PastQuestions.com.ng
- NECO Past Questions 2021
December 18, 2023 Victoria Jackson NECO , Past Questions 0

NECO Past Questions 2021 – NECO past questions and answers for all subjects are now available for download in PDF format. NECO past questions will help boost your score and overall performance in the 2021 NECO examination. These past questions and answers are created to help all prospective undergraduate students who are searching for NECO past questions for both science and art subjects.
NECO exam’s past questions and answers are provided here for download. If you are one of them, then we wish you good luck as you embark on your journey to write this year’s examination. We have uploaded up-to-date NECO past questions to help you during your preparation.


Why you need NECO Past Questions 2021
The more you practice with past exam questions, the more you sharpen your skills. These papers help you to recall what you have been learning in your class. Besides, it allows you to apply the concepts you have been mastering during your study times. Therefore, past exam papers are a perfect option in your revision for your upcoming exams.
NECO Past Questions 2021 Pattern
NECO Past Questions 2021 follow the original pattern, usually in multiple-choice format. We have made it very easy for you. We bring all the questions for many years and put them together but indicate the specific years of their occurrence. We provide the correct answers to save you time.
All you need to do is devote quality time to studying the NECO Past Questions 2021 and watch yourself change the narrative by scoring better than you expected in the examination.
Sample of NECO Past Questions 2021
A. development of secondary sexual characteristics B. onset of the heat period C. milk let–down after parturition D. development of udder
A. 0.83 kg B. 1.21 kg C. 1.28 kg D. 2. 25kg
A. the eggs cannot be candled B. the chicks are less healthy C. the brooding hens sometimes abandon the eggs D. it takes a longer time for eggs to hatch
A. increase water intake B. provide bulk to feed C Reduce microbial activity D. provide deficient nutrients
A. Aspergillosis B. Acidosis C. Milk fever D. Rickets
A. potential energy B. mechanical energy C. nuclear energy D. solar energy E. kinetic energy
A. harrow B. ridge C. cultivator D. planter E. plough
The complete NECO Past Questions 2021 with accurate answers is N2,000.
Delivery Assurance
How are you sure we will deliver the past question to you after payment?
Our services are based on honesty and integrity. That is why we are very popular.
For us (ExamsGuru Team), we have been in business since 2012 and have been delivering honest and trusted services to our valued customers.
Since we started, we have not had any negative comments from our customers, instead, all of them are happy with us.
Our past questions and answers are original and from the source. So, your money is in the right hands and we promise to deliver it once we confirm your payment.
Each year, thousands of students gain admission into their schools of choice with the help of our past questions and answers. Pastquestions.com.ng
7 Tips to Prepare for NECO Exams
- Don’t make reading your hobby: A lot of people put reading as a hobby in their CV, they might be right because they have finished schooling. But “You” are still schooling, so reading should be a top priority, not a hobby. Read far and wide to enhance your level of aptitude
- Get Exams Preparation Materials: These involve textbooks, dictionaries, Babcock University Post UTME Past Questions and Answers, mock questions, and others. These materials will enhance your mastery of the scope of the exams you are expecting.
- Attend Extramural Classes: Register and attend extramural classes at your location. This class will help you refresh your memory and boost your classroom understanding and discoveries of new knowledge.
- Sleep when you feel like: When you are preparing for any exams, sleeping is very important because it helps in the consolidation of memory. Caution: Only sleep when you feel like it and don’t oversleep.
- Make sure you are healthy: Sickness can cause excessive feelings of tiredness and fatigue and will not allow you to concentrate on reading. If you are feeling as if you are not well, report to your parent, a nurse, or a doctor. Make sure you are well.
- Eat when you feel like it: During the exam preparation period, you are advised not to overeat, and to avoid sleep. You need to eat little and light food whenever you feel like eating. Eat more fruits, drink milk and glucose. This will help you enhance retention.
- Reduce your time on social media: Some people live their entire lives on Facebook, Twitter, WhatsApp, Messenger chat. This is so bad and catastrophic if you are preparing for exams. Try and reduce your time spent on social media during this time. Maybe after the exams, you can go back and sleep in it.
If you like these tips, consider sharing them with your friends and relatives. Do you have a question or comments? Put it on the comment form below. We will be pleased to hear from you and help you score as high as possible. myPastQuestion.com .
We wish you good luck!
- NECO Past Questions
- PAST QUESTIONS
- Past Questions 2021
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Copyright © 2024 | WordPress Theme by MH Themes
You cannot copy content of this page
NECO Chemistry Past Questions and Answers | Theory and Objectives –Download PDF.
NECO Chemistry Past Questions and Answers | Theory and Objectives –Download PDF. The National Examination Council of Nigeria (NECO) Examination is around the corner. This article provides the readers with the information on how to download NECO Chemistry Past Questions and Answers. NECO Chemistry Questions are essential for you to know if you want to pass the NECO SSCE Chemistry Exams 2023.
The NECO Chemistry past questions and answers is a compiled material in PDF that contains past questions from NECO Chemistry Exams of previous years. The material comes with answers and is easily downloadable.
- NECO Commerce Past Questions and Answers | Theory and Objectives –Download PDF.
- NECO English Past Questions and Answers –Download PDF.
You can download the materials here. The instructions on how to download the NECO Chemistry can be found below. You are expected to follow the instructions in order for you to succeed. The material does not only contain information on NECO Chemistry Questions and answers but how to pass exams as well. You can see this article on the 21 indispensable steps to pass any exams with high grade .
Download also: WAEC past Questions and Answers .
NECO Chemistry Sample Questions and Answers
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination.
Related Posts:
- NECO CRS Past Questions and Answers | Theory, and…
- NECO Physics Past Questions and Answers | Theory and…
- NECO Biology Past Questions and Answers | Theory and…
- NECO Government Past Questions and Answers | Theory…
- NECO Economics Past Questions and Answers | Theory…
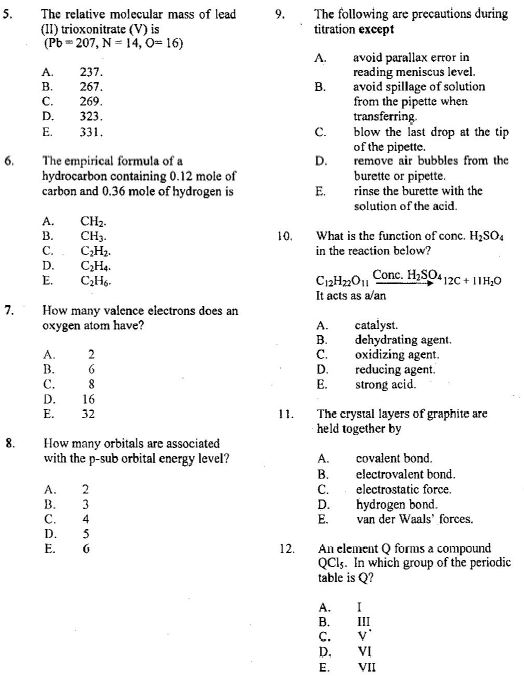
1. The minimum amount of energy required for effective collisions between reacting particles is known A) Activation energy B) Bond energy C) Kinetic energy D) Potential energy
2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is A) Dative B) Covalent C) Electrovalent D) Ionic
3. An element XX forms the following oxides X2O,XOX2O,XO and XO2.XO2. This phenomenon illustrates the law of ________. A) Conservation of mass B) Definite proportion C) Mass action D) Multiple proportion
4.. How many moles of oxygen would contain 1.204×10241.204×1024 molecules? NB: Avogadro’s constant (NA) =6.02×1023=6.02×1023 A) 1 B) 2 C) 3 D) 4
5. Which of the following statements about solids is correct? A) Solid particles are less orderly than those of a liquid B) Solid have lower densities than liquids C) Solid particles have greater kinetic energies than those of liquids D) Solid particles cannot be easily compressed
6. Which of the following apparatus can be used to measure a specific volume of a liquid accurately? A) Beaker B) Conical flask C) Measuring cyclinder D) Pipette
7. The general gas equation PVT=KPVT=K is a combination of A) Boyle’s and Charles’ laws B) Boyle’s and Graham’s laws C) Charles’ and Graham’s laws D) Dalton’s and Graham’s laws
8. The spreading of the scent of a flower in a garden is an example of? A) Brownian motion B) Diffusion C) Osmosis D) Tynadal effect
9. Propane and carbon (IV) oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] Options A) They are both gases B) Their molecules contain carbon C) They have the same relative molecular mass D) Both are denser than air
1O. The energy which accompanies the addition of an electron to an isolated gaseous atom is A) Atomization B) Electronegativity C) Electron affinity D) Ionization
11. A sample of hard water contains some calcium sulphate and calcium hydrogen carbonate. The total hardness may therefore be removed by A. boiling the water B. adding excess calcium hydroxide C. adding a calculated amount of calcium hydroxide D. adding sodium carbonate E. adding magnesium hydroxide
12. During the electrolysis of copper II sulphate between platinum electrodes, if litmus solution is added to the anode compartment, A. the litmus turns blue but no gas is evolved B. the litmus turns blue and oxygen is evolved C. the litmus turns blue and hydrogen is evolved D. the litmus turns red and oxygen is evolved E. the litmus turns red and then becomes colourless
13. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as; A. saponification B. dehydration C. esterification D. hydrolysis E. hydration
14. The IUPAC names of the compounds CH3COOH and CH2=CH2 are respectively; A. acetic acid and ethane B. ethanoic acid and ethene C. methanoic acid and ethylene D. ethanol and ethene E. acetic acid and ethylene
15. If 30cm3 of oxygen diffuses through a porous pot in 7 seconds, how long will it take 60cm3 of chlorine to diffuse through the same pot, if the vapour densities of oxygen and chlorine are 16 and 36 respectively? A. 9.3 sec B. 14 sec C. 21 sec D. 28 sec E. 30.3 sec
16. When heat is absorbed during a chemical reaction, the reaction is said to be A. thermodynamic B. exothermic C. isothermal D. endothermic E. thermostatic
17. When large hydrocarbon molecules are heated at high temperature in the presence of a catalyst to give smaller molecules, the process is known as A. disintegration B. polymerization C. cracking D. degradation E. distillation
18. The pH of four solutions W, X, Y, Z are 4, 6, 8, 10 respectively, therefore A. none of these solutions is acidic B. the pH of Y is made more acidic by addition of distilled water C. Z is the most acidic solution D. W is the most acidic solution E. X is neutral
19. When each of the nitrates of Potassium, Magnesium and iron is heated, A. all the nitrates decompose to their oxides B. the nitrate of magnesium gives the nitrite and oxygen C. the nitrates of iron magnesium and iron give the oxides D. the nitrate of iron gives the nitrite and oxygen E. the nitrate of the magnesium is not decomposed
2O. Which of the following metals cannot replace hydrogen from water or steam? A. Sodium B. Magnesium C. Iron D. Calcium E. Copper
21. small quantity of solid ammonium chloride (NH4Cl) was heated gently in a test tube, the solid gradually disappears to produce two gases. Later, a white cloudy deposit was observed on the cooler part of the test tube. The ammonium chloride is said to have undergone A. distillation B. sublimation C. precipitation D. evaporation E. decomposition
22. Elements P, Q, R, S have 6, 11, 15, 17 electrons respectively, therefore, A. P will form an electrovalent bond with R B. Q will form a covalent bond with S C. R will form an electrovalent bond with S D. Q will form an electrovalent bond with S E. Q will form a covalent bond with R
23. An element X forms the following compounds with chlorine; XCl4, XCl3, XCl2. This illustrates the A. law of multiple proportions B. law of chemical proportions C. law of simple proportions D. law of conservation of mass E. law of definite proportions
24. The oxidation state of chlorine in potassium chlorate is A. +1 B. +2 C. +3 D. +5 E. +7
25. 10 When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are; A. nitrogen and carbondioxide B. the rare gases C. nitrogen and oxygen D. nitrogen and the rare gases E. nitrogen, carbondioxide and the rare
26. Which of the following statements is NOT correct? A. The average kinetic energy of a gas is directly proportional to its temperature B. At constant tempearture, the volume of a gas increases as the pressure increases C. The pressure of a gas is inversely proportional to its volume D. The temperature of a gas is directly proportional to its volume E. The collisions of molecules with each other are inelastic
27. Zinc Oxide is a A. Basic Oxide B. Acidic Oxide C. Amphoteric Oxide D. Neutral Oxide E. Reactive Oxide
28. When sodium chloride and metallic sodium are each dissolved in water A. both processes are exothermic B. both processes are endothermic C. the dissolution of metallic sodium is endothermic D. the dissolution of metallic sodium is exothermic E. the dissolution of sodium chloride is explosive
29. The periodic classification of elements is an arrangement of the elements in order of their A. Atomic Weights B. Isotopic Weights C. Molecular Weights D. Atomic Numbers E. Atomic Masses
3O. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid? A. 5cm3 B. 10cm3 C. 20cm3 D. 25cm3 E. 50cm3
How to Download NECO Chemistry past Questions and Answers
Here are the instructions to be followed in order to download the NECO Chemistry Questions and answers without any issue. Please follow our instructions and guide very well.
The cost of the newly updated NECO Past Questions and Answers is 2,000.00 naira only for One Subject e.g
- Use of English 1000 naira
- Biology 1000 naira
- Chemistry 1000 naira
- Physics 1000 naira
See The Payment Detail Below.
To get NECO Past Questions and Answers follow the steps below.
Pay into the account below.
Account Details Account Name: ETUK, OTO-OBONG EMMANUEL
Account Number : 0318379097 Bank : GTBANK .
After Payment , send the following
(1) Depositors name,
(2) Teller number,
(3) Amount paid to 07063986527 on whatsapp or [email protected]
The purchased NECO Past Questions and Answer will be uploaded immediately into your email address within the next 24 hours.
If you have purchased yours, kindly drop a testimony in the comment box below.
CALL or WhatsApp EXAMSTUTS: 07063986527. Send all your mails to [email protected]
Tips on How to Pass NECO Examination 2023
If you want to Past the NECO Examination this year, you will need to follow the instructions below.
- Practice daily with NECO Past Questions and Answers
- Write out your subjects, the dates and time.
- Engage in general revision few days to the examination and dwell more on the subjects you find difficult.
- Focus on only your first paper a week to the exam. This would make it sweet and you will have energy to read for other papers.
- Don’t read any other subject when it is two days to a particular subject.
- When you have two papers that are separated by only one day, read the second one first before going to the subject you have first.
- Ensure to be in the NECO examination venue at least two hours to the examination so that you would be balanced.
- Try to be up to date in cases of changes in the timetable.
- Have a personal copy of the NECO timetable and syllabus.
- Go through your timetable daily so that you don’t miss any paper.
- Don’t go to the hall when you are not having paper that day.
Disclaimer: Please note these are not the actual questions and answers that you will find in the NECO Examination this year, but they are the likely questions. to get the actual NECO Past Questions and answers, please call 07063986527.
Download Actual NECO Past Questions and Answers.
If this article on NECO Chemistry Questions and Answers has been helpful Please share and Like us on Facebook@Examstuts
If You Want More Information, please subscribe to our email list and leave a comment with your number below, also Follow us on Facebook, Twitter, and Linkedin
For Inquiries on all Past Questions Call 07063986527 or Chat on WhatsApp.
Disclaimer: Please note that we are not in any way affiliated with any of the organizations or institutions here. All articles here are for the sole purpose of providing information. All Past questions are gotten from previous years’ examinations and likely questions from the Internet and related exams. We are not in any way promising that what you find in the past questions is what you will find in your examination. We are not in any way responsible for how the reader uses the information here. Please do consult an expert or professional in your field should the need arise. Copyrights Infringement: No article from this website should be copied without a proper reference and link to the page picked from. Anything otherwise will lead to legal action of copyright infringement. All articles on this website are products of painstaking research from our writers and journalist. Should you find any material bearing semblance here to any material on your page, please quickly notify us by sending a mail to [email protected] and we will immediately commence the process of taking it down.
NECO Data Science Past Questions and Answers | Theory, and Objectives –Download PDF.
Neco past questions and answers for all subjects | free pdf download, related articles, neco further mathematics past questions and answers | theory and objectives –download pdf., neco timetable 2023 pdf download (june/july)- download here., neco economics past questions and answers | theory and objectives –download pdf., neco yoruba past questions and answers | theory and objectives –download pdf..

NECO Geography Past Questions and Answers | Theory and Objectives –Download PDF.
- NECO Result Checker 2023 (June/July)- https://result.neco.gov.ng | Check NECO Results
NECO Chemistry Questions and Answers 2022/2023 Theory & OBJ Expo
- March 29, 2022
Getting NECO Chemistry Questions 2021/2022 with solved NECO chemistry answers 2021 before the exam had been a big dream for candidates looking for NECO Chemistry questions and answers in 2021
That is the reason we are sharing this NECO chemistry expo 2021 with candidates so that they will not be stranded during the exam.
Proper usage of this material will guarantee you sure insights on the kind of NECO questions for chemistry 2021 that you should expect and how to draft expo answers for them.
OBJ (Paper 1)NECO Questions and Answers for Chemistry 2021.
The chemistry questions and answers for NECO 2021 will be in two papers. The first paper (paper 1) is the OBJ (objective) and the second paper is paper 2 (theory, essay, calculations and show working).
So in this aspect, I will supply you with the basic NECO chemistry questions 2021 samples with correct NECO chemistry answers 2021 to the NECO 2021 chemistry questions and answers samples.
Check out the sample solved Questions and Answers below.
- Solved Chemistry Answers
- Practice Chemistry Questions and answers
- Secret on how to pass NECO 2021
Sample OBJ Chemistry Questions and Answers for NECO 2021

Download NECO 2021 Syllabus for Chemistry
1. Who found the Periodic table?
- Period Dickson
- John Dalton
The correct answer is D. Mendeleev
2. Periodic table is arranged in ____and _____
- Lanthanide and Actinide
- Groups and periods
- Groups and electronic configuration
- Atomic number and outer shell
- Transition and atomic shell
The correct answer is B. Groups and periods
3. Which of these is not a constituent of Urine.
The answer is E. Bacteria
4. Bronze is made up of _____ and _____?
- Iron and copper
- Tin and iron
- Copper and tin
- Nickel and iron
- Zinc and copper
The answer is copper and tin
5. Which of the following is not a metalloids
The correct answer is D. Nickel
6. The most reactive non-metal is_____?
The correct answer is A. Fluorine
7. What is the I.U.P.A.C name of CH 3 CH 2 OH?
The correct answer is D. Ethanol
8. Which of these is not an aromatic compound
- Acetophenone
The correct answer is E. Tulane
9. The chemical symbol for Silver is____?
The answer is B. Ag
10. An element with an atomic number of 16 will likely belong to group_____ on the periodic table.
The answer is E.6
11. Group 7 elements are also known as _______?
- Alkali metals
- Transition elements.
- Alkali earth metals.
The correct answer is A. Halogens
Get answers for chemistry practical paper
Theory Paper 2, Chemistry Questions and answers 2021 Samples
These are NECO 2021 theory Chemistry Questions and answers 2021 Samples for paper 2. This is the second paper (paper 2) and it is an “essay, calculations and show working section”.
Practice these questions because they might come up in the exam.
1. a. State Faraday’s first law of electrolysis.
Faraday’s first law of electrolysis states that the mass (m) of a substance liberated at an electrode during electrolysis is directly proportional to the quantity of electricity (Q) passing through the electrolyte.
Mathematically;
Where quantity of electricity = current (I) x time (t).
i.e Q = It.
ii. Explain an electrolytic cell.
An electrolytic cell consists of a container of electrolytes with two electrodes connected to a suitable direct current supply.
2a. List 4 constituents of Air.
- Carbon (iv) oxide
- Rare gases.
2b. Write the IUPAC name of CH 2 OH CHOHCHO: Ans. 2, 3-Dihydroxy propanal.
3. When 4.5g of liquid water was formed by burning hydrogen gas in oxygen, -72KJ of heat was given off. Calculate the standard heat of the formation of water.
Equation of reaction.
H 2(g) + 1 / 2 O 2(g) → H 2 O(l) ∆H © f = ? KJmol -1 .
4.5g of liquid water produces -72KJ of heat
1.0g of liquid water produces – 72
= -16.0KJ of heat
Therefore, 18g of liquid water will produce: (16.0 x 18) KJ of heat = -288KJmol -1
4. Define the following terms:
- Bomb calorimeter
- Heat of fusion
- Spontaneous reaction
- Gibbs free energy.
Bomb calorimeter: A bomb calorimeter consists of a strong cylindrical steel vessel lined with enamel to prevent corrosion.
The heat of fusion: This is the heat change when one mole of solid is melted.
Entropy: Entropy is a degree of disorderliness or randomness of a system.
Spontaneous reaction: A spontaneous reaction is a reaction that occurs on its own without the assistance of an external agent.
Gibbs free energy: The free energy (G), of a chemical system is the energy that is available for doing work.
NECO Expo for Chemistry exam 2021 (Important terms)
Now that you have gotten some sample NECO chemistry theory and OBJ questions for 2021 to practice with, let me give you a quick and common expo hint for Chemistry exam 2021 (Important terms to note)
IUPAC: IUPAC means, International Union of Pure and Applied Chemistry.
Nucleus: The Nucleus is made up of Proton and neutron.
Laws of Chemical combination includes;
- Law of conservation of mass
- The law of definite proportions or constant composition.
- Law of multiple proportions.
Chemical symbol: A chemical symbol of an element represents an atom of the element.
Radicals: Radicals are groups of an atom carrying charges that keep their identity and react as a single unit.
Empirical formula: Empirical formula is the simplest possible formula giving the ratio of atoms in a molecule of the compound.
Mole: A mole is the amount of substance that contains as many elementary entities as that in a 12g of carbon.
Isotope: An isotope occurs in elements with the same atomic number (Number of protons) but different mass numbers (because of varying numbers of neutrons).
- The Avogadro’s Number (N A ) is 6.02×1023
PH scale: the PH scale is numbered from 0 – 14. From 0 to 6 indicates the degree of acidity, 7 is neutral while, above 7 to 14 indicates the degree of alkalinity.
I am sure these NECO Chemistry Questions and answers 2021 material will help you to perform better in this exam.
Please, treat the above questions as sample questions and not as the main exam questions.
Thanks a lot
you’re welcome.
Thanks for your help
Thanks so much for your help
Nice page of learning
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
NECO Accounting Questions and Answers 2022/2023 (Essay and Objectives)
Neco civic education questions and answers 2021/2022 (theory & obj), you may also like, waec syllabus for mathematics 2022/2023 & textbook download pdf.
- December 20, 2021
NECO Government Questions and Answers 2022/2023-Theory &OBJ Expo
- April 7, 2022
NECO Commerce Questions and Answers 2022/2023: Theory & OBJ Expo
- February 23, 2022

Download NECO Syllabus for Chemistry 2020/2021 & Textbooks PDF
- April 25, 2022

NECO Syllabus For Further Mathematics 2020/2021 PDF & Textbooks
- April 19, 2022
WAEC GCE Mathematics Questions, Answers 2020/2021 & Syllabus
- April 26, 2022
Jamb Admission
Latest nigerian school news, neco chemistry questions and answers 2021/2022 expo.

NECO Chemistry Per Subject
To subscribe for Chemistry send MTN recharge card of N700 now to 08133139104. wait for confirmation message. After you will see the questions and answers directly to your phone. We will not post free neco questions. See screenshots of Past NECO,WAEC, GCE students we’ve helped so far
To subscribe for our 2021/2022 Neco assistance program, it cost N5000 for all subjects and payment is made directly to our account number. We currently have the correct complete and verified Chemistry Questions and Answers available. We assure you of A in this Chemistry Paper and all other papers you will write in your NECO examination. All NECO subjects Questions and Answers [Objectives and theory] has been posted here. Click here to see them
Once you’ve subscribe to our Neco assistance program expo runs you will be added to our official Whatsapp group chat where you will be getting daily questions and answers, we guarantee you A’s and B’s in all your subjects. Click Here To See NECO 2021/2022 EXPO Chemistry Objective & Essay Questions And Answers
To subscribe to our 2021/2022 NECO may/June Expo runs assistance program kindly contact jambadmission.com.ng via; SMS and Whatsapp only 08133139104. We repond very well to whatsapp. Please do not contact us asking for free questions and Answers cause we won’t reply you.
How to subscribe for NECO 2021/2022 EXPO Chemistry Objective & Essay Questions And Answers
Pay a one-time fees of N5000 for all subjects To Our bank Account below Forward Your Name and bank depositors name, List of Subjects, your WhatsApp Phone number to: 08133139104 We will send Chemistry Objective & Essay directly to your submitted phone numbers 9hours before the exam day. We assure you a flying colours in all your papers.
Free NECO 2021/2022 EXPO Chemistry Objective & Essay Questions And Answers
NECO 2021/2022 Chemistry Essay is available and the content is locked. To unlock this content kindly share this page to 15 whatsApp group chat, Facebook and twitter profile using the share button below this post. After then you should be able to see the full questions and answers. If you don’t see any questions and answers kindly carry out the above instructions.
Invite your friends to jambadmission.com.ng for NECO Free Questions and Answers
Content Locked: Chemistry questions and answers are now available, To unlock fast, kindly comment your phone number and your list of subjects, after doing this we will forward Chemistry to your inbox through SMS or on this very page. So do not bother subscribing to any other site, always revisit this site cause we are the best examination portal for WAEC JAMB NECO NABTEB.
We have provided answers to your questions like; Neco 2021/2022 Chemistry examination runz, Free website to get Chemistry Neco expo questions and Answers directly to your inbox, 2021/2022 Chemistry exam runs, Neco 2021/2022 nov/dec 100% correct expo, Neco 2021/2022 100% real expo/runz, best Neco 2021/2022 expo site. 2021/2022 Neco/Ssce Chemistry (Obj And Essay) Expo/ Questions/ Answers Free. Free Neco 2010 Chemistry (Obj And Essay) Questions And Answers, 2021/2022 Free Neco Chemistry Answer, 100% 2021/2022 Essay And Objective Question And Answers, Download 2021/2022 Neco Chemistry (Obj And Essay) Essay And Objective Answers Neco Answer, Neco 2021/2022 Free No Payment Essay And Objective answers Chemistry (Obj And Essay) Essay And Objective Answer For Neco 2021/2022, Chemistry 2021/2022 Neco Expo For Chemistry (Obj And Essay) Science Theory/ Essay And Obj/Objective Answers
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Copyright © 2023 | All Rights Reserved.
| |
Register • Login
Chemistry neco past questions and answers.
- Classroom
- Jamb
- Waec
- Neco
- Post Utme
- Bece
- Ncee
- Mock
| White is NOT a property of metals? Ductile: Malleable Conduct heat and electricity Have many oxidation states High melting points anti boiling points The Atomic theory model which explains that electrons in an atom revolve round quantized energy levels is attributed to ____________ Becquerel. Bohr. De Broglie. Rutherford. Thompson. Which compound will have the lowest boiling point? CH OH CH COOH CH CHOH C H (CH ) CH The molar mass of a compound X is 56. its empirical formula is CH2. X is ____________ Butene. Butyne. Ethene. Propene. Propyne The Kinetic theory can NOT be used to explain Allotropy. Brownian movement. Diffusion. Tyndal effect. Vaporization. | |







IMAGES
COMMENTS
CLICK TO SEE ANSWERS. (Chemistry - NECO 2021) Direct Mobile/SMS: N800 MTN CARD. Password/Link: N500 MTN CARD. Forward Subject Name, MTN CARD PINS, Phone number to: 08074021548 via TEXT MESSAGE ...
Besides, it allows you to apply the concepts you have been mastering during your study times. Therefore, past exam papers are a perfect option in your revision for your upcoming exams. NECO Past Questions 2021 Pattern. NECO Past Questions 2021 follow the original pattern, usually in multiple-choice format. We have made it very easy for you.
#NECO CHEMISTRY ANSWER 2021 #ZAMGIST #NECO 2021CLICK HERE - https://zamgist.com.ngAnswer page - https://lite.zamgist.com.ngNECO 2021 CHEMISTRY ESSAY AND OBJE...
NECO Chemistry Sample Questions and Answers. The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. 2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is.
The chemistry questions and answers for NECO 2021 will be in two papers. The first paper (paper 1) is the OBJ (objective) and the second paper is paper 2 (theory, essay, calculations and show working). So in this aspect, I will supply you with the basic NECO chemistry questions 2021 samples with correct NECO chemistry answers 2021 to the NECO ...
NECO 2021/2022 Chemistry Essay is available and the content is locked. To unlock this content kindly share this page to 15 whatsApp group chat, Facebook and twitter profile using the share button below this post. After then you should be able to see the full questions and answers. If you don't see any questions and answers kindly carry out ...
NECO 2021 Chemistry Essay and Objective Answers (28th July, 2021) Wednesday 28th July, 2021. Paper III & II: Objective & Essay - Chemistry 10:00am - 1:00pm. OBJECTIVE ANSWERS 1-10 EECBBAEBBB 11-20...
C. H 2 2 O 2 2. D. NO 2 2. View Answer & Discuss (3) WAEC 2021. 3. The ratio of carbon atoms of hydrogen atoms in a hydrocarbon is 1:2. If its molecular mass is 56, what is its molecular formula? A. C 3 3 H 6 6.
Question 1 : NECO 2001. White is NOT a property of metals? A. Ductile: B. Malleable. C. Conduct heat and electricity. D. Have many oxidation states. E. High melting points anti boiling points.
Download Now! National Examination Council NECO Chemistry Past Questions; Paper 2 - Theory/Essay Questions; Paper 3 - Objective Test Questions
The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm. This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write. Here, we will be posting the neco chemistry questions for candidates that will ...
This is a perfect guide for NECO practical chemistry examination. The question has been perfectly answered targeting all possible questions and answers
Undiluted Chemistry neco expo runz from best expo site 2021 (1) ( Reply ) Free Robotics Course For Beginners By University Of Sheffield / 2021 WAEC GCE Registration Form - Instructions And Guidelines [august/september / The Easy Way To Install Windows 11 On Unsupported Cpus
Nairaland Forum / Nairaland / General / Education / 2021 Neco Chemistry Questions And Answers, Chemistry Obj And Essay Answers (332 Views) 2023 NECO Chemistry Practical | Questions & Answers 1 / Neco 2020 Chemistry Obj And Theory Available / 2020 Waec Sure Success Answers ( Chemistry).. (2) (3) (4) Accurate NECO 2021 Chemistry answer, NECO ssce ...
Equilibrium is said to be attained in reversible reaction when. A. all the reactants have been used up. B. all the products have been formed. C. there is no further change in temperature. D. the rates of the forward and backward reactions are equal. E. the rate of formation of the products decreases with time. View Answer & Discuss (7) WAEC 1988.
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. A) Activation energy. B) Bond energy. C) Kinetic energy. D) Potential energy. 2.
Chemistry Paper 3 (III) Objective Test Questions. Each question is followed by five options lettered A - E. 1. The raw material used in cement production is. A. calcium trioxocarbonate (IV). B. hydrochloric acid. C. sodium trioxocarbonate (IV). D. tetraoxosulphate (VI) acid. E. trioxocarbonate (IV) acid.
The answers to Chemistry Practical,NECO 2021. Complete answers with detailed explanation.
Nairaland Forum / Nairaland / General / Education / Neco 2021 Chemistry(obj And Essay) Chemistry Questions And Answers Now Posted (166 Views) 2021 Neco Chemistry (obj And Essay)chemistry Questions And Answers, Chemistry / Neco 2020 Chemistry Obj And Theory Available / WAESSCE 2020/2021 Chemistry Practical Instructions (1)
Check out Physics Practical Solution here. According to the NECO Exam Timetable, the Chemistry Essay & Objective has been scheduled to take place on Wednesday 28th July, 2021 from 10:00 am to 01:00 pm. Receive the correct NECO Chemistry Essay & Objective Expo 2021, NECO Chemistry Essay & Objective Expo Answers 2021 midnight before your exam.
NECO 2024 Chemistry essay and OBJ answers is now available. If you are writhing this subject, Here is 2024 NECO Chemistry Questions June/July verified solution. Thd news reaching us is that the exam will take place on 12th of July, 2024. The National Examinations Council (NECO) is a Nigerian examination body responsible for conducting and ...
View Answer & Discuss (3) NECO 2009 167 What volume of carbon (IV) oxide in dm 3 is produced at s.t.p. when 2.50g of CaCO 3 reacts with excess acid according to the following equation?