Sleep Terrors: An Updated Review
Affiliations.
- 1 Department of Pediatrics, The University of Calgary and The Alberta Children’s Hospital, Calgary, Alberta, Canada
- 2 Department of Family Medicine, The University of Alberta, Edmonton, Alberta, Canada
- 3 Department of Family Medicine, The University of Calgary, Calgary, Alberta, Canada
- 4 Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong
- 5 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong
- PMID: 31612833
- PMCID: PMC8193803
- DOI: 10.2174/1573396315666191014152136
Background: Sleep terrors are common, frightening, but fortunately benign events. Familiarity with this condition is important so that an accurate diagnosis can be made.
Objective: To familiarize physicians with the clinical manifestations, diagnosis, and management of children with sleep terrors.
Methods: A PubMed search was completed in Clinical Queries using the key terms "sleep terrors" OR "night terrors". The search strategy included meta-analyses, randomized controlled trials, clinical trials, observational studies, and reviews. Only papers published in the English literature were included in this review. The information retrieved from the above search was used in the compilation of the present article.
Results: It is estimated that sleep terrors occur in 1 to 6.5% of children 1 to 12 years of age. Sleep terrors typically occur in children between 4 and 12 years of age, with a peak between 5 and 7 years of age. The exact etiology is not known. Developmental, environmental, organic, psychological, and genetic factors have been identified as a potential cause of sleep terrors. Sleep terrors tend to occur within the first three hours of the major sleep episode, during arousal from stage three or four non-rapid eye movement (NREM) sleep. In a typical attack, the child awakens abruptly from sleep, sits upright in bed or jumps out of bed, screams in terror and intense fear, is panicky, and has a frightened expression. The child is confused and incoherent: verbalization is generally present but disorganized. Autonomic hyperactivity is manifested by tachycardia, tachypnea, diaphoresis, flushed face, dilated pupils, agitation, tremulousness, and increased muscle tone. The child is difficult to arouse and console and may express feelings of anxiety or doom. In the majority of cases, the patient does not awaken fully and settles back to quiet and deep sleep. There is retrograde amnesia for the attack the following morning. Attempts to interrupt a sleep terror episode should be avoided. As sleep deprivation can predispose to sleep terrors, it is important that the child has good sleep hygiene and an appropriate sleeping environment. Medical intervention is usually not necessary, but clonazepam may be considered on a short-term basis at bedtime if sleep terrors are frequent and severe or are associated with functional impairment, such as fatigue, daytime sleepiness, and distress. Anticipatory awakening, performed approximately half an hour before the child is most likely to experience a sleep terror episode, is often effective for the treatment of frequently occurring sleep terrors.
Conclusion: Most children outgrow the disorder by late adolescence. In the majority of cases, there is no specific treatment other than reassurance and parental education. Underlying conditions, however, should be treated if possible and precipitating factors should be avoided.
Keywords: Impaired arousal; night terrors; nightmares; non-rapid eye movement sleep; parasomnias; pavor nocturnus.
Copyright© Bentham Science Publishers; For any queries, please email at [email protected].

Publication types
- Child, Preschool
- Diagnosis, Differential
- Night Terrors / diagnosis*
- Night Terrors / epidemiology
- Night Terrors / etiology
- Night Terrors / therapy*
- Sleep / physiology
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Neuropsychopharmacology Reviews
- Published: 23 August 2019
Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms
- Anne Richards 1 , 2 na1 ,
- Jennifer C. Kanady 1 , 2 na1 &
- Thomas C. Neylan 1 , 2
Neuropsychopharmacology volume 45 , pages 55–73 ( 2020 ) Cite this article
12k Accesses
96 Citations
33 Altmetric
Metrics details
- Neuroscience
A Correction to this article was published on 07 October 2019
This article has been updated
The current report provides an updated review of sleep disturbance in posttraumatic stress disorder and anxiety-related disorders. First, this review provides a summary description of the unique and overlapping clinical characteristics and physiological features of sleep disturbance in specific DSM anxiety-related disorders. Second, this review presents evidence of a bidirectional relationship between sleep disturbance and anxiety-related disorders, and provides a model to explain this relationship by integrating research on psychological and neurocognitive processes with a current understanding of neurobiological pathways. A heuristic neurobiological framework for understanding the bidirectional relationship between abnormalities in sleep and anxiety-related brain pathways is presented. Directions for future research are suggested.
You have full access to this article via your institution.
Similar content being viewed by others
Overanxious and underslept
Integrating sleep, neuroimaging, and computational approaches for precision psychiatry
Investigating the effect of a nap following experimental trauma on analogue PTSD symptoms
Introduction.
Sleep is a vital physiological process, disruptions of which affect performance in multiple domains of functioning [ 1 ], including but not restricted to cognitive [ 2 , 3 ], emotional [ 4 ], metabolic [ 5 ], and immunologic [ 6 ]. Focusing on posttraumatic stress disorder (PTSD) and other anxiety-related disorders specifically, published research points to a bidirectional relationship between disturbances in sleep and anxiety-related disorders. Neurobiological research ranging from animal research to human neuroimaging and polysomnography-based research, alongside clinical treatment research, provide insights about this relationship, while also highlighting questions that remain to be answered. A heuristic neurobiological framework for this relationship is proposed to contribute to ongoing research in this area.
The study of sleep–wake regulation and the neurobiology of sleep disturbance in humans: an introductory overview
Both human and basic animal research have driven a surge in our understanding of sleep neurobiology in recent decades. In the 1950’s and 1960’s, researchers coined the terms REM and NREM sleep (REMS, NREMS) after observing that humans cycled through periods of sleep with unique electroencephalography (EEG) signatures combined with distinct eye movement patterns and muscle activity levels [ 7 , 8 , 9 ]. Since then, the field of sleep research has started to unravel the significance of sleep and its different visually scored stages (NREM N1, N2, N3, and REMS) for various essential functions, including but not limited to synaptic homeostasis [ 10 , 11 ], cognitive function [ 2 , 12 ], emotion regulation [ 4 ], memory processing and consolidation [ 10 , 13 , 14 , 15 ], glucose metabolism [ 5 ], immunity [ 6 ], and more [ 1 ]. Since the mid-20th century as well, numerous studies have also established that circadian and homeostatic processes regulate sleep in humans [ 16 , 17 ]. Although understanding the functional neuroanatomy of sleep regulation in humans is still impeded by the challenges of imaging the human brain during sleep, animal research has greatly advanced our grasp of sleep/wake neurocircuitry in mammals, and in combination with pharmacological, EEG, and imaging studies in humans, provides clues regarding the relevance of different brain nuclei and circuits in human sleep.
The existing research points to distinct nuclei and brain regions with prominent and preferential roles in promoting NREMS (which includes slow-wave sleep (SWS/N3 sleep), REMS, and the wake state. Although the most-recent evidence challenges the relative simplicity of sleep/wake neurocircuitry models developed in recent decades (e.g., see [ 18 ]), the extant research strongly indicates that specific brain regions and cell types are preferentially active in NREMS, REMS, and/or the wake state, and that these nuclei and cell types influence each other via positive and negative feedback mechanisms in the service of regulating sleep and wake. Figure 1 depicts a current understanding of important brain nuclei involved in NREMS, REMS, and wake regulation. Mutually inhibitory network interactions between NREMS promoting neurons in the ventrolateral preoptic area (VLPO in rodent model; intermediate nucleus (IN) in humans) and wake-promoting neurons in the brainstem and hypothalamus regulate sleep–wake rhythms in a fashion analogous to the engineering concept of a flip–flop switch [ 19 , 20 , 21 , 22 , 23 ]. This model was recently updated to emphasize the importance of fast neurotransmitters, glutamate and GABA [ 24 ]. In addition to the VLPO, GABAergic neurons in the medullary parafacial zone (PZ) are also critical for slow-wave sleep (SWS) homeostasis, shown by anatomic, electrophysiologic, chemogenetic and optogenetic studies in mice [ 25 , 26 ]. For example, lesions in GABAergic neurons in PZ increased wakefulness by 50% and severely disrupted sleep, including a marked reduction of SWS [ 25 ]. Brain regions specifically or preferentially active during REMS have also been identified (Fig. 1 ). Of critical importance in the context of a discussion of anxiety disorders is that arousal/wake centers also broadly innervate the cerebral cortex, and the cerebral cortex reciprocally innervates arousal centers [ 24 , 27 ]. This means that in the context of salient information to the cortex, for example from the amygdala, arousal may be elicited despite competing sleep-promoting signals from sleep-specific centers of the brain.
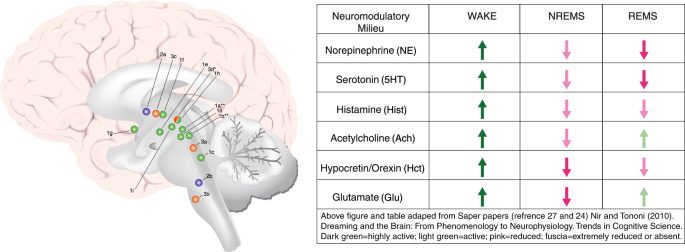
Important wake, NREM sleep, and REM sleep-regulating brain structures and/or nuclei, and associated neuromodulatory milieus. Wake-promoting regions, in green 1 a – i ; NREMS (including SWS) promoting regions in blue 2a – b ; REM sleep-regulating regions in orange ( 3a –d). Wake (green): 1 a : lateraldorsal tegmentum (LDT; Ach); 1b : pedunculopontine tegmentum (PPT; Ach); 1c : locus coeruleus (LC; NE); 1d : dorsal raphe nucleus (DRN; 5HT); 1e : tuberomammillary nucleus (TMN; Hist); 1f : hypocretin neurons of Lateral Hypothalamus (LH; Hct); 1g : cholinergic neurons of basal forebrain (BF; Ach); 1h : ventrolateral periaqueductal gray (vlPAG; DA); 1i : ventral tegmental area (VTA, DA). NREMS (blue): 2a : ventrolateral preoptic nucleus (VLPO; GABA, galanin; human homolog: intermediate nucleus (IN)); 2b : parafacial zone (PZ; GABA). REMS (orange): 3a : sublaterodorsal tegmental nucleus (SDT; Glu); 3b : ventral gigantocellular reticular nuclei (mediate REM-related muscle atonia; GABA/glycine); 3c : MCH neurons of lateral hypothalamus (LH; MCH/GABA); 3d : ventrolateral periaqueductal gray (vlPAG; GABA), *which is REM-suppressing. **LDT and PPT Ach neurons are also active during and may promote REMS, but may not play a central regulatory role
With respect to the study of sleep in psychiatric disorders, scalp-EEG-based measurement of cortical physiology remains the most-commonly utilized tool for studying the neurophysiology of sleep disturbance. Some researchers have also successfully probed the deeper functional neuroanatomy of human sleep disturbance in anxiety-related disorders utilizing neuroimaging techniques [ 28 , 29 , 30 ], but such studies are still relatively few. Figure 2 depicts the polysomnographic characteristics associated with different stages of sleep, along with a list of their associated clinical features and proposed or evidence-based functions, selected for their pertinence to anxiety disorders. Anxiety-disorder-related findings that will be discussed in greater detail in the following sections include reductions in sleep duration and sleep continuity, reductions in SWS, increases in lighter stages of sleep (NREM N1 and/or N2), and a variety of abnormalities in REMS, including disrupted REMS in anxiety subjects relative to controls. Based on the proposed functions of these various stages of sleep, one can infer numerous neurocognitive and psychological consequences relevant to anxiety, including disturbances in cognitive control, emotion regulation, memory consolidation, and emotional memory processing.
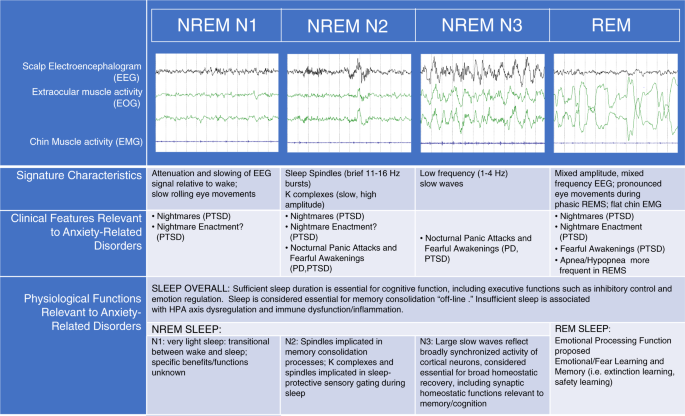
Polysomnographic characteristics of different sleep stages in humans, along with a list of their associated clinical features and proposed or evidence-based functions selected for their pertinence to anxiety disorders. Some studies have studied the effects of sleep overall (especially sleep duration) on performance in various domains, such as executive cognitive function and emotion regulation, whereas other studies have examined the role of specific sleep stages on performance of various functions more specifically
Neurobiology of anxiety-related disorders: a brief overview of neurocircuitry
An overview of the neurobiology of anxiety-related disorders must begin with a definition of these disorders. Given the ever-changing landscape of DSM-defined anxiety disorders, and given that all mental disorders may contain or produce a pronounced anxiety component (take paranoid schizophrenia, for example, in which patients may experience core psychotic symptoms resulting in profound and debilitating anxiety), we refer here to anxiety-related disorders as disorders in which fear and/or anxiety are considered primary and core features of the disorder. As such, we limit our detailed review of sleep disturbance to PTSD, Generalized Anxiety Disorder (GAD), Panic Disorder (PD), Social Phobia (SP), and Specific Phobias (SP). We also include obsessive–compulsive disorder (OCD) owing to its recent (DSM-IV) categorization as an anxiety disorder, because there are significant anxiety components to this disorder, and because we believe this will be of interest to readers.
With these definitions in mind, neuroimaging research in humans has provided relatively greater insight (relative to sleep disturbance in humans) into the functional neuroanatomy and neurocircuitry of anxiety-related disorders [ 31 , 32 , 33 , 34 , 35 ]. Figure 3 depicts the main brain regions broadly implicated in anxiety-related disorders. The amygdala and insula, key structures involved in the processing of aversive stimuli and in the expression of fear and anxiety, most consistently display hyperactivation in (aversive) task-based neuroimaging studies of anxiety-related disorders (see Duval et al., [ 34 ] for a review including disorders of interest minus OCD). In contrast, the medial prefrontal cortex (mPFC) tends to display hypoactivation in anxiety-related disorders, especially PTSD and GAD, and treatment of anxiety-related disorders has been associated with increases in mPFC activation that relate to symptom improvement [ 34 ]. In addition, the dorsal anterior cingulate cortex (dACC), with some exceptions, shows increased activation in anxiety-related disorders, which is thought to reflect the role of dACC in processing and expression of fear and anxiety. In contrast, the rostral ACC (rACC) is proposed to have a fear-modulating role based on neuroimaging findings. Functional connectivity studies in anxiety-related disorders generally indicate decreased connectivity between regions that modulate negative emotions (mPFC/rACC) and emotion-processing regions (amygdala, insula).
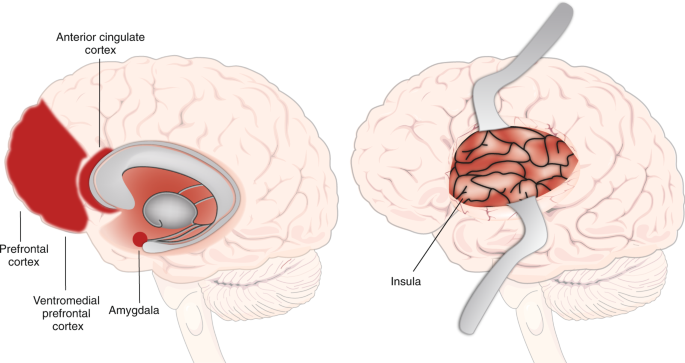
Important brain regions involved in fear, threat, and anxiety expression and modulation. Neuroimaging research indicates that the amygdala and insula are involved in the expression of fear, threat and anxiety. The dorsal anterior cingulate cortex (dACC) is more involved in the processing and expression of anxiety and fear, whereas the rostral ACC (rACC) is more involved in their modulation. The medial prefrontal cortex (mPFC) is involved in their modulation, especially the ventromedial PFC (vmPFC)
Other regions are considered to be relevant to anxiety-related disorders, some of which are less well understood or are associated with less consistent or broadly generalizable findings [ 34 ]. For example, the hippocampus is considered to play an emotion-modulating role in fear and anxiety, and often shows hyperactivation in imaging studies of anxiety-related disorders. The dorsolateral prefrontal cortex (dlPFC), implicated in emotion modulation and attention control, has shown patterns of hyper- and hypoactivation in anxiety-related disorders. Finally, the thalamus has been shown to display excess activation in task-based neuroimaging studies in PTSD, Social Anxiety Disorder (SAD), and SD relative to controls. The thalamus is also a key structure involved in the generation of SWS as well as the downregulation of sensory processing during sleep.
Anxiety and sleep circuitry: partially overlapping, intercommunicating neurocircuits
Although we describe two distinct sets of brain regions implicated in sleep/wake regulation and anxiety states, there is clear evidence of interaction between them, the extent and details of which are only beginning to be uncovered. There are obvious ways in which these communicate, including the aforementioned tight connection between the arousal centers and the cerebral cortex. This shared broad connectivity with the cerebral cortex provides a concrete neurobiological pathway for interconnectivity, but also provides an anatomical context for the cognitive hyperarousal that may link anxiety to sleep disturbance. In addition, the locus coeruleus, the main generator of norepinephrine in the central nervous system, plays both an important role in sleep/wake regulation as well as in anxiety-related processes through its inputs to and from the amygdala as well as the cerebral cortex. We will return to these neurobiological pathways later. A closer look at the clinical and sleep-physiological findings pertaining to the disorders of interest will create a phenomenological background for a subsequent discussion of these neurobiological pathways.
Sleep disturbance in anxiety-related disorders: a review of clinical features
The term “sleep disturbance” in published research on sleep and anxiety-related disorders refers to a range of subjective complaints, sleep disorder diagnoses, and alterations in subjective and objective measures of sleep when clinical samples are compared with controls. Several types of clinical sleep disturbance stand out in this literature; including DSM insomnia disorder diagnoses or symptoms per se, more broadly defined disturbances in subjective sleep quality as measured using various self-report surveys, nightmares, fearful awakenings, nocturnal panic attacks, obstructive sleep apnea (OSA), and abnormal movements during sleep ranging from periodic limb movements to dream enactment behaviors. Certain types of sleep disturbance are more common across anxiety-related disorders (e.g., insomnia and/or broadly defined subjective sleep quality disturbances [ 36 , 37 , 38 , 39 , 40 ] than others (e.g., nightmares) and individuals with anxiety-related disorders often experience more than one type of sleep disturbance. A recent comprehensive publication by Cox and Olatunji provides a highly informative review of sleep disturbance in anxiety-related disorders and the clinical features are given relatively brief review here, where we focus on elements that are less emphasized in that review [ 41 ].
Clinical features of sleep disturbance: PTSD
Subjective sleep disturbance is highly comorbid with PTSD and is often considered a “hallmark” feature of the disorder [ 42 , 43 ]. Subjective sleep disturbance and recurrent nightmares are listed as diagnostic criteria with prevalence rates as high as 90% [ 36 , 44 , 45 , 46 ]. It is therefore rare to encounter a patient with clinically significant non-sleep symptoms of PTSD who does not also report non-restorative and/or disrupted sleep, or distressing dreams. Sleep disturbance in PTSD may also be the most well-studied as compared with sleep disturbance in other anxiety-related disorders, and therefore may provide the best clues for understanding the sleep–anxiety relationship [ 41 ].
Although comorbid insomnia symptoms and poor subjective sleep quality are pervasive features of PTSD, nightmares stand out as a particularly unique feature of this disorder. Prevalence rates for nightmares vary widely, ranging from 19 to 96% [ 39 , 47 , 48 ], however ample research indicates that the prevalence of nightmares is significantly higher in individuals with PTSD than the general population [ 45 , 49 ] and other psychiatric conditions [ 47 , 48 , 50 , 51 ]. The wide range is likely attributable to differences in data collection methods, differences in study sample characteristics, and differences in trauma nightmare definitions. For example, most research uses retrospective measures, which are likely to underestimate nightmare frequency compared with prospective log assessments [ 52 ]. In addition, the definition of trauma nightmares has changed with various iterations of the DSM and varies widely across studies. Research has demonstrated higher rates of trauma nightmares in females compared with males [ 53 , 54 ] and nightmares may be more frequent in the context of recent trauma compared with chronic PTSD [ 55 ].
In addition to the highly prevalent disturbances of insomnia and nightmares, it is now well-established that other sleep disturbances also occur following trauma and/or in PTSD [ 56 , 57 , 58 , 59 , 60 ]. For example, several studies have indicated that panicked awakenings from sleep with poor or no recall of dream content are common in PTSD [ 59 ]. Several studies have also reported increases in physical movement during sleep when compared with controls, including periodic leg movements [ 59 ] (although see Woodward et al. [ 61 ] reporting decreased movement in PTSD sleep). Nightmare enactment is also commonly reported in clinical practice, although prevalence rates are poorly established. Nightmare enactment has typically been observed in younger individuals and is more common immediately following trauma. However, most studies have been conducted in combat veterans, for whom age and time since trauma are difficult to disentangle (e.g., military personnel are exposed at a young age), and thus warrants study in other populations.
Based on the unique combination of sleep symptoms following trauma, Mysliwiec et al. [ 57 , 62 ] have proposed a new disorder, “trauma-associated sleep disorder”. The proposed disorder encompasses disruptive nocturnal behaviors such as thrashing movements panic-like, or startled awakenings, and dream enactment that are proposed to occur out of both REM and NREM sleep. Further research is necessary to determine whether these sleep symptoms represent an early and severe form of sleep response to trauma that later evolves into the more typically described sleep presentation (e.g., insomnia and nightmares), or whether this trajectory is distinct, and possibly even related to neurodegenerative risk. For example, dream enactment is well-described in REM Behavior Disorder (RBD) and is considered a prodromal marker of Parkinson’s Disease. It remains to be seen whether dream enactment following trauma is unique or is related to the dream enactment characteristic of RBD.
Clinical features of sleep disturbance: GAD
GAD is the other anxiety-related disorder whose DSM diagnostic criteria specifically include sleep disturbance [ 46 ]. Despite the inclusion of sleep disturbance in the nosological definition, empirical investigations of sleep disturbance in GAD are relatively limited. Nonetheless, existing published research fairly consistently indicates that subjects with GAD report worse subjective sleep than healthy controls [ 41 ]. Sleep disturbance has been reported in up to 75% of individuals with GAD [ 63 , 64 ]. Adults with GAD report poorer subjective sleep quality [ 65 , 66 , 67 ] and have been found to be more likely to have a sleep disorder (vaguely defined, as per chart review) when compared with control groups [ 68 ].
Clinical features of sleep disturbance: PD
The extant research has demonstrated that subjective sleep complaints are common in PD, with controlled studies indicating greater difficulties with sleep initiation, sleep maintenance, early morning awakenings and more general sleep quality disturbances in PD subjects than healthy controls [ 41 , 69 , 70 , 71 , 72 , 73 ].
A PD-specific sleep disturbance that may overlap with panicked awakenings seen in PTSD are nocturnal panic attacks. Nocturnal panic attacks are characterized by abrupt awakenings from sleep with panic attack symptoms [ 74 ]. These can be distinguished from sleep terrors, which are a parasomnia involving severe autonomic arousal and fearful behavior during NREM sleep, and which are generally not recalled subsequently [ 75 ]. Although epidemiological studies have not been conducted, survey data indicate that lifetime prevalence rates for at least one nocturnal panic attack in individuals with PD are as high as 71% [ 74 , 76 , 77 , 78 , 79 ]. Based on one report with a small sample, it appears that nocturnal panic attacks in PD emerge during the transition from lighter to deeper NREM sleep, either in visually scored N2 preceded by EEG slowing (indicating a transition to deeper sleep) or in early N3 sleep [ 80 ]. There is some evidence to suggest that individuals with PD and nocturnal panic suffer from higher rates of depression [ 80 ] and suicidality [ 81 ] compared with individuals with PD without nocturnal panic. Individuals with nocturnal panic may also suffer from more-frequent daytime panic attacks and greater somatic symptoms during daytime attacks [ 76 ]. These findings have led researchers to postulate that nocturnal panic attacks may represent a more severe variant of PD.
Clinical features of sleep disturbance: specific phobia and SAD
Limited research exists examining sleep disturbance in phobias and SAD [ 41 ]. To summarize, two studies using community samples found that the presence of phobias and SAD was associated with increased likelihood of having sleep disturbances, as measured by the PSQI [ 40 ] and WHO Composite International Diagnostic Interview (WHO CIDI) [ 82 ].
Clinical features of sleep disturbance: OCD
Several studies have examined subjective sleep disturbance in adults with OCD. One study found no differences in sleep parameters when comparing an OCD and controls [ 83 ]. The second study found greater subjective reports of sleep disorders and poorer subjective sleep quality in individuals with OCD compared with healthy controls [ 84 ]. Similar to other anxiety-related disorders, greater OCD symptom severity may be associated with greater sleep disturbance severity; a cross-sectional study demonstrated that short sleep duration predicted OCD in a Korean community sample (Park et al. [ 85 ]) and another cross-sectional study found that greater OCD symptom severity was associated with shorter sleep duration and decreased sleep efficiency in 13 adults with an OCD diagnosis [ 86 ]. Conversely, Marcks et al. [ 87 ] failed to find an association between OCD diagnosis and sleep disturbance in a sample of primary care patients. In a nationally representative sample, Cox and Olatunji [ 41 ] found that individuals reporting insomnia symptoms reported increased OCD symptoms relative to those who did not.
Obstructive sleep apnea
Recently, there has been a growing interest in the relationship between OSA and anxiety-related disorders, particularly PTSD. OSA is a sleep disorder characterized by repeated upper airway obstruction during sleep, leading to breathing interruptions and sleep fragmentation [ 88 ]. The underlying pathophysiology of OSA is multifactorial and complex and may vary considerably across individuals. Important components of OSA pathophysiology include upper airway anatomy, the ability of upper airway dilator muscles to respond to respiratory challenges during sleep, arousal thresholds during sleep, the stability of the respiratory control system, and state-related changes in lung volume [ 89 , 90 ]. Major risk factors for OSA include obesity, older age, male sex, smoking, and use of central nervous system depressants (e.g., alcohol). The apnea hypopnea index (AHI) is the number of apneas or hypopneas recorded per hour of sleep. The AHI is often used as a diagnostic tool and is a marker of OSA severity.
OSA prevalence rates appear to be higher in PTSD samples compared with the general population [ 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 ]. Greater PTSD symptom severity increases the risk of screening positive for OSA [ 91 ]. On the other hand, including both veteran and non-veteran studies, prevalence rates of OSA in PTSD vary considerably [ 92 , 100 , 101 , 102 , 103 , 104 , 105 ]. Differences may result from differences in diagnostic criteria and sample characteristics such as sample size, sex, age, ethnicity, and health status compositions. It is also important to note that some studies generated prevalence estimates from samples specifically recruited for OSA-high-risk features (e.g., sleep disturbance), which is likely to artificially inflate prevalence estimates.
There is insufficient evidence to conclude that OSA rates in non-PTSD anxiety disorders differ relative to controls. One cross-sectional study using data from outpatient records of the Veteran Health Administration found that an OSA diagnosis increased the odds of having an anxiety disorder diagnosis in veterans [ 106 ]. With respect to PD, the majority of work elucidating associations between PD and OSA has been limited to case studies [ 107 , 108 , 109 ], studies with small sample sizes [ 110 , 111 ], and studies without an appropriate control group [ 111 ]. To our knowledge, only one study has examined OSA prevalence rates in PD and GAD; this study was conducted in a community sample of OSA-high-risk participants and reported an OSA prevalence rate of 58.8% in PD and 57.1% in GAD [ 112 ]. Another study demonstrated that individuals with PD demonstrated significantly greater rates of microapneas during sleep than controls [ 110 ].
In summary, although high rates of OSA are reported in PTSD, these are not convincingly demonstrated in other anxiety-related disorders, and the mechanism explaining the relationship between OSA and PTSD remains to be clarified [ 98 ]. The increasingly high rates of obesity in veterans [ 113 ] and people with PTSD [ 114 ] may be a major factor contributing to this surge in OSA prevalence.
Physiological and functional neuroanatomical features of sleep disturbance in anxiety-related disorders
A number of polysomnography (PSG) studies along with a few neuroimaging studies have begun to identify physiological and functional neuroanatomical features that may underly the subjective complaints of sleep disturbance in anxiety-related disorders. We focus here on polysomnography-based studies, and also include a brief presentation of neuroimaging and neuroendocrine research. Of note, we do not discuss actigraphy-based findings in detail here, and instead focus on direct measures of physiology during sleep in anxiety disorders. Overall, actigraphy-based findings demonstrate objective sleep abnormalities in anxiety-related disorders, although there are inconsistencies in results within and across disorders [ 41 , 84 ].
Sleep physiological findings: PTSD
Several studies, including two meta-analyses, now provide convincing evidence of PSG-measured sleep abnormalities in PTSD [ 41 , 115 , 116 ]. In a meta-analysis incorporating 20 studies, Kobayashi and colleagues found that PTSD was associated with more stage N1 percent, and lower SWS percent as compared with a mix of controls, including healthy subjects, trauma-exposed, or non-exposed [ 116 ]. Authors of a more-recent meta-analysis, which only incorporated 13 studies owing to more-restrictive inclusion criteria, and which incorporated new studies not reviewed by Kobayashi et al., also reported less SWS duration in PTSD as compared with a mix of controls [ 115 ]. The latter study found no differences in N1 or N2 sleep duration. On the other hand, they report that PTSD was associated with a reduction in PSG sleep continuity as compared with controls, as defined by a composite measure including sleep latency, sleep efficiency, and/or number of awakenings [ 115 ].
Needless to say, the above meta-analyses were performed in the context of inconsistencies in the literature. Some clinical studies observed no differences in PSG-based sleep measures in PTSD vs. control groups (all trauma exposed) [ 99 , 117 ], whereas other studies have found reduced SWS, increased stage 1 sleep, reduced sleep efficiency, reduced sleep duration, and/or greater number of awakenings in PTSD relative to trauma-exposed [ 118 ] or healthy and depressed controls [ 119 ]. Inconsistencies have been attributed to moderating third variables such as age, sex [ 116 , 120 ], comorbid diagnoses, and substance use disorders [ 116 ]. For example, the Kobayashi et al. meta-analysis also found an effect of PTSD on total sleep time (shorter in PTSD) in studies with male samples only. Finally, laboratory-based studies of PTSD subjects suggest that PTSD subjects may in fact sleep better in the supervised environment of the sleep laboratory. This may obfuscate differences in objective sleep quality in PTSD relative to controls.
In terms of REM sleep, both of the above-cited meta-analyses reported that PTSD was associated with greater REM density (REMD), an index of eye movements during REM sleep, than controls [ 115 , 116 ]. The implications of REMD are not entirely clear, although some research indicates that REMD is an indicator of phasic hyperarousal during sleep. Interruptions or fragmentation of REMS have also been reported in PTSD and/or trauma survivors relative to controls [ 56 , 121 , 122 ], including PTSD-positive vs. PTSD-negative veterans [ 56 ], and PTSD-positive vs. normal healthy civilians [ 121 ]. In a subsample of a representative community-based sample Breslau et al. reported that more frequent arousals from REMS was associated with a history of lifetime PTSD [ 122 ]. Mellman and colleagues [ 123 ] have also found that REMS fragmentation in the early aftermath of trauma predicted subsequent development of PTSD symptoms. Complicating the REM/PTSD story, Mellman et al. [ 124 ] also reported that duration of PTSD was associated with higher REM percent, longer REM duration, and shorter REM latency. Mellman et al. [ 124 ] suggested that the linear association between REM parameters and the course of PTSD may reflect an adaptive process in chronic patients, as REM sleep is implicated in emotional processing. On the other hand, Ross suggests that this “reconstituted” REM sleep in chronic PTSD is actually pathological [ 125 ]. Richards et al. found longer duration of REMS in PTSD relative to exposed controls in women only [ 120 ]. Studies in animal models have fairly consistently demonstrated alterations in REMS in rodent models of acute and chronic stress, which may be relevant across anxiety-related disorders, but these have included both disruptions and prolongations of REMS. Altogether, these findings provoke ongoing interest in REMS but limited definitive conclusions about patterns of disturbance [ 126 , 127 , 128 , 129 , 130 , 131 ].
Although few neuroimaging studies have been performed to understand sleep disturbance in anxiety-related disorders, a number of studies have been conducted in PTSD. For example, Germain and colleagues [ 132 ] performed FDG PET imaging in military veterans with and without PTSD and found hypermetabolism in both wake and REM sleep in PTSD-positive, relative to PTSD-negative subjects, in regions involved in emotion expression and emotion regulation. These included the mPFC, the (right) amygdala, and hippocampus as well as brainstem regions overlapping with the raphe nuclei and (right) locus coeruleus. They also saw elevated activity in the thalamus in PTSD relative to controls. In another study, Germain and colleagues reported hyperactivity in NREMS in PTSD relative to control subjects, as well as increased elevation in NREMS relative to wake, in PTSD more than controls [ 29 ]. Although these reports indicate hyperarousal of both cortical and subcortical brain regions during sleep, other studies contribute to a more complex story with less straightforward interpretation [ 30 , 133 , 134 ].
Other approaches have been utilized to examine the evidence for physiological hyperarousal during sleep in PTSD by measuring peripheral indicators of neuroendocrine activity. Indices of hyperarousal such as nighttime excretion of norepinephrine metabolites and hypothalamic-pituitary-adrenal (HPA) axis activity have indeed been associated sleep disturbance in PTSD [ 135 , 136 , 137 , 138 ].
Sleep physiological findings: GAD
Studies examining physiological sleep characteristics in GAD are limited and therefore it is difficult to draw conclusions [ 41 ]. Nonetheless, PSG-based differences in GAD vs. controls are reported. compared with control groups, individuals with GAD exhibit decreased sleep duration [ 139 , 140 ], increased sleep onset latency [ 139 ], increased wake after sleep onset and reduced sleep efficiency [ 140 ], reduced NREM N2 sleep and reduced SWS [ 140 ]. Two studies found no differences in objective sleep efficiency between GAD and controls [ 139 , 141 ]. A separate study found that individuals with GAD exhibit increased REM latency compared with individuals without GAD [ 141 ].
Sleep physiological findings: PD
Objective reports of PSG sleep abnormalities in PD are less clear. Some studies have found that individuals with PD exhibit decreased sleep efficiency [ 80 , 142 , 143 , 144 ], increased onset latency [ 80 , 142 , 143 ], and reduced sleep duration [ 78 , 80 ] compared with controls. Another study found no differences in PSG measures of sleep disturbance, despite subjective differences in sleep quality across a PD and control group [ 73 ]. A recent meta-analysis based on four studies concluded that PD is associated with poorer sleep efficiency and marginally significant longer sleep onset latency when compared with individuals without PD [ 115 ].
An older and small body of research exists examining sleep physiological characteristics in PD [ 41 ]. The existing literature has demonstrated decreased REM latency [ 142 , 145 ], decreased number of REM periods [ 80 ], decreased REM density [ 145 ], increased stage 1% [ 146 ], decreased stage 2 duration [ 143 ], and deceased SWS % [ 78 , 144 ]. Contrarily, Dube et al. [ 147 ] found no differences in sleep physiology when comparing PD to healthy controls.
Sleep physiological findings in OCD
A small number of studies have found differences in NREM sleep parameters in individuals with OCD compared with healthy controls [ 41 ]. More specifically, studies have reported decreased sleep duration [ 148 , 149 ], reduced sleep efficiency [ 149 , 150 ], and greater number of nocturnal and early morning awakenings [ 148 , 149 , 150 ] in individuals with OCD compared with healthy controls. Studies also indicate increased N1 sleep [ 148 ], decreased N2 sleep [ 148 ], and decreased SWS [ 148 , 151 ] in individuals with OCD. There is also evidence of altered REM parameters in OCD, though results are mixed. Some studies have demonstrated reduced REM latency [ 148 ], higher REM density in the first REM period [ 149 ], and a trend towards reduced REM efficiency ( p = 0.06; [ 148 ]) in individuals with OCD. In contrast, other studies have found no differences in REM parameters between OCD and control groups [ 86 , 150 ]. A recent meta-analysis that combined data from PSG, self-report and observer reports found that sleep duration was also reduced in OCD relative to healthy controls [ 152 ].
Summary of Sleep Physiological Findings
In summary, published sleep-physiological studies overall indicate hyperarousal during sleep in anxiety disorders, with effects on sleep architecture and continuity that may impact some important functions of sleep. It is possible that publication bias has reduced the number of negative published results, favoring publications detecting differences between anxiety disorders and controls. On the flip-side, the generalized but subtle pattern of disruption may indicate that measuring hyperarousal during sleep is more complex than current methods are able to discern, and more sophisticated methods of measurement and analysis such as quantitative EEG analysis [ 120 , 153 , 154 , 155 ] and brain imaging during sleep [ 156 ] are indicated.
Evidence for a bidirectional relationship between sleep disturbance and anxiety-related disorders
Most studies demonstrating high levels of sleep disturbance in anxiety-related disorders are cross-sectional, therefore the causal relationship between sleep disturbance and anxiety-related disorders cannot be discerned. However, a number of studies strongly indicate that sleep disturbance enhances risk for future anxiety-related disorders, especially PTSD, and there is also empirical evidence from human studies demonstrating that that anxiety-related disturbances can precede and increase risk for sleep disturbances. We briefly review studies indicating this bidirectional relationship here.
Prospective studies have convincingly demonstrated that sleep disturbance both prior to and following trauma is an important predictor of subsequent PTSD development [ 117 , 157 , 158 ]. Van Liempt and colleagues [ 159 ] found that nightmares, although not insomnia symptoms, prior to military deployment predicted PTSD subsequent to deployment. Furthermore, treatment research indicates that treatment of insomnia using standard cognitive-behavioral therapy for insomnia (CBT-I) results in an improvement in daytime PTSD symptoms [ 160 , 161 ], indicating that insomnia may be a driver of PTSD symptoms, which, when treated, results in a diminution of PTSD symptoms. A recent meta-analysis by Hertenstein et al. [ 162 ] also demonstrated that insomnia symptoms prospectively predicted anxiety symptoms in the short-term (12–24 months) and long-term (>24 months). In the other direction, Wright et al., cited above, found that PTSD symptoms 4 months after trauma did not predict insomnia symptoms at 12 months. In contrast, an interesting recent study used ecological momentary assessments of PTSD and insomnia symptoms and demonstrated that daytime anxiety predicted (temporally preceded) poorer sleep quality and efficiency, even when controlling for baseline PTSD symptoms [ 163 ]. This study also found that daytime PTSD symptoms and fear of sleep predicted subsequent nightmares. Findings from a large community-based sample of adolescents also showed that having any anxiety disorder was prospectively associated with an increased risk of insomnia [ 164 ]. In that study, insomnia did not predict the future development of anxiety disorder. Although our review is mostly focused on sleep and anxiety in adults, research in children and adolescents, when these disorders first present, may be particularly informative with respect to the chicken-and-egg question.
To our knowledge, studies that prospectively examine the relationship between sleep and non-PTSD anxiety disorders is limited. As described above, in a study in adolescents aged 13–17, anxiety disorder predicted future insomnia, whereas insomnia did not predict future anxiety [ 164 ]. One prospective study utilizing self-report measures found that sleep disturbance was associated with anxiety symptoms (though not GAD specifically) or a diagnosis of PD 4 years later [ 165 ]. CBT for GAD reduces sleep disturbance symptoms, but one study demonstrated that 33% of individuals had an insomnia disorder diagnosis and 63% had clinically significant sleep disturbance following treatment, indicating that insomnia is not a result of GAD alone, and/or that insomnia that may have resulted from GAD has taken on a life of its own, and is therefore unresponsive to GAD treatment. Several laboratory studies using total sleep deprivation paradigms have demonstrated that sleep deprivation increases the vulnerability to subsequent daytime panic attacks [ 166 , 167 ]. It is not clear how these experimental results generalize to sleep disruption in the real world. Additional work has demonstrated that sleep disturbance symptoms persist following successful PD treatment [ 168 , 169 ], which suggests that sleep disturbance is not a secondary symptom of PD or that sleep disturbance has become independent of original PD precipitants.
Explaining the relationship between sleep disturbance and anxiety-related disorders: psychological and psychophysiological models
Although the extant research indicates that sleep disturbances and anxiety-related processes mutually influence each other, the mechanisms through which this occurs is not yet clear. The dominant psychological and psychophysiological models of insomnia disorder certainly provide clues about these mechanisms.
Insomnia disorder is a sleep disorder characterized by subjective difficulties with sleep onset and/or sleep maintenance, despite adequate opportunity to sleep, with associated daytime impairment or distress [ 46 ]. A wealth of research has advanced our understanding of insomnia as a condition resulting from and reinforced by maladaptive, sleep-focused cognitions and maladaptive behaviors and coping strategies. Genetic and biological/physiological traits may predispose individuals to the development of insomnia, and physiological disturbances also result from maladaptive cognitions and behaviors. The interaction of cognitive, behavioral, and physiological factors all contribute to a vicious cycle of sleep disturbance and associated distress. The profound value of these models and testimony to their validity is that the CBT-I that is based on these models is the first-line therapy for insomnia disorder, with proven efficacy in insomnia alone and comorbid with a multitude of psychiatric and medical conditions [ 170 , 171 ]. CBT-I has also demonstrated benefits for anxiety symptoms in several studies [ 161 , 172 ].
The diathesis-stress model states that there are three interrelated and sequential factors involved in the pathogenesis of insomnia: predisposing, precipitating, and perpetuating factors [ 173 ]. Predisposing factors are individual genetic, physiological, or psychological traits that produce differing levels of susceptibility for developing insomnia (e.g., sex, chronotype, genetic polymorphisms affecting arousal regulation or cognition). Precipitating factors are physiological, environmental, or psychological stressors that trigger the onset of disrupted sleep patterns and include factors ranging from physical injury, work-related stress, or a traumatic stressor. They may or may not be psychological in nature. Perpetuating factors are maladaptive strategies for coping with disrupted sleep that contribute to insomnia symptoms and result in chronic insomnia if sustained. They generally reflect efforts to compensate for insufficient nighttime sleep and are comprised of behaviors such as sleeping in on weekends, taking daytime naps, and increasing time in bed in an effort to obtain the desired amount of sleep. The perpetuating influence of the latter behaviors is explained by the stimulus-control model proposed by Bootzin [ 174 ], and is anchored in classical conditioning theory. Increased time in bed in the absence of actual sleep eventually results in increased frustration in bed (i.e., cognitive and physiological arousal), which effectively reduces the likelihood of sleep and results in a further perpetuation of wakefulness rather than sleep. Their perpetuating effect is enhanced by the fact that the compensatory mechanisms result in efforts to sleep at physiologically suboptimal times (i.e., in the absence of homeostatic and circadian pressures to sleep).
Cognitive models of insomnia focus on thoughts, beliefs, and/or feelings that may interfere with sleep and lead to maladaptive coping behaviors [ 175 ]. According to cognitive models of insomnia, excessive worry and rumination lead to arousal and distress, all of which precipitate and perpetuate insomnia symptoms [ 175 ]. Harvey hypothesized that insomnia is driven by inappropriate worry about sleep and sleep-related consequences. This worry subsequently leads to physiological arousal, selective attention to sleep-related threats (e.g., ambient noise), and the adoption of maladaptive safety behaviors. Another cognitive model proposed by Espie proposes that psychological and/or physiological stress leads to selective attention towards stressors, which inhibits the natural “de-arousal” that is necessary to initiate sleep [ 176 ].
Riemann and colleagues have advanced a hyperarousal model of insomnia that incorporates the elements of the above models while also emphasizing a fundamental contribution of genetic and physiological vulnerability to sleep/wake regulatory problems and to hyperarousal at a physiological as well as cognitive level. In their words, they conceptualize insomnia as “a final common pathway resulting from the interplay between a genetic vulnerability for an imbalance between arousing and sleep-inducing brain activity, psychosocial/medical stressors and perpetuating mechanisms including dysfunctional sleep-related behavior, learned sleep preventing associations, and other cognitive factors like tendency to worry/ruminate” [ 177 ]. They cite evidence indicating that hyperarousal processes from the molecular to higher system levels play a role in the pathophysiology of insomnia [ 177 ]. Several other researcher groups have demonstrated that hyperarousal in insomnia exists in cognitive (e.g., sleep-related worries [ 178 , 179 ], somatic/physiologic (e.g., increased heart rate; [ 178 , 179 ]), and cortical (e.g., high frequency EEG) [ 179 ] domains. Riemann et al. [ 177 ] have pointed out that while standard visual scoring of EEG has often failed to show differences between insomnia and controls, more sophisticated approaches to EEG analysis are pointing to subtle and dynamic features that distinguish good from poor sleepers. These methods are now often used in insomnia disorder research but have only been used in a small number of studies of sleep in anxiety-related disorders [ 120 , 153 , 154 ].
Altogether, research on insomnia provides strong evidence for an interplay between biological predispositions, sleep-disrupting life events, and maladaptive coping behaviors that result in cognitive and physiological hyperarousal, that then perpetuate the cycle of sleep disturbance. The model described by Riemann et al. proposes both cognitive-behavioral and neurobiological pathways through which biological and cognitive risk factors may lead to insomnia as well as anxiety disorders, that then positively reinforce sleep-disturbance in a vicious-cyclic pattern.
Applicability of the dominant insomnia models to PTSD
Consistent with the above models, a traumatic event amounts to a classic and severe insomnia “precipitating factor” that may result in disrupted sleep owing to a variety of factors [ 180 ]. From a psychological perspective, nighttime arousal that occurs immediately following trauma may be adaptive in settings of increased predatory threat (e.g., combat), or owing to one’s increased vulnerability when asleep [ 181 , 182 ]. Unfortunately, whether initially adaptive or not, compensatory behaviors (e.g., naps) to compensate for nighttime sleep deficits will result in mistimed sleep, and therefore wakefulness in bed even in the absence of psychological distress, and thereby contribute to conditioned arousal. On top of this, nighttime trauma-related psychological distress, such as distressing trauma memories and ruminations, and frightful awakenings occurring either spontaneously or from nightmares, are likely to contribute to conditioned arousal in the sleep environment. Although the insomnia models emphasize sleep-related cognitions as a precipitant of hyperarousal, the essential ingredient is that worry in the bedroom, at night, and in the sleep environment precipitates hyperarousal. In this context, the application of insomnia models to PTSD is therefore straightforward, and the success of standard CBT-I for treating sleep disturbance in PTSD provides strong support for its relevance to PTSD [ 160 ].
Fear of sleep is common in PTSD and distinguishes sleep-related cognitions in PTSD from insomnia (in which individuals desire rather than fear sleep). Evidence suggests that fear of sleep is driven by both fear of having a nightmare and fear of the vulnerability that occurs during sleep [ 163 , 183 , 184 , 185 ]. Fear of sleep may result in individuals avoiding sleep and/or in difficulties with sleep initiation. Some studies have elucidated an association between fear of sleep and insomnia symptoms [ 163 , 185 , 186 ], whereas other studies have found no such association [ 183 ]. One highly credible explanation for the latter findings is that over time, insomnia is no longer driven by fear of sleep and is instead maintained by a pattern of learned maladaptive coping behaviors that are no longer dependent on the initial precipitating causes [ 183 ].
Applicability of the dominant insomnia models to GAD
Cognitive and hyperarousal models of insomnia may be particularly applicable for insomnia pathogenesis in GAD. A cardinal feature of GAD is excessive cognitive activity, specifically worry and rumination. Although not performed specifically in GAD subject, one study demonstrated that sleep-related rumination was associated with both trait and state arousal in insomnia disorder [ 187 ]. Several studies have also demonstrated associations between worry, rumination, and sleep disturbance, though most of these studies are limited to undergraduate samples and results may not be generalizable [ 188 , 189 , 190 , 191 , 192 ]. On the other hand, some studies examining trait worry and rumination failed to find significant associations with sleep disturbance [ 193 ]. O’Kearney et al. [ 194 ] found that worry was associated with insomnia symptoms as measured by the ISI, but not specific sleep continuity parameters.
Applicability of Dominant Insomnia Models to PD
Within the insomnia model framework, nocturnal panic attacks can be considered a precipitating factor for insomnia, disrupting sleep, and contributing to sleep–environment arousal and fear of having another panic attack [ 79 ]. Repeated nocturnal panic attacks produce conditioned fear (e.g., hyperarousal) and avoidance of sleep [ 79 ], which results in the adoption of maladaptive sleep-related behaviors (e.g., sleeping in brightly lit and very loud environments to obtain rest but avoid deep sleep). Conditioned fear, avoidance of sleep, and maladaptive safety behaviors are perpetuating factors and maintain insomnia symptoms in the absence of nocturnal panic attacks.
Two studies have demonstrated the presence of insomnia symptoms in the absence of nocturnal panic attacks, suggesting that other factors may be involved in insomnia pathogenesis in PD [ 72 , 195 , 196 ]. Anxiety sensitivity—a tendency to experience excessive fear of anxiety-related sensations based on the fear that these sensations are harmful [ 197 ] has been associated with sleep onset difficulties in PD [ 198 ]. Anxiety sensitivity may amplify the conditioned hyperarousal characteristic of insomnia by leading to a hyper-focus on physiological symptoms. Related to this, two studies manipulating pre-sleep appraisal of sleep physiology support this theory by demonstrating that individuals with PD and nocturnal panic report more distress when they believe that physiological signals are abnormal [ 199 , 200 ].
Contrasting with this idea of anxiety sensitivity, Craske and Tsao [ 74 ] also propose that nocturnal panic in PD is driven in part by fear of loss of vigilance. Evidence cited to support this model included studies demonstrating higher physiological and self-reported emotional reactivity to relaxation and imagery exercises, greater self-reports of panic attacks following relaxation and fatigue, and greater self-reported discomfort during relaxation exercises in individuals with PD and nocturnal panic compared with individuals with PD without nocturnal panic [ 80 , 201 , 202 ].
Applicability of dominant insomnia models to OCD
A recent study exploring associations between insomnia symptoms and OCD symptoms in an undergraduate sample found that insomnia symptoms were associated with obsessions, but not compulsions [ 203 ]. This finding suggests that similar to other anxiety-related disorders, hyperarousal and cognitive activity may be driving insomnia in individuals with OCD as obsessions are characterized by heightened arousal and compulsions serve to alleviate anxiety.
In summary, the above-described findings implicate fear, threat, and anxiety-related cognitions occurring at night or in the sleep environment, or in a trait-like fashion and therefore likely to occur at night, in sleep disturbance associated with anxiety-related disorders. Whether they are focused on anxiety about missed sleep (insomnia) or any other range of concerns may be less important than the fact that they reflect overall cognitive hyperarousal in the sleep environment and as such result in physiological arousal as well as all the downstream compensatory and conditioning phenomena typical of insomnia disorder. Overall, this reflects a transdiagnostic process, consistent with a transdiagnostic model of insomnia relevant across psychiatric disorders.
Affective and cognitive neuroscience of sleep inform the understanding of sleep disturbance in anxiety-related disorders: emotion regulation and emotional memory
Insomnia models satisfactorily explain many of the subjective sleep complaints and insomnia manifestations per se in anxiety-related disorders, but they do not fully or explicitly incorporate the effects of objective disruptions of sleep integrity on two aspects of brain functioning that may be elemental to the disorders discussed here: emotion regulation and emotional memory.
Sleep disturbance and emotion regulation
Emotion regulation generally refers to the ability to modulate emotional experiences in a way that is responsive to the context of a situation as well as an organism’s long-term objectives [ 187 ]. In anxiety disorders, emotion-regulatory problems manifest as the maladaptive expression of fearful and anxious emotions and emotional responses. Emotion regulation may be considered an aspect of cognitive executive function [ 4 ]. The prefrontal cortex (PFC) has been demonstrated to play an important role in emotion regulation [ 204 , 205 ], and the frontal lobes may be particularly vulnerable to sleep loss. Gruber and Cassoff [ 4 ] recently published an informative review of empirical findings and a conceptual framework for understanding the importance of sleep for executive functioning and emotion regulation more specifically.
Related to this, it has been reported that deficits in cognitive inhibition (e.g.,“tuning out” irrelevant stimuli) [ 206 ] and emotion regulation [ 187 ] were associated with sleep-related rumination in individuals with insomnia disorder. Particular to GAD, one study found that difficulties with emotion regulation mediated the association between GAD diagnosis and insomnia symptoms [ 207 ]. A recent study in PD found that poorer performance on a cognitive inhibition task was associated with longer sleep onset latency and reduced sleep quality in individuals with PD [ 208 ].
A recent imaging study provides neurobiological evidence for the role of emotion regulation in the anxiety–sleep relationship. Pace-Schott et al. found that compared with GAD and insomnia disorder groups, a good sleeper group exhibited greater resting-state functional connectivity between the left amygdala and a bilateral region of the rACC. As described earlier, the rACC is part of a prefrontal network believed to exert top-down control over amygdala activity and may constitute an emotion-regulatory circuit. These findings support the idea that deficits in emotion regulation may play a role in both anxiety and insomnia pathogenesis [ 209 ].
These studies do not explain causal relationships, and do not necessarily distinguish between objective sleep disruption and insomnia symptoms/disorder per se, yet they provide evidence that deficits in cognitive functions, which may result from sleep disturbance or be a manifestation of an anxiety disorder, are mechanistically involved in the sleep-disturbance-anxiety disorder cycle.
Sleep and memory: relevance to anxiety-related disorders
A sizable body of research now provides strong evidence that sleep has an important role in memory consolidation [ 10 , 11 , 210 , 211 , 212 , 213 ]. The “off-line” state of sleep provides a unique opportunity to consolidate information considered relevant to the organism through the reinforcement of key synaptic connections in the relative absence of environmental inputs and high energy-expenditure needs [ 10 ]. Although the most-compelling evidence currently points to NREMS as the major driver of sleep-dependent memory consolidation, both NREMS and REMS have been shown to be relevant for consolidation of memory of various sorts [ 11 , 210 , 214 ]. Research on sleep in anxiety-related disorders, and PTSD in particular, has honed in on REMS as an important player in the processing of fearful, threatening, or distressing experiences in a way that results in selective and adaptive consolidation of emotional memories [ 215 , 216 ].
Fear conditioning and related protocols in both animal models and human subjects constitute the dominant approach for studying the role of sleep and REMS in emotional learning relevant to anxiety-related disorders [ 13 , 217 , 218 ]. Fear conditioning involves the transformation of a neutral stimulus, such as a neutral visual image or context, into a frightening stimulus through its repetitive pairing with an aversive experience, such as an electric shock. Via classical conditioning, the neutral stimulus becomes a conditioned stimulus eliciting a fearful emotional response, usually measured using electrophysiology techniques such as skin conductance response or electromyography responses in humans. Once established, conditioned fear can be extinguished through subsequent repetitive presentation of the conditioned stimulus in the absence of the aversive stimulus. Modifications of the simplest fear-conditioning protocols exist, including addition of “safety” stimuli that are never paired with an aversive stimulus, the responses to which can be compared with conditioned fear responses to assess the subject’s ability to discriminate between fear and safety cues. Neuroimaging research indicates that fear extinction is mediated in large part by the mPFC, in particular the ventromedial PFC and rACC, which are structures implicated in anxiety-related disorders [ 13 , 219 ].
Although findings are not entirely consistent across studies, which utilize different measures of REMS consolidation and/or different outcome measures, recent findings in humans indicate that intact REMS is important for adaptive extinction of conditioned fear, including retention of extinction over time, and the ability to distinguish between safety and fear signals [ 218 , 220 ]. This has important implications for psychotherapies since extinction and safety learning are psychological models underlying psychotherapy for anxiety. Although not focused on REMS, there is indeed some evidence indicating that sleep may enhance the efficacy of phobia and SAD treatment [ 221 ]. Exposure therapy followed by a period of sleep is associated with decreases in heart rate [ 222 ], skin conductance [ 222 ], and self-reported fear [ 223 ] when confronted with the feared stimulus compared with sessions followed by wake. Poorer sleep at baseline was associated with poorer outcome following CBT treatment for SAD and more restful sleep following sessions was associated with a greater reduction in SAD symptoms [ 224 ].
Although fear conditioning is a form of implicit emotional learning, some researchers have also found REMS associations with declarative forms of emotional memory [ 14 , 225 , 226 ]. These studies may be most pertinent to PTSD, the symptoms of which revolve around a traumatic emotional memory and include persistent, intrusive emotional memories [ 227 ]. For example, Walker et al. found that higher REMS duration correlated with enhanced emotional, vs. neutral, recall in a nap paradigm involving recall for emotionally distressing vs. neutral images [ 225 ]. Walker and others have proposed an emotional processing function to REMS, wherein REMS enhances the declarative recall of emotional memories while diffusing the negative emotional valence [ 216 ]. On the other hand, several studies reported findings indicating that REMS in healthy subjected either heightened, or did not affect, the emotional tone associated with memories [ 15 , 226 , 228 , 229 ]. Furthermore, a REM-deprivation study found that selective REMS deprivation did not impact emotional memory. Another study found that while sleep after learning did enhance emotional memory recall relative to a non-sleep group, selective REM deprivation had no impact on negative vs. neutral recall [ 230 ]. These varied findings leave researchers with many unanswered puzzles, and suggest that REM/NREM distinctions may be inadequate for pinpointing the complexities of sleep effects on emotional memory.
Sleep spindles deserve brief mention because sleep spindles reflect memory consolidation processes involving the coordinated oscillations of the thalamo-cortical circuitry, the hippocampus and the cerebral cortex that together result in the transfer of information from temporary storage in the hippocampus to more durable cortical storage [ 211 , 231 ]. In a recent laboratory study in which subjects viewed a trauma film prior to either overnight sleep, a day of wake, or sleep deprivation, Kleim and colleagues [ 232 ] found that greater N2 sleep and more parietal spindles (13–15 Hz) in the sleep group were associated with fewer intrusive memories in the week following the laboratory exposure. They also found that increased N1, wake-after-sleep-onset, and REMD were associated with higher intrusions, and that sleep was protective relative to both the other groups. Although few studies have examined spindles in emotional memory, these findings may be relevant in the context of anxiety-related disorders, especially PTSD.
What about nightmares?
Nightmares continue to be an elusive and intriguing phenomenon, which have generated a host of theories regarding etiology and possible function. A long history of dream theory based primarily in psychoanalytic work, but also in peer-reviewed dream research, has espoused a psychological, sleep-protective, and/or emotional processing function for dreams [ 57 , 233 ]. These theories are generally anchored in the notion that emotional dreams are REMS phenomena [ 234 ], that REMS is important for emotional processing (Walker, [ 212 ]; Walker & van der Helm, [ 216 ]), and that trauma nightmares result from a disruption in the normal emotional processing and consolidation functions of REMS [ 235 , 236 , 237 ]. These conceptualizations are grounded in compelling reasoning, but require bolstering by empirical evidence. Further, early research increasingly indicates that trauma nightmares are not an exclusively REMS phenomenon; nightmare awakenings occur out of both REM and NREM sleep [ 59 , 238 , 239 ].
In the context of REM sleep models, it has also been proposed that abnormally elevated adrenergic tone during REMS contributes to the distress of REMS dreams and/or awakenings from REMS [ 240 ]. Our current understanding of central nervous system pathways involved in sleep and arousal are generally consistent with this hypothesis: norepinephrine-generating locus coeruleus activity is low during normal sleep, and essentially absent during normal REMS. Excess activation of the locus coeruleus could perturb REMS. The hypothesis that excessive adrenergic tone during sleep contributes to nightmares seems reasonable and could apply regardless of sleep stage context. We are not aware of studies that have examined this hypothesis. Results from one recent study demonstrated that reduced respiratory sinus arrhythmia during sleep, a measure of parasympathetic tone, was a predictor of nightmare endorsement the following morning [ 241 ]. This may indicate that a disruption in the normal balance of parasympathetic vs. sympathetic tone contributes to nightmares, but is not direct evidence of enhanced sympathetic activity.
Despite our limited understanding of the neurobiology underlying the generation of nightmares, nightmares likely contribute to the maintenance of PTSD and sleep disturbance in a manner consistent with the insomnia processes discussed previously. Nightmares are likely to contribute to fear of sleep and sleep avoidance, factors that contribute to altered sleep-related behaviors and insomnia. Short et al. also demonstrated that daytime PTSD symptoms and fear of sleep were prospectively related to nightmares [ 163 ]. The effects of repeated activation of trauma-related thoughts during sleep are unknown, but one might speculate that they contribute to reinforcement of memory-containing neural networks (the PTSD “fear network”), thereby consolidating those memories in association with distressing emotions.
Abnormal movements during sleep: from muscle twitches to nightmare enactment
Abnormal movements during sleep in anxiety-related disorders are also poorly understood phenomena, and are primarily reported in PSTD, as opposed to other anxiety-related disorders. They are grouped together here because they involve abnormal motor behavior during sleep, rather than due to a clearly understood neurobiological overlap. Muscle twitching in the context of REMS atonia is normal phenomena, and there is even evidence that it plays a role in sensorimotor development [ 214 ]. However, indications that muscle movements are increased in PTSD relative to normal are consistent with the overall concept of hyperarousal during sleep. Carwile and colleagues proposed that RBD-like phenomena in PTSD (nightmare enactment) may be due to abnormalities in LC NE firing. However, in contrast with the idea that NE activity is elevated in REMS, they propose that it is a high “turnover” and resulting depletion of NE in the LC of PTSD patients that disrupts REM atonia, just like degeneration of LC neurons occurs in neurogenerative diseases [ 242 ]. Further research is clearly indicated to better understand the neurobiology of these phenomena and their implications for outcomes in anxiety-related disorders. Regardless of etiology, like other sleep-disrupting events described in this report, they are likely to contribute to the many downstream effects of objective sleep disturbance and the cycle of behavioral, cognitive, and physiological problems that characterize the sleep–anxiety relationship.
Sleep disturbance and anxiety-related disorders: underlying neuromodulatory context
Figure 4 provides a schematic diagram modeling the psychological and neurocognitive factors that link sleep disturbance and anxiety-related disorders in a bidirectional fashion. This relationship occurs in the setting of heightened activity in known neuromodulatory pathways. Animal and pharmacology studies point to important neuromodulators, such as norepinephrine and hypocretin, that play an important role in this dynamic.
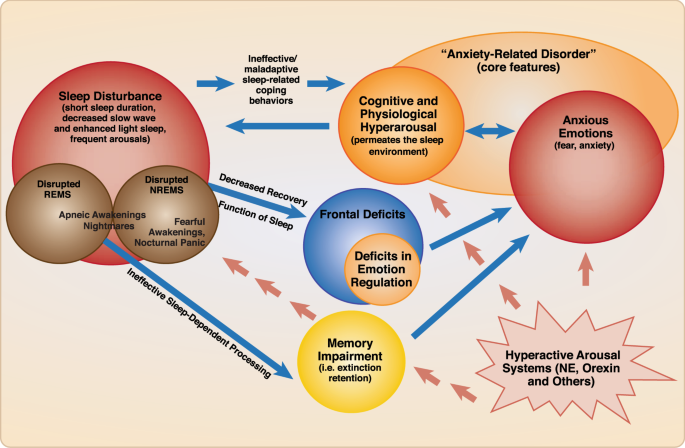
Schematic diagram including the psychological and neurocognitive factors that link sleep disturbance and anxiety-related disorders in a bidirectional fashion. Sleep disturbance, regardless of cause, may result in maladaptive sleep-related compensatory behaviors, resulting in sleep timing that is out-of-synchrony with sleep drive, and therefore wake time in bed. This results in hyperarousal (i.e., cognitive and emotional) in the sleep environment, promoting a vicious cycle of sleep disturbance, compensatory behaviors, hyperarousal cognitions and anxious emotions. Sleep disturbance also has deleterious effects on cognitive functions, due to insufficient sleep-dependent recovery of neural substrates that carry out said functions during wake; this results in widespread deficits including deficits in emotion regulation (i.e., reduced ability to modulate anxious emotions). Sleep disturbance also disrupts processes thought to be carried out during sleep , such as adaptive memory functions (e.g., fear extinction retention; safety learning); this also promotes or maintains anxiety/fear emotions. Anxious emotions and cognitive hyperarousal are mutually reinforcing, and feed back into the sleep disturbance cycle. The specific role of REMS and NREMS in recovery and sleep-dependent processes is a topic of investigation. The nature of mechanistic connections between anxiety-related events such as nightmares and panicked awakenings, and NREM or REM sleep-dependent processes, remains to be demonstrated with empirical evidence. At the very least, their promotion of cognitive hyperarousal and anxious emotions can be inferred from their contribution to sleep disruption and the fearful emotions they generate. A neuromodulatory milieu involving heightened arousal signals, e.g., norepinephrine, and/or hypocretin, drives and underlies this relationship
Major neuromodulators in sleep–wake regulation and anxiety-related disorders
Norepinephrine.
Norepinephrine is released both peripherally (adrenal medulla) and in the central nervous system as a neurotransmitter and plays a central role in both sleep regulation and anxiety (See Fig. 1 ). Although its nearly exclusive CNS source, the locus coeruleus, seems to be too small to be captured by classic neuroimaging techniques (although see PET studies above and fMRI findings by Naegeli et al. [ 243 ] in PTSD), it is clearly central to anxiety- and fear-related processes in the CNS through its direct innervation of the amygdala and its inhibitory inputs to the VLPO, among a multitude of other projections (e.g., cerebral cortex). Normally, NE activity is high during wake, low during NREMS, and nearly completely quiescent during REMS [ 244 ]. Human studies have provided some evidence of elevated sympathetic tone and/or LC activity in anxiety-disordered sleep [ 29 , 136 , 245 ].
Pharmacological research has studied various noradrenergic blockers for treatment of anxiety-related disorders. For example, the selective alpha-1-adrenergic blocker prazosin has demonstrated benefits in multiple small to medium-sized RCTs for PTSD symptoms and trauma nightmares [ 246 , 247 , 248 ]. Disappointingly, a recent large RCT of prazosin for PTSD in veterans demonstrated no benefit relative to placebo and no benefit overall for either PTSD symptoms overall or for nightmares [ 249 , 250 ]. One recent study indicates that higher baseline systolic blood pressure predicts greater therapeutic response to prazosin for nightmares and sleep disturbance [ 251 ]. Doxazosin, a medication with the same alpha-1 blocking effects of prazosin, but with a longer half-life, has shown some benefit in preliminary studies and is currently being studied as an alternative to prazosin [ 252 ]. Interestingly, it is thought that doxazosin is less able to cross the blood–brain barrier than prazosin. This raises questions about whether PTSD is associated with greater blood–brain barrier permeability, thus allowing entry of less lipophilic agents, or whether some of the CNS benefits are mediated via peripheral adrenergic nervous system responses. Finally, the alpha-2-agonist clonidine has shown some benefit in PTSD nightmares, however it has not been extensively studied [ 253 ].
The neurotransmitter serotonin (5-hydroxytryptophan, 5HT) is a neurotransmitter that has an important role in arousal (see Fig. 1 ) and which also plays an important role in the regulation of anxiety and mood states. It is produced in the dorsal raphe nucleus, which has broad axonal projections including projections to the VLPO (inhibitory) and the cerebral cortex. The arousal effect of 5HT is mediated in part by 5HT-2A receptor activity, based on animal studies as well as clinical research demonstrating that agents with high 5HT-2A blocking effects are sedating (i.e., trazodone, quetiapine, amitriptyline). Agents that selectively inhibit the reuptake of serotonin into neurons (selective serotonin reuptake inhibitors, (SSRI)) and thereby increase 5HT in the synapse, are first-line agents for anxiety and mood-related disorders, including all of the disorders described in this review. Serotonin levels are high during wake, low during NREMS and at their lowest during REMS [ 244 ]. One can therefore see how agents that enhance 5HT across 24 hours (SSRI’s and SNRI’s, which also block norepinephrine reuptake) might have untoward effects on sleep even if beneficial for anxiety. On the other hand, downregulation of 5HT-2A receptors with prolonged SSRI administration may be associated with resolution of initial activating effects of these drugs.
The role of histamine in sleep/wake circuitry is demonstrated in basic research and is supported by the broad use, though limited controlled research, of various drugs with anti-histaminergic properties (e.g., trazodone, quetiapine, amitriptyline, doxepin, diphenhydramine, and cetirizine). It has been proposed that histamine release is under circadian control, and is released earlier than other neurotransmitters. Although circadian disruptions are a major topic that is hardly covered in this review, the possibility the role of circadian timing of histamine release may be relevant to early morning awakening in anxiety-related disorders. Anti-histamines are commonly used off-label for the treatment of anxiety, although evidence for specificity as an anxiolytic, as opposed to a sedating agent, is weak.
Acetylcholine
Acetylcholine is a neurotransmitter synthesized in several brain regions/nuclei, including the pedunculopontine tegmentum (pons), the lateraldorsal tegmentum (pons) and the basal forebrain and is also an important arousal neurotransmitter. In contrast to the other major neuromodulators described above, CNS cholinergic activity is normally high in both wake and REMS, although decreased in NREMS [ 244 ]. Walker and colleagues [ 212 , 216 ] have postulated that elevated cholinergic activity in REMS, combined with maximal suppression of noradrenergic activity during this stage, contributes to the emotion and emotional memory processing function of REMS. Although this remains highly theoretical, Walker and colleagues propose that the memory-promoting function of acetylcholine in REMS acts to consolidate the declarative memories (an adaptive process) in the absence of the NE-promoted emotional “tag”.
Several other medications with effects on these neurotransmitters systems have been used to treat sleep disturbance in anxiety-related disorders. Trazodone, for example, is used widely in public health settings [ 254 ] despite limited controlled data on efficacy and is known to block receptors for histamine, serotonin (5HT-2A/2C), and alpha-adrenergic receptors, [ 255 ]. A recent study of cyclobenzaprine, a tricyclic molecule structurally similar to amitriptyline, which like trazodone blocks histamine, alpha-adrenergic, and serotonergic 5-HT2A receptors, was halted during a Phase III development trial because of inadequate separation from placebo for the primary sleep endpoint in military-related PTSD (Clinicaltrials.gov: NCT03062540).
Dopamine, produced in the ventral tegmental area and the ventrolateral periaqueductal gray, is also an important arousal-promoting neurotransmitter. While mostly known for its central involvement in reward neurocircuitry and disorders of addiction, a recent study of quetiapine for PTSD lends some support for dopamine-receptor blockade in the treatment of anxiety-related disorders, and quetiapine is often used off-label for the treatment of insomnia [ 256 , 257 ]. Quetiapine’s broad inhibition of multiple arousal-promoting neurotransmitters, including serotonin (5HT-2A), histamine, and NE (alpha-1) do raise questions about dopamine-specific mechanisms.
Hypocretin/orexin
The hypocretins, also known as orexins, including hypocretin-1 and 2, are neuropeptides with a well-established role in promoting and stabilizing arousal [ 258 , 259 , 260 ]. Recent publications present the growing evidence implicating the hypocretins in anxiety-related disorders, and the pathways through which they act [ 261 , 262 ]. Hypocretin is produced in the hypothalamus, and hypocretin-producing neurons have widespread excitatory projections, including to the main wake-promoting centers (see Fig. 1a ), the cerebral cortex, and the amygdala. Overall, the research indicates that hyperactivity of the orexin system contributes to anxiety-related behavior. While most research has been performed in animal models, one recent study demonstrated elevated CSF orexin in patients with PD. An interesting recent study found that hypocretin was related to panicked awakenings in a rodent model. These findings may be highly relevant to understanding nocturnal panic attacks and frightful awakenings in the context of PTSD and PD and deserve further study.
The dual-orexin antagonist suvorexant is FDA-approved for the treatment of insomnia. At present there are no published trials of hypocretin/orexin antagonists in anxiety-related disorders, though a few studies are underway. Theoretically, hypocretin/orexin antagonists would target more-discrete pathways than commonly used GABAergic hypnotics, and ideally would be suitable for individuals who demonstrate abnormally increased hypocretin activity [ 263 ]. There is preclinical evidence that orexin antagonism can block stress-induced sleep disturbance but have no effect on baseline autonomic and autonomic activity [ 264 ]. The implication is that it is possible to develop compounds that only affect stress-related over-activation of arousal pathways without interfering with normal activity in healthy individuals.
GABA receptors are ubiquitous in the CNS and GABA-mediated CNS inhibition has a huge role in sleep promotion through specific pathways. On the other hand, endogenous GABA-mediated promotion of sleep involves a targeted suppression of arousal-promoting nuclei, rather than a more global inhibition of the cerebral cortex (See Fig. 1 ). Despite some theoretical value of targeting GABAergic function in anxiety-related disorders, the potential for adverse effects (tolerance, dependence, cognitive side-effects, ataxia, respiratory depression) are substantial given widespread distribution of GABAergic inhibitory neurons in the brainstem, cerebellum, and cortex [ 265 ]. At least in PTSD, there is also limited evidence of diminished GABA brain activity [ 266 ]. Furthermore, GABA also plays a role in arousal through inhibition of inhibitory interneurons in the cerebral cortex. This may contribute to paradoxical disinhibition in response to GABAergic drugs in some individuals. Despite concerns, benzodiazepines have demonstrated benefits for anxiety symptoms, and their use continues to be widespread [ 267 ]. There are only a few published controlled studies focused on GABAergic hypnotics in PTSD [ 268 , 269 ], and the extant evidence is insufficient for recommending a GABAergic approach.
The so-called non-benzodiazepine receptor agonists, or “z-drugs,” were developed to specifically target subtypes of the GABA-A receptor, with the aim of reducing adverse effects. One study of eszopiclone, for example, which targets the alpha-3 subunit specifically, provides preliminary evidence of benefit for both sleep disturbance and PTSD [ 269 ]. The advantages of z-drugs over the benzodiazepines in sleep disturbance in anxiety-related disorders has not been well-established.
The HPA axis and CRF
Research in humans and rodent models indicate that stress-induced alterations in corticotropin-releasing factor (CRF) and the HPA axis may result in objective sleep quality disturbances, including decreased delta sleep in human subjects, a quantitative measure of slow-wave activity [ 137 , 153 ] and REMS disturbances in rodents [ 127 ]. CRF functions as a neurotransmitter in the amygdala, locus coeruleus, and bed nucleus of the stria terminalis, and has an arousing effect on the cortex. Theoretically, CRF antagonism would be a good target for treating anxiety-related sleep disturbance, however trials of such agents in psychiatric disorders, including PTSD, have been disappointing [ 270 ]. Nonetheless, these studies represent an attempt to specifically engage in a target pathway implicated in fear and anxiety states. Future trials with agents that are more specifically targeted to abnormal pathways in PTSD and other anxiety disorders await much-needed advances for understanding the underpinning neurobiology [ 256 ].
Other diverse and emerging players: cytokines, adenosine, cannabinoids
Research is beginning to cover the role of multiple other players in sleep-regulatory processes that may be relevant to the relationship reviewed here. Adenosine is a major player in the homeostatic sleep drive (the drive to sleep that increases as a function of time awake and/or physiologic recovery need), and cytokines IL-1 and TNF influence adenosine-mediated effects [ 271 ]. Likewise, overnight cytokine activity has been demonstrated to be altered in anxiety, including PTSD [ 272 ], indicating that abnormalities in these inflammatory factors may contribute to sleep disturbances in anxiety.
One example of an approach that targets a signaling pathway outside the intrinsic sleep or wake-promoting networks is a small trial of nabilone, a synthetic cannabinoid with agonist properties for the CB1 receptor. Cannabis, which contains hundreds of cannabinoids, is widely used as self-medication for anxiety and sleep problems, but the existing evidence of long-term therapeutic benefit is scarce and overall unfavorable, at least for PTSD [ 273 , 274 ]. On the other hand, cannabidiol, as opposed to tetrahydrocannabinol, shows some promise in preliminary research for sleep disturbance [ 273 ]. A trial of nabilone was predicated on evidence from PET imaging for elevated brain cannabinoid CB1 receptor availability in the amygdala of PTSD, which was associated with attentional bias to threat [ 275 ]. This small trial, which showed some efficacy for improving sleep in military-related PTSD represents a proof of concept trial of a sleep-related therapeutic targeting a pathway putatively known to be abnormal in the specific clinical population. Another potential target in the endocannabinoid system is fatty acid amide hydrolase, which metabolizes endogenous cannabinoids [ 276 ].
Summary of Important Neuromodulators in Sleep and Anxiety
The above-described CNS neuromodulators have well-described roles in sleep regulation and/or anxiety, and may be targets for pharmacotherapy of sleep disturbance in anxiety-related disorders. Norepinephrine and hypocretin stand out as obvious targets that are currently under study, but novel research is pointing to a host of other neuromodulators worthy of study as potential drug targets.
A heuristic neurobiological framework for understanding sleep disturbance in anxiety disorders
Given what is known about the brain pathways (see introduction) and neuromodulators regulating sleep and anxiety, a logical assumption is that sleep disturbance and anxiety-related disorders can emanate from distinct innate or acquired neurobiological abnormalities which can then impact each other in mutually reinforcing ways [ 196 ]. Figure 5 presents a heuristic model for considering the neurobiological dynamics underlying the sleep–anxiety relationship modeled in Fig. 4 . For example, the emergence of anxiety-driven sleep disturbance (i.e., anxiety first) results from the input of anxiety circuitry on sleep–wake regulatory neurocircuitry. These anxiety-related arousal pathways are likely to emanate from brain regions involved in fear and/or threat responses. The end result is disruption of NREM-generating circuitry, especially nodes most important for the generation of deep, restorative (i.e., N3/slow-wave) sleep, or the over-riding of sleep-promoting signals [ 277 ]. The effects of anxiety circuitry inputs on REMS circuitry remain to be clarified, as research in humans and animals indicates disruptions as well as increases in REMS in response to stress or in PTSD subjects [ 120 , 125 , 127 , 128 , 278 , 279 ].
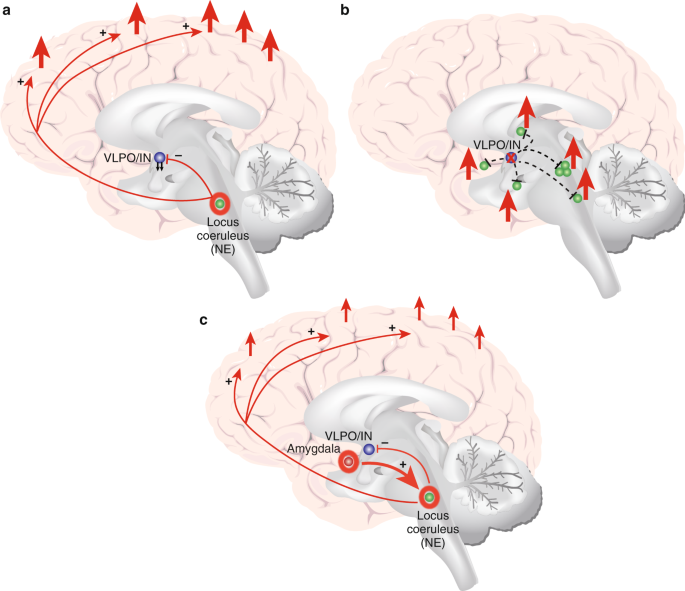
Heuristic neurobiological framework for the sleep disturbance and anxiety disorder relationship. Abnormal activation in wake, NREMS, and/or anxiety regions may generate sleep disturbance in anxiety-related disorders and promote a bidirectional sleep-disturbance-anxiety relationship. a Sleep disturbance emerges from hyperactivation in intrinsic wake circuitry. For example, here, excess arousal in NE-generating LC (green with orange halo) results in inhibition of VLPO/Intermediate Nucleus (IN) (blue) and hyperactivation of cerebral cortex. b Sleep disturbance emerges from dysfuncton in central (NREM) sleep-promoting region. For example, here failure of VLPO GABA signaling (blue, with orange “X”) disinhibits arousal centers (green). c Sleep disturbance emerges from hyperactivation of fear/threat/anxiety regions. For example, here amygdala hyperactivation (red, with orange halo) sends excitatory inputs to LC, which then inhibits VLPO-mediated sleep promotion and sends excitatory inputs to cerebral cortex, resulting in cortical hyperarousal, as in a . A common node in these examples is LC and NE signaling, but aberrant activity in other wake, NREMS, REMS, and anxiety regions may produce analogous effects
In the other direction, abnormalities in sleep–wake regulatory circuitry in the form of heightened arousal signals or deficits in NREM sleep circuitry may impact anxiety-related neurocircuitry directly. They will also act indirectly via the cerebral cortex to generate cortical hyperarousal during wake and sleep, while at the same time impeding sleep-recovery processes that reinforce frontal-lobe-mediated functions such as emotion regulation and adaptive emotional memory processes
Finally, disruptions in neurocircuitry and modulators with fundamental roles in both sleep regulation and anxiety-related circuitry, such as NE, CRF, and hypocretin, can affect both types of disturbance. Regardless of the source cause, pathological processes emanating from either source influence each other and can become mutually reinforcing.
Summary, important undiscussed topics, future directions
Summarizing the above findings, the clinical and epidemiological research indicates that anxiety disorders and subjective/clinical sleep disturbance are highly co-occurring. The objective physiological data provide a mixed picture, but overall suggest cortical hyperarousal during sleep in anxiety-related disorders, characterized by interrupted or shortened sleep, increased lighter stages of sleep (NREM N1 and N2), reductions in SWS (N3), and higher REM density (in PTSD). More-sophisticated analysis of existing data that move beyond simple sleep-stage analysis are likely necessary to better understand hyperarousal in anxiety-related sleep disturbance. The dominant psychological models of insomnia, which underscore the mutually reinforcing effects of fear and anxiety-based cognitive hyperarousal in the sleep environment and maladaptive behavioral coping, go a long way toward explaining bidirectional mechanisms driving the sleep-disturbance anxiety–disorder relationship. Insomnia models emphasizing multi-domain hyperarousal as a risk factor and endpoint in insomnia are consistent with various sleep neurobiological indicators of arousal in insomnia and are consistent with findings in anxiety-disordered sleep. Beyond insomnia models, abnormalities in cognitive inhibition, emotion regulation, and memory consolidation result from sleep disturbance and are features of anxiety disorders, and therefore have implications for bidirectional mechanisms in the anxiety–disorder–sleep–disturbance relationship. Finally, animal and human translational research as well as pharmacological treatment research provide clues to important neuromodulatory pathways that may be relevant to this relationship. A heuristic neurobiological framework indicates how sleep/wake regulatory centers may be affected during sleep in anxiety-related disorders, and suggests direct and indirect pathways through which sleep and anxiety neurobiology may affect each other.
In this review, we broadly defined anxiety-related disorders as DSM disorders in which fear and/or anxiety are considered to play a core and primary role. This reflects an attempt to maintain a faithful connection to the clinical phenomena and the human research, which has been conducted, until recently, using case/controls approaches, while also recognizing that an approach guided by current knowledge of neurocircuitry (i.e., mediating arousal, fear and anxiety processes) in psychopathology has many advantages. The Research Domain Criteria (RDoC) encourages researchers to focus on transdiagnostic mental processes that correspond better with known neurocircuitry than do DSM disorders because they are more likely to advance our understanding of psychopathology. Therefore, rather than recruiting uniquely for a specific anxiety-related disorder (e.g., PTSD) or sleep disorder (e.g., insomnia), investigators should consider recruiting for a specific domain or symptom presentation (e.g., individuals who report high-anxiety sensitivity, individuals with poor sleep continuity). The RDoC also emphasizes the importance of integrating many levels of information (e.g., genetics, neurobiological data from human and animal subjects, subjective-report data). In line with this model, future studies will benefit from multimodal assessments in order to better understand the intricacies of relevant mechanisms.
Although reviewing this vast topic, it is important to acknowledge items not covered in this review that are important when considering sleep and anxiety-related disorders. First, the effects of circadian rhythm and circadian disruption on sleep and anxiety-related disorders are outside of the scope of this review, but are fundamental contributors to the disturbances in question. For example, there is evidence that circadian factors impact anxiety symptom expression, extinction memory, and exposure therapy efficacy, although discrimination between circadian and homeostatic contributors is not clear in most studies [ 280 , 281 ]. We refer readers to existing studies which address this topic [ 83 , 152 , 282 , 283 , 284 , 285 , 286 , 287 ] and encourage the consideration of circadian factors in sleep research, and anxiety research, in general. In addition, we have focused on anxiety disorders, but the comorbidity and overlap with mood disorders is considerable [ 288 , 289 ]. The effects reviewed in this report often, but not always, accounted for the effects of mood disorders such as major depressive disorder. Finally, the genetics underlying the above-described relationships are outside of the scope of the current review but genetics research is essential for understanding shared and overlapping etiologies.
The future of therapeutic progress in improving outcomes for individuals with sleep disturbance in anxiety-related disorders will involve expanding upon existing excellent cognitive-behavioral treatments and integrating these with tools developed based on a deeper grasp of neurobiology. Dias and colleagues [ 290 ] predict that advanced technologies that target specific disturbed pathways in the human body, in the way that optogenetic manipulations already affect anxiety in animal models, are on the horizon. Combining the least invasive biological methods with the many advantages of psychological treatments should be the objective of medical science in sleep and anxiety-related disorders into the future.
Funding and Disclosure
Dr. Neylan is a consultant for Jazz Pharmaceuticals. Dr. Kanady and Dr. Richards declare nopotential conflicts of interest.
Change history
07 october 2019.
The HTML and PDF versions of this Article have been updated to replace Figure 3 and Figure 5 and amend the Figure 3 legend.
A Correction to this paper has been published: https://doi.org/10.1038/s41386-019-0529-y
Van Someren, EJW et al. Disrupted sleep: from molecules to cognition. J Neurosci. 2015;35:13889–95.
Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339.
Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-29.
Gruber R, Cassoff J. The interplay between sleep and emotion regulation: conceptual framework empirical evidence and future directions. Curr Psychiatry Rep. 2014;16:500.
Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66.
Ibarra-Coronado EG, et al. The Bidirectional Relationship between Sleep and Immunity against Infections. J Immunol Res. 2015;2015:678164.
Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–74
Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–90.
Jouvet M. The States of Sleep. Sci Am. 1967;216:62–8.
Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34.
Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62.
Richards A, et al. Sleep and cognitive performance from teens to old age: more is not better. Sleep. 2017;40: https://doi.org/10.1093/sleep/zsw029 .
Pace-Schott EF, Germain A, Milad MR. Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull. 2015; https://doi.org/10.1037/bul0000014 .
Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013; https://doi.org/10.1016/j.nlm.2012.10.006 .
Groch S, Zinke K, Wilhelm I, Born J. Dissociating the contributions of slow-wave sleep and rapid eye movement sleep to emotional item and source memory. Neurobiol Learn Mem. 2015; https://doi.org/10.1016/j.nlm.2014.08.013 .
Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81.
CAS PubMed Google Scholar
Borbely AA. A two process model of sleep regulation. Hum Neurobiol 1982;1:195–204.
Oh J. et al. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2018; https://doi.org/10.1038/s41380-018-0291-2 .
Schwartz MD, Kilduff TS. The neurobiology of sleep and wakefulness. Psychiatr Clin North Am. 2015; https://doi.org/10.1016/j.psc.2015.07.002 .
Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63.
Behn CGD, Brown EN, Scammell TE, Kopell NJ. Mathematical model of network dynamics governing mouse sleep–wake behavior. J Neurophysiol 2007;97:3828–40.
PubMed Google Scholar
Phillips AJK, Robinson PA. A quantitative model of sleep-wake dynamics based on the physiology of the brainstem ascending arousal system. J Biol Rhythms. 2007;22:167–79.
Rempe MJ, Best J, Terman D. A mathematical model of the sleep/wake cycle. J Math Biol 2010;60:615–44.
Saper CB, Fuller PM. wake-sleep circuitry: an overview. Curr Opin Neurobiol 2017;44:186–92.
CAS PubMed PubMed Central Google Scholar
Anaclet C, et al. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci 2012;32:17970–6.
Anaclet C, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci 2014;17:1217–24.
Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron. 2010; https://doi.org/10.1016/j.neuron.2010.11.032 .
Nofzinger EA, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry. 2004; https://doi.org/10.1176/appi.ajp.161.11.2126 .
Germain A, Stocker R, Ebdlahad S, Mammen O. Functional neuroimaging of NREM sleep in combat veterans with and without posttraumatic stress disorder (PTSD). Sleep. 2013;211:176–79.
Ebdlahad S, Stocker R, Mammen O, Nofzinger E, Germain A. Neural correlates of insomnia comorbid with PTSD during wakefulness and nrem sleep. Sleep. 2013;36:A303.
Etkin A, Gyurak A, O’Hara, R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin. Neurosci. 2013;15:419–429.
Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007; https://doi.org/10.1176/appi.ajp.2007.07030504 .
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010; https://doi.org/10.1038/npp.2009.83 .
Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 2015; https://doi.org/10.2147/TCRM.S48528 .
Norton PJ, Paulus DJ. Transdiagnostic models of anxiety disorder: theoretical and empirical underpinnings. Clin Psychol Rev 2017;56:122–37.
Neylan TC, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam Veterans. Am J Psychiatry. 1998;155:929–33.
Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med Rev 2000;4:263–76.
Stewart R, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7.
Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res 2002;36:449–52.
Ramsawh HJ, Stein MB, Belik SL, Jacobi F, Sareen J. Relationship of anxiety disorders, sleep quality, and functional impairment in a community sample. J Psychiatr Res 2009;43:926–33.
Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord 2016;37:104–29.
Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–82.
PubMed PubMed Central Google Scholar
Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the Hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707.
Roszell DK, McFall ME, Malas KL. Frequency of symptoms and concurrent psychiatric disorder in Vietnam veterans with chronic PTSD. Hosp Community Psychiatry. 1991;42:293–6.
Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41:469–78.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc. 2013; https://doi.org/10.1176/appi.books.9780890425596.744053
Creamer JL, Brock MS, Mysliwiec V. Nightmares in United States military personnel are multifactorial and require further astudy. J Clin Sleep Med 2018;14:1275–6.
Sandman N, et al. Nightmares: prevalence among the Finnish general adult population and war veterans during 1972-2007. Sleep. 2013;36:1041–50.
Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–62.
Bjorvatn B, Grønli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med 2010;11:1031–4.
Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. 2010;33:774–80.
Robert G, Zadra A. Measuring nightmare and bad dream frequency: Impact of retrospective and prospective instruments. J Sleep Res 2008;17:132–9.
Kobayashi I, Delahanty DL. Gender differences in subjective sleep after trauma and the development of posttraumatic stress disorder symptoms: sa pilot study. J Trauma Stress. 2013;26:467–74.
Milanak ME, et al. Traumatic event exposure, posttraumatic stress disorder, and sleep disturbances in a national sample of U.S. adults. J Trauma Stress. 2019;32:14–22.
Woodward SH, Arsenault NJ, Murray C, Bliwise DL. Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol Psychiatry. 2000;48:1081–7.
Insana SP, Hall M, Buysse DJ, Germain A. Validation of the pittsburgh sleep quality index addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J Trauma Stress. 2013;26:192–200.
Mysliwiec V, et al. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev 2018;37:94–104.
Brown TM, Boudewyns PA. Periodic limb movements of sleep in combat veterans with posttraumatic stress disorder. J Trauma Stress. 1996;9:129–36.
Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:110–5.
Ross RJ, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17:723–32.
Woodward SH, Michell G, Santerre C. The psychophysiology of PTSD nightmares. in Sleep and Combat-Related Post Traumatic Stress Disorder (2017). https://doi.org/10.1007/978-1-4939-7148-0_20 .
Mysliwiec V, et al. Trauma associated sleep disorder: sa proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med 2014;10:1143–8.
Bélanger L, Morin CM, Langlois F, Ladouceur R. Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for gad on insomnia symptoms. J Anxiety Disord 2004;18:561–71.
Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–36.
Brenes GA, et al. Insomnia in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2009;17:465–72.
Tempesta D, et al. Neuropsychological functioning in young subjects with generalized anxiety disorder with and without pharmacotherapy. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;45:236–41.
CAS Google Scholar
Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18:622–7.
Berger A, et al. Patterns of healthcare utilization in patients with generalized anxiety disorder in general practice in Germany. Eur J Psychiat. 2009; 23:90–100.
Mellman TA, Uhde TW. Patients with frequent sleep panic: clinical findings and response to medication treatment. J Clin Psychiatry. 1990;51:513–26.
Park HJ, et al. Association between sleep disorder and panic disorder in South Korea: nationwide nested case-control study of data from 2004 to 2013. Psychiatry Res 2018;260:286–91.
Sheehan DV, Ballenger J, Jacobsen G. Treatment of endogenous anxiety with phobic, hysterical, and hypochondriacal symptoms. Arch Gen Psychiatry. 1980;37:51–59.
Overbeek T, Van Diest R, Schruers K, Kruizinga F, Griez E. Sleep complaints in panic disorder patients. J Nerv Ment Dis 2005;193:488–93.
Todder D, Baune BT. Quality of sleep in escitalopram-treated female patients with panic disorder. Hum Psychopharmacol. 2010;25:167–73.
Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Med Rev 2005;9:173–84.
Castelnovo A, Lopez R, Proserpio P, Nobili L, Dauvilliers Y. NREM sleep parasomnias as disorders of sleep-state dissociation. Nat Rev Neurol. 2018; https://doi.org/10.1038/s41582-018-0030-y .
Craske MG, Barlow DH. Nocturnal panic. J Nerv Ment Dis 1989;173:160–7.
Google Scholar
Krystal JH, Woods SW, Hill CL, Charney DS. Characteristics of panic attack subtypes: sassessment of spontaneous panic, situational panic, sleep panic, and limited symptom attacks. Compr Psychiatry. 1991;32:474–80.
Stein MB, Enns MW, Kryger MH. Sleep in nondepressed patients with panic disorder: II. Polysomnographic assessment of sleep architecture and sleep continuity. J Affect Disord 1993;28:1–6.
Uhde TW, Anxiety Disorders. in Principles and Practice of Sleep Medicine,. Kryger Meir H; Roth, TDWC (ed.) 871–98 (Saunders Co., 1994).
Mellman TA, Uhde TW. Electroencephalographic sleep in panic disorder: a focus on sleep-related panic attacks. Arch Gen Psychiatry. 1989;46:178–84.
Aǧargün MY, Kara H. Recurrent sleep panic, insomnia, and suicidal behavior in patients with panic disorder. Compr Psychiatry. 1998;39:149–51.
Roth T, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60:1364–71.
Bobdey M, Fineberg N, Gale TM, Patel A, Davies HA. Reported sleep patterns in obsessive compulsive disorder (OCD). Int J Psychiatry Clin Pract 2002;6:15–21.
Donse L, Sack AT, Fitzgerald PB, Arns M. Sleep disturbances in obsessive-compulsive disorder: association with non-response to repetitive transcranial magnetic stimulation (rTMS). J Anxiety Disord. 2017; https://doi.org/10.1016/j.janxdis.2017.03.006 .
Park S, et al. Relationships of sleep duration with sociodemographic and health-related factors, psychiatric disorders and sleep disturbances in a community sample of Korean adults. J Sleep Res 2010;19:567–77.
Robinson D, Walsleben J, Pollack S, Lerner G. Nocturnal polysomnography in obsessive-compulsive disorder. Psychiatry Res 1998;80:257–63.
Marcks BA, Weisberg RB, Edelen MO, Keller MB. The relationship between sleep disturbance and the course of anxiety disorders in primary care patients. Psychiatry Res 2010;178:487–92.
Daroff RB. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. Neurology. 2012; https://doi.org/10.1212/wnl.41.1.160 .
Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; https://doi.org/10.1513/pats.200707-114mg .
Younes M. Pathogenesis of obstructive sleep Apnea. Clin Chest Med. 2019; https://doi.org/10.1016/j.ccm.2019.02.008 .
Colvonen PJ, et al. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med 2015;11:513–8.
Yesavage JA, et al. Sleep-disordered breathing in vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry. 2012;20:199–204.
Krakow B, et al. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis 2002;190:442–52.
Krakow B, et al. Complex insomnia: Insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49:948–53.
Ocasio-Tascón ME, Alicea-Colón E, Torres-Palacios A, Rodríguez-Cintrón W. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 2006;10:70–75.
Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: saecondary symptom or core feature? Sleep Med Rev 2008;12:169–84.
Youakim JM, Doghramji K, Schutte SL. Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics. 1998;39:168–71.
Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. 2015; https://doi.org/10.1016/j.smrv.2014.11.001 .
Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biol Psychiatry. 2000;47:520–5.
Kinoshita LM, et al. Modeling the effects of obstructive sleep apnea and hypertension in Vietnam veterans with PTSD. Sleep Breath 2012;16:1201–9.
Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med 2015;11:165–75.
Mysliwiec V, et al. Sleep disorders and sassociated medical comorbidities in sactive duty military personnel. Sleep. 2013;36:167–74.
Mysliwiec V, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144:549–57.
van Liempt, S, Westenberg, HG, Arends, J, Vermetten, E. Obstructive sleep apnea in combat-related posttraumatic stress disorder: a controlled polysomnography study. Eur J Psychotraumatol 2011;2:10.3402/ejpt.v2i0.8451.
Zhang Y, Weed JG, Ren R, Tang X, Zhang W. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: a meta-analysis. Sleep Med 2017;36:125–32.
Babson KA, Del Re AC, Bonn-Miller MO, Woodward SH. The comorbidity of sleep apnea and mood, anxiety, and substance use disorders among obese military veterans within the veterans health administration. J Clin Sleep Med 2013;9:1253–8.
Pevernagie D, et al. Behavioural hyperventilation as a novel clinical condition associated with central sleep apnoea: a report of three cases. Sleep Med 2012;13:1317–20.
Trajanovic NN, Rasool MS, Voloh I, Shapiro CM. Sleep-disordered breathing, cardiac arrhythmia, and panic disorder. J Clin Psychol Med Settings. 2005;1:288–9.
Enns MW, Stein M, Kryger M. Successful treatment of comorbid panic disorder and sleep apnea with continuous positive airway pressure. Psychosomatics. 1995;36:585–6.
Stein MB, Millar TW, Larsen DK, Kryger MH. Irregular breathing during sleep in patients with panic disorder. Am J Psychiatry. 1995;152:1168–73.
Edlund MJ, McNamara ME, Millman RP. Sleep apnea and panic attacks. Compr Psychiatry. 1991;32:130–2.
Hrubos-Strøm H, et al. Sleep apnoea, anxiety, depression and somatoform pain: sa community-based high-risk sample. Eur Respir J 2012;40:400–7.
Breland JY, et al. The obesity epidemic in the veterans health administration: prevalence samong key populations of women and men veterans. J Gen Intern Med. 2017; https://doi.org/10.1007/s11606-016-3962-1 .
Rosenbaum S, et al. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism 2015;64:926–33.
Baglioni C, et al. Sleep and mental disorders: sa meta-analysis of polysomnographic research. Psychol Bull 2016;142:969–90.
Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9.
Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7.
Fuller KH, Waters WF, Scott O. An investigation of slow-wave sleep processes in chronic PTSD patients. J Anxiety Disord 1994;8:227–36.
Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20:46–51.
Richards A, et al. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J Sleep Res 2013;22:679–87.
Habukawa M, Uchimura N, Maeda M, Kotorii N, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol Psychiatry. 2007;62:1179–82.
Breslau N, et al. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61:508–16.
Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002; https://doi.org/10.1176/appi.ajp.159.10.1696 .
Mellman TA, Kobayashi I, Lavela J, Wilson B, Hall Brown TS. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. 2014;1:1321–6.
Ross RJ. The changing REM sleep signature of posttraumatic satress disorder. Sleep. 2014;37:1281–2.
Sanford LD, Suchecki D, Meerlo P. Stress, arousal, and sleep. Curr Top Behav Neurosci. 2015; https://doi.org/10.1007/7854_2014_314 .
Wellman LL, Yang L, Ambrozewicz MA, Machida M, Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. Sleep. 2013; https://doi.org/10.5665/sleep.2526 .
Vanderheyden WM, et al. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp Brain Res 2015;233:2335–46.
Vanderheyden W, Urpa L, Poe G. Increase in rem sleep following trauma exposure. Sleep Med. 2014; https://doi.org/10.1016/j.sleep.2013.11.718 .
Suchecki D, Tiba PA, Machado RB. REM sleep rebound as an adaptive response to stressful situations. Front Neurol. 2012; https://doi.org/10.3389/fneur.2012.00041 .
Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008; https://doi.org/10.1016/j.neubiorev.2007.06.001 .
Germain A, James JA, Mammen O, Price J, Nofzinger E. Functional neuroimaging of rem sleep in returning veterans with ptsd: san [18F]-FDG PET study. Sleep. 2011;A242:34.
Suter D, Mammen O, Insana S, Nofzinger E, Germain A. Neurobiological effects of prazosin on NREM sleep in Veterans with PTSD. in SLEEP - Abstract Supplement, Volume 37 (2014).
Germain A, et al. Prazosin increases brain glucose metabolism in regions involved in fear extinction learning and memory during REM sleep in combat exposed Veterans with PTSD. in SLEEP - Abstract Supplement, Volume 37 (2014).
Van Wyk M, Thomas KGF, Solms M, Lipinska G. Prominence of hyperarousal symptoms explains variability of sleep disruption in posttraumatic stress disorder. Psychol Trauma. 2016;8:688–96.
Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–9.
Inslicht SS, et al. Sleep and hypothalamic pituitary adrenal axis responses to metyrapone in posttraumatic stress disorder. Psychoneuroendocrinology. 2018;88:136–43.
Van Liempt S, et al. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38:155–65.
Papadimitriou GN, Kerkhofs M, Kempenaers C, Mendlewicz J. EEG sleep studies in patients with generalized anxiety disorder. Psychiatry Res 1988;26:183–90.
Arriaga F, Paiva T. Clinical and EEG sleep changes in primary dysthymia and generalized anxiety: a comparison with normal controls. Neuropsychobiology. 1990;24:109–14.
Lund HG, Bech P, Eplov L, Jennum P, Wildschiødtz G. An epidemiological study of REM latency and psychiatric disorders. J Affect Disord 1991;23:107–12.
Lauer CJ, Krieg JC, Garcia-Borreguero D, Özdaglar A, Holsboer F. Panic disorder and major depression: a comparative electroencephalographic sleep study. Psychiatry Res 1992;44:41–54.
Lydiard RB, et al. Electroencephalography during sleep of patients with panic disorder. J Neuropsychiatry Clin Neurosci 1989;1:372–6.
Sloan EP, et al. Nocturnal and daytime panic attacks comparison of sleep architecture, heart rate variability, and response to sodium lactate challenge. Biol Psychiatry. 1999;45:1313–20.
Uhde TW, et al. The sleep of patients with panic disorder: sa preliminary report. Psychiatry Res 1984;12:251–9.
Ferini-Strambi L, et al. Cyclic alternating pattern of sleep electroencephalogram in patients with panic disorder. Biol Psychiatry. 1996;40:225–6.
Dubé S, et al. Interface of panic and depression: clinical and sleep EEG correlates. Psychiatry Res 1986;19:119–33.
Insel TR, et al. The sleep of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1982;39:1372–7.
Voderholzer U, et al. Sleep in obsessive compulsive disorder: Polysomnographic studies under baseline conditions and after experimentally induced serotonin deficiency. Eur Arch Psychiatry Clin Neurosci 2007;257:173–82.
Hohagen F, et al. Sleep EEG of patients with obsessive-compulsive disorder. Eur Arch Psychiatry Clin Neurosci 1994;243:273–8.
Kluge M, et al. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J Psychiatr Res 2007;41:928–33.
Nota JA, Sharkey KM, Coles ME. Sleep, arousal, and circadian rhythms in adults with obsessive-compulsive disorder: sa meta-analysis. Neurosci Biobehav Rev.2015; https://doi.org/10.1016/j.neubiorev.2015.01.002 .
Otte C, et al. Effects of Metyrapone on hypothalamic-pituitary-adrenal axis and sleep in women with post-traumatic stress disorder. Biol Psychiatry. 2007, https://doi.org/10.1016/j.biopsych.2006.08.018 .
Woodward SH, Murburg MM, Bliwise DL. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000, https://doi.org/10.1016/S0031-9384(00)00271-7 .
Wei Y, et al. Sleep stage transition dynamics reveal specific stage 2 vulnerability in insomnia. Sleep 2017; https://doi.org/10.1093/sleep/zsx117 .
Nofzinger EA, et al. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Res Protoc. 1998; https://doi.org/10.1016/S1385-299X(97)00042-1 .
Gehrman P, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36:1009–18.
Wright KM, et al. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol 2011;67:1240–58.
Van Liempt S, Van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: sa prospective longitudinal cohort study. Depress Anxiety. 2013; https://doi.org/10.1002/da.22054 .
Talbot LS, et al. Cognitive behavioral therapy for insomnia in posttraumatic satress disorder: a randomized controlled trial. Sleep. 2014;37:327–41.
Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016; https://doi.org/10.1016/j.cpr.2015.09.005 .
Hertenstein E, et al. Insomnia as a predictor of mental disorders: sa systematic review and meta-analysis. Sleep Med Rev. 2019;. https://doi.org/10.1016/j.smrv.2018.10.006 .
Short NA, Allan NP, Stentz L, Portero AK, Schmidt NB. Predictors of insomnia symptoms and nightmares among individuals with post-traumatic stress disorder: san ecological momentary assessment study. J Sleep Res 2017;27:64–72.
Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006; https://doi.org/10.1016/j.jpsychires.2006.07.008 .
Batterham PJ, Glozier N, Christensen H. Sleep disturbance, personality and the onset of depression and anxiety: prospective cohort study. Aust N Z J Psychiatry. 2012;46:1089–98.
Roy Byrne PP, Uhde TW, Post RM. Effects of one night’s sleep deprivation on mood and behavior in panic disorder: patients with panic disorder compared with depressed patients and normal controls. Arch Gen Psychiatry. 1986;43:895–9.
Babson KA, Feldner MT, Trainor CD, Smith RC. An experimental investigation of the effects of acute sleep deprivation on panic-relevant biological challenge responding. Behav Ther 2009;40:239–50.
Cousineau H, et al. Insomnia symptoms following treatment for comorbid panic disorder with agoraphobia and generalized anxiety disorder. J Nerv Ment Dis 2016;204:267–73.
Cervena K, Matousek M, Prasko J, Brunovsky M, Paskova B. Sleep disturbances in patients treated for panic disorder. Sleep Med 2005;6:149–53.
Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R. National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans. Int J Geriatr Psychiatry. 2015; https://doi.org/10.1002/gps.4143 .
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med 2016;165:125–33.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme M-E. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev 2011; https://doi.org/10.1016/j.cpr.2011.02.004 .
Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541–53.
Bootzin R. Stimulus control treatment for insomnia. Proc Am Psychol Assoc 1972;7:395–6.
Harvey AG. A cognitive model of insomnia. Behav Res Ther 2002;40:869–93.
Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol 2002;53:215–43.
Riemann D et al. The hyperarousal model of insomnia: sa review of the concept and its evidence. Sleep Med Rev. 2010; https://doi.org/10.1016/j.smrv.2009.04.002 .
Perlis ML, Smith MT, Pigeon WR. Etiology and Pathophysiology of Insomnia. in Principles and Practice of Sleep Medicine, 714–25 (Elsevier Inc., 2005). https://doi.org/10.1016/B0-72-160797-7/50067-7 .
Pigeon WR. Diagnosis, prevalence, pathways, consequences, treatment of insomnia. Indian J Med Res 2010;131:321–32.
Sinha SS. Trauma-induced insomnia: sa novel model for trauma and sleep research. Sleep Med Rev 2016;25:74–83.
Lima SL, Rattenborg NC, Lesku JA, Amlaner CJ. Sleeping under the risk of predation. Anim Behav 2005;70:723–36.
Eban-Rothschild A, Giardino WJ, de Lecea L. To sleep or not to sleep: neuronal and ecological insights. Curr Opin Neurobiol 2017;44:132–8.
Kanady JC, et al. Cognitive behavioral therapy for insomnia reduces fear of sleep in individuals with posttraumatic stress disorder. J Clin Sleep Med 2018;14:1193–203.
Krakow B, et al. An open-label trial of evidence-based cognitive behavior therapy for nightmares and insomnia in crime victims with PTSD. Am J Psychiatry. 2001;158:2043–7.
Pruiksma KE, et al. A psychometric study of the Fear of Sleep Inventory-Short Form (FoSI-SF). J Clin Sleep Med 2014;10:551–8.
Hall Brown T, Mellman TA. The influence of PTSD, sleep fears, and neighborhood stress on insomnia and short sleep duration in urban, young adult, african americans. Behav Sleep Med 2014;12:198–206.
Palagini L, Moretto U, Dell’Osso L, Carney C. Sleep-related cognitive processes, arousal, and emotion dysregulation in insomnia disorder: the role of insomnia-specific rumination. Sleep Med 2017;30:97–104.
Palagini L, et al. Daytime rumination as a feature of insomnia disorder: sleep related cognition is not merely a problem of the night. Arch Ital Biol 2015;153:239–47.
Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Heal. 2010;46:124–32.
Takano K, Iijima Y, Tanno Y. Repetitive thought and self-reported sleep disturbance. Behav Ther 2012;43:779–89.
Thomsen DK, Mehlsen MY, Christensen S, Zachariae R. Rumination - relationship with negative mood and sleep quality. Pers Individ Dif. 2003;34:1293–301.
Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med 2009;71:771–5.
Lancee J, Eisma MC, van Zanten KB, Topper M. When thinking impairs sleep: trait, daytime and nighttime repetitive thinking in insomnia. Behav Sleep Med 2017;15:53–69.
O’Kearney R, Pech M. General and sleep-specific worry in insomnia. Sleep Biol Rhythms. 2014;12:212–5.
Singareddy R, Uhde TW. Nocturnal sleep panic and depression: relationship to subjective sleep in panic disorder. J Affect Disord 2009;112:262–6.
Uhde TW, Cortese BM, Vedeniapin A. Anxiety and sleep problems: emerging concepts and theoretical treatment implications. Curr Psychiatry Rep 2009;11:269–76.
Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther 1986;24:1–8.
Hoge EA, et al. The role of anxiety sensitivity in sleep disturbance in panic disorder. J Anxiety Disord 2011;25:536–8.
Craske MG, et al. Presleep attributions about arousal during sleep: nocturnal panic. J Abnorm Psychol 2002;111:53–62.
Craske MG, Freed S. Expectations about sarousal and nocturnal panic. J Abnorm Psychol 1995;104:567–75.
Craske MG, Lang AJ, Tsao JC, Mystkowski JL, Rowe MK. Reactivity to interoceptive cues in nocturnal panic. J Behav Ther Exp Psychiatry. 2001;32:173–90.
Tsao JCI, Craske MG. Reactivity to imagery and nocturnal panic attacks. Depress Anxiety. 2003;18:205–13.
Timpano KR, Carbonella JY, Bernert RA, Schmidt NB. Obsessive compulsive symptoms and sleep difficulties: exploring the unique relationship between insomnia and obsessions. J Psychiatr Res 2014;57:101–7.
Del Río-Casanova L, González A, Páramo M, Van Dijke A, Brenlla J. Emotion regulation strategies in trauma-related disorders: Pathways linking neurobiology and clinical manifestations. Rev Neurosci. 2016; https://doi.org/10.1515/revneuro-2015-0045 .
Cohen Kadosh K, et al. Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. Neuroimage. 2016; https://doi.org/10.1016/j.neuroimage.2015.09.070 .
Ballesio A, Ottaviani C, Lombardo C. Poor cognitive inhibition predicts rumination about insomnia in a clinical sample. Behav Sleep Med 2018;20:1–10.
Tsypes A, Aldao A, Mennin DS. Emotion dysregulation and sleep difficulties in generalized anxiety disorder. J Anxiety Disord 2013;27:197–203.
Hovland A, et al. Subjective sleep quality in relation to inhibition and heart rate variability in patients with panic disorder. J Affect Disord 2013;150:152–5.
Pace-Schott EF, et al. Resting state functional connectivity in primary insomnia, generalized anxiety disorder and controls. Psychiatry Res - Neuroimaging. 2017;265:26–34.
Diekelmann S, Born J. Slow-wave sleep takes the leading role in memory reorganization. Nat. Rev. Neurosci. 2010; https://doi.org/10.1038/nrn2762-c2 .
McDevitt EA, Krishnan GP, Bazhenov M, Mednick SC. Cognitive Neuroscience of Memory Consolidation, Studies in Neuroscience, Psychology and Behavioral Economics. 2017; https://doi.org/10.1007/978-3-319-45066-7_13 .
Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci 2009;1156:168–97.
Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; https://doi.org/10.1152/physrev.00032.2012 .
Peever J, Fuller PM. The biology of REM sleep. Curr Biol. 2017; https://doi.org/10.1016/j.cub.2017.10.026 .
Colvonen PJ, Straus LD, Acheson D, Gehrman P. A Review of the relationship between emotional learning and memory, sleep, and PTSD. Curr Psychiatry Rep. 2019; https://doi.org/10.1007/s11920-019-0987-2 .
Walker MP, van der Helm E. Overnight Therapy? The Role of Sleep in Emotional Brain Processing. Psychol Bull 2009;135:731–48.
Pace-Schott EF, Germain A, Milad MR. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord. 2015; https://doi.org/10.1186/s13587-015-0018-9 .
Straus LD, Norman SB, Risbrough VB, Acheson DT, Drummond SPA. REM sleep and safety signal learning in posttraumatic stress disorder: a preliminary study in military veterans. Neurobiol Stres. 2018; https://doi.org/10.1016/j.ynstr.2018.07.001 .
Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci. 2011; https://doi.org/10.3389/fnbeh.2011.00044 .
Marshall AJ, Acheson DT, Risbrough VB, Straus LD, Drummond, SPA. Fear conditioning, safety learning, and sleep in humans. J Neurosci. 2014; https://doi.org/10.1523/jneurosci.0478-14.2014 .
Pace-Schott EF et al. Effects of post-exposure naps on exposure therapy for social anxiety. Psychiatry Res. 2018; https://doi.org/10.1016/j.psychres.2018.10.015 .
Pace-Schott EF, Verga PW, Bennett TS, Spencer RMC. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res 2012;46:1036–44.
Kleim B, et al. Sleep enhances exposure therapy. Psychol Med 2014;44:1511–9.
Zalta AK, et al. Sleep quality predicts treatment outcome in CBT for social anxiety disorder. Depress Anxiety. 2013;30:1114–20.
Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009; https://doi.org/10.1093/cercor/bhn155 .
Wiesner CD, et al. The effect of selective REM-sleep deprivation on the consolidation and affective evaluation of emotional memories. Neurobiol Learn Mem. 2015; https://doi.org/10.1016/j.nlm.2015.02.008 .
van Marle H. PTSD as a memory disorder. Eur J Psychotraumatol. 2015; https://doi.org/10.3402/ejpt.v6.27633 .
Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002; https://doi.org/10.1097/01.PSY.0000021940.35402.51 .
Baran B, Pace-Schott EF, Ericson C, Spencer RMC, Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012; https://doi.org/10.1523/JNEUROSCI.2532-11.2012 .
Morgenthaler J, et al. Selective REM-sleep deprivation does not diminish emotional memory consolidation in young healthy subjects. PLoS ONE. 2014;9:e89849.
Latchoumane CFV, Ngo HVV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95:424–35.e6.
Kleim, B, Wysokowsky, J, Schmid, N, Seifritz, E, Rasch, B. Effects of sleep after experimental trauma on intrusive emotional memories. Sleep. 2016;39:2125–32.
Fisher C, Byrne J, Edwards A, Kahn E. A psychophysiological study of nightmares. J Am Psychoanal Assoc 1970;18:747–82.
Ross RJ, et al. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202.
Kobayashi I, Mellman TA, Altaee D, Howell MK, Lavela J. Sleep and processing of trauma memories. J Trauma Stress. 2016;29:568–71.
Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull 2007;133:482–528.
Rothbaum BO, Mellman TA. Dreams and exposure therapy in PTSD. J Trauma Stress. 2001;14:481–90.
Phelps AJ, et al. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep. 2018;41: https://doi.org/10.1093/sleep/zsx188 .
Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: a critical review of phenomenology, psychophysiology and treatment. Psychother Psychosom 2007;76:25–39.
Spoormaker VI. Sleep and combat-related post traumatic stress disorder (Springer, 2018).
Miller KE, Jamison AL, Gala S, Woodward SH. Two independent predictors of nightmares in posttraumatic stress disorder. J Clin Sleep Med 2018;14:1921–7.
Husain AM, Miller PP, Carwile ST. REM sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol 2001;18:148–57.
Naegeli C, et al. Locus coeruleus activity mediates hyperresponsiveness in posttraumatic satress disorder. Biol Psychiatry 2018;83:254–62.
Nir Y, Tononi G. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn Sci. 2010;14:88–100.
Germain A, et al. A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Res. 2013;211:176–9.
Germain A, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res 2012;72:89–96.
Raskind MA, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–3.
Taylor FB, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–32.
Raskind MA, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med 2018;378:507–17.
Morgenthaler T, et al. Position paper for the treatment of nightmare disorder in adults: an american academy of sleep medicine position paper. J Clin Sleep Med 2018;14:1041–55.
Raskind MA, et al. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016;80:736–42.
Richards A, et al. No access an open-label study of doxazosin extended-release for PTSD: findings and recommendations for future research on doxazosin. J lifelong Learn psychiatry. 2018;16:67–73.
Aurora RN, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6:389–401.
Krystal JH, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82:e51–e59.
Hertzberg MA, Feldman ME, Beckham JC, Davidson JRT. Trial of trazodone for posttraumatic stress disorder using a multiple baseline group design. J Clin Psychopharmacol. 1996;16:294–8.
Villarreal G, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173:1205–12.
Kozaric-Kovacic D, Pivac N. Quetiapine treatment in an open trial in combat-related post-traumatic stress disorder with psychotic features. Int J Neuropsychopharmacol. 2007;10:253–61.
De Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res. 2012;198:15–24.
Tao R, et al. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101-5.
Bonnavion P, De Lecea L. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 2010;10:174–9.
Grafe LA, Bhatnagar S. Orexins and stress. Front Neuroendocrinol. 2018;51:132–45.
Carter M, De Lecea L. Hyperarousal and post-traumatic stress disorder: a role for the hypocretin system. In: LeDoux J., Keane T., Shiromani P. (eds) Post-Traumatic Stress Disorder (Humana Press, 2009). https://doi.org/10.1007/978-1-60327-329-9_9 .
Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekkar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–61.
Bonaventure P, et al. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther 2015;352:590–601.
Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther 2002;300:2–8.
Quadrelli S, Mountford C, Ramadan S. Systematic review of in-vivo neuro magnetic resonance spectroscopy for the assessment of posttraumatic stress disorder. Psychiatry Res. 2018;282:110–25.
Starcevic V. The reappraisal of benzodiazepines in the treatment of anxiety and related disorders. Expert Rev Neurother. 2014;14:1275–86.
Guina J, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract 2015;21:281–303.
Pollack MH, et al. Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72:892–7.
Dunlop BW, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82:866–74.
Davis CJ, Krueger JM. Sleep and cytokines. Sleep Medicine Clinics. 2012;7:517–527.
Küffer A, et al. Altered overnight levels of pro-inflammatory cytokines in men and women with posttraumatic stress disorder. Psychoneuroendocrinology. 2019;102:114–20.
Babson KA, Sottile J, Morabito D. Cannabis, Cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:23.
Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn-Miller MO. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict. 2013;22:277–84.
Pietrzak RH, et al. Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology. 2014;39:2519–28.
Ney LJ, Matthews A, Bruno R, Felmingham KL. Cannabinoid interventions for PTSD: where to next? Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;93:124–40.
Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J. Neurosci. 2008;28:10167–84.
Mellman TA, David D, Kulick-Bell R, Hebding J, Nolan B. Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. Am J Psychiatry. 1995;152:1659–63.
Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901.
Zuj DV, et al. Impaired fear extinction associated with ptsd increases with hours-since-waking. Depress Anxiety (2016). https://doi.org/10.1002/da.22463 .
Pace-Schott EF, et al. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J Psychiatr Res. 2013;47:1776–84.
Schuch JB, Genro JP, Bastos CR, Ghisleni G, Tovo-Rodrigues L. The role of CLOCK gene in psychiatric disorders: evidence from human and animal research. Am J Med Genet Part B Neuropsychiatr Genet. 2018;177:181–98.
Liu C, Chung M. Genetics and epigenetics of circadian rhythms and their potential roles in neuropsychiatric disorders. Neurosci Bull. 2015;31:141–59.
Mukhopadhyay S, et al. Delayed sleep phase in severe obsessive-compulsive disorder: a systematic case-report survey. CNS Spectr 2008;13:406–13.
Turner J, et al. A prospective study of delayed sleep phase syndrome in patients with severe resistant obsessive-compulsive disorder. World Psychiatry. 2007;6:108–11.
Coles ME, Schubert JR, Sharkey KM. Delayed bedtimes and obsessive-compulsive symptoms. Behav Sleep Med 2012;10:258–65.
Cox RC, Tuck B, Olatunji BO. The role of eveningness in obsessive-compulsive symptoms: cross-sectional and prospective approaches. J Affect Disord. 2018;235:448–55.
Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000. https://doi.org/10.1002/1520-6394 (2000)12:1+<69::AID-DA9>3.0.CO;2-K.
Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61:22–32.
Dias BG, Banerjee SB, Goodman JV, Ressler KJ. Towards new approaches to disorders of fear and anxiety. Curr Opin Neurobiol. 2013;23:346-52.
Download references
Author information
These authors contributed equally: Dr. Richards, Dr. Kanady
Authors and Affiliations
The San Francisco VA Health Care System, San Francisco, CA, USA
Anne Richards, Jennifer C. Kanady & Thomas C. Neylan
The University of California, San Francisco, San Francisco, CA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anne Richards .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Richards, A., Kanady, J.C. & Neylan, T.C. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacol. 45 , 55–73 (2020). https://doi.org/10.1038/s41386-019-0486-5
Download citation
Received : 17 March 2019
Revised : 09 August 2019
Accepted : 12 August 2019
Published : 23 August 2019
Issue Date : 01 January 2020
DOI : https://doi.org/10.1038/s41386-019-0486-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Associations between 24-h movement behaviors and indicators of mental health and well-being across the lifespan: a systematic review.
- Claire I. Groves
- Christopher Huong
- Denver M. Y. Brown
Journal of Activity, Sedentary and Sleep Behaviors (2024)
Investigating the Leisure Patterns and the Well-being of Healthcare Workers in High-Risk Environments: Insights from a Pandemic Landscape
- Hsiao-Hsien Lin
- Yi-Han Tseng
- Chih-Hsiang Hung
Journal of the Knowledge Economy (2024)
The relationship between living alone or not and depressive symptoms in older adults: a parallel mediation effect of sleep quality and anxiety
- Zhanpeng Guo
BMC Geriatrics (2023)
From childhood adversity to latent stress vulnerability in adulthood: the mediating roles of sleep disturbances and HPA axis dysfunction
Neuropsychopharmacology (2023)
Rate and correlates of post-traumatic stress disorder (PTSD) following the Beirut blast and the economic crisis among Lebanese University students: a cross-sectional study
- Christian-Joseph El Zouki
- Abdallah Chahine
- Souheil Hallit
BMC Psychiatry (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Patient Care & Health Information
- Diseases & Conditions
- Sleep terrors (night terrors)
Sleep terrors are times of screaming or crying, intense fear, and sometimes waving arms and legs when not fully awake. Also known as night terrors, sleep terrors may lead to sleepwalking. Like sleepwalking, sleep terrors are a type of parasomnia. Parasomnias are disturbing or strange behaviors or experiences during sleep. A sleep terror usually lasts from seconds to a few minutes, but it may last longer.
Sleep terrors may happen in children between the ages of 1 and 12 years. They happen much less often in adults. Although sleep terrors can be frightening to those around the person with sleep terrors, they aren't usually a cause for concern. Most children outgrow sleep terrors by their teenage years.
Sleep terrors may need treatment if they cause problems with getting enough sleep or cause a safety risk.
Sleep terrors differ from nightmares. A nightmare is a bad dream. The person who has a nightmare wakes up from the dream and may remember details. A person who has a sleep terror remains asleep. Children usually don't remember anything about their sleep terrors in the morning. Adults may recall part of a dream they had during the sleep terrors.
Sleep terrors generally happen in the first part of sleep time, and rarely during naps. A sleep terror may lead to sleepwalking.
During a sleep terror, a person may:
- Start by screaming, shouting or crying.
- Sit up in bed and look scared.
- Stare wide-eyed.
- Sweat, breathe heavily, and have a racing pulse, flushed face and enlarged pupils.
- Kick and thrash.
- Be hard to wake up and be confused if awakened.
- Not be comforted or soothed.
- Have no or little memory of the event the next morning.
- Possibly, get out of bed and run around the house or have aggressive behavior if blocked or held back.
When to see a doctor
Occasional sleep terrors aren't usually a cause for concern. If your child has sleep terrors, you can simply mention them at a routine well-child exam. But if you have concerns for you or your child, talk to your doctor or other healthcare professional sooner, especially if sleep terrors:
- Happen more often.
- Regularly disrupt the sleep of the person with sleep terrors or other family members.
- Lead to safety concerns or injury.
- Result in daytime symptoms of extreme sleepiness or problems with daily activities.
- Continue beyond the teen years or start as an adult.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Sleep terrors are a type of parasomnia. A parasomnia is a disturbing or strange behavior or experience during sleep. People who have sleep terrors don't completely wake up from sleep during the episodes. Their appearance may suggest they are awake, but they remain partially asleep.
Several issues can contribute to sleep terrors, such as:
- Serious lack of sleep and extreme tiredness.
- Sleep schedule changes, travel or sleep interruptions.
Sleep terrors sometimes can be triggered by conditions that interfere with sleep, such as:
- Sleep-disordered breathing — a group of disorders that include breathing patterns that are not typical during sleep. The most common type of sleep-disordered breathing is obstructive sleep apnea.
- Restless legs syndrome.
- Some medicines.
- Mood disorders, such as depression and anxiety.
- Alcohol use.
Risk factors
Sleep terrors are more common if family members have a history of sleep terrors or sleepwalking.
Complications
Some complications that may result from sleep terrors include:
- Being too sleepy during the day, which can lead to problems at school or work or with everyday tasks.
- Disturbed sleep.
- Embarrassment about the sleep terrors or problems with relationships.
- Injury to the person having a sleep terror or, rarely, to someone nearby.
- Sateia M. Sleep terrors. In: International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014. https://learn.aasm.org/Listing/a1341000002XmRvAAK. Accessed March 1, 2023.
- Kryger M, et al., eds. Disorders of arousal. In: Principles and Practice of Sleep Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed March 1, 2023.
- Parasomnias. Merck Manual Professional Version. https://www.merckmanuals.com/professional/neurologic-disorders/sleep-and-wakefulness-disorders/parasomnias. March 3, 2023.
- Sleep-wake disorders. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5-TR. 5th ed. American Psychiatric Association; 2022. https://dsm.psychiatryonline.org. Accessed. March 2, 2023.
- Leung AKC, et al. Sleep terrors: An updated review. Current Pediatric Reviews. 2020; doi:10.2174/1573396315666191014152136.
- Bruni O, et al. The parasomnias. Child and Adolescent Psychiatric Clinics of North America. 2021; doi:10.1016/j.chc.2020.08.007.
- Olson EJ (expert opinion). Mayo Clinic. March 10, 2023.
Associated Procedures
- Biofeedback
- Cognitive behavioral therapy
- Polysomnography (sleep study)
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book

Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Search Menu
- Volume 12, Issue 1, 2024 (In Progress)
- Volume 11, Issue 1, 2023
- Advance articles
- Editor's Choice
- Virtual Issues
- Clinical Briefs
- ISEMPH Prizes
- Author Guidelines
- Submission Site
- Open Access
- Calls for Papers
- Why submit?
- About Evolution, Medicine, and Public Health
- About the International Society for Evolution, Medicine and Public Health
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- For Reviewers
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, historical versus modern environments: the mismatch hypothesis, attachment theory, extensions, testable predictions and limitations, acknowledgements.
- < Previous
An evolutionary perspective on night terrors
- Article contents
- Figures & tables
- Supplementary Data
Sean D Boyden, Martha Pott, Philip T Starks, An evolutionary perspective on night terrors, Evolution, Medicine, and Public Health , Volume 2018, Issue 1, 2018, Pages 100–105, https://doi.org/10.1093/emph/eoy010
- Permissions Icon Permissions
Night terrors, also known as sleep terrors, are an early childhood parasomnia characterized by screams or cries, behavioral manifestations of extreme fear, difficulty waking and inconsolability upon awakening. The mechanism causing night terrors is unknown, and a consistently successful treatment has yet to be documented. Here, we argue that cultural practices have moved us away from an ultimate solution: cosleeping. Cosleeping is the norm for closely related primates and for humans in non-Western cultures. In recent years, however, cosleeping has been discouraged by the Western medical community. From an evolutionary perspective, cosleeping provides health and safety benefits for developing children. We discuss night terrors, and immediate and long-term health features, with respect to cosleeping, room-sharing and solitary sleeping. We suggest that cosleeping with children (≥1-year-old) may prevent night terrors and that, under certain circumstances, cosleeping with infants (≤11-months-old) is preferable to room-sharing, and both are preferable to solitary sleeping.
Night terrors are an early childhood parasomnia associated with disturbance from non-REM, slow-wave sleep [ 1 ]. According to the American Academy of Sleep Medicine’s (AASM) International Classification of Sleep Disorders , night terrors (also known as sleep terrors) are defined as ‘a cry or piercing scream, accompanied by autonomic nervous system and behavioral manifestations of intense fear. … Sometimes there is prolonged inconsolability associated with a [night] terror’ [ 2 ]. Notably, night terrors are distinguishable from less severe nightmares by difficulty in waking the child [ 3 ]. These events are stressful and disturbing for the child experiencing them, the parents of the child and other family members [ 4 ].
The prevalence of night terrors in children is difficult to assess. Research has yielded discrepant results regarding the likelihood of experiencing night terrors with measurements ranging from 1.7% to almost 56% of individuals and ages ranging from 18 months to adolescence [ 4–6 ]. (Night terrors also occur in adults, but rarely so.) Prevalence has most frequently been assessed in school-age children, although research has demonstrated that night terrors are most common in children between 1 and 5 years of age [ 1 , 4 , 5 ]. The difficulties in determining prevalence are probably due to the varying definitions of night terrors used in research studies. This underscores a broader finding by Hublin and colleagues that the general public does not have one clear definition of night terrors, and thus often confuses them with simple nightmares [ 7 ]. Because of this, we use the AASM’s definition of night terrors provided above [ 2 ].
Attempts to treat night terrors have yet to establish an effective remedy for the condition. Currently, the recommended treatment for night terrors is to leave the child alone; parents are encouraged to let the terror proceed uninterrupted, as the child is unlikely to respond to attempts to be woken and is often inconsolable upon awakening [ 4 ]. Some sedative medications have proven effective in case studies [ 8 , 9 ]; however, such medications may lead to tolerance or dependence in children, and the notion of treating children with sedatives is troubling to many. Scheduled awakenings, performed around the time when the child transitions from non-REM to REM sleep, have also been used as treatment for night terrors [ 10 ].
One practice that has not been investigated in the context of night terrors is cosleeping. One of us (PTS) has a personal anecdote on this topic. By the summer of 2014, PTS’ 3-year-old child had been experiencing four to seven night terrors weekly for several months. Finding this behavior disturbing, PTS standardized the child’s schedule, modified his diet, and monitored for suitability the images and stories to which he was exposed. When these failed to have any discernable effect, PTS subjected the child to scheduled awakenings, which similarly had little impact. Finally, and possibly due to exhaustion, PTS began cosleeping with the child; the child’s night terrors rapidly ceased and have not reoccurred.
Cosleeping is not a novel behavior. Cosleeping is observed in all closely related primates and in many current human societies. It is traditionally defined as caregivers sharing a bed with offspring, and this is the definition we use here. In addition to the potential benefit to children (≥1-year-old) suggested by the anecdote above, cosleeping has demonstrated physiological effects on infants (≤11-months-old) [ 11 , 12 ]. Moreover, the impact on a mothers’ sleep duration or stage of sleep is negligible [ 13 ]. In spite of this, cosleeping with infants has been advised against by the American Academy of Pediatrics [ 14 ].
Here, we present an argument that night terrors are the result of an environmental mismatch between evolved behavior and the modern cultural practice of solitary sleeping. Using an environmental mismatch approach, attachment theory and research on the physiological, behavioral and psychosocial impacts of cosleeping, we argue that cosleeping is beneficial for children and may prevent or greatly reduce night terrors. We further suggest that under certain circumstances cosleeping with infants is preferable to room-sharing, and both are preferable to solitary sleeping.
In recent years, the environmental mismatch hypothesis—the idea that specific traits evolved to maximize their fitness in an environment very different from the one in which they are expressed today—has become a growing model for the study of the evolutionary basis of disease [ 15 ]. Many disorders have been studied through an environmental mismatch lens. One popular example is obesity, a particularly concerning condition due to its links with diabetes and cardiovascular disease. Several environmental mismatch hypotheses have been proposed for the rising prevalence of obesity; for example, genes linked with increased fat storage would have been evolutionarily favorable for Paleolithic hunter-gatherers, for whom food was not always readily available. However, as humans progressed into developed, sedentary societies with consistent access to food, this adaptation has become a pathology: average body weight and obesity have increased throughout the world [ 16 ].
The mismatch approach has also been applied to the study of human sleep behavior and sleep disorders. Significant changes to human sleep patterns have occurred throughout evolutionary time, including a decrease in the amount of time humans sleep relative to other primates [ 17 ]. Such changes have been explored as potential factors in sleep disorders ranging from insomnia to sleep apnea [ 18 ]. Additional research has shown an increase in sleep disorders in recent years; this increase has been partially attributed to the increase in light pollution and constant mental stimulation of developed societies [ 15 , 19 , 20 ].
The likely relative behavioral and physiological effects of solitary sleeping, cosleeping and room-sharing between a caregiver and an infant (≤11-months-old) a
(Proper bedding and surrounds are assumed for all conditions.) Solitary sleeping is considered the standard (–). ↑ indicates a beneficial effect, ↓ indicates a detrimental effect and – indicates no change in situation for the infant relative to the standard. Multiple designations are provided when the outcome is in question. From a cost-benefit approach, solitary sleeping fairs very poorly and cosleeping is preferable whenever the risk of smothering (purple) is lower than the added benefits over room-sharing (orange).
The practice of cosleeping is also supported by attachment theory, which addresses the prolonged period of helplessness in human infants and the infant’s need to elicit the mother’s (or other caregiver’s) protection and care [ 28 ]. These behaviors are rooted in evolution, providing a survival advantage by increasing caregiver-infant proximity. They include infant rooting and signaling (e.g. crying) and caregiver responsivity (meeting the infant’s need) and sensitivity (meeting the need in a timely fashion). The attachment system is activated in the presence of stress, either internally or externally derived. Evidence of the system can be seen in the first few weeks of life, when the infant begins signaling and the caregiver responds. It peaks at about 1 year of age, the time when the child typically develops independent locomotion and can get away from the mother, and continues at high intensity throughout the years of dependency in early childhood [ 29 ]. It is of interest that the age range during which attachment behaviors are strongest is the age range when night terrors first present [ 4 , 5 ]. Indeed, one Swiss study found that cosleeping, while uncommon in children below 1 year of age (<10%), increased during ages when night terrors are most common [ 30 ].
Primates and cosleeping: an ancient and modern practice
Cosleeping is observed in all closely related primates, as well as a significant portion of human populations. Barry and colleagues collected data on sleeping arrangements for 90 cultures, and found that mother and infant slept in the same bed in 41 of them (46%); mother slept in the same room with the infant but in an unspecified bed in 30 (33%), and in the same room in separate beds in 19 (21%). In none of 90 cultures did the mother and infant sleep in a separate room [ 31 ]. Despite this, cosleeping has been discouraged by the American Academy of Pediatrics due to a stated link with sleep-related infant deaths [ 25 ]. Research on the rates of cosleeping in the US has shown that, although cosleeping increased from 6.5% to 13.0% from 1993 to 2010, no significant increase was observed in white families from 2001 to 2010; these findings suggest that recommendations against cosleeping are not uniformly followed across cultural groups [ 32 ].
Cosleeping has, however, persisted in small-scale, high-fertility/high-mortality cultures that characterized human societies for much of our evolutionary history. A study on the Aka hunter-gatherers and Ngandu farmers of central Africa by Hewlett and Roulett found that an overwhelming majority of offspring coslept with their parents from infancy through adolescence, although rates decreased as children aged [ 33 ]. Reasons for cosleeping include limitations in space, protection from predators and shared heat sources (e.g. body heat), similar adaptive benefits that likely promoted cosleeping in early human evolution [ 33 ].
Attitudes about cosleeping are beginning to change on a broad scale in Western nations. In particular, the UK, which formerly held views similar to those in the US that discourage cosleeping, has begun to embrace parents’ choice to cosleep [ 34 ]. Overall, the UK has become more open to parents’ decisions on infant cosleeping; the same cannot be said for the US [ 14 , 35 ]. Data suggest that this may be unfortunate.
The adaptive benefits of cosleeping in humans can easily be seen through the physiological effects on parent and infants [ 27 ]. We summarize some of these and other findings on the study of different sleeping practices on parent and infant behavior and physiology in Table 1 . Although the American Academy of Pediatrics has reported risks of cosleeping over solitary sleeping or room-sharing, including overheating and smothering [ 25 ], they may have overemphasized these risks or ignored factors contributing to them [ 36 ]. In addition, the reported risks may be balanced by the benefits provided by cosleeping, including improvements in thermoregulation and respiratory regulation, increased breastfeeding and easier arousal of both infants and parents (see Table 1 ).
The relative psychosocial effects of solitary sleeping and cosleeping between a caregiver and child (ages ranging from 2 to 13 years of age across studies) a
Solitary sleeping is considered the standard (–). ↑ indicates a beneficial effect and – indicates no change in situation for the child relative to the standard. We have excluded room sharing due to the lack of data and to difficulties predicting outcomes. Cosleeping appears to be preferable to solitary sleeping.
For many families, solitary sleeping has replaced cosleeping, and is often accomplished through the practice of sleep training. Sleep training involves parents leaving an infant or child alone in a separate room at night, and limiting responsiveness to its cries to the point of extinction, thus encouraging it to self-settle [ 43 ]. These practices are arguably disturbing for the infant or child, who is responding to the separation from its caregiver, and the cries are stressful for parents [ 44 ]. Although very popular and widely recommended [ 45 ], sleep training is counterintuitive to attachment theory and other evolutionary tenets of a responsive parent-offspring relationship.
Although formally a proponent of solitary sleeping [ 14 ], the American Academy of Pediatrics has recently revised its recommendations for safe infant sleep to include room-sharing, but not bed-sharing, of the parents and infant [ 25 ]. These recommendations posit that room-sharing with an infant increases the ability of the parent to quickly respond to the infant, while minimizing the risk of suffocation or overheating. These revisions are a move in the right direction; however, cosleeping in a safe environment appears to have increased benefits on infant ( Table 1 ), and thus could offer more benefits than simply sharing the same room. The difference in short-term ( Table 1 ) and long-term ( Table 2 ) benefits between cosleeping, room-sharing, and solitary sleeping merits increased attention from sleep researchers.
We propose that cosleeping with children may reduce or possibly prevent night terrors. Accordingly, we are referring to the post-infancy stage with respect to this parasomnia. We include the infancy data, however, because there appear to be benefits for cosleeping with infants and because cosleeping with infants is likely to lead to cosleeping with children. It is important to note that not all children develop night terrors, and many are able to sleep in rooms separate from their parents nightly. The argument presented in this commentary is not, however, to suggest that night terrors will always result from separation of a parent and child at night; rather, we propose night terrors to be an extreme outcome of this separation, one that likely works together with other physiological and/or psychosocial factors.
Several testable predictions, both observational and interventional, arise from the argument made here. One observational prediction is that children who cosleep will have a lower prevalence of night terrors compared with children who sleep solitarily. A comparison between the prevalence of night terrors in children who cosleep and children who sleep in the same room with parents should be undertaken to determine the degree of physical proximity with the parent necessary to influence positive change, should it occur. Previous research suggests that cosleeping and room-sharing have beneficial effects for infants (see Table 1 ), but it is unclear if either approach is superior with respect to parasomnias in young children.
One interventional prediction of our hypothesis is that children with night terrors would experience a decrease in incidence once they began cosleeping with their parents. There are currently no findings on the correlation between these phenomena beyond our anecdotal report (see above), but an interventional, prospective study could easily test this prediction. However, possible complications could arise as a result of the lack of understanding surrounding night terrors in the lay public and scientific community alike [ 7 ]. Any study undertaken to assess cosleeping as an intervention for night terrors would have to take care in defining night terror symptoms (intensity, duration, etc.) as well as defining cosleeping (bed-sharing vs room-sharing). We are currently beginning work on such a study.
Alternative hypotheses
We have presented a mechanistic hypothesis with an evolutionary basis: the lack of cosleeping could reasonably trigger night terrors. This does not necessarily mean that cosleeping currently provides a fitness advantage.
One could hypothesize that solitary sleeping fosters a child’s independence from its parents. Fitness benefits may accrue from this if children who learn separation from their parents earlier display better adjustment or self-reliance in adulthood. We do not favor this hypothesis because, to our knowledge, there is no evidence that solitary sleeping leads to better adjustment. In fact, attachment theory suggests the opposite; caregiver sensitivity and responsivity lead to secure attachment in children. Secure attachment is associated with child compliance (increases safety), increased and better social relationships (survival and reproductive advantage) [ 28 , 29 ]. Long-term psychosocial benefits due to cosleeping, which are indicative of secure attachment, can be found in Table 2 .
One could hypothesize a direct fitness cost to cosleeping: cosleeping may present significant risk to the child in the form of accidental smothering while the parent is sleeping. This is the core argument the medical field uses against cosleeping [ 25 ]. We do not favor this hypothesis for caregiver-child cosleeping: we could not locate any studies of accidental smothering deaths in children beyond infancy.
Cosleeping may present a direct fitness cost for infants. It is unclear, however, how great this risk is or how costly the absence of cosleeping is to infants. Smothering rates are very low (0.1 per 1000 live births, as described in a New Zealand population study [ 46 ]), and many cases of smothering involve drug and/or alcohol abuse, or some other extenuating circumstance, that prevents the parent from waking up to their offspring’s cries or movements [ 47 , 48 , 49 ]. It may be that, for responsible, sober parents, the benefits to an infant for cosleeping with a caregiver on an appropriately designed bed outweighs the risk of smothering ( Table 1 ). This warrants future exploration.
In short, we are unaware of any fitness benefit to the child (or parent) for solitary sleeping. Since our most closely related primates all cosleep with their young, and since individuals from many non-Western cultures cosleep with their young, solitary sleeping is clearly a culturally derived trait. At best, solitary sleeping may be selectively neutral, but data on SIDS rates and the American Academy of Pediatrics’ recent recommendations against it [ 25 ], suggest that solitary sleeping is maladaptive.
To our knowledge, this paper is the first to propose that night terrors are an extreme response to a novel environment by children who sleep apart from their parents. We have addressed the many benefits of cosleeping to the infant ( Table 1 ), and this paper extends the argument into the early childhood years ( Table 2 ), showing that physiological and psychosocial benefits for infants (i.e. survival, protection) give way to physiological and psychosocial benefits for young children (i.e. survival, protection and relational dependence). This bio-behavioral scaffolding is precisely what contributes to the child feeling protected and thus safe in a sleeping environment that includes the caregiver. Cosleeping, however, is currently discouraged in Western cultures. Further research is needed to understand if the discouragement is warranted. It may be that a return to cosleeping practices in Western cultures will lead to better child health overall, including a reduction in the prevalence of night terrors.
We thank Caroline Blackie, Rachael Bonoan, Sara Lewis and Avalon Owens for helpful comments on a draft of this manuscript.
The authors declare no research funding for this project, however PTS received a Tufts University FRAC award to cover the open access fees.
Conflict of interest : None declared.
Petit D , Pennestri M-H , Paquet J et al. Childhood sleepwalking and sleep terrors: a longitudinal study of prevalence and familial aggregation . JAMA Pediatr 2015 ; 169 : 653 – 8 .
Google Scholar
American Academy of Sleep Medicine . International Classification of Sleep Disorders , 3rd edn. Darien, IL : American Academy of Sleep Medicine , 2014 .
Google Preview
Haupt M , Sheldon SH , Loghmanee D. Just a scary dream? a brief review of sleep terrors, nightmares, and rapid eye movement sleep behavior disorder . Pediatr Ann 2013 ; 42 : e221 – 6 .
Moreno MA. Sleep terrors and sleepwalking: common parasomnias of childhood . JAMA Pediatr 2015 ; 169 : 704.
Nguyen BH , Perusse DA , Paquet J et al. Sleep terrors in children: a prospective study of twins . Pediatrics 2008 ; 122 : e1164 .
Simonds JF , Parraga H. Prevalence of sleep disorders and sleep behaviors in children and adolescents . J Am Academy Child Psych 1982 ; 21 : 383 – 8 .
Hublin C , Kaprio K , Partinen M et al. Limits of self-report in assessing sleep terrors in a population survey . Sleep 1999 ; 22 : 89 – 93 .
Sasayama D , Washizuka S , Honda H. Effective treatment of night terrors and sleepwalking with Ramelteon . J Child Adolesc Psychopharmacol 2016 ; 26 : 948.
Popoviciu L , Corfariu O. Efficacy and safety of midazolam in treatment of night terrors in children . Br J Clin Pharmacol 1983 ; 16 : 97S – 102S .
Harris-Carlson SA. Sleepwalking and Night Terrors in Children: Treatment with Scheduled Awakenings and Circadian Rhythm Management . Michigan : Central Michigan University ProQuest Dissertations Publishing , 1983 ,
McKenna JJ , McDade T. Why babies should never sleep alone: a review of the co-sleeping controversy in relation to SIDS, bedsharing and breast feeding . Paediatr Respir Rev 2005 ; 6 : 134 – 52 .
Cortesi F , Giannotti F , Sebastiani T et al. Cosleeping versus solitary sleeping in chidren with bedtime problems: child emotional problems and parental distress . Behav Sleep Med 2008 ; 6 : 89 – 105 .
Mosko S , Richard C , McKenna J. Maternal sleep and arousals during bedsharing with infants . Sleep 1997 ; 20 : 142 – 50 .
American Academy of Pediatrics Task Force on Infant Sleep Position and Sudden Infant Death Syndrome . Changing concepts of Sudden Infant Death Syndrome: implications for infant sleeping environment and sleep position . Pediatrics 2000 ; 105 : 650 – 6 .
Gluckman P , Beedle A , Hanson M. Principles of Evolutionary Medicine . New York : Oxford University Press , 2009 .
Albuquerque D , Stice E , Rodriguez LR et al. Current review of genetics of human obesity from molecular mechanisms to an evolutionary perspective . Mol Genet Genomics 2015 ; 290 : 1191 – 21 .
Nunn C , Samson D. Sleep in a comparative context: investigating how human sleep differs from sleep in other primates. Am J Phys Anthropol . 2018 ; 2018 : 1 – 12 .
Nunn CL , Samson DK , Krystal AD. Shining evolutionary light on human sleep and sleep disorders . Evol Med Public Health 2016 ; 2016 : 227 – 43 .
Kronholm E , Partonen T , Harma M et al. Prevalence of insomnia-related symptoms continues to increase in the Finnish working-age population . J Sleep Res 2016 ; 25 : 454 – 7 .
Pallesen S , Sivertsen B , Nordhus IH et al. A 10-year trend of insomnia prevalence in the adult Norweigan population . Sleep Med 2014 ; 15 : 173 – 9 .
Mason TB , Pack AI. Sleep terrors in childhood . J Pediatr 2005 ; 147 : 388 – 92 .
St James-Roberts I , Alvarez M , Csipke E et al. Infant crying and sleeping in London, Copenhagen and when parents adopt a “proximal” form of care . Pediatrics 2006 ; 117 : e1146 – 55 .
Baddock SA , Galland BC , Bolton DP et al. Differences in infant and parent behaviors during routine bed sharing compared with cot sleeping in the home setting . Pediatrics 2006 ; 117 : 1599 – 607 .
McKenna JJ , Mosko SS , Richard CA. Bedsharing promotes breastfeeding . Pediatrics 1997 ; 100 : 214 – 9 .
Moon RY , Darnall RA , Feldman-Winter L et al. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment . Pediatrics 2016 ; 138 : e20162938.
Thomas KA , Burr R. Preterm infant temperature circadian rhythm: possible effect of parental cosleeping . Biol Res Nurs 2002 ; 3 : 150 – 9 .
McKenna JJ , Thoman EB , Anders TF et al. Infant-parent co-sleeping in an evolutionary perspective: implications for understanding infant sleep development and the sudden infant death syndrome . Sleep 1993 ; 16 : 263 – 82 .
Cassidy J , Shaver P. Handbook of Attachment: Theory, Research, and Clinical Applications , 3rd edn. New York : The Guilford Press , 2016 .
Bowlby J. Attachment and Loss . New York : Basic Books , 1982 .
Jenni OG , Fuhrer HZ , Iglowstein I et al. A longitudinal study of bed sharing and sleeping problems among swiss children in the first 10 years of life . Pedatrics 2005 ; 115 : 233 – 40 .
Barry H , Bacon MK et al. Definitions , Ratings and Bibliographic Sources for Child Training Practices of 110 Cultures . Cross-Cultural Approaches . C.S. Ford. New Haven : HRAF Press , 1967 .
Colson ER , Willinger M , Rybin D et al. Trends and factors associated with infant bed sharing, 1993–2010: the national infant sleep position study . JAMA Pediatr 2013 ; 167 : 1032 – 7 .
Hewlett BS , Roulette JW et al. Cosleeping beyond infancy: culture, ecology, and evolutionary biology of bedspring among Aka foragers and Ngandu farmers of central Africa. In: Narvaez (eds.). Ancestral Landscapes in Human Evolution: Culture, Childrearing, and Social Wellbeing. New York : Oxford Press , 2014 , 129 – 63 .
Ball H , Howel D , Bryant A et al. Bed-sharing by breastfeeding mothers: who bed-shares and what is the relationship with breastfeeding duration? Acta Paediatr 2016 ; 105 : 628 – 34 .
Ball H. The Atlantic divide; contrasting U.K. and U.S. Recommendations on cosleeping and bed-sharing . J Hum Lact 2017 ; 33 : 765 – 9 .
Gessner BD , Porter TJ. Bed sharing with unimpaired parents is not an important risk for sudden infant death syndrome . Pediatrics 2006 ; 117 : 990 – 1 . 994-6.
McKenna JJ , Gettler LT. Cultural influences on infant sleep biology and the science that studies it: toward a more inclusive paradigm, part II. In: Loughlin G , Carroll J , Marcus C (eds.). Sleep and Breathing in Children: A Developmental Approach , New York : Marcel Dekker , 2008 , 183 – 221 .
Heron P. Nonreactive co-sleeping and child behavior: getting a good night’s sleep all night every night, Masters’ Thesis . Bristol, UK: University of Bristol 1994 .
Lewis RJ , Janda LH. The relationship between adult sexual adjustment and childhood experience regarding exposure to nudity, sleeping in the parental bed, and parental attitudes toward sexuality . Arch Sex Behav 1988 ; 17 : 349 – 63 .
Crawford M. Parenting practices in the Basque country: implications of lnfant and childhood sleeping location for personality development . Ethos 1994 ; 22 : 42 – 82 .
Mosenkis J. The effects of childhood coslceping on later life development, Masters’ Thesis . Chicago, IL : University of Chicago 1998 .
Okami P , Weisner I , Olmstead R. Outcome correlates of parent-child bedsharing: an eighteen-year longitudinal study . Dev Behav Pediatr 2002 ; 23 : 244 – 53 .
Ferber R. Solve Your Child's Sleep Problems, Rev. Touchstone: S. & S. May 2006 .
Middlemiss W , Granger DA , Goldberg WA , Nathans L. Asynchrony of mother-infant hypothalamic-pituitary-adrenal axis activity following extinction of infant crying responses induced during the transition to sleep . Early Human Dev 2012 ; 88 : 227 – 32 .
Mindell JA , Kuhn B , Lewin DS et al. A behavioral treatment of bedtime problems and night wakings in infants and young children: an American Academy of Sleep Medicine review . Sleep 2006 ; 29 : 1263 – 76 .
Hayman RM , McDonald G , Baker NJ et al. Infant suffocation in place of sleep; New Zealand national data 2002–2009 . Arch Dis Childhood 2015 ; 100 : 610 – 4 .
Britten N. Baby smothered by drunk mother. The Telegraph . http://www.telegraph.co.uk/news/uknews/1499621/Baby-smothered-by-drunk-mother.html ( 24 April 2018, date last accessed).
Lapoint T. Parents’ intoxication results in baby’s death, but coroner blames co-sleeping. Inquisitr . https://www.inquisitr.com/1396828/parents-intoxication-results-in-babys-death-but-coroner-blames-co-sleeping/ (24 April 2018, date last accessed).
Blair PS , Sidebotham P , Pease A , Fleming PJ. Bed-sharing in the absence of hazardous circumstances: is there a risk of sudden infant death syndrome? An analysis from two case-control studies conducted in the UK . PLoS One 2014 ; 9 : e107799. doi:10.1371/journal.pone.0107799
- night terrors
- cries pain scale
- co-sleeping
Email alerts
Citing articles via, affiliations.
- Online ISSN 2050-6201
- Copyright © 2024 International Society for Evolution, Medicine, and Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Introduction
- Conclusions
- Article Information
From the peak prevalence of 34.4% at age 1 1 / 2 years, the number of new cases of sleep terrors (orange squares) decreased rapidly each year to reach 10% at age 7 years. Conversely, the number of new cases of sleepwalking (open circles) increased steadily until age 12 years. Assessment of sleep terrors begins at age 2 1 / 2 years and assessment of sleepwalking at 3 1 / 2 years because the data presented here are the new cases reported after the first assessment time. Error bars represent 95% CIs.
- Sleep Terrors and Sleep Walking JAMA Pediatrics JAMA Pediatrics Patient Page July 1, 2015 Megan A. Moreno, MD, MSEd, MPH
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Petit D , Pennestri M , Paquet J, et al. Childhood Sleepwalking and Sleep Terrors : A Longitudinal Study of Prevalence and Familial Aggregation . JAMA Pediatr. 2015;169(7):653–658. doi:10.1001/jamapediatrics.2015.127
Manage citations:
© 2024
- Permissions
Childhood Sleepwalking and Sleep Terrors : A Longitudinal Study of Prevalence and Familial Aggregation
- 1 Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Cœur de Montréal, Montreal, Quebec, Canada
- 2 Department of Psychiatry, Université de Montréal, Montreal, Quebec, Canada
- 3 Douglas Mental Health University Institute, McGill University, Montreal, Quebec, Canada
- 4 Department of Neurosciences, Université de Montréal, Montreal, Quebec, Canada
- 5 Service of Neurology, Hôpital du Sacré-Coeur de Montréal, Montreal, Quebec, Canada
- 6 Department of Psychology, Université de Montréal, Montreal, Quebec, Canada
- 7 School of Psychoeducation, Université de Montréal, Montreal, Quebec, Canada
- 8 Research Unit on Children’s Psychosocial Maladjustment, Université de Montréal, Montreal, Quebec, Canada
- 9 Department of Pediatrics, Université de Montréal, Montreal, Quebec, Canada
- 10 School of Public Health, Physiotherapy and Population Science, University College Dublin, Dublin, Ireland
- 11 Research Unit on Children’s Psychosocial Maladjustment, Laval University, Quebec City, Quebec, Canada
- 12 Institute of Genetic, Neurobiological, and Social Foundations of Child Development, Tomsk State University, Tomsk, Tomsk Oblast, Russian Federation
- JAMA Pediatrics Patient Page Sleep Terrors and Sleep Walking Megan A. Moreno, MD, MSEd, MPH JAMA Pediatrics
Importance Childhood sleepwalking and sleep terrors are 2 parasomnias with a risk of serious injury for which familial aggregation has been shown.
Objectives To assess the prevalence of sleepwalking and sleep terrors during childhood; to investigate the link between early sleep terrors and sleepwalking later in childhood; and to evaluate the degree of association between parental history of sleepwalking and presence of somnambulism and sleep terrors in children.
Design, Setting, and Participants Sleep data from a large prospective longitudinal cohort (the Quebec Longitudinal Study of Child Development) of 1940 children born in 1997 and 1998 in the province were studied from March 1999 to March 2011.
Main Outcomes and Measures Prevalence of sleep terrors and sleepwalking was assessed yearly from ages 1 1 / 2 and 2 1 / 2 years, respectively, to age 13 years through a questionnaire completed by the mother. Parental history of sleepwalking was also queried.
Results The peak of prevalence was observed at 1 1 / 2 years for sleep terrors (34.4% of children; 95% CI, 32.3%-36.5%) and at age 10 years for sleepwalking (13.4%; 95% CI, 11.3%-15.5%). As many as one-third of the children who had early childhood sleep terrors developed sleepwalking later in childhood. The prevalence of childhood sleepwalking increases with the degree of parental history of sleepwalking: 22.5% (95% CI, 19.2%-25.8%) for children without a parental history of sleepwalking, 47.4% (95% CI, 38.9%-55.9%) for children who had 1 parent with a history of sleepwalking, and 61.5% (95% CI, 42.8%-80.2%) for children whose mother and father had a history of sleepwalking. Moreover, parental history of sleepwalking predicted the incidence of sleep terrors in children as well as the persistent nature of sleep terrors.
Conclusions and Relevance These findings substantiate the strong familial aggregation for the 2 parasomnias and lend support to the notion that sleepwalking and sleep terrors represent 2 manifestations of the same underlying pathophysiological entity.
Sleepwalking is a common childhood parasomnia that usually disappears during adolescence.However, it can persist, or appear de novo , in adulthood. In the third edition of the International Classification of Sleep Disorders , sleepwalking is defined as “complex behaviors that are usually initiated during partial arousals from slow-wave sleep.…The sleepwalking individual is disoriented in time and space, with slow speech, with severely diminished mentation, and blunted response to questions or requests. There is often prominent anterograde and retrograde memory impairment,” 1 (p230-231) but not always. 2 Sleep terrors, an early childhood parasomnia, also consist of partial arousals from slow-wave sleep “often accompanied by a cry or piercing scream, accompanied by autonomic nervous system and behavioral manifestations of intense fear.…Sometimes there is prolonged inconsolability associated with a sleep terror.” 1 (p231) For most children, these sleep disorders are relatively benign; however, in some cases, there is a high potential for injury, not to mention parental sleep disruption.
These 2 parasomnias share many characteristics. They are generally characterized by relative unresponsiveness to external stimuli as well as mental confusion. 1 Both kinds of episodes arise mainly from slow-wave sleep, and their occurrence is facilitated by the same factors, including sleep deprivation, 3 - 5 noise, 6 , 7 fever (temperature, >38.3°C), 8 , 9 medication, 10 and sleep-related respiratory events. 11 , 12 Treatment is also the same for the 2 disorders: scheduled awakenings is the recommended approach in children. Consequently, there is reason to believe that these parasomnias represent different phenotypic expressions of the same underlying disorder rather than distinct entities. Another convincing argument in favor of this view is the cosegregation of these parasomnias within families. It was shown (although in a small sample) that about 80% of sleepwalkers and 96% of people with sleep terrors have at least 1 family member affected by sleepwalking, sleep terrors, or both. 13
The prevalence of sleep terrors during childhood has never been accurately assessed. The estimations reported are variable (from about 1% to 14.7%). 14 - 17 Some reasons for these varying estimates are that (1) some studies considered only cases for which the sleep terrors caused a functional effect; (2) the definition of a sleep terror was variable among studies; (3) the age range investigated was significantly different among studies, both in width and in targeted ages; (4) some studies were performed in adults 14 or adolescents 15 ; and (5) some sample sizes were too small to be conclusive. 16 , 17 Moreover, studies rarely include children aged 2 years or younger even though sleep terrors were historically thought to begin at 18 months. Although the prevalence of sleep terrors during childhood is not known with precision, it is greater in children of parents with a history of sleep terrors. 13 , 18
Furthermore, studies in twins have consistently documented a possible genetic underpinning for these parasomnias. A model-fitting analysis found that early childhood sleep terrors were in large part explained by additive genetic effects. 19 Hublin and colleagues 20 conducted a retrospective study using an adult Finnish population of twins and found a concordance rate 1.5 times higher in monozygotic than in dizygotic pairs for childhood sleepwalking and 5 times higher in monozygotic than in dizygotic pairs for adult sleepwalking. Using the same cohort, Hublin and colleagues 14 also reported a higher polychoric correlation for childhood sleep terrors in monozygotic twins than in dizygotic twins.
Hence, since most studies on the familial aggregation of sleepwalking and sleep terrors were either conducted retrospectively or in a small sample of probands and none was longitudinal in nature, the aims of the present study were to assess the prevalence of sleepwalking and sleep terrors during childhood in a large prospective longitudinal sample of children; assess the probability of developing somnambulism later in childhood for children who had early sleep terrors; and assess the degree of association between parental history of sleepwalking and presence of sleep terrors and somnambulism in children.
At a Glance
This large prospective cohort study examines the prevalence of sleep terrors and sleepwalking and association of these with parental history.
The peak of prevalence was observed at age 1 1 / 2 years for sleep terrors (34.4%) and at age 10 years for sleepwalking (13.4%).
As many as one-third of children who had early childhood sleep terrors developed sleepwalking later in childhood.
The prevalence of childhood sleepwalking increases with parental history of sleepwalking: 22.5% for children without parental history, 47.4% for children with 1 parent with a history of sleepwalking, and 61.5% for children with both parents with a history of sleepwalking.
These findings point to a strong genetic influence on sleepwalking and, to a lesser degree, sleep terrors.
This study was conducted from March 1999 to March 2011 as part of the Quebec Longitudinal Study of Child Development. All children were recruited from the Quebec Master Birth Registry managed by the Ministry of Health and Social Services. A randomized, 3-level, stratified survey design was used to study a representative sample of infants who were born in 1997 and 1998 in the province of Quebec, Canada. The 3 levels were geographic regions of Quebec, each region subdivided into areas that were representative of the number of births in the region, and number of children selected per area proportional to the number of births and to the sex ratio of this area. Families who lived in the northern part of the province of Quebec, Inuit territories, and First Nations reserves were excluded for technical reasons. Children with known neurologic conditions were excluded from the cohort. All families received detailed information by mail on the aims and procedures of the research program, and parents signed a consent form before each assessment. The protocol was approved by the Institut de la Statistique du Québec Ethics Committee.
At the inception of the Quebec Longitudinal Study of Child Development (March 1998), 2223 children aged 5 months were included. Throughout the years, some attrition occurred. In all, 1940 children (87.3% of the initial sample) were included at the onset of the present study, but there was attrition at each assessment time point. The majority of the sample was white (92.8%). Black African, Native Amerindian, Arab, and Asian individuals each represented less than 2% of the sample. Moreover, the numbers may vary from one analysis to another because of missing data on specific questions or at certain assessment times or because of the number of missing values allowed in specific analyses.
The presence of sleep terrors and sleepwalking was assessed yearly from age 1 1 / 2 years (for sleep terrors) or 2 1 / 2 years (for sleepwalking) to age 13 years using single questions included in the self-administered questionnaire for the mother of the child. The question for sleep terrors was, “Does your child have night terrors (wakes up suddenly, crying, sometimes drenched in sweat and confused)?” whereas the question assessing sleepwalking was, “Does your child walk in his/her sleep?” Response choices were never, sometimes, often, and always. Since these 2 parasomnias are not necessarily characterized by a daily or even weekly occurrence, a child was considered as showing the parasomnia if the answer was sometimes, often, or always. When the child was 10 years old, the mother also had to report whether she (if she was the biological mother) or the biological father, or both, had a history of sleepwalking during either childhood or adulthood.
All prevalence data were adjusted through a weighted variable (according to the 3-level survey design) at each time point so that results could be generalized to the target population of the Quebec Longitudinal Study of Child Development. The effect of sex of the children on the prevalence of sleep terrors and sleepwalking was evaluated using univariate logistic regression. Given that no relationship between sex and either sleep terrors or sleepwalking was found, the association between early childhood sleep terrors (between 1 1 / 2 and 3 1 / 2 years, the typical period of occurrence of sleep terrors) and sleepwalking later in childhood (from ages 5 to 13 years) was also evaluated using univariate logistic regression without adjusting for sex of the children. For this analysis, data for all 3 time points of early childhood had to be present, but 3 missing data points on sleepwalking were allowed for ages 5 to 13 years.
Univariate logistic regression was also used to evaluate the association between presence of lifetime sleep terrors and somnambulism in children and their parents’ history of sleepwalking. In the case of lifetime presence of sleep terrors (ages 1 1 / 2 to 13 years) or sleepwalking (ages 2 1 / 2 to 13 years) in children, some missing data were allowed to avoid too much attrition. For sleep terrors, the data at age 1 1 / 2 years were required (peak of prevalence) and 5 of the other 10 yearly data points were needed to include the participants. Similarly for sleepwalking, 5 of the other 10 yearly data points were needed (from ages 2 1 / 2 to 13 years), and the presence of the data at age 10 years (peak of prevalence) was ensured by the fact that the question regarding parental history of sleepwalking was asked at that age. Finally, multivariable logistic regressions were used to predict sleep terrors and sleepwalking, adjusting for confounding variables (sex and presence of snoring).
All prevalences and unadjusted and adjusted odds ratios (ORs) are reported with their corresponding 95% CIs. Statistical analyses were conducted using SPSS, version 21 (IBM).
The prevalence of sleep terrors (total and by sex) from ages 1 1 / 2 to 13 years is illustrated in Table 1 . This large cohort and prospective study reveals a high prevalence for sleep terrors of 34.4% at 1 1 / 2 years (sleep terrors were not assessed at 5 months). This prevalence rapidly decreased to 13.4% at age 5 years and slowly tapered to 5.3% at age 13 years. Corroborating that sleep terrors are an early childhood parasomnia, few new cases appeared after age 5 years ( Figure ). The overall childhood prevalence of sleep terrors (ages 1 1 / 2 to 13 years; 1654 children) was 56.2% (95% CI, 53.8%-58.6%). Sex was not associated with the occurrence of sleep terrors during childhood ( Table 2 ).
By contrast, sleepwalking was relatively infrequent during the preschool period but increased steadily to reach 13.4% by age 10 years ( Table 1 ). Its prevalence then remained at approximately 13% until age 13 years (12.7% at 12 years and 12.8% at 13 years). The percentage of new cases slowly increased until age 12 years ( Figure ). The overall childhood prevalence of sleepwalking (ages 2 1 / 2 to 13; 1524 children) was 29.1% (95% CI, 26.8%-31.4%). In general, sex was not associated with the occurrence of sleepwalking during childhood ( Table 2 ).
Children who experienced sleep terrors during early childhood (from 1 1 / 2 to 3 1 / 2 years; 546 children) were more likely to develop somnambulism later in childhood (≥5 years) than were the children (n = 631) who did not experience sleep terrors in early childhood (34.4% vs 21.7%; OR, 1.89; 95% CI, 1.46-2.45). Among children who had early childhood sleep terrors, 41.7% (95% CI, 37.6%-45.8%) continued to experience them from age 5 years onward. By comparison, only 16.5% (95% CI, 13.6%-19.4%) of children without sleep terrors before age 4 years started experiencing them at age 5 years or older (OR, 3.61; 95% CI, 2.75-4.73). In a more general fashion, the presence of early childhood sleep terrors was associated with childhood sleepwalking ( Table 2 ); children with sleep terrors were almost twice as likely to also experience sleepwalking.
A response on the parental history of sleepwalking was obtained for 1051 mothers and 801 fathers when the children were aged 10 years. There were slightly more parents with a history of sleepwalking in the group of children with sleep terrors compared with children who never had sleep terrors (31.6% [95% CI, 27.4%-35.8%] vs 25.0% [95% CI, 20.6%-29.4%]; OR, 1.39; 95% CI, 1.03-1.88).
Moreover, our data suggest that parental history of sleepwalking was associated with the transient or persistent nature of sleep terrors in children. Transient was defined as having sleep terrors before age 4 years and none thereafter, whereas persistent meant that children had sleep terrors before age 4 years and still had them after the age of 5 years. Twice as many children with a parental history of sleepwalking had persistent sleep terrors than children without such a parental history (32.0% [95% CI, 23.8%-40.2%] vs 16.8% [95% CI, 12.2%-21.4%]; OR, 2.33; 95% CI, 1.41-3.85).
There were more than twice as many parents who had experienced sleepwalking among children who sleepwalked than among children who had never sleepwalked from 2 1 / 2 to 13 years. Similarly, there were twice as many children who sleepwalked than those who had never sleepwalked who had either a mother or father who sleepwalked. Overall, we found that the odds of sleepwalking in a child increased with the number of parents with a history of sleepwalking ( Table 2 ): children with 1 parent with a history of sleepwalking had 3 times the odds of becoming a sleepwalker, and children with both parents with a history had 7 times the odds (adjusted model) of becoming a sleepwalker compared with children with no parental history of sleepwalking. In prevalence terms, 22.5% (95% CI, 19.2%-25.8%) of children without a parental history of sleepwalking developed sleepwalking, 47.4% (95% CI, 38.9%-55.9%) of children who had 1 parent who was a sleepwalker developed sleepwalking, and 61.5% (95% CI, 42.8%-80.2%) of children developed sleepwalking when both the mother and father were sleepwalkers.
We also investigated the association between parental history of sleepwalking and age at onset of sleepwalking in children in a subset of 132 sleepwalking children with complete data from ages 2 1 / 2 to 13 years. There was no difference in age at onset between children of parents with no history of sleepwalking and children of parents with a history of sleepwalking.
Finally, there was no association between the presence of snoring and either sleep terrors (1819 children, measured at peak prevalence) or sleepwalking (916 children, measured at peak prevalence).
Although sleep terrors are known to occur during early childhood, their prevalence had never been estimated with precision or during the entire period of childhood. We found a high prevalence (almost 35%) for sleep terrors (at least occasionally) at 1 1 / 2 years, with a progressive decline thereafter. A similar prevalence (36.9%) was found in 390 pairs of 18-month-old twins, 19 which also declined to approximately 20% at 30 months. In our study, the overall prevalence during the entire childhood (1 1 / 2 to 13 years) was even greater at approximately 56%. However, in both studies, the presence or absence of sleep terrors at each time point was derived from the mother’s responses on a self-administered questionnaire. Consequently, both the overall and age-specific prevalences of sleep terrors may be overestimated in the current sample, but it is nonetheless considerably higher than what was previously reported.
Our study revealed, in a large prospective and representative sample of children, that the prevalence of sleepwalking is approximately 29% for the entire childhood period (2 1 / 2 to 13 years) and that it peaks at approximately 13% around ages 10 to 13 years. The only study that investigated childhood somnambulism in a prospective and longitudinal manner (from ages 6 to 16 years), also based on a questionnaire completed by the mother, was conducted in 31 girls and 44 boys with somnambulism from a reference sample with an unknown total number of children. 21 It found a peak prevalence of 16.7% at age 12 years with no sex difference and with a progressive decline to approximately 7.5% at age 16 years. To our knowledge, the evolution of early childhood sleep terrors had never been investigated in a large prospective study. We showed that sleep terrors persist in more than 40% of children after age 5 years and, perhaps more important, that they are associated with sleepwalking later in childhood in one-third of children (with or without concomitant sleep terrors).
Our study also adds to the literature showing that the likelihood of being a sleepwalker as a child is largely associated with parental history (past or present) of sleepwalking. The percentages of children found to experience sleepwalking as a function of the absence or presence of parental history are similar to what was reported by Kales et al 13 on a much smaller sample. With our large cohort, we were able to estimate that children of parents who are or were sleepwalkers are 3 to 7 times more likely to be sleepwalkers themselves depending on whether 1 or both parents had the sleep disorder. In this disorder however, parental history of sleepwalking does not seem to elicit an earlier age of onset in offspring as is observed in other diseases, such as Alzheimer disease 22 or some cancers. 23
A similar association with parental history for sleep terrors was reported in the literature. 13 Unfortunately, parental history of sleep terrors was not documented in our study. However, we showed that a parental history of sleepwalking can also predict the emergence of sleep terrors in children. These findings lend support to the notion that sleepwalking and sleep terrors represent 2 manifestations of the same underlying pathophysiological condition.
Sleep-disordered breathing can be a triggering factor for sleepwalking 12 and shows familial aggregation. 24 It has been suggested that the genetic predisposition for sleepwalking and sleep terrors could be shared with sleep-disordered breathing. 24 The fact that both childhood parasomnias had a strong association with parental history, while not associated with snoring in the child, suggests that this theory is not the case.
This study has some limitations. The assessment of sleepwalking and sleep terrors was not derived from physicians’ diagnoses or from objective sleep laboratory assessments. Our data were obtained from parental reports and, although recognizing sleepwalking is usually not difficult for parents, the identification of sleep terrors can be more problematic. Our questionnaire contained an operational definition for sleep terrors, but it is nevertheless possible that some parents mistook nightmares for sleep terrors and vice versa.
These findings point to a strong genetic influence on sleepwalking and, to a lesser degree, sleep terrors. This effect may occur through polymorphisms in the genes involved in slow-wave sleep generation or sleep depth. 25 - 27 Parents who have been sleepwalkers in the past, particularly in cases where both parents have been sleepwalkers, can expect their children to sleepwalk and thus should prepare adequately. Although genetics establish predisposition, there are triggering environmental factors that may be involved as moderators. Preventive measures include avoiding sleep deprivation, irregular sleep schedules, and noisy sleeping environments. In more serious cases, house alarms may be needed to prevent children from leaving the house in their sleep. As stated in the third edition of the International Classification of Sleep Disorders , “With the development of sophisticated genetic testing and neuroimaging, direct research into the causes and mechanisms of disorders of arousal is anticipated.” 1 (p238)
Accepted for Publication: January 14, 2015.
Corresponding Author : Jacques Montplaisir, MD, PhD, Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Cœur de Montréal, 5400 Blvd Gouin Ouest, Montréal, QC H4J 1C5, Canada ( [email protected] ).
Published Online: May 4, 2015. doi:10.1001/jamapediatrics.2015.127.
Author Contributions: Dr Montplaisir had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Petit, Tremblay, Montplaisir.
Acquisition, analysis, or interpretation of data: Petit, Pennestri, Paquet, Desautels, Zadra, Vitaro, Boivin, Montplaisir.
Drafting of the manuscript: Petit.
Critical revision of the manuscript for important intellectual content: Pennestri, Paquet, Desautels, Zadra, Vitaro, Tremblay, Boivin, Montplaisir.
Statistical analysis: Petit, Pennestri, Paquet, Vitaro.
Obtained funding: Zadra, Vitaro, Tremblay, Boivin.
Administrative, technical, or material support: Vitaro.
Study supervision: Zadra, Tremblay, Boivin, Montplaisir.
Conflict of Interest Disclosures: Dr Desautels reported receiving research grants from GlaxoSmithKline and Novartis Pharma as well as honoraria from speaking engagements from UCB and Paladin Labs. Dr Montplaisir reported receiving grants or support from Merck and GlaxoSmithKline; serving as an advisor for Sanofi, Servier, Merck, Jazz Pharmaceuticals, Valeant Pharmaceuticals, and Impax Laboratories; and receiving honoraria for speaking engagements from Valeant Pharmaceuticals and Otsuka Pharmaceutical. No other disclosures were reported.
Funding/Support: Funding was obtained from Quebec’s Department of Health and Social Services; the Canadian Institutes of Health Research; the Social Sciences and Humanities Research Council of Canada; the Quebec Fund for Research on Society and Culture; the Quebec Fund for Research on Nature and Technology; the Health Research Fund of Quebec; Quebec’s Ministry of Research, Science and Technology; Human Resources Development Canada; Health Canada; the University of Montreal; Laval University; and McGill University. This funding was obtained throughout the years for the Quebec Longitudinal Study of Child Development as a whole (design and data collection) but not for the specific purpose of the present study.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank the children and families whose ongoing participation made this study possible. We also acknowledge the considerable contribution of the coordinators of the Quebec Longitudinal Study of Child Development and the Quebec Institute of Statistics. The contributors were not compensated.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
See our updated masking policy »
- Doctors, Clinics & Locations, Conditions & Treatments
- Patients & Visitors
- Medical Records
- Support Groups
- Help Paying Your Bill
- COVID-19 Resource Center
- Locations and Parking
- Visitor Policy
- Hospital Check-in
- Video Visits
- International Patients
View the changes to our visitor policy »
View information for Guest Services »
New to MyHealth?
Manage Your Care From Anywhere.
Access your health information from any device with MyHealth. You can message your clinic, view lab results, schedule an appointment, and pay your bill.
ALREADY HAVE AN ACCESS CODE?
Don't have an access code, need more details.
Learn More about MyHealth » Learn More about Video Visits »
MyHealth for Mobile
Get the iPhone MyHealth app » Get the Android MyHealth app »
WELCOME BACK
Sleep terrors.
Also called "night terrors", these episodes are characterized by extreme terror and a temporary inability to attain full consciousness. The person may abruptly exhibit behaviors of fear, panic, confusion, or an apparent desire to escape. There is no response to soothing from others. They may experience gasping, moaning or screaming. However, the person is not fully awake, and once the episode passes, often returns to normal sleep without ever fully waking up. In most cases, there is no recollection of the episode in the morning.
Like sleepwalking , night terror episodes usually occur during NREM delta (slow wave) sleep. They are most likely to occur during the first part of the night. The timing of the events helps differentiate the episodes from nightmares, which occur during the last third of the sleep period.
While sleep terrors are more common in children, they can occur at any age. Research has shown that a predisposition to night terrors may be hereditary. Emotional stress during the day, fatigue or an irregular routine are thought to trigger episodes. Ensuring a child has the proper amount of sleep, as well as addressing any daytime stresses, will help reduce terrors.
Sleep Medicine Center
Meet a team of experts who focus on you and your condition. Visit the clinic to make an appointment.

Stanford Medicine Outpatient Center
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Statistical Analyses
Conclusions, sleep terrors in children: a prospective study of twins.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Bich Hong Nguyen , Daniel Pérusse , Jean Paquet , Dominique Petit , Michel Boivin , Richard E. Tremblay , Jacques Montplaisir; Sleep Terrors in Children: A Prospective Study of Twins. Pediatrics December 2008; 122 (6): e1164–e1167. 10.1542/peds.2008-1303
Download citation file:
- Ris (Zotero)
- Reference Manager
OBJECTIVE. There is growing evidence that genetic factors are involved in the occurrence of sleep terrors. Twin studies provide invaluable information regarding genetic and environmental factors that can affect the manifestation of the disease; however, most previous twin studies on sleep terrors were performed retrospectively or with a sample that was too small to yield conclusive results. The aim of this large prospective study was to clarify the genetic and environmental contributions to sleep terrors in childhood.
METHODS. In all, 390 pairs of monozygotic and dizygotic twins were recruited at birth for a longitudinal study. The prevalence and frequency of sleep terrors were assessed at 18 and 30 months of age with a questionnaire administered to the biological mother of the twins. Zygosity was determined by a questionnaire and genotyping. The prevalence and polychoric correlation for each type of twins were calculated. Structural-equation modeling was used to determine the proportion of variance attributable to additive genetic, shared, and nonshared environmental factors.
RESULTS. The prevalence of sleep terrors was 36.9% at 18 months and 19.7% at 30 months; 49% of affected children were boys, and 51% were girls. At 18 months, the polychoric correlations were 0.63 for monozygotic and 0.36 for the dizygotic twins. These were 0.68 (monozygotic) and 0.24 (dizygotic) at 30 months. Our model-fitting analysis showed that sleep terrors were explained by a 2-component model at 18 months (43.7% additive genetic effects and 56.3% nonshared environment) and at 30 months (41.5% additive genetic effects and 58.5% nonshared environment).
CONCLUSIONS. These results strongly support the heritability of sleep terrors. There also seems to be continuity in genetic effects with the persistence of sleep-terror symptoms.
Sleep terrors (also referred to as night terrors, pavor nocturnus [in children], and incubus attacks [in adults]) are a common childhood parasomnia. They represent the most dramatic disorder of arousal. 1 The onset of sleep terrors is abrupt and frightening, usually sudden arousal with screaming. Sleep terrors are associated with intense autonomic discharge: tachycardia, tachypnea, flushing, diaphoresis, and mydriasis. The universal feature of this disorder is inconsolability. 1 During these events, children seem confused and disoriented. Any attempt to awaken them may increase their agitation and prolong their episode. These events are brief and cease abruptly, and the child returns to a deep sleep and is amnesic about the episode. These events usually occur within 2 hours of sleep onset and are a result of a partial arousal from deep slow-wave sleep.
Diagnosis is based on the identification of these symptoms and exclusion of organic pathologies. The exact prevalence of sleep terrors is unknown. There are major discrepancies in the literature (as a result of different sampling methods, sample age, and definitions of sleep terrors). 2 – 8 One study reported an overall prevalence of 19.2% for children between 4 and 9 years of age. 9 In a population-based study of parasomnias in children, the overall prevalence of sleep terrors between the ages of 3 and 10 years (measured retrospectively) was estimated at 14.7% for children. 10 Prevalence was estimated prospectively thereafter to be 3.8% at 11 years, 2.3% at 12 years, and 1.2% at 13 years. 10 The peak of prevalence occurs during childhood, and the resolution of sleep terrors is typically before adolescence; however, few studies, if any, studied prospectively the prevalence of sleep terrors in very early childhood.
Precipitating factors of sleep terrors are physical stress such as fever, nocturnal asthma, gastroesophageal reflux, sleep deprivation, and central nervous system medications. 11 – 13 There is, however, growing evidence that genetic factors are involved in the occurrence of sleep terrors. Familial aggregation is found, 14 , 15 and these studies suggest an autosomal dominant mode of inheritance. Kales et al 15 reported that the prevalence of sleepwalking and sleep terrors was ∼10 times greater in first-degree relatives of affected patients than in the general population. Twins studies can provide invaluable information regarding the relative contribution of genetic and environmental factors. Three studies looked specifically at the prevalence and concordance of sleep terrors in twins, and only 1 studied children directly. Hublin et al 16 conducted a retrospective study by using an adult Finnish twin population and reported a higher polychoric correlation for childhood sleep terrors in monozygotic twins (males: 0.38; females: 0.35) than in dizygotic twins (males: 0.17; females: 0.18). In a retrospective study of 881 pairs of junior high school Japanese monozygotic and dizygotic twins, results showed a moderate to strong contribution of genetic factors for sleep talking, sleepwalking, and sleep terrors. 17 Finally, a higher concordance rate was found for sleep terrors among 47 pairs of monozygotic twins than for the 14 pairs of dizygotic twins (15% vs 0%; P = .05) in a sample of 3- to 8-year-old children. 18
The objective of this study was to determine the relative contribution of genetic and environmental factors to the manifestation of sleep terrors in a large cohort of preschool twins who were followed prospectively. This study will also allow determination of the prevalence of sleep terrors at the age at which they are more likely to emerge (∼1.5–3.0 years).
Subjects for this study were monozygotic and dizygotic twins who were born between November 1995 and July 1998 in Montreal, Canada. These participants were part of an ongoing longitudinal study, the Quebec Newborn Twin Study. The descriptive statistics on prevalence were based on 887 twins at 18 months and 796 twins at 30 months. Our genetic–environment model-fitting analyses were based on results that were obtained from 161 pairs of monozygotic and 229 pairs of dizygotic twins at 18 months of age and 140 pairs of monozygotic and 207 pairs of dizygotic twins at 30 months of age (pairs for whom we had no missing data on the main variable). For same-gender twin pairs, zygosity was evaluated at 5 and 18 months of age by using a physical resemblance questionnaire and genotyping of 8 to 10 highly polymorphic genetic markers. 19 Eighty-four percent of the families were of European descent, 3% were of African descent, 2% were of Asian descent, 2% were Native North Americans, and the remaining families (9%) did not provide ethnic information; 49% of the subjects are boys. All twin pairs were reared together.
Before participating in the study, all families had received detailed information by mail on the aims and procedures of the research program and had signed a consent form. The study was approved by the ethics committee of the Sacre-Coeur Hospital of Montreal (affiliated with the University of Montreal). This research was conducted in accordance with the ethical standards of International Conference on Harmonisation and with the Declaration of Helsinki.
The sleep portion of the Quebec Newborn Twin Study contained 18 sleep-related questions. Sleep terrors were assessed by asking the biological mother to determine the frequency of sleep terrors experienced by the twins prospectively at 18 and 30 months of age: “Does your child have sleep terrors (this means sudden arousal with screams, sometimes with confusion and sweating)? Please circle one of the following answers: 1-never, 2-sometimes, 3-often, or 4-always.”
The prevalence of sleep terrors was first calculated at 18 and 30 months of age. The categories “often” and “always” were combined together as “often-always” for statistical reporting (Table 1 ). Age and gender effects were assessed with χ 2 tests. Then, the correlation in disorder tendency between the 2 members of each type of twin pairs (monozygotic and dizygotic) was computed by using SAS 9.1 (SAS Institute, Inc, Cary, NC) and represents the polychoric correlation. The polychoric correlation estimates the bivariate normal distribution that most closely approximates the cell probabilities from a contingency table. 20 – 22 Finally, a structural equation modeling by using a maximum likelihood fit function applied to the twin variances-covariances matrices was performed to obtain a formal estimation of the genetic and environmental parameters. 21 , 22 Under the current design of twins reared together, it is possible to model 3 different parameters: an additive genetic component (A), shared or common environmental components (family, living conditions, physical environment) (C) and nonshared (or unique to a single twin) environmental components (eg, hospitalization, illness) (E) that could explain the variation in the tendency to express the disorder (phenotypic variance). One can, thus, test different models that represent different combinations of factors (genetic, shared environmental, and nonshared environmental; genetic and nonshared environmental; shared environmental and nonshared environmental; and nonshared environmental). In the first series of analyses, such univariate estimates of variance decomposition were calculated by using the Mx statistical package. 22 , 23 To determine the best-fitting and most parsimonious model given the pattern of intercorrelations observed within twin pairs, the model fit was assessed on the basis of the χ 2 statistics and the Akaike information criterion (AIC). 21 , 22 The AIC was based on the likelihood and not on the χ 2 statistics. The lowest AIC value indicates the best combination of model fitting and parsimony.
The prevalence of sleep terrors (ie, the mother responded as either sometimes, often, or always) was 36.9% at 18 months of age and 19.7% at 30 months of age. The number and percentage of children in each frequency of occurrence category are reported in Table 1 for both 18 and 30 months. There was a significant age effect (χ 2 = 60.2, P < .0001) on the reported prevalence of sleep terrors; this parasomnia was almost twice as frequent at 18 months than at 30 months. Among the 140 cases that presented sleep terrors at 30 months (and for whom the data were available for 18 months), 52 (37.1%) were new cases and 88 (62.9%) were persistent cases; however, no gender difference was found at either 18 (χ 2 = 0.07, P = .80) or 30 (χ 2 = 2.51, P = .11) months of age.
The polychoric correlation was 0.63 for the monozygotic and 0.36 for the dizygotic twins at 18 months of age. At 30 months of age, the polychoric correlation was 0.68 for the monozygotic and 0.24 for the dizygotic twins. Results for the model-fitting analysis are shown in Table 2 . Sleep terrors were best explained by a 2-component model (genetic and nonshared environmental). At 18 months, genetic accounted for 43.7% and nonshared environmental accounted for 56.3% of the phenotypic variance. At 30 months, genetic and nonshared environmental similarly accounted for 41.5% and 58.5% of the variance, respectively.
To our knowledge, this is the largest prospective twin cohort (390 pairs of twins) ever studied on the genetic–environmental etiology of sleep terrors. In addition, no study of twins has ever reported the prevalence of sleep terrors so early in life. The prevalence of sleep terrors is high in infants (36.9% at 18 months) but decreases to 19.7% by 30 months of age. Our prevalence at 30 months is similar to the prevalence found in a smaller sample of twins aged 3 to 8 years (19.1%). 18 It is also similar to that of a recent study, 24 which found a prevalence of 19.9% in a population of 2.5-year-old single-birth children also from the province of Quebec, Canada. Thus, twins do not show a different prevalence of sleep terrors than single-birth children.
The results of this study also confirm that sleep terrors are a partially hereditary parasomnia. Genetic factors play an important role in the manifestation of sleep terror at a very young age. Of the total phenotypic variance in sleep terrors, the proportions that were attributable genetic influences were >40% for both 18- and 30-month-old twins. The polychoric correlations found for our cohort are higher than those published by Hublin et al 16 (monozygotic male: 0.38, female: 0.35; dizygotic male: 0.17, female: 0.18); however the latter was a retrospective study, and information bias may have accounted for the difference in the polychoric correlations observed. In addition, the childhood period in that study covers up to ∼15 years of age, whereas our results are for preschool children. Our polychoric correlations were also higher than those found in the study by Abe et al. 18 Again, the children in the latter study were older than those of this study, and the results were based on small samples of monozygotic and dizygotic twins. On the basis of heritability, this study suggests that there also seems to be continuity in genetic effects with the persistence of sleep-terror symptoms (at least to the age of 30 months).
The role of nonshared environmental factors was also significant in our study (>55% of the variance), although this percentage also includes measurement error. In our cohort of twins, all pairs were reared together; therefore, they mostly shared the same postnatal environment. One of the twins may still have undergone a prolonged hospitalization, may have had a concomitant disease, may have received medication, may have been be a poorer sleeper, or may have been of smaller birth weight than the other twin (nonshared environment). It must be kept in mind that the way that an individual reacts to a given common environmental event (eg, both twins may have been hospitalized) may also be a factor in the equation of why 1 twin only would develop sleep terrors and not the other twin. To that effect, Laberge et al 10 found an association between anxiety and the occurrence of sleep terrors.
This study has some limitations. Our data were obtained from parental reports, and children with a history of sleep terrors did not have objective sleep laboratory evaluations to validate their diagnosis. Although our questionnaire contained an operational definition for sleep terrors, it is nevertheless possible that some parents mistook nightmares for sleep terrors, and vice versa. It is also possible that some sudden awakenings with crying were mistaken for sleep terrors. The results therefore should be interpreted with caution, and the prevalence of sleep terrors in early childhood should be reconfirmed in large prospective studies.
Despite these limitations, the large sample size allowed the testing of models that assessed the role of genetic and environmental factors. Our results show that there is a substantial effect of genetics factors in sleep terrors. The findings also demonstrate that half of the children who experience sleep terrors at the age of 18 months will not experience this problem at 30 months, although the gene–environment etiology was found to be essentially the same at both times. To date, no specific genes have been identified for sleep terrors. Additional studies are needed; however, this large prospective twin study is the first step toward identifying susceptibility genes for sleep terrors.
Frequency of Sleep Terrors According to Age
Model-Fitting Results for Analysis of Liability to Sleep Terrors Among Twin Pairs for 18 and 30 Months
df indicates degrees of freedom; a 2 , variance attributed to additive genetic influence (heritability); c 2 , variance attributed to common environment; e 2 , variance attributed to unique environment; ACE, the phenotype is attributed to genetic influence, common environment, and unique environment; AE, the phenotype is attributed to both genetic influence and unique environment; CE, the phenotype is attributed to both common environment and unique environment (no genetic influence); E, the phenotype is attributed to the unique environment only.
The authors have indicated they have no financial relationships relevant to this article to disclose.
What's Known on This Subject
There is growing evidence that genetic factors are involved in the occurrence of sleep terrors. Three studies have examined the prevalence and concordance of sleep terrors in twins, and only 1 dealt with children specifically.
What This Study Adds
We determined the precise contribution of genetic and environmental factors to the manifestation of sleep terrors in a large cohort of early-childhood twins followed prospectively. In addition, we determined the prevalence of sleep terrors at the age at which they emerge.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Night Terrors in Children and Adults
Screaming and physical episodes happen while still asleep
Night Terrors in Children
- Sleep Routine
- Mental Health
- When a Child Has a Night Terror
Night terrors, also called sleep terrors, are a type of sleep disorder. During a sleep terror, you might scream or cry while asleep, or it may seem like you’re acting out a bad dream. These episodes can affect children or adults, but they’re more common during early childhood.
Generally, sleep terrors are not considered to be harmful to your physical or psychological health, but sometimes they can be a sign of underlying anxiety. If you think that you or your child has sleep terrors, you should rest assured that they can be well managed with lifestyle changes and medical care.
In this article, learn about the causes of sleep terrors and how to deal with them in adults and children.
Sleep terrors are not common, but they are not rare either. They are more common among young children under age 5 than any other age group. One research article published in 2022 estimated the frequency of sleep terrors during early childhood to be between 16.7% and 20.5%.
There is a genetic predisposition to night terrors, but not everyone with this sleep disorder has a family member who also has had them.
Causes of sleep terrors during childhood include:
- Fatigue, sleep deprivation
- Sleep disruption
- Waking up during sleep
- Behavioral problems
Night terrors typically occur during transitions between the phases that cycle throughout sleep . Children are more prone to waking up between sleep phases than adults are. Sleep normally becomes more regulated as the brain matures, and night terrors are rare during adulthood.
Sleep Phases ofr Night Terrors
Night terrors occur during non-rapid eye movement (non-REM) sleep, during stage 3 (slow-wave) sleep. Dreams are normally part of rapid eye-movement (REM) sleep . During the REM dream phase of sleep, people are unable to move, cry, scream, or speak. Unlike nightmares, night terrors happen when a person is not dreaming, which is why physical movements, screaming, and crying can occur during sleep terror episodes.
Some Adults Experience Night Terrors Too
While the most common age for night terrors is early childhood, these episodes can continue or begin during adolescence or adulthood.
Having night terrors at any age does not indicate a psychiatric condition, and there is no reason to be embarrassed or concerned if you or your child is having them. However, if you experience night terrors at any age, it is important to get a medical evaluation. These episodes can sometimes be related to underlying health conditions.
Risk factors for adult-onset night terrors include:
- Sleep deprivation
- Sleep disorders, such as obstructive sleep apnea
- Medication side effects, especially antihistamines and antidepressants
If you have been experiencing sleep terrors, it would be beneficial to learn whether you have an underlying medical condition so that you can get appropriate treatment. Treatment will help the underlying condition and lower the risk of recurrent sleep terrors.
An anxiety disorder can cause anxiety symptoms, but that’s not always the case. Many people can experience periods of anxiety due to stress without having an anxiety disorder.
People Unaware of Night Terror Episodes
One of the key characteristics of sleep terrors is that people are not aware that they are having them and are unable to recall the episodes.
Symptoms: Night Terrors, Nightmares, or Nightmare Disorder?
Night terrors occur during sleep, and people who are experiencing these episodes are unaware that the episodes are occurring. When a person has night terrors, they may cry, scream, or punch while they appear to be sleeping.
Symptoms and characteristics of night terrors include:
- Making sounds or movements that indicate distress during sleep
- Rapid breathing
- Rapid heart rate
- Dilated pupils
- Muscle tension
- Not responding to the speech of other people in the room during the episode
- Not being able to remember or describe what happened
After having a night terror, sometimes people can recall having experienced some anxiety during the night or might describe a sense of doom.
Other Parasomnias
Night terrors are a type of parasomnia . A parasomnia is an unpleasant sleep experience, such as a nightmare . However, night terrors are not the same as nightmares or other parasomnias.
How other parasomnias compare to night terrors include:
- Nightmares are bad dreams, and people usually remember some of the content of a nightmare. Unlike sleep terrors, people do not act out during a nightmare, and others who are in the room usually don’t notice any changes in movement or behavior.
- Sleepwalking is a type of coordinated physical movement that occurs during sleep. Sleepwalking does not occur during the dream stage of sleep, and people experiencing them will not recall sleepwalking. They are more common among children than adults.
- Sleep paralysis is a terrifying experience during which you are unable to physically move any part of your own body even though you feel you are awake. Most people remember sleep paralysis episodes, and others who might be present in the room typically do not see any altered behavior.
- Sleep talking is when people talk when they are asleep. This can happen during any stage of sleep, and it isn't usually unpleasant.
Sleep Routines to Stop Night Terrors
If you have been experiencing sleep terrors, there are some ways to prevent them from occurring. You should start by seeing a healthcare provider, who will evaluate your overall health and consider underlying psychological issues (especially anxiety) and health conditions that could be putting you at risk.
Some recommendations for preventing recurrent sleep terrors include lifestyle adjustments.
Measures you can take to avoid sleep terrors include:
- Avoiding alcohol, caffeine, and other stimulants (such as medications with stimulant action), especially before bedtime
- Avoiding disturbing content, such as frightening books, media, or discussions, especially before bed
- Getting enough sleep if you have not been sleeping well
- Regulating your sleep schedule to sleep and wake up at approximately the same time every day
Additionally, consider going over the following with your healthcare provider:
- A review of your medication list to detect whether you have been taking any medications that could be causing sleep terrors as a side effect
- Whether you may have anxiety that could be overwhelming for you, and how to get help and support with distressful issues
- Whether a sleep evaluation is needed to identify an underlying sleep disorder that needs an assessment and treatment
For a child with ongoing night terrors that occur at a regular time each night, a healthcare provider may recommend scheduled awakenings. In this process, the usual time of the night terror is noted over the course of two weeks.
The parent gently wakes the child 15 to 30 minutes before that time each night and allows them to return to sleep. This is done for two to four weeks.
Mental Health and Night Terrors
Sometimes people who experience sleep terrors become concerned about whether these events could be an indication of an underlying mental health problem. Older research on this subject has not shown a strong association between sleep terrors and psychiatric conditions.
In general, people who have psychiatric diagnoses, such as post-traumatic stress disorder (PTSD), anxiety disorders, borderline personality disorder, or schizophrenia, may be at a slightly higher risk of experiencing sleep terrors or other parasomnias. However, having sleep terrors is not an indication of an underlying or undiagnosed psychiatric condition.
For Parents: When You See Your Child Having Night Terrors
If you’ve experienced night terrors in your children, you might be concerned that your child could be having a seizure or a panic attack. It can be difficult for parents to know the difference between night terrors and psychiatric illnesses or neurological conditions.
It could be helpful for you to video or audio record the episodes so that you can share the recordings with your child’s pediatrician when you take them in for an evaluation.
During a Night Terror
If your child is having a night terror, it’s best not to wake them up, not to move them, and not to interact with them. When they wake up, be sure to allow them to talk about any distress they’re experiencing, and offer gentle reassurance.
Many children become stressed about a variety of things in life, ranging from exaggerated anxiety about issues that they don’t have control over to serious concerns about issues like parental fighting or bullying at school.
If you feel that you are not able to address your child’s anxiety, it could be helpful to seek professional help from someone who is experienced in counseling children and families with young children.
Night terrors, also called sleep terrors, are more common among young children than any other age group, but they can occur at any age. Sometimes sleep disruption, sleep deprivation, or daytime anxiety can contribute to the risk of having night terrors, and they can also occur as a medication side effect.
Night terrors are episodes that involve acting out a sense of terror during sleep, which can be alarming to other people but does not cause distress to the person who is experiencing the episode. Some people may feel a sense of doom or anxiety before or after a night terror. The key feature of night terrors is that people do not remember having them.
If you or your child has been experiencing night terrors, it will be helpful to see a healthcare provider who can try to identify the underlying cause and provide some guidance to help with management.
Leung AKC, Leung AAM, Wong AHC, Hon KL. Sleep terrors: An updated review . Curr Pediatr Rev. 2020;16(3):176-182. doi:10.2174/1573396315666191014152136
Laganière C, Gaudreau H, Pokhvisneva I, Kenny S, Bouvette-Turcot AA, Meaney M, Pennestri MH. Sleep terrors in early childhood and associated emotional-behavioral problems . J Clin Sleep Med . 2022;18(9):2253-2260. doi:10.5664/jcsm.10080
Petit D, Pennestri MH, Paquet J, et al. Childhood sleepwalking and sleep terrors: a longitudinal study of prevalence and familial aggregation . JAMA Pediatr. 2015;169(7):653-8. doi:10.1001/jamapediatrics.2015.127
Futenma K, Inoue Y, Saso A, Takaesu Y, Yamashiro Y, Matsuura M. Three cases of parasomnias similar to sleep terrors occurring during sleep-wake transitions from REM sleep . J Clin Sleep Med . 2022;18(2):669-675. doi:10.5664/jcsm.9666
Silber MH. Parasomnias occurring in non-rapid eye movement sleep . Continuum (Minneap Minn). 2020;26(4):946-962. doi:10.1212/CON.0000000000000877
Ting CY, Thomas B. Behavioural sleep problems in children . Singapore Med J . 2023. doi:10.4103/singaporemedj.SMJ-2021-102
Gau SF, Soong WT. Psychiatric comorbidity of adolescents with sleep terrors or sleepwalking: a case-control study . Aust N Z J Psychiatry. 1999;33(5):734-9. doi:10.1080/j.1440-1614.1999.00610.x This is the best study that could be found, although older.
By Heidi Moawad, MD Dr. Moawad is a neurologist and expert in brain health. She regularly writes and edits health content for medical books and publications.
Sleep Loss and Emotion: A Systematic Review and Meta-Analysis
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
Sleep and emotion are fundamentally intertwined, both being universal human experiences that profoundly shape our daily functioning and well-being. Our emotional states influence every facet of our lives, from our physical health and cognitive performance to our social relationships and overall life satisfaction. Similarly, sleep plays a vital role in regulating our emotional processing , reactivity, and mood.

- This meta-analysis synthesized over 50 years of experimental research on sleep loss and emotion, examining the effects of sleep deprivation, sleep restriction, and sleep fragmentation on various emotional outcomes.
- Sleep loss resulted in reduced positive affect (SMD = -0.27 to -1.14), increased anxiety symptoms (SMD = 0.57-0.63), and blunted arousal in response to emotional stimuli (SMD = -0.20 to -0.53). Findings for negative affect, emotional valence, and depressive symptoms varied based on the type of sleep loss.
- Nonlinear dose-response effects were found for the amount of sleep loss on some emotional outcomes. Losing REM sleep had a stronger effect on unpleasant emotional reactivity compared to losing slow-wave sleep.
- While the research sheds light on the detrimental effects of sleep loss on emotions, it has some limitations such as relying predominantly on young adult samples and potential expectancy effects.
- The pervasiveness of insufficient sleep worldwide makes this an important public health issue with significant implications for emotional well-being and risk for psychiatric disorders.
Sleep loss is common in modern society, with large segments of the population regularly failing to obtain adequate sleep (Hafner et al., 2017).
Poor sleep is known to catalyze the development of emotional difficulties and affective disorders across the lifespan (Goldstein & Walker, 2014; Gregory et al., 2009).
While an increasing number of experimental studies have demonstrated the adverse effects of sleep loss on emotion in recent decades (Palmer & Alfano, 2017; Tempesta et al., 2018), findings have been mixed and a comprehensive quantitative synthesis was needed to integrate results across the heterogeneous research designs and consolidate conclusions.
This meta-analysis aimed to quantify the effects of various forms of experimental sleep loss (deprivation, restriction, fragmentation) on multiple aspects of emotional experience and elucidate factors that may influence these effects.
The researchers conducted a preregistered systematic review and meta-analysis following PRISMA guidelines.
They searched PubMed/MEDLINE, PsychINFO, and Web of Science databases for experimental studies examining the effects of sleep deprivation, sleep restriction, or sleep fragmentation on positive affect, negative affect, mood disturbance, emotional reactivity, anxiety symptoms and/or depressive symptoms in healthy populations.
Additional unpublished data was sought via listservs and contacting authors. Inclusion criteria required studies to have healthy samples, an experimental sleep loss manipulation, an adequate control/baseline condition, and an emotion-related dependent variable.
Two independent coders screened and extracted data from eligible studies.
Search strategy and terms
The search criteria included human studies mentioning experimental sleep manipulations and any emotion-related outcomes in the title/abstract. There were no restrictions on language, location or date.
Inclusion and exclusion criteria
Included studies had to 1) use healthy samples without psychiatric, sleep or medical disorders that impact sleep/emotions, 2) experimentally manipulate nighttime sleep deprivation, restriction or fragmentation, 3) have an adequate control/baseline condition, 4) assess an emotion-related dependent variable after the sleep manipulation.
Studies were excluded if they used specialized samples, nap/circadian protocols, or had an intervention prior to the emotional assessment that could affect outcomes.
Statistical measures
Standardized mean differences (Hedges’ g) were calculated for each eligible outcome. Multivariate multilevel random effects models were used to estimate overall effects for each sleep loss type and emotional outcome, accounting for non-independent effect sizes.
Mixed effects models examined potential moderators. Heterogeneity, outliers, and indices of publication bias were assessed.
The meta-analysis included 154 studies (N=5,717) yielding 1,338 effect sizes.
For sleep deprivation (k=599), significant effects were found for reduced positive affect (SMD=-0.86), increased negative affect (SMD=0.37), mood disturbance (SMD=0.71), blunted arousal (SMD=-0.53), and anxiety (SMD=0.63). Nonlinear dose-response effects showed negative affect, mood disturbance and anxiety peaking at 30-60 hours of wakefulness.
For sleep restriction (k=483), effects were significant for reduced positive affect (SMD=-0.56), increased negative affect (SMD=0.20), mood disturbance (SMD=0.56), greater unpleasantness (SMD=0.23), blunted arousal (SMD=-0.20), anxiety (SMD=0.57) and depression (SMD=0.46). Nonlinear dose-response effects found the largest deficits for positive affect and unpleasantness around 4 hours of sleep.
Sleep fragmentation (k=256) significantly reduced positive affect (SMD=-0.40) and blunted arousal (SMD=-0.36). There were no significant effects on negative affect, mood, or valence.
Some moderating effects of age, sex, and study characteristics emerged, though not consistently across outcomes. Importantly, losing REM sleep had a stronger effect than losing slow-wave sleep on ratings of unpleasantness to emotional stimuli.
This meta-analysis provides the most comprehensive picture to date of how sleep loss impacts human emotions.
It demonstrates that multiple aspects of emotional functioning are significantly altered by sleep deprivation, restriction, and fragmentation.
The most robust effects across all three types of sleep loss were found for reductions in positive affect, suggesting that inadequate sleep may be particularly detrimental for experiences and expressions of positive emotions.
This could have important mental health implications given the role of positive emotionality in psychological well-being and resilience (Fredrickson, 2001). The blunting of emotional arousal also seen after sleep loss may reflect impairments in top-down emotional processing.
In contrast, sleep loss effects on negative affect were smaller and less consistent across studies. The evolutionary importance of negative emotions for signaling threats and promoting survival could make negative affective responses more resistant to the effects of sleep loss compared to positive affect.
However, anxiety symptoms were consistently increased by sleep loss, indicating heightened feelings of apprehension and worry. The dose-response findings suggest these detrimental effects on negative affect and anxiety are exacerbated by more extreme sleep deprivation.
An intriguing finding was that losing REM sleep impacted ratings of emotional stimuli more than losing slow-wave sleep. This aligns with theories proposing a key role of REM sleep in emotional memory consolidation and maintaining emotional reactivity (Walker & van der Helm, 2009).
More targeted research comparing the effects of selective REM and slow-wave sleep deprivation on emotional reactivity and regulation is needed.
While only a small number of studies included children or older adults, some moderating effects of age did emerge, with sleep loss having stronger effects on mood disturbances in older individuals. Examining sleep-emotion dynamics in developmental and lifespan contexts is an important future direction.
Sex differences were also found for some outcomes, but not consistently, highlighting the need for more studies powered to detect potential gender differences in emotional vulnerability to sleep loss.
Overall, these findings underscore the consequences of insufficient sleep for affective experience and functioning. They provide an important foundation for further investigating the mechanisms linking sleep and emotion and developing interventions to mitigate the risks of sleep loss for emotional health.
- Preregistration of the study design and analytical plan
- Adherence to PRISMA guidelines for transparent reporting
- Comprehensive search strategy across multiple databases
- Inclusion of unpublished data to mitigate publication bias
- Rigorous coding procedures with two independent coders
- Examination of multiple types of sleep loss and emotional outcomes
- Advanced statistical methods accounting for non-independent effect sizes
- Assessment of heterogeneity, outliers, and publication bias indices
Limitations
- Most studies used young adult samples, limiting generalizability to other ages
- There was a lack of geographical diversity, with studies predominantly from Western countries
- Masking participants to sleep loss conditions is not possible, so expectancy effects may have influenced results
- Some analyses for specific emotional outcomes or sleep types had a small number of studies
- Variability in emotional assessments and sleep manipulation procedures across studies
Implications
The finding that even relatively modest amounts of sleep loss can have significant negative repercussions for emotional well-being has important real-world implications.
With up to one-third of the general adult population reporting insufficient sleep (Liu et al., 2016), a substantial proportion of people may be at heightened risk for emotional difficulties and affective disorders as a result of inadequate sleep.
This makes sleep a critical target for public health interventions aimed at promoting mental health.
The differential impacts of REM versus slow-wave sleep loss on emotional reactivity also have clinical relevance, suggesting sleep stage-specific interventions may be warranted for certain emotional issues.
The nonlinear dose-response effects for several outcomes indicate that sleeping less than 4-5 hours and/or being continuously awake for over 24 hours may represent particularly dangerous thresholds for emotional health.
Organizations and occupations where sleep loss is common (e.g., military, healthcare, shift work) need to be aware of the risks to emotional well-being and implement strategies to mitigate these effects.
Public policies regulating work hours and school start times should prioritize sleep to reduce adverse emotional consequences at the population level.
With the high rates of insufficient sleep among adolescents (Basch et al., 2014) and emerging mood disorders during this developmental period, optimizing sleep could be an important avenue for youth mental health promotion.
More broadly, initiatives to increase public awareness about the importance of sleep for emotional health, address barriers to adequate sleep, and promote evidence-based sleep hygiene practices have the potential to make a substantial and much-needed impact on psychological well-being and public health.
The current findings underscore the affective benefits of prioritizing sleep and the dangers of sacrificing it.
Primary reference
Palmer, C. A., Bower, J. L., Cho, K. W., Clementi, M. A., Lau, S., Oosterhoff, B., & Alfano, C. A. (2024). Sleep loss and emotion: A systematic review and meta-analysis of over 50 years of experimental research. Psychological Bulletin, 150 (4), 440–463. https://doi.org/10.1037/bul0000410
Other references
Basch, C. E., Basch, C. H., Ruggles, K. V., & Rajan, S. (2014). Prevalence of sleep duration on an average school night among 4 nationally representative successive samples of American high school students, 2007–2013. Preventing Chronic Disease, 11 , Article 140383. https://doi.org/10.5888/pcd11.140383
Fredrickson, B. L. (2001). The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. American Psychologist, 56 (3), 218–226. https://doi.org/10.1037/0003-066X.56.3.218
Goldstein, A. N., & Walker, M. P. (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679-708. https://doi.org/10.1146/annurev-clinpsy-032813-153716
Gregory, A. M., Rijsdijk, F. V., Lau, J. Y., Dahl, R. E., & Eley, T. C. (2009). The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep, 32 (2), 189-199. https://doi.org/10.1093/sleep/32.2.189
Hafner, M., Stepanek, M., Taylor, J., Troxel, W. M., & van Stolk, C. (2017). Why sleep matters—the economic costs of insufficient sleep: a cross-country comparative analysis. Rand Health Quarterly, 6 (4), 11. https://doi.org/10.7249/RB9962
Liu, Y., Wheaton, A. G., Chapman, D. P., Cunningham, T. J., Lu, H., & Croft, J. B. (2016). Prevalence of healthy sleep duration among adults — United States, 2014. MMWR. Morbidity and Mortality Weekly Report, 65 (6), 137–141. https://doi.org/10.15585/mmwr.mm6506a1
Palmer, C. A., & Alfano, C. A. (2017). Sleep and emotion regulation: An organizing, integrative review. Sleep Medicine Reviews, 31, 6-16. https://doi.org/10.1016/j.smrv.2015.12.006
Tempesta, D., Socci, V., De Gennaro, L., & Ferrara, M. (2018). Sleep and emotional processing. Sleep Medicine Reviews, 40, 183-195. https://doi.org/10.1016/j.smrv.2017.12.005
Walker, M. P., & van der Helm, E. (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin, 135 (5), 731–748. https://doi.org/10.1037/a0016570
Keep Learning
- How does this meta-analysis advance our understanding of the relationship between sleep and emotion compared to previous individual studies? What are the advantages of synthesizing results across multiple studies?
- The strongest effects of sleep loss were found for positive affect. Why might inadequate sleep be particularly detrimental for positive emotions from an evolutionary or neurobiological perspective? What are the potential mental health implications of this finding?
- The study found some moderating effects of age on the sleep loss-emotion relationship. How might the effects of sleep loss on emotional functioning differ across the lifespan from childhood to older adulthood? What developmental factors could influence this?
- Losing REM sleep impacted emotional reactivity to unpleasant stimuli more than losing slow-wave sleep. What are the potential mechanisms that could explain this finding? How does it relate to theories about the role of REM sleep in emotional memory processing?
- Given the pervasiveness of insufficient sleep in the population, what public health strategies or policies could help mitigate the negative emotional consequences of sleep loss at a societal level? How could this research inform interventions for at-risk groups?
- What are some of the limitations of experimental sleep research for understanding real-world emotional functioning? How well do these controlled laboratory studies generalize to chronic partial sleep loss as it’s experienced in daily life?
- Where should sleep and emotion research go from here? What are the most pressing unanswered questions or promising future directions based on the current state of the science? What types of studies or methodologies are needed to advance the field?
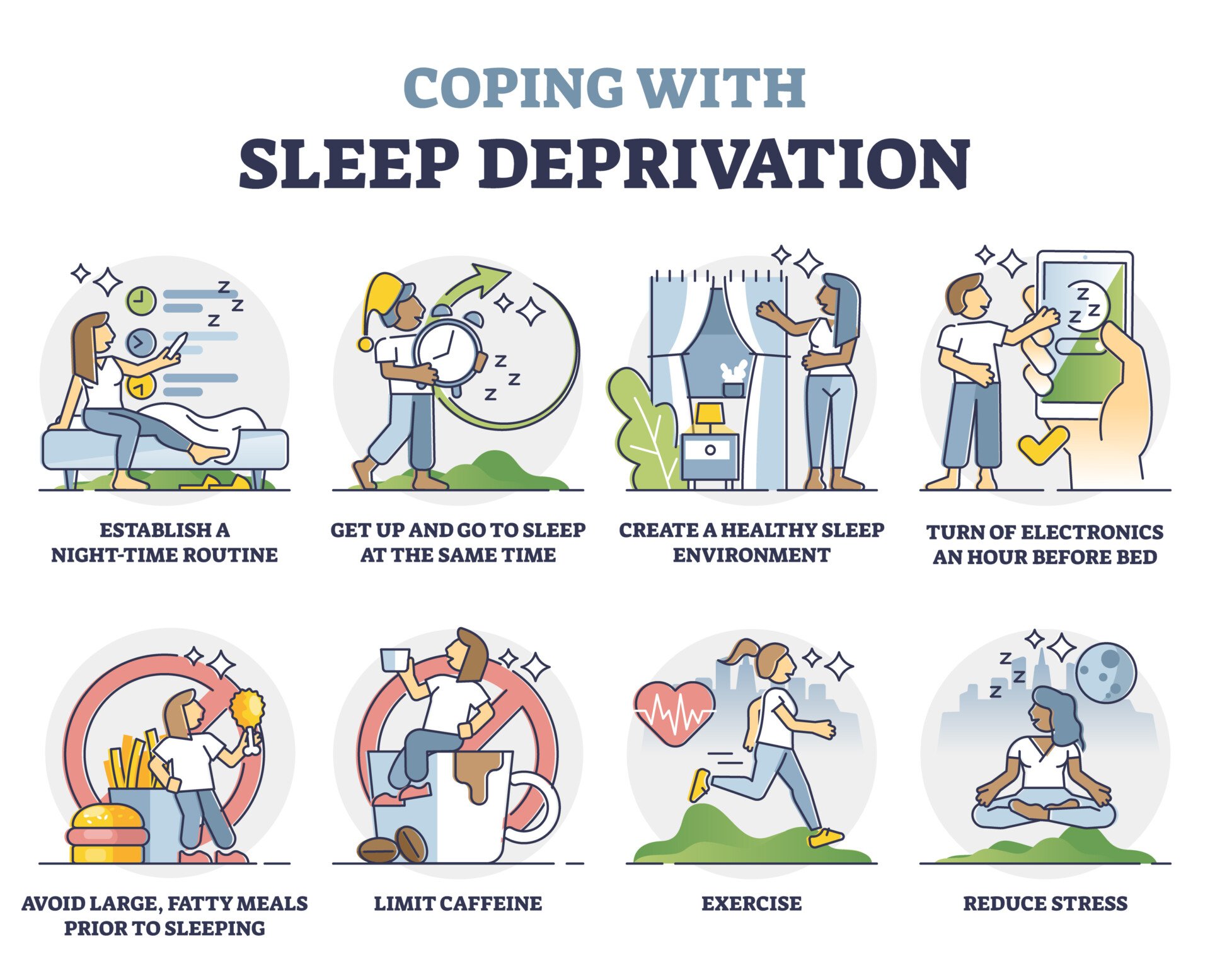
Related Articles

Clinical Psychology
A Study Of Social Anxiety And Perceived Social Support

Psychological Impact Of Health Misinformation: A Systematic Review

Family History Of Autism And ADHD Vary With Recruitment Approach And SES

Measuring And Validating Autistic Burnout
A Systematic Review of Grief and Depression in Adults

Anxiety in Early Adolescents During the Covid-19 Pandemic

Sleep terrors (night terrors) in children
D o you know a child who screams and jumps out of bed at night while still asleep? The child might be experiencing sleep terrors -- a frightening event for those who see it, but usually not a cause for concern.
Also known as night terrors, sleep terrors usually affect children between the ages of about 4 to 12. Some adults may have them too. An episode usually lasts a few minutes but may last up to 30 minutes or more.
What are sleep terrors?
During a sleep terror, a child might:
- Sit up in bed with a loud scream.
- Have a red face and look sweaty.
- Be restless.
- Ignore your attempts to offer comfort.
- Jump out of bed as if running from a threat. However, if the child leaves the bed and walks or moves away from it, this is considered sleepwalking. Sleepwalking is a condition similar to sleep terrors and often runs in families.
People often confuse sleep terrors with nightmares. But they're not the same.
Sleep terrors usually:
- Happen early in the night.
- Aren't remembered by a child in the morning.
Fortunately, most children outgrow sleep terrors on their own.
Coping with sleep terrors
If a child has occasional sleep terrors:
- Just wait it out. It's upsetting to watch, but it doesn't harm the child and the episode will stop on its own.
- Speak softly and calmly and try not to wake the child.
- Secure all windows and doors. Consider putting locks or alarms on them.
- Place the child's mattress on the floor. Do not have the child sleep in a top bunk bed.
- Consider having a child sleep on the first floor in homes with more than one level. This may help reduce the chance of falling down stairs.
- Move electrical cords or anything else the child could trip over.
- Place sharp or fragile objects out of reach.
Remember that sleep terrors typically aren't a serious condition and usually go away on their own.
Treatment for sleep terrors
Most children do not need treatment for sleep terrors. But if sleep terrors happen more than twice a month, pose a safety threat or cause extreme daytime sleepiness, a child might need treatment. The options include:
- Treating another sleep problem, such as pediatric obstructive sleep apnea or restless legs syndrome, if that's a factor.
- Relaxation techniques and hypnosis.
- A prescription medicine, such as a mild sleep aid or an antidepressant.
How to prevent sleep terrors
Sleep terrors can disrupt everyone's sleep. Simple steps might help prevent them. Try to:
- Make sure a child gets enough sleep. Being overtired can trigger episodes.
- Establish a bedtime routine. Do something quiet and calming with the child -- such as reading books or having a warm bath -- before bed. Do not use smartphones, TVs and other electronics close to bedtime. Have the child go to bed and wake up at the same time every day, even on the weekends.
- Schedule a wakeup. If a child has night terrors at a consistent time, you can try waking the child 15 to 20 minutes before that time. Wake the child just long enough for the child to change position or briefly speak or make a sound. Although research on this method is limited, it may be worth trying as a way to prevent sleep terrors. Occasionally it may prompt a sleep terror episode to happen later in the night.
Sleep terrors usually aren't serious and typically go away on their own. But if a child's symptoms get worse or happen more than a couple of times a month, talk with a healthcare professional.
©2024 Mayo Foundation for Medical Education and Research (MRMER). All rights reserved.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About Sleep
- Sleep Facts
- Sleep Resources
Sleep Facts and Stats
At a glance.
- The amount of sleep you need changes as you age.
- Several national surveys are used to study insufficient sleep (or short sleep duration) in U.S. children, high school students, and adults.
- These surveys show that not getting enough sleep has varied by age, location, racial/ethnic group, and over time in the United States.

Populations

FastStats: Sleep in Children

FastStats: Sleep in High School Students

FastStats: Sleep in Adults
By sharing information and resources, CDC raises awareness about the importance of sleep health and its effect on public health.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Psychiatry
Disturbed Sleep in PTSD: Thinking Beyond Nightmares
Marike lancel.
1 Centre of Expertise on Sleep and Psychiatry, GGZ Drenthe Mental Health Institute, Assen, Netherlands
2 Department of Clinical Psychology and Experimental Psychopathology, University of Groningen, Groningen, Netherlands
Hein J. F. van Marle
3 Department of Psychiatry, Amsterdam Neuroscience, Vrije Universiteit, Amsterdam UMC, Amsterdam, Netherlands
4 GGZ InGeest Specialized Mental Health Care, Amsterdam, Netherlands
Maaike M. Van Veen
Annette m. van schagen.
5 ARQ Centrum'45, Oegstgeest, Netherlands
Associated Data
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Sleep disturbances frequently co-occur with posttraumatic stress disorder (PTSD). Insomnia and nightmares are viewed as core symptoms of PTSD. Yet, relations between disturbed sleep and PTSD are far more complex: PTSD is linked to a broad range of sleep disorders and disturbed sleep markedly affects PTSD-outcome. This article provides a concise overview of the literature on prevalent comorbid sleep disorders, their reciprocal relation with PTSD and possible underlying neurophysiological mechanisms. Furthermore, diagnostic procedures, standard interventions—particularly first choice non-pharmacological therapies—and practical problems that often arise in the assessment and treatment of sleep disturbances in PTSD are described. Finally, we will present some perspectives on future multidisciplinary clinical and experimental research to develop new, more effective sleep therapies to improve both sleep and PTSD.
Introduction
Sleep disturbances frequently occur in posttraumatic stress disorder (PTSD) and are reported by 70–90% of patients ( 1 ). Nightmares (intrusions) and difficulties sleeping (hyperarousal) are specifically included in the diagnostic (DSM-5) criteria of the disorder ( 2 ). In addition, various other sleep disorders are common in PTSD [e.g., ( 3 , 4 )]. It has long been thought that interventions focusing on trauma itself would eventually reduce disturbed sleep, but accumulating evidence shows that sleep disorders play a central role in both the development and maintenance of PTSD [e.g., ( 5 , 6 )] and therefore require particular clinical attention.
In this paper we provide an overview of prevalent sleep disorders in PTSD, the reciprocal association of sleep disturbances and PTSD and its underlying mechanisms, as well as information on accurate assessment and treatment of disturbed sleep tailored to the PTSD patient population. Finally, our perspectives for future research directed at more effective sleep-targeted interventions and integrated treatment strategies are described. Our aim is to enhance awareness of clinical practitioners of the importance of targeting sleep in PTSD treatment.
PTSD and Sleep Disturbances
The majority of patients with PTSD, about 50–70%, suffer from recurrent distressing nightmares (see Table 1 for an overview of the most frequently occurring sleep disorders in PTSD, their characteristics, ways of assessment and treatment). These can be exact replications or more symbolic representations of traumatic experiences, and primarily occur during rapid eye movement sleep (REMS) ( 7 ). Insomnia symptoms, reported by ~70% of patients ( 8 ), are often related to increased autonomic arousal and fear of sleep: fear of loss of control and/or of having nightmares ( 9 , 10 ). PTSD is also associated with obstructive sleep apnea (OSA), concerning 40–90% of PTSD patients ( 11 ). The repeated OSA events lead to frequent oxygen desaturations and arousals. Insomnia, nightmares and OSA may trigger and exacerbate each other, forming a vicious cycle ( 1 , 4 ). In addition, multiple studies found a high proportion (around 33%) of periodic limb movement disorder (PLMD) in PTSD patients ( 12 ). The limb movements during sleep are associated with arousals/awakenings. Also relatively prevalent in PTSD are periods of sleep paralysis, typically occurring during (REM) sleep-wake transitions, which are often accompanied by distressing experiences, referred to as hypnagogic or hypnopompic hallucinations ( 13 ). Although the exact frequency is unclear, PTSD is also linked to remarkable disruptive nocturnal behaviors, including abnormal vocalizations and complex body movements. These parasomnias are generally thought to occur during non-REMS (confusional arousals, night terrors, sleepwalking), but may also take place during REMS, implying REMS behavior disorder (RBD)-like periods of REMS without the usual muscle atonia (RWA) with dream enactment. Mysliwiec et al. ( 14 ) proposed a distinct trauma-associated sleep disorder (TASD), conceptualized as a parasomnia encompassing nightmares, disruptive nocturnal behaviors as well as RWA [see ( 15 ) for an illustrative case study]. In support of this idea, a recent study in a large sample of veterans found self-reported dream enactment in nearly 40%. However polysomnography (PSG) showed no RWA in 80% of this group, indicating a non-REMS parasomnia, rather than a REMS phenomenon ( 16 ). Furthermore, in those veterans with RWA, RBD appeared related to PTSD (prevalence rate 15%) and even more so to the combination of PTSD and traumatic brain injury (prevalence rate 21%). Therefore, it is still controversial whether TASD really represents a separate sleep disorder ( 17 , 18 ).
Overview of frequently occurring sleep disorders in PTSD: characteristics, assessment and treatments.
Interrelations Between PTSD and Sleep Disturbances
Research strongly indicates that disturbed sleep is not merely a symptom or consequence of PTSD, but constitutes a predisposing, precipitating and perpetuating factor for PTSD. Sleep disturbances prior to and/or shortly after trauma increase the risk for PTSD ( 5 , 12 ). For instance, in patients admitted to an emergency department after a motor vehicle collision both pre-trauma insomnia and nightmares predicted subsequent PTSD development ( 19 ). Furthermore, sleep disturbances affect the clinical course of PTSD: poor sleep quality is associated with reduced responsiveness to trauma-focused therapy [e.g., ( 20 , 21 )], while interventions targeting insomnia, nightmares or OSA improve sleep quality and ameliorate daytime PTSD symptoms ( 22 ). For example, Kanady et al. ( 9 ) observed that cognitive behavioral treatment of insomnia (CBT-I) in patients with PTSD and insomnia significantly decreased hypervigilance as well as PTSD symptom severity, and both were related to persistent reductions in fear of sleep. Moreover, sleep disturbances often persist after trauma-focused therapy ( 10 ). For example, Walters et al. ( 23 ) recently showed that prolonged exposure therapy improved daytime PTSD symptoms, but did not ameliorate insomnia and nightmares in veterans with PTSD. Residual insomnia has been shown to be an important risk factor for the development of and relapse in diverse mental disorders [e.g., ( 24 )].
The reciprocal relations between sleep disturbances and PTSD suggest that disturbed sleep constitutes a causal factor in PTSD ( 25 , 26 ). This causality is partly based on sleep's role in memory consolidation and emotion regulation ( 27 , 28 ). While memory consolidation takes place during both slow wave sleep (SWS; deep non-REMS) and REMS, the processing of emotional memories is thought to happen primarily during REMS ( 29 ). In PTSD, traumatic memories arise in part from a failure in extinction learning, i.e., learning that the previously conditioned stimulus no longer represents a threat ( 30 , 31 ). It is postulated that REMS disturbances, resulting from the noradrenergic hyperactivation typical of PTSD, hamper the consolidation of extinction memory, leading to a failure of the extinction memory to persist and generalize ( 32 ). So far the experimental support for this idea is limited, but nonetheless growing. Sleep disturbances following a traumatic event, including fragmented REMS, predict the development of PTSD ( 33 – 35 ). In polysomnographic studies, PTSD is characterized by reduced SWS and increased REM density ( 36 , 37 ) as well as REMS fragmentation ( 38 ). These characteristics may well result from increased noradrenergic tone during (REM) sleep in PTSD patients ( 39 , 40 ). Focusing on the role of sleep in the treatment of PTSD, a recent study found that the level of SWS and REM density positively predict treatment outcome ( 41 ). This and other clinical studies point toward an additional role of non-REMS disturbances, particularly a shortage of SWS, in the development and perpetuation of PTSD. Furthermore, shared neuromodulatory pathways may also underlie the relationship between PTSD and disturbed sleep. Especially (hyperactive) noradrenergic projections from the locus coeruleus (LC), as part of both the sleep-wake and PTSD-related circuitry, could form a final common pathway in generating the state of hyperarousal typical for both PTSD and disturbed sleep ( 32 ). Insomnia ( 42 ), nightmares ( 26 ) and most other sleep disorders discussed in this perspective are characterized by hyperarousal, frequent disruptions in REMS and aberrant LC-firing. In case of OSA, trauma-related hyperarousal may promote sleep disordered breathing ( 43 ). Vice versa, untreated OSA may contribute to development of PTSD, being a continuous stressor leading to sympathetic overactivity and disruption of sleep ( 44 ). As OSA events often occur during REMS, it is the brain's capacity to process negative emotions during REMS that is most likely affected.
Assessment of Sleep Disturbances in PTSD
Sleep disturbances can be screened and assessed with a clinical interview and objectified with other measures such as actigraphy and PSG. An actigraph and/or smartwatch can be helpful in detecting nightly arousals and limb movements, as well as daily rhythms in sleep and activity, and estimating sleep onset latency, total sleep time and sleep efficiency ( 45 ). PSG (with/without overnight video recording) provides an accurate picture of multiple physiological parameters related to sleep and wakefulness. PSG is less suitable as a screening tool, because it is an elaborate measurement which might not be readily accessible and financially feasible.
For an accurate diagnosis of PTSD according to DSM-5 criteria ( 2 ), the Clinician Administered PTSD Scale (CAPS-5) ( 46 ) can be used. It is a structured interview to diagnose current and life-time PTSD. However, the CAPS-5 is not sufficient for assessing the presence of sleep disorders, as it contains only two questions regarding sleep problems, considering nightmares and sleep disturbance in general. Diagnoses of sleep disorders are easily missed if specific diagnostic criteria are not inquired about. Therefore an accurate clinical assessment according to the International Classification of Sleep Disorders 3 (ICSD-3) ( 47 ) of sleep history, present sleep quality, sleep-wake behavior (preferably including information from the bedpartner to get a more accurate report of nightly behaviors) and screening for sleep disorders is essential.
We recommend an extensive clinical interview as there is no comprehensive questionnaire for screening diverse sleep disturbances in PTSD available. The diagnostic procedure should include an assessment of daily routines, diet, substance (ab)use, medication, mental state, presence of diseases and/or pain (or other physical limitations that compromise sleep), activity levels during night and day, and sleep behaviors including fear of sleep ( 10 ) [see ( 48 ) for a comprehensive review of the assessment and treatment guidelines of insomnia].
In PTSD the following events should be evaluated. (1) Presence of trauma-related triggers associated with sleep, the bedroom, nighttime and/or darkness, as these triggers might maintain a high arousal level, thereby hampering sleep onset and sleep maintenance. (2) Evaluation of circadian rhythm sleep-wake disorders in (uniformed) personnel working irregular hours (military personnel, police officers, fire-fighters, first responders). (3) Presence of parasomnias and distinguishing the different parasomnias, which is important for psychoeducation as well as treatment indication. For the detection of nightmares, which occur primarily during REMS, screening questionnaires such as the Nightmare Disorder Index (NDI) might be useful ( 49 ). However, both patients with PTSD and clinicians tend to misinterpret all nightly behaviors/experiences as nightmares. As the NDI does not cover other parasomnias, the clinician should always ask further about the experiences. Non-REMS parasomnias, such as confusional arousals, night terrors and sleepwalking, are often misdiagnosed as nightmares. Experiences during non-REMS parasomnias are generally not remembered well. The associated emotional distress can therefore be different from nightmares that are typically remembered vividly. It is important to ask patients to describe their nightmares in detail: What is the story in the dream? Is this trauma-related or more symbolic? What is the emotional intensity? Other parasomnias, such as sleep paralysis with or without hypnagogic and/or hypnopompic hallucinations, can be distressing, but they are not the same as nightmares. (4) Patient and bedpartner need to be asked about snoring, breathing stops, arousals and other symptoms to screen for OSA. One should take into account that the usually reported excessive daytime sleepiness is often not experienced by PTSD patients with OSA, possibly due to hyperarousal, yielding low scores on a screening questionnaire such as the Epworth Sleepiness Scale ( 50 ). An overnight audio-recording can be a useful tool to screen for sleep-related breathing problems. However, a PSG is the most objective measurement to assess OSA and its severity ( 51 ). (5) Patients and bedpartner can be asked about movements during sleep, and if present these movements can be objectified and interpreted with video-assisted PSG.
NON-Pharmacological Treatment of Sleep Disturbances in PTSD
With or without PTSD, non-pharmacological interventions are first choice in the treatment of insomnia, nightmares and other (non-REMS) parasomnias ( 48 , 52 – 54 ). In line with this, a recent meta-analysis on studies in PTSD patients found that PTSD symptoms and sleep both improve across all PTSD and sleep treatments. Yet, sleep improved the most after sleep-focused interventions, especially psychotherapy approaches ( 55 ).
For insomnia CBT-I has shown the most evidence of efficacy ( 56 ). CBT-I consists of several therapeutic components targeting different aspects of the sleep disorder: psychoeducation about sleep and sleep hygiene, relaxation training, behavioral interventions such as stimulus control (focus on re-connection of bed/bedroom with sleep) and sleep restriction (focus on reduction of time in bed to total sleep time), and cognitive therapy ( 48 ). Drawn from clinical experience and the cognitive behavioral model of PTSD, the following interventions within CBT-I require specific attention in PTSD: relaxation training because of hyperarousal ( 57 ); treatment of trauma-related triggers associated with sleep, the bed and/or bedroom, with exposure in vivo , EMDR and/or cognitive therapy. Furthermore, other interventions promoting the feeling of safety, such as a photograph of a loved one next to the bed, sleeping with a dim light, soothing music or white noise can be helpful. An increasing number of studies in patients with both PTSD and insomnia show positive effects of CBT-I on sleep efficiency, time awake after sleep onset, self-reported insomnia severity and fear of sleep ( 58 ). Another practice based intervention is the use of weighted blankets, some patients benefit from it. It is a simple non-invasive intervention and a first trial shows promising results ( 59 ). However, the presence of OSA is a contra-indication.
If nightmares are particularly prominent and perpetuate fear of sleep and insomnia, one can decide to treat nightmares before starting trauma-focused therapy. Most evidence is found for imagery rehearsal therapy, a technique for rescripting the nightmare story toward a better ending ( 60 ). The new dream is subsequently rehearsed through imagination. Imaginal exposure to the nightmare story is another effective, however, less studied intervention ( 53 ). There are no studies on EMDR for nightmares, even though it can be argued that desensitization of the nightmare image might be helpful.
Night Terrors or Arousals
If the patient has night terror-induced arousals, the bedpartner can sooth the patient with a soft and low voice, directing him/her back to bed and to sleep. Do not force awakening, ensure safety and trust that the patient will have no recollection of the event. If the arousals occur often and generally at the same time of the night it can be helpful to awaken the patient 15–30 mins before the expected arousal to prevent its occurrence ( 61 ).
Obstructive Sleep Apnea
Continuous positive airway pressure (CPAP) and mandibular repositioning appliance (MRA) can be used, and show most evidence in the treatment of OSA syndrome. CPAP has been shown to successfully reduce PTSD symptoms, including nightmare frequency, possibly by stabilizing the arousal system ( 43 ). In veterans with subclinical PTSD, non-compliance to CPAP therapy leads to increased PTSD symptoms, implying that optimal OSA-treatment prevents progression to clinical PTSD ( 44 ). If OSA-treatment adherence, e.g., wearing a CPAP-mask or MRA, is complicated by trauma-related anxiety, this needs to be specifically addressed, for example with cognitive therapy or EMDR. Other treatment options may be considered, such as weight reduction.
Timing of Sleep Interventions
There is no guideline available for the timing of sleep-targeted interventions in PTSD in relation to trauma-focused therapy. Because of the reciprocal relation between PTSD and sleep disturbances one can argue that the sequence of interventions should be determined by the most prominent symptoms. Moreover, regarding the heterogeneity of PTSD symptoms, it is unlikely that a “one size fits all” treatment will be found. Therefore, we recommend focusing the treatment on the most distressing symptoms and/or administer two different treatments, e.g., EMDR for PTSD and CBT-I for sleep disturbances, side by side. Through monitoring the treatment process, the treatment plan can be adjusted when necessary.
Pharmacological Treatment of Sleep Disturbances in PTSD
Several types of drugs have been specifically evaluated in PTSD-related sleep disorders ( 51 ). Alpha1-receptor antagonists such as prazosin are best supported by evidence, showing improvement in nightmares as well as insomnia ( 62 , 63 ). Both sedating antipsychotics and antidepressants have been found beneficial in the treatment of PTSD, including specific positive effects on sleep quality and nightmares, but need close monitoring of negative effects such as hang-over, metabolic dysregulation, and induction/elevation of restless legs syndrome (RLS), PLMD and nightmares ( 64 ). The use of benzodiazepine-receptor agonists is controversial in patients with PTSD, not just because of generally known adverse effects, but specific negative outcomes such as worse therapy outcomes and increased risk of developing PTSD when used directly following trauma ( 65 ). Considering current evidence, pharmacological treatment of insomnia and nightmares in PTSD should be regarded as temporary and additional, rather than alternative, to psychological interventions.
Conclusions and Perspectives
Research convincingly demonstrates that PTSD is frequently associated with multiple and diverse sleep disorders that impact both PTSD development, maintenance and recovery. Thus, an early and comprehensive assessment of comorbid sleep disorders as well as their timely treatment is of high clinical relevance for patients with trauma and PTSD. In our opinion, centers providing (mental) health care to patients with PTSD should, therefore, include at least one clinician trained in sleep medicine and establish close collaboration with a sleep center for accurate assessment and (interdisciplinary) treatment of co-occurring sleep disorders.
Yet, there are clear gaps in the knowledge on the links between PTSD and sleep and to optimize PTSD-outcome further research and innovations are warranted. For both research and clinical practice, it would be helpful to develop a screening instrument to more accurately assess all sleep disturbances and contributing factors relevant in PTSD populations, ultimately leading to a guideline for the assessment of sleep disorders in PTSD. Prospective studies of large, naturalistic cohorts suffering from trauma implementing both subjective and objective sleep measures, would be highly informative for instance with respect to delineating the sleep-related protective as well as risk factors in the development of PTSD. Furthermore, evidence on the efficacy of integrated PTSD and sleep treatment is limited to small samples, specific patient groups (veterans) and only a few sleep disorders (insomnia and nightmares) and interventions. Research needs to be expanded to include larger and more diverse groups of traumatized/PTSD patients (to entangle general and population-specific factors) and diverse, both pharmacological and non-pharmacological, treatment strategies for all relevant sleep disorders. Moreover, novel developments in the neuroscience of sleep may also guide PTSD treatment. Combining for instance trauma-focused treatment with new EEG-based techniques to deepen and lengthen SWS ( 66 , 67 ) could have a synergistic effect through enhanced consolidation of the traumatic memories altered in therapy. Due to faster and more complex oscillatory dynamics, such sleep-based interventions are harder to perform during REMS. Alternatively, novel behavioral methods to strengthen memories during sleep (known as targeted memory reactivation, TMR) ( 68 , 69 ) could in theory be used in PTSD during post-treatment sleep to augment treatment outcome ( 70 ).
Data Availability Statement
Author contributions.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Go to: 3. PREVALENCE. Sleep terrors typically occur in children between 4 and 12 years of age, with a peak between 5 and 7 years of age [ 20 - 22 ]. It is estimated that sleep terrors occur in 1 to 6.5% of children 1 to 12 years of age, although a prevalence of 14% or higher has also been reported [ 5, 17, 21 - 28 ].
Abstract. Night terrors, also known as sleep terrors, are an early childhood parasomnia characterized by screams or cries, behavioral manifestations of extreme fear, difficulty waking and inconsolability upon awakening. The mechanism causing night terrors is unknown, and a consistently successful treatment has yet to be documented.
What Causes Night Terrors? Night terrors are thought to occur when someone partially wakes up, but remains in a mixed state of consciousness between sleep and wakefulness. Trusted Source UpToDate More than 2 million healthcare providers around the world choose UpToDate to help make appropriate care decisions and drive better health outcomes. UpToDate delivers evidence-based clinical decision ...
Sleep terrors typically occur in children between 4 and 12 years of age, with a peak between 5 and 7 years of age. The exact etiology is not known. Developmental, environmental, organic, psychological, and genetic factors have been identified as a potential cause of sleep terrors. Sleep terrors tend to occur within the first three hours of the ...
Night terrors can cause severe distress, followed by a state of panic and a sensation of helplessness. Most episodes last 45-90 minutes and are most common as the individual passes through stages 3 and 4 non-rapid eye movement sleep. Night terrors are most common in between ages 4 until puberty. Go to:
Results It is estimated that sleep terrors occur in 1 to 6.5% of children 1 to 12 years of age. Sleep terrors typically occur in children between 4 and 12 years of age, with a peak between 5 and 7 ...
BRIEF SUMMARY. Current Knowledge/Study Rationale: While the prevalence of sleep terrors peaks in toddlerhood, most studies investigating the association between sleep terrors and emotional-behavioral problems focus on school-aged children, adolescents, and adults. Moreover, longitudinal studies are lacking. Study Impact: This longitudinal study shows that sleep terrors in toddlerhood are ...
Children typically outgrow sleep terrors. Treatment may be needed if the sleep terrors cause a safety risk, interfere with sleep, don't go away with time or happen more often. Being embarrassed or disrupting the sleep of others may lead some people to seek treatment. Treatment generally focuses on plans for safety and getting rid of causes or ...
These can be distinguished from sleep terrors, which are a parasomnia involving severe autonomic arousal and fearful behavior during NREM ... Research on sleep in anxiety-related disorders, ...
Sleep terrors, categorized under disorders of arousal, more prevalent in pediatric population, generally are self-limited but sometimes can persist or occur in adulthood. These are primed by factors enhancing homeostatic drive on backdrop of developmental predisposition and are precipitated by factors increasing sleep fragmentation resulting in dissociated state of sleep with some cerebral ...
During a sleep terror, a person may: Start by screaming, shouting or crying. Sit up in bed and look scared. Stare wide-eyed. Sweat, breathe heavily, and have a racing pulse, flushed face and enlarged pupils. Kick and thrash. Be hard to wake up and be confused if awakened.
Night terrors often cause children to kick, scream, and thrash about, but, because night terrors do not occur during REM sleep, most children do not remember them. "Night terrors are a phenomenon of the deepest parts of non-REM sleep, when the brain is less active," says Barrett. "In a night terror, a child awakens with heart pounding.
Sleep terrors (STs) are sleep disorders characterized by abrupt arousal from sleep with autonomic hyperactivity and inappropriate behavior. Though a common condition in childhood that usually affects children between 4 and 12 years of age, STs, however, may be present even in adulthood. The exact etiology of STs is not known yet, however ...
"Sleep terrors and sleepwalking … are closely related … On the basis of their many similarities, these two conditions have been considered recently to be part of the same nosologic continuum." ... Patrick Simon Moffett Levy is a Research Associate at the University of Dundee. He has taught philosophy at both Dundee and the University of ...
INTRODUCTION. Night terrors are an early childhood parasomnia associated with disturbance from non-REM, slow-wave sleep [].According to the American Academy of Sleep Medicine's (AASM) International Classification of Sleep Disorders, night terrors (also known as sleep terrors) are defined as 'a cry or piercing scream, accompanied by autonomic nervous system and behavioral manifestations of ...
Night terrors, or sleep terrors, are common terms for episodes that cause fear at night, especially in children. They are different from nightmares and may stem from many factors like stress, a ...
Recent sleep research on enuresis nocturna, sleep walking, sleep terrors and confusional arousals: a review of dissociative awakening disorders in slow wave sleep. ... some missing data were allowed to avoid too much attrition. For sleep terrors, the data at age 1 1 / 2 years were required (peak of prevalence) and 5 of the other 10 yearly data ...
Research has shown that a predisposition to night terrors may be hereditary. Emotional stress during the day, fatigue or an irregular routine are thought to trigger episodes. Ensuring a child has the proper amount of sleep, as well as addressing any daytime stresses, will help reduce terrors.
A systematic study on young adults with a diagnosis of sleep terrors showed a 58% average recall rate of mental imagery upon awakening after episodes, although recall was often fragmentary and consisting of a single scene or thought.15 Later, occasional reports confirmed the presence of some kind of activity including perceptual, cognitive, and ...
OBJECTIVE. There is growing evidence that genetic factors are involved in the occurrence of sleep terrors. Twin studies provide invaluable information regarding genetic and environmental factors that can affect the manifestation of the disease; however, most previous twin studies on sleep terrors were performed retrospectively or with a sample that was too small to yield conclusive results ...
Night Terrors in Children . Sleep terrors are not common, but they are not rare either. They are more common among young children under age 5 than any other age group. One research article published in 2022 estimated the frequency of sleep terrors during early childhood to be between 16.7% and 20.5%.
Included studies had to 1) use healthy samples without psychiatric, sleep or medical disorders that impact sleep/emotions, 2) experimentally manipulate nighttime sleep deprivation, restriction or fragmentation, 3) have an adequate control/baseline condition, 4) assess an emotion-related dependent variable after the sleep manipulation.
Sleep terrors can be upsetting to watch but usually aren't a cause for concern. Learn how to recognize and cope with sleep terrors. ... Although research on this method is limited, it may be worth ...
At a glance. The amount of sleep you need changes as you age. Several national surveys are used to study insufficient sleep (or short sleep duration) in U.S. children, high school students, and adults. These surveys show that not getting enough sleep has varied by age, location, racial/ethnic group, and over time in the United States.
A new study in mice finds that awake time cleans the brain more than when asleep or under anesthesia. The study observed that 30% less fluorescent dye — standing in for toxins and metabolites ...
PTSD and Sleep Disturbances. The majority of patients with PTSD, about 50-70%, suffer from recurrent distressing nightmares (see Table 1 for an overview of the most frequently occurring sleep disorders in PTSD, their characteristics, ways of assessment and treatment). These can be exact replications or more symbolic representations of traumatic experiences, and primarily occur during rapid ...
Many undecided voters, stunned by the EU's high-handedness, broke for Leave. Brussels had, in the most spectacular way, vindicated the central Eurosceptic contention, namely that being a member ...