doi:10.1186/s13690-020-00497-w
Belgrave, D. C., Buchan, I., Bishop, C., Lowe, L., Simpson, A., and Custovic, A. (2014). Trajectories of lung function during childhood. Am. J. Respir. Crit. Care Med. 189 (9), 1101–1109. doi:10.1164/rccm.201309-1700OC
Bergen, N., and Labonte, R. (2020). “Everything is perfect, and we have No problems”: detecting and limiting social desirability bias in qualitative research. Qual. Health Res. 30 (5), 783–792. doi:10.1177/1049732319889354
Cameron, K. A., Song, J., Manheim, L. M., and Dunlop, D. D. (2010). Gender disparities in health and healthcare use among older adults. J. Womens Health (Larchmt) 19 (9), 1643–1650. doi:10.1089/jwh.2009.1701
Carrillo Zuniga, G., Kirk, S., Mier, N., Garza, N. I., Lucio, R. L., and Zuniga, M. A. (2012). The impact of asthma health education for parents of children attending head start centers. J. Community Health 37 (6), 1296–1300. doi:10.1007/s10900-012-9571-y
Cave, A. J., and Atkinson, L. L. (2014). Asthma in preschool children: a review of the diagnostic challenges. J. Am. Board Fam. Med. 27 (4), 538–548. doi:10.3122/jabfm.2014.04.130276
Clouston, S. A. P., Manganello, J. A., and Richards, M. (2017). A life course approach to health literacy: the role of gender, educational attainment and lifetime cognitive capability. Age Ageing 46 (3), 493–499. doi:10.1093/ageing/afw229
DeWalt, D. A., Dilling, M. H., Rosenthal, M. S., and Pignone, M. P. (2007). Low parental literacy is associated with worse asthma care measures in children. Ambul. Pediatr. 7 (1), 25–31. doi:10.1016/j.ambp.2006.10.001
Ducharme, F. M., Dell, S. D., Radhakrishnan, D., Grad, R. M., Watson, W. T., Yang, C. L., et al. (2015). Diagnosis and management of asthma in preschoolers: a Canadian Thoracic Society and Canadian Paediatric Society position paper. Can. Respir. J. 22 (3), 135–143. doi:10.1155/2015/101572
Gerald, L. B., Gerald, J. K., Gibson, L., Patel, K., Zhang, S., and McClure, L. A. (2009). Changes in environmental tobacco smoke exposure and asthma morbidity among urban school children. Chest 135 (4), 911–916. doi:10.1378/chest.08-1869
Global Initiative for Asthma (2021). Global Strategy for asthma management and prevention, updated 2021 . Fontana: Global Initiative for Asthma .
Google Scholar
Global Initiative for Asthma (2023). Global Strategy for asthma management and prevention, updated 2023 . Fontana: Global Initiative for Asthma .
Gong, T., Lundholm, C., Rejno, G., Mood, C., Langstrom, N., and Almqvist, C. (2014). Parental socioeconomic status, childhood asthma and medication use--a population-based study. PLoS One 9 (9), e106579. doi:10.1371/journal.pone.0106579
Healthcare Improvement Scotland (2019). SIGN158. British guideline on the management of asthma . Edinburgh: Healthcare Improvement Scotland .
Hijazi, N., Abalkhail, B., and Seaton, A. (2000). Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax 55 (9), 775–779. doi:10.1136/thorax.55.9.775
Ho, J., Bender, B. G., Gavin, L. A., O’Connor, S. L., Wamboldt, M. Z., and Wamboldt, F. S. (2003). Relations among asthma knowledge, treatment adherence, and outcome. J. Allergy Clin. Immunol. 111 (3), 498–502. doi:10.1067/mai.2003.160
Kaplan, A., and Price, D. (2020). Treatment adherence in adolescents with asthma. J. Asthma Allergy 13, 39–49. doi:10.2147/JAA.S233268
Kiely, K. M., Brady, B., and Byles, J. (2019). Gender, mental health and ageing. Maturitas 129, 76–84. doi:10.1016/j.maturitas.2019.09.004
Klok, T., Kaptein, A. A., Duiverman, E. J., and Brand, P. L. (2015). Long-term adherence to inhaled corticosteroids in children with asthma: observational study. Respir. Med. 109 (9), 1114–1119. doi:10.1016/j.rmed.2015.07.016
Koster, E. S., Philbert, D., Winters, N. A., and Bouvy, M. L. (2015). Adolescents’ inhaled corticosteroid adherence: the importance of treatment perceptions and medication knowledge. J. Asthma 52 (4), 431–436. doi:10.3109/02770903.2014.979366
Lajunen, K., Kalliola, S., Kotaniemi-Syrjanen, A., Malmberg, L. P., Pelkonen, A. S., and Makela, M. J. (2019). Environmental tobacco smoke affects lung function of preschoolers with asthma even after a decade. Am. J. Respir. Crit. Care Med. 199 (4), 534–536. doi:10.1164/rccm.201809-1729LE
Lee, H. Y., Lee, J., and Kim, N. K. (2015). Gender differences in health literacy among Korean adults: do women have a higher level of health literacy than men? Am. J. Mens. Health 9 (5), 370–379. doi:10.1177/1557988314545485
Mauvais-Jarvis, F., Bairey Merz, N., Barnes, P. J., Brinton, R. D., Carrero, J. J., DeMeo, D. L., et al. (2020). Sex and gender: modifiers of health, disease, and medicine. Lancet 396 (10250), 565–582. doi:10.1016/S0140-6736(20)31561-0
Montuschi, P., and Barnes, P. J. (2011). New perspectives in pharmacological treatment of mild persistent asthma. Drug Discov. Today 16 (23-24), 1084–1091. doi:10.1016/j.drudis.2011.09.005
Morisky, D. E., Ang, A., Krousel-Wood, M., and Ward, H. J. (2008). Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. (Greenwich) 10 (5), 348–354. doi:10.1111/j.1751-7176.2008.07572.x
National Collaborative Group on Pediatric Asthma (2004). Comparison of prevalence of childhood bronchial asthma between 2000 and 1990 [J]. Chin. J. Tuberc. Respir. Med. 27 (2), 112–116. doi:10.3760/j:issn:1001-0939.204.02.014
National Asthma, E., and Prevention, P. (2007). Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J. Allergy Clin. Immunol. 120 (5 Suppl. l), S94–S138. doi:10.1016/j.jaci.2007.09.043
Noureddin, A. A., Shaaban, K. M., Mohamed, S. O. O., Abdalla, A. A., Mahmoud, A. A. A., and Salman, M. S. T. (2019). The knowledge attitude and practice (KAP) of mothers of asthmatic children toward asthma in Khartoum asthma clinics. Sci. Rep. 9 (1), 12120. doi:10.1038/s41598-019-48622-2
Orrell-Valente, J. K., Jarlsberg, L. G., Rait, M. A., Thyne, S. M., Rubash, T., and Cabana, M. D. (2007). Parents’ specific concerns about daily asthma medications for children. J. Asthma 44 (5), 385–390. doi:10.1080/02770900701364221
Papi, A., Brightling, C., Pedersen, S. E., and Reddel, H. K. (2018). Asthma. Lancet 391 (10122), 783–800. doi:10.1016/S0140-6736(17)33311-1
Pearce, C. J., and Fleming, L. (2018). Adherence to medication in children and adolescents with asthma: methods for monitoring and intervention. Expert Rev. Clin. Immunol. 14 (12), 1055–1063. doi:10.1080/1744666X.2018.1532290
Ponieman, D., Wisnivesky, J. P., Leventhal, H., Musumeci-Szabo, T. J., and Halm, E. A. (2009). Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann. Allergy Asthma Immunol. 103 (1), 38–42. doi:10.1016/S1081-1206(10)60141-X
Soussan, D., Liard, R., Zureik, M., Touron, D., Rogeaux, Y., and Neukirch, F. (2003). Treatment compliance, passive smoking, and asthma control: a three year cohort study. Arch. Dis. Child. 88 (3), 229–233. doi:10.1136/adc.88.3.229
Trivedi, M., and Denton, E. (2019). Asthma in children and adults-what are the differences and what can they tell us about asthma? Front. Pediatr. 7, 256. doi:10.3389/fped.2019.00256
van Dellen, Q. M., Stronks, K., Bindels, P. J., Ory, F. G., van Aalderen, W. M., and Group, P. S. (2008). Adherence to inhaled corticosteroids in children with asthma and their parents. Respir. Med. 102 (5), 755–763. doi:10.1016/j.rmed.2007.12.005
Wong, C. H., and Mortin, R. W. (2021). Monitoring adherence in children with asthma. Paed Child. Health 31 (7), 284–289. doi:10.1016/j.paed.2021.04.004
World Health Organization (2008). Advocacy, communication and social mobilization for TB control: a guide to developing knowledge, attitude and practice surveys. Available at: http://whqlibdoc.who.int/publications/2008/9789241596176_eng.pdf (Accessed November 22, 2022).
Xiaoyan, S., and Shuxia, L. (2020). Observation of clinical effect of mecobalamin combined withMudan granules in the treatment of type 2 diabetic withPeripheral neuropathy. Clin. Res. 28 (6), 108–109.
Yan, J., You, L. M., Yang, Q., Liu, B., Jin, S., Zhou, J., et al. (2014). Translation and validation of a Chinese version of the 8-item Morisky medication adherence scale in myocardial infarction patients. J. Eval. Clin. Pract. 20 (4), 311–317. doi:10.1111/jep.12125
Yang, C. L., Hicks, E. A., Mitchell, P., Reisman, J., Podgers, D., Hayward, K. M., et al. (2021). Canadian Thoracic Society 2021 Guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can. J. Resp. Crit. Care Sleep. Med. 5 (6), 348–361. doi:10.1080/24745332.2021.1945887
Zahran, H. S., Bailey, C. M., Damon, S. A., Garbe, P. L., and Breysse, P. N. (2018). Vital signs: asthma in children - United States, 2001-2016. MMWR Morb. Mortal. Wkly. Rep. 67 (5), 149–155. doi:10.15585/mmwr.mm6705e1
Zhao, J., Shen, K., Xiang, L., Zhang, G., Xie, M., Bai, J., et al. (2013). The knowledge, attitudes and practices of parents of children with asthma in 29 cities of China: a multi-center study. BMC Pediatr. 13, 20. doi:10.1186/1471-2431-13-20
Zhao, X., Furber, S., and Bauman, A. (2002). Asthma knowledge and medication compliance among parents of asthmatic children in Nanjing, China. J. Asthma 39 (8), 743–747. doi:10.1081/jas-120015798
Keywords: knowledge, attitudes, practice, asthma, children, treatment adherence and compliance, cross-sectional study
Citation: Tang J, Zhao Z, Guo R, Niu C, Zhang R, Wang L and Luo N (2024) Preschool children’s asthma medication: parental knowledge, attitudes, practices, and adherence. Front. Pharmacol. 15:1292308. doi: 10.3389/fphar.2024.1292308
Received: 11 September 2023; Accepted: 16 January 2024; Published: 03 April 2024.
Reviewed by:
Copyright © 2024 Tang, Zhao, Guo, Niu, Zhang, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Guo, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study ), this bullying is related directly to the food allergy. From a medical perspective, there are little published data ...
A recently published study examined the effect of long-term treatment with inhaled budesonide on adult height in children with asthma. 7 This study observed a cohort of 142 children with asthma who had received a mean dose of inhaled budesonide of 412 μg daily. The final adult height of children with asthma was compared with that of control ...
April 23, 2024. Joncita Todechine, a mother of four who lives on the Navajo Nation, knows all too well what can trigger asthma symptoms in her daughter Ashley. But she didn't always. She recalls a time in 2013, living in Phoenix and attending medical assistant school, when she rushed her then-three-year-old to the Indian Medical Center.
Case 9-2013 — A 9-Year ... This child was acutely ill with a respiratory process. Infectious diseases were primary considerations, and Dr. El Saleeby will discuss the differential diagnosis from ...
This case study was prepared for CDC by Dr. LaMar Palmer of MAS Consultants. The purpose of the case study is to share the experience of one community as they attempt to address the problem of asthma. It does not represent an endorsement of this approach by CDC. YES WE CAN Children's Asthma Program: Patient and Family Education
Dr. Wechsler: This case highlights the problem of asthma-associated morbidity and mortality, with more than 4000 deaths from asthma occurring annually in the United States. 1 This patient's ...
Summary. This chapter presents a case study of a 10-year-old girl with moderate intermittent asthma diagnosed at age 4 and was admitted to the pediatric intensive care unit with status asthmaticus. The case study includes details about history of present illness, past medical history, past surgical history, family history, and current status.
Asthma is a complex disease that involves physiological, environmental, and psychosocial factors. This paper reviews childhood asthma case management by social service professionals, lay health workers, and nurses, and it presents a new randomized controlled study using nurse case management in a local community coalition. Evidence suggests the common factor for success involves case managers ...
Clinical presentation (recurrent, episodic attacks of wheezing, cough, dyspnea, itchy red eyes, nasal discharge, stuffiness, and chest tightness), and PFT findings (FEV1/FVC of 0.65, FEV1 is 60% of predictive and post-bronchodilator therapy the FEV1 increases to 74% of predictive) are consistent with the diagnosis of asthma.
Patient Presentation. A 12 year old girl, Annie, enters the Pediatrician's office with complaints of dyspnea, wheezing, and chest tightness. The history of the illness from the family includes: A recent upper respiratory infection with cough, congestion and runny nose with a low grade fever of 100.8 degrees Fahrenheit.
Key Points. Question Is there an association between early life exposure to air pollution and the risk of asthma by early and middle childhood, and is this association modified by individual and community-level characteristics?. Findings In this cohort study of 5279 children, mean fine particulate matter (PM 2.5) and mean nitrogen dioxide (NO 2) air pollution during the first 3 years of life ...
Abstract. Asthma is a complex disease that involves physiological, environmental, and psychosocial factors. This paper reviews childhood asthma case management by social service professionals, lay health workers, and nurses, and it presents a new randomized controlled study using nurse case management in a local community coalition.
Wheezing. Recurrent wheezing, including: during sleep with triggers such as activity, laughing, crying exposure to tobacco smoke or air pollution Difficult or heavy breathing or shortness of breath. Occurring with exercise , laughing, or crying Reduced activity.
Asthma is the the most common chronic respiratory condition of childhood worldwide, with around 14% of children and young people affected. Despite the high prevalence, paediatric asthma outcomes are inadequate, and there are several avoidable deaths each year. Characteristic asthma features include wheeze, shortness of breath and cough, which are typically triggered by a number of possible ...
This case study was prepared for CDC by Dr. LaMar Palmer of MAS Consultants. The purpose of the case study is to share the experience of one community as they attempt to address the problem of asthma. It does not represent an endorsement of this approach by CDC. YES WE CAN Children's Asthma Program: Operation of the Program Overview
YES WE CAN Children's Asthma Program. This case study was prepared for CDC by Dr. LaMar Palmer of MAS Consultants. The purpose of the case study is to share the experience of one community as they attempt to address the problem of asthma. It does not represent an endorsement of this approach by CDC.
The case study presented here is reported separately because it provides an exemplar of nursing leading integrated whole-system service provision for children with asthma. Background The growth of networked and partnership collaborations arises from the recognition that a number of the most pressing public health problems cannot be addressed by ...
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
Asthma Case Study. Asthma affects about 6.1 million children in the US under 18 years of age, making it one of the most common chronic childhood disorders (American Lung Association, 2021). Asthma occurs as a result of a stimulus which can range from allergens, cigarette smoke, changes in temperature, stress, or exercise. ...
Methods: A case control study with 30 cases of childhood asthma and 30 gender- and aged-matched controls selected from the paediatric outpatient department and paediatric ward of a tertiary hospital. The primary caregiver was interviewed to capture sociodemographic details, prenatal and birth history, and history of exposure to environmental ...
Case study Progression of asthma in childhood Ronina A. Covar, MD,a,b Carlyne Cool, MD,c and Stanley J. Szefler, MDa,b Denver, Colo In general, asthma runs a variable course. However, there are children with asthma who are at risk for progressive disease that can lead to failure to attain peak lung growth and
Case study 1: Improving the experience of children with asthma. The PFCC team at Walsall Manor Hospital chose to work to improve the experience of children with asthma, following a patient safety incident some years earlier. The team felt that nationally, management of asthma could be better, and they wanted to work on this as a local priority ...
Some studies suggested that exposure to secondary smoke is a significant risk factor for asthma development in children (National Asthma and Prevention, 2007; Healthcare Improvement Scotland, 2019; Global Initiative for Asthma, 2023) and has a negative effect on asthma control (Soussan et al., 2003; Gerald et al., 2009).