Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
- Writing Strong Research Questions | Criteria & Examples

Writing Strong Research Questions | Criteria & Examples
Published on October 26, 2022 by Shona McCombes . Revised on November 21, 2023.
A research question pinpoints exactly what you want to find out in your work. A good research question is essential to guide your research paper , dissertation , or thesis .
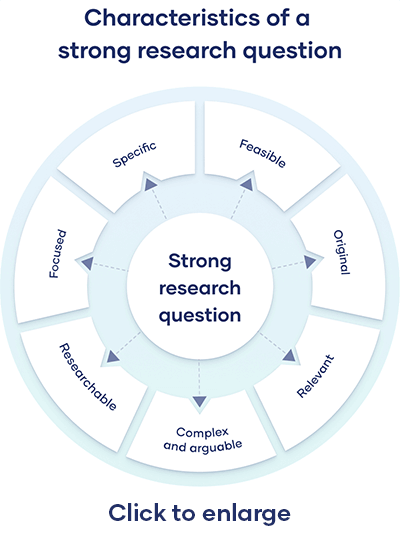
All research questions should be:
- Focused on a single problem or issue
- Researchable using primary and/or secondary sources
- Feasible to answer within the timeframe and practical constraints
- Specific enough to answer thoroughly
- Complex enough to develop the answer over the space of a paper or thesis
- Relevant to your field of study and/or society more broadly
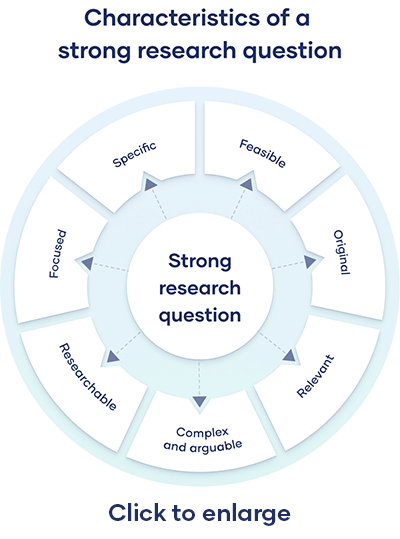
Table of contents
How to write a research question, what makes a strong research question, using sub-questions to strengthen your main research question, research questions quiz, other interesting articles, frequently asked questions about research questions.
You can follow these steps to develop a strong research question:
- Choose your topic
- Do some preliminary reading about the current state of the field
- Narrow your focus to a specific niche
- Identify the research problem that you will address
The way you frame your question depends on what your research aims to achieve. The table below shows some examples of how you might formulate questions for different purposes.
| Research question formulations | |
|---|---|
| Describing and exploring | |
| Explaining and testing | |
| Evaluating and acting | is X |
Using your research problem to develop your research question
| Example research problem | Example research question(s) |
|---|---|
| Teachers at the school do not have the skills to recognize or properly guide gifted children in the classroom. | What practical techniques can teachers use to better identify and guide gifted children? |
| Young people increasingly engage in the “gig economy,” rather than traditional full-time employment. However, it is unclear why they choose to do so. | What are the main factors influencing young people’s decisions to engage in the gig economy? |
Note that while most research questions can be answered with various types of research , the way you frame your question should help determine your choices.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Research questions anchor your whole project, so it’s important to spend some time refining them. The criteria below can help you evaluate the strength of your research question.
Focused and researchable
| Criteria | Explanation |
|---|---|
| Focused on a single topic | Your central research question should work together with your research problem to keep your work focused. If you have multiple questions, they should all clearly tie back to your central aim. |
| Answerable using | Your question must be answerable using and/or , or by reading scholarly sources on the to develop your argument. If such data is impossible to access, you likely need to rethink your question. |
| Not based on value judgements | Avoid subjective words like , , and . These do not give clear criteria for answering the question. |
Feasible and specific
| Criteria | Explanation |
|---|---|
| Answerable within practical constraints | Make sure you have enough time and resources to do all research required to answer your question. If it seems you will not be able to gain access to the data you need, consider narrowing down your question to be more specific. |
| Uses specific, well-defined concepts | All the terms you use in the research question should have clear meanings. Avoid vague language, jargon, and too-broad ideas. |
| Does not demand a conclusive solution, policy, or course of action | Research is about informing, not instructing. Even if your project is focused on a practical problem, it should aim to improve understanding rather than demand a ready-made solution. If ready-made solutions are necessary, consider conducting instead. Action research is a research method that aims to simultaneously investigate an issue as it is solved. In other words, as its name suggests, action research conducts research and takes action at the same time. |
Complex and arguable
| Criteria | Explanation |
|---|---|
| Cannot be answered with or | Closed-ended, / questions are too simple to work as good research questions—they don’t provide enough for robust investigation and discussion. |
| Cannot be answered with easily-found facts | If you can answer the question through a single Google search, book, or article, it is probably not complex enough. A good research question requires original data, synthesis of multiple sources, and original interpretation and argumentation prior to providing an answer. |
Relevant and original
| Criteria | Explanation |
|---|---|
| Addresses a relevant problem | Your research question should be developed based on initial reading around your . It should focus on addressing a problem or gap in the existing knowledge in your field or discipline. |
| Contributes to a timely social or academic debate | The question should aim to contribute to an existing and current debate in your field or in society at large. It should produce knowledge that future researchers or practitioners can later build on. |
| Has not already been answered | You don’t have to ask something that nobody has ever thought of before, but your question should have some aspect of originality. For example, you can focus on a specific location, or explore a new angle. |
Chances are that your main research question likely can’t be answered all at once. That’s why sub-questions are important: they allow you to answer your main question in a step-by-step manner.
Good sub-questions should be:
- Less complex than the main question
- Focused only on 1 type of research
- Presented in a logical order
Here are a few examples of descriptive and framing questions:
- Descriptive: According to current government arguments, how should a European bank tax be implemented?
- Descriptive: Which countries have a bank tax/levy on financial transactions?
- Framing: How should a bank tax/levy on financial transactions look at a European level?
Keep in mind that sub-questions are by no means mandatory. They should only be asked if you need the findings to answer your main question. If your main question is simple enough to stand on its own, it’s okay to skip the sub-question part. As a rule of thumb, the more complex your subject, the more sub-questions you’ll need.
Try to limit yourself to 4 or 5 sub-questions, maximum. If you feel you need more than this, it may be indication that your main research question is not sufficiently specific. In this case, it’s is better to revisit your problem statement and try to tighten your main question up.
Prevent plagiarism. Run a free check.
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
The way you present your research problem in your introduction varies depending on the nature of your research paper . A research paper that presents a sustained argument will usually encapsulate this argument in a thesis statement .
A research paper designed to present the results of empirical research tends to present a research question that it seeks to answer. It may also include a hypothesis —a prediction that will be confirmed or disproved by your research.
As you cannot possibly read every source related to your topic, it’s important to evaluate sources to assess their relevance. Use preliminary evaluation to determine whether a source is worth examining in more depth.
This involves:
- Reading abstracts , prefaces, introductions , and conclusions
- Looking at the table of contents to determine the scope of the work
- Consulting the index for key terms or the names of important scholars
A research hypothesis is your proposed answer to your research question. The research hypothesis usually includes an explanation (“ x affects y because …”).
A statistical hypothesis, on the other hand, is a mathematical statement about a population parameter. Statistical hypotheses always come in pairs: the null and alternative hypotheses . In a well-designed study , the statistical hypotheses correspond logically to the research hypothesis.
Formulating a main research question can be a difficult task. Overall, your question should contribute to solving the problem that you have defined in your problem statement .
However, it should also fulfill criteria in three main areas:
- Researchability
- Feasibility and specificity
- Relevance and originality
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 21). Writing Strong Research Questions | Criteria & Examples. Scribbr. Retrieved July 1, 2024, from https://www.scribbr.com/research-process/research-questions/
Is this article helpful?
Shona McCombes
Other students also liked, how to define a research problem | ideas & examples, how to write a problem statement | guide & examples, 10 research question examples to guide your research project, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
Module 1: Research and the Writing Process
Steps in developing a research proposal, learning objectives.
- Identify the steps in developing a research proposal.
- Choose a topic and formulate a research question and working thesis.
- Develop a research proposal.
Writing a good research paper takes time, thought, and effort. Although this assignment is challenging, it is manageable. Focusing on one step at a time will help you develop a thoughtful, informative, well-supported research paper.
Your first step is to choose a topic and then to develop research questions, a working thesis, and a written research proposal. Set aside adequate time for this part of the process. Fully exploring ideas will help you build a solid foundation for your paper.
Choosing a Topic
When you choose a topic for a research paper, you are making a major commitment. Your choice will help determine whether you enjoy the lengthy process of research and writing—and whether your final paper fulfills the assignment requirements. If you choose your topic hastily, you may later find it difficult to work with your topic. By taking your time and choosing carefully, you can ensure that this assignment is not only challenging but also rewarding.
Writers understand the importance of choosing a topic that fulfills the assignment requirements and fits the assignment’s purpose and audience. (For more information about purpose and audience, see Chapter 6 “Writing Paragraphs: Separating Ideas and Shaping Content”.) Choosing a topic that interests you is also crucial. You instructor may provide a list of suggested topics or ask that you develop a topic on your own. In either case, try to identify topics that genuinely interest you.
After identifying potential topic ideas, you will need to evaluate your ideas and choose one topic to pursue. Will you be able to find enough information about the topic? Can you develop a paper about this topic that presents and supports your original ideas? Is the topic too broad or too narrow for the scope of the assignment? If so, can you modify it so it is more manageable? You will ask these questions during this preliminary phase of the research process.
Identifying Potential Topics
Sometimes, your instructor may provide a list of suggested topics. If so, you may benefit from identifying several possibilities before committing to one idea. It is important to know how to narrow down your ideas into a concise, manageable thesis. You may also use the list as a starting point to help you identify additional, related topics. Discussing your ideas with your instructor will help ensure that you choose a manageable topic that fits the requirements of the assignment.
In this chapter, you will follow a writer named Jorge, who is studying health care administration, as he prepares a research paper. You will also plan, research, and draft your own research paper.
Jorge was assigned to write a research paper on health and the media for an introductory course in health care. Although a general topic was selected for the students, Jorge had to decide which specific issues interested him. He brainstormed a list of possibilities.
If you are writing a research paper for a specialized course, look back through your notes and course activities. Identify reading assignments and class discussions that especially engaged you. Doing so can help you identify topics to pursue.

Set a timer for five minutes. Use brainstorming or idea mapping to create a list of topics you would be interested in researching for a paper about the influence of the Internet on social networking. Do you closely follow the media coverage of a particular website, such as Twitter? Would you like to learn more about a certain industry, such as online dating? Which social networking sites do you and your friends use? List as many ideas related to this topic as you can.
Narrowing Your Topic
Once you have a list of potential topics, you will need to choose one as the focus of your essay. You will also need to narrow your topic. Most writers find that the topics they listed during brainstorming or idea mapping are broad—too broad for the scope of the assignment. Working with an overly broad topic, such as sexual education programs or popularized diets, can be frustrating and overwhelming. Each topic has so many facets that it would be impossible to cover them all in a college research paper. However, more specific choices, such as the pros and cons of sexual education in kids’ television programs or the physical effects of the South Beach diet, are specific enough to write about without being too narrow to sustain an entire research paper.
A good research paper provides focused, in-depth information and analysis. If your topic is too broad, you will find it difficult to do more than skim the surface when you research it and write about it. Narrowing your focus is essential to making your topic manageable. To narrow your focus, explore your topic in writing, conduct preliminary research, and discuss both the topic and the research with others.
Exploring Your Topic in Writing
“How am I supposed to narrow my topic when I haven’t even begun researching yet?” In fact, you may already know more than you realize. Review your list and identify your top two or three topics. Set aside some time to explore each one through freewriting. (For more information about freewriting, see Chapter 8 “The Writing Process: How Do I Begin?”.) Simply taking the time to focus on your topic may yield fresh angles.
Jorge knew that he was especially interested in the topic of diet fads, but he also knew that it was much too broad for his assignment. He used freewriting to explore his thoughts so he could narrow his topic. Read Jorge’s ideas.

Conducting Preliminary Research
Another way writers may focus a topic is to conduct preliminary research . Like freewriting, exploratory reading can help you identify interesting angles. Surfing the web and browsing through newspaper and magazine articles are good ways to start. Find out what people are saying about your topic on blogs and online discussion groups. Discussing your topic with others can also inspire you. Talk about your ideas with your classmates, your friends, or your instructor.
Jorge’s freewriting exercise helped him realize that the assigned topic of health and the media intersected with a few of his interests—diet, nutrition, and obesity. Preliminary online research and discussions with his classmates strengthened his impression that many people are confused or misled by media coverage of these subjects.
Jorge decided to focus his paper on a topic that had garnered a great deal of media attention—low-carbohydrate diets. He wanted to find out whether low-carbohydrate diets were as effective as their proponents claimed.
Writing at Work
At work, you may need to research a topic quickly to find general information. This information can be useful in understanding trends in a given industry or generating competition. For example, a company may research a competitor’s prices and use the information when pricing their own product. You may find it useful to skim a variety of reliable sources and take notes on your findings.
The reliability of online sources varies greatly. In this exploratory phase of your research, you do not need to evaluate sources as closely as you will later. However, use common sense as you refine your paper topic. If you read a fascinating blog comment that gives you a new idea for your paper, be sure to check out other, more reliable sources as well to make sure the idea is worth pursuing.
Review the list of topics you created in Note 11.18 “Exercise 1” and identify two or three topics you would like to explore further. For each of these topics, spend five to ten minutes writing about the topic without stopping. Then review your writing to identify possible areas of focus.
Set aside time to conduct preliminary research about your potential topics. Then choose a topic to pursue for your research paper.
Collaboration
Please share your topic list with a classmate. Select one or two topics on his or her list that you would like to learn more about and return it to him or her. Discuss why you found the topics interesting, and learn which of your topics your classmate selected and why.
A Plan for Research
Your freewriting and preliminary research have helped you choose a focused, manageable topic for your research paper. To work with your topic successfully, you will need to determine what exactly you want to learn about it—and later, what you want to say about it. Before you begin conducting in-depth research, you will further define your focus by developing a research question , a working thesis, and a research proposal.
Formulating a Research Question
In forming a research question, you are setting a goal for your research. Your main research question should be substantial enough to form the guiding principle of your paper—but focused enough to guide your research. A strong research question requires you not only to find information but also to put together different pieces of information, interpret and analyze them, and figure out what you think. As you consider potential research questions, ask yourself whether they would be too hard or too easy to answer.
To determine your research question, review the freewriting you completed earlier. Skim through books, articles, and websites and list the questions you have. (You may wish to use the 5WH strategy to help you formulate questions. See Chapter 8 “The Writing Process: How Do I Begin?” for more information about 5WH questions.) Include simple, factual questions and more complex questions that would require analysis and interpretation. Determine your main question—the primary focus of your paper—and several subquestions that you will need to research to answer your main question.
Here are the research questions Jorge will use to focus his research. Notice that his main research question has no obvious, straightforward answer. Jorge will need to research his subquestions, which address narrower topics, to answer his main question.

Using the topic you selected in Note 11.24 “Exercise 2”, write your main research question and at least four to five subquestions. Check that your main research question is appropriately complex for your assignment.
Constructing a Working ThesIs
A working thesis concisely states a writer’s initial answer to the main research question. It does not merely state a fact or present a subjective opinion. Instead, it expresses a debatable idea or claim that you hope to prove through additional research. Your working thesis is called a working thesis for a reason—it is subject to change. As you learn more about your topic, you may change your thinking in light of your research findings. Let your working thesis serve as a guide to your research, but do not be afraid to modify it based on what you learn.
Jorge began his research with a strong point of view based on his preliminary writing and research. Read his working thesis statement, which presents the point he will argue. Notice how it states Jorge’s tentative answer to his research question.

One way to determine your working thesis is to consider how you would complete sentences such as I believe or My opinion is . However, keep in mind that academic writing generally does not use first-person pronouns. These statements are useful starting points, but formal research papers use an objective voice.
Write a working thesis statement that presents your preliminary answer to the research question you wrote in Note 11.27 “Exercise 3”. Check that your working thesis statement presents an idea or claim that could be supported or refuted by evidence from research.

Creating a Research Proposal
A research proposal is a brief document—no more than one typed page—that summarizes the preliminary work you have completed. Your purpose in writing it is to formalize your plan for research and present it to your instructor for feedback. In your research proposal, you will present your main research question, related subquestions, and working thesis. You will also briefly discuss the value of researching this topic and indicate how you plan to gather information.
When Jorge began drafting his research proposal, he realized that he had already created most of the pieces he needed. However, he knew he also had to explain how his research would be relevant to other future health care professionals. In addition, he wanted to form a general plan for doing the research and identifying potentially useful sources. Read Jorge’s research proposal.

Before you begin a new project at work, you may have to develop a project summary document that states the purpose of the project, explains why it would be a wise use of company resources, and briefly outlines the steps involved in completing the project. This type of document is similar to a research proposal. Both documents define and limit a project, explain its value, discuss how to proceed, and identify what resources you will use.
Writing Your Own Research Proposal
Now you may write your own research proposal, if you have not done so already. Follow the guidelines provided in this lesson.
Key Takeaways
- Developing a research proposal involves the following preliminary steps: identifying potential ideas, choosing ideas to explore further, choosing and narrowing a topic, formulating a research question, and developing a working thesis.
- A good topic for a research paper interests the writer and fulfills the requirements of the assignment.
- Defining and narrowing a topic helps writers conduct focused, in-depth research.
- Writers conduct preliminary research to identify possible topics and research questions and to develop a working thesis.
- A good research question interests readers, is neither too broad nor too narrow, and has no obvious answer.
- A good working thesis expresses a debatable idea or claim that can be supported with evidence from research.
- Writers create a research proposal to present their topic, main research question, subquestions, and working thesis to an instructor for approval or feedback.
- Successful Writing Section 11.2, Steps in Developing a Research Proposal. Authored by : Anonymous. Located at : http://2012books.lardbucket.org/books/successful-writing/s15-02-steps-in-developing-a-research.html . License : CC BY-NC-SA: Attribution-NonCommercial-ShareAlike

PHILO-notes
Free Online Learning Materials
How to Write the Background of the Study in Research (Part 1)
Background of the Study in Research: Definition and the Core Elements it Contains
Before we embark on a detailed discussion on how to write the background of the study of your proposed research or thesis, it is important to first discuss its meaning and the core elements that it should contain. This is obviously because understanding the nature of the background of the study in research and knowing exactly what to include in it allow us to have both greater control and clear direction of the writing process.
So, what really is the background of the study and what are the core elements that it should contain?
The background of the study, which usually forms the first section of the introduction to a research paper or thesis, provides the overview of the study. In other words, it is that section of the research paper or thesis that establishes the context of the study. Its main function is to explain why the proposed research is important and essential to understanding the main aspects of the study.
The background of the study, therefore, is the section of the research paper or thesis that identifies the problem or gap of the study that needs to addressed and justifies the need for conducting the study. It also articulates the main goal of the study and the thesis statement, that is, the main claim or argument of the paper.
Given this brief understanding of the background of the study, we can anticipate what readers or thesis committee members expect from it. As we can see, the background of the study should contain the following major points:
1) brief discussion on what is known about the topic under investigation; 2) An articulation of the research gap or problem that needs to be addressed; 3) What the researcher would like to do or aim to achieve in the study ( research goal); 4) The thesis statement, that is, the main argument or contention of the paper (which also serves as the reason why the researcher would want to pursue the study); 5) The major significance or contribution of the study to a particular discipline; and 6) Depending on the nature of the study, an articulation of the hypothesis of the study.
Thus, when writing the background of the study, you should plan and structure it based on the major points just mentioned. With this, you will have a clear picture of the flow of the tasks that need to be completed in writing this section of your research or thesis proposal.
Now, how do you go about writing the background of the study in your proposed research or thesis?
The next lessons will address this question.
How to Write the Opening Paragraphs of the Background of the Study?
To begin with, let us assume that you already have conducted a preliminary research on your chosen topic, that is, you already have read a lot of literature and gathered relevant information for writing the background of your study. Let us also assume that you already have identified the gap of your proposed research and have already developed the research questions and thesis statement. If you have not yet identified the gap in your proposed research, you might as well go back to our lesson on how to identify a research gap.
So, we will just put together everything that you have researched into a background of the study (assuming, again, that you already have the necessary information). But in this lesson, let’s just focus on writing the opening paragraphs.
It is important to note at this point that there are different styles of writing the background of the study. Hence, what I will be sharing with you here is not just “the” only way of writing the background of the study. As a matter of fact, there is no “one-size-fits-all” style of writing this part of the research or thesis. At the end of the day, you are free to develop your own. However, whatever style it would be, it always starts with a plan which structures the writing process into stages or steps. The steps that I will share with below are just some of the most effective ways of writing the background of the study in research.
So, let’s begin.
It is always a good idea to begin the background of your study by giving an overview of your research topic. This may include providing a definition of the key concepts of your research or highlighting the main developments of the research topic.
Let us suppose that the topic of your study is the “lived experiences of students with mathematical anxiety”.
Here, you may start the background of your study with a discussion on the meaning, nature, and dynamics of the term “mathematical anxiety”. The reason for this is too obvious: “mathematical anxiety” is a highly technical term that is specific to mathematics. Hence, this term is not readily understandable to non-specialists in this field.
So, you may write the opening paragraph of your background of the study with this:
“Mathematical anxiety refers to the individual’s unpleasant emotional mood responses when confronted with a mathematical situation.”
Since you do not invent the definition of the term “mathematical anxiety”, then you need to provide a citation to the source of the material from which you are quoting. For example, you may now say:
“Mathematical anxiety refers to the individual’s unpleasant emotional mood responses when confronted with a mathematical situation (Eliot, 2020).”
And then you may proceed with the discussion on the nature and dynamics of the term “mathematical anxiety”. You may say:
“Lou (2019) specifically identifies some of the manifestations of this type of anxiety, which include, but not limited to, depression, helplessness, nervousness and fearfulness in doing mathematical and numerical tasks.”
After explaining to your readers the meaning, nature, and dynamics (as well as some historical development if you wish to) of the term “mathematical anxiety”, you may now proceed to showing the problem or gap of the study. As you may already know, the research gap is the problem that needs to be addressed in the study. This is important because no research activity is possible without the research gap.
Let us suppose that your research problem or gap is: “Mathematical anxiety can negatively affect not just the academic achievement of the students but also their future career plans and total well-being. Also, there are no known studies that deal with the mathematical anxiety of junior high school students in New Zealand.” With this, you may say:
“If left unchecked, as Shapiro (2019) claims, this problem will expand and create a total avoidance pattern on the part of the students, which can be expressed most visibly in the form of cutting classes and habitual absenteeism. As we can see, this will negatively affect the performance of students in mathematics. In fact, the study conducted by Luttenberger and Wimmer (2018) revealed that the outcomes of mathematical anxiety do not only negatively affect the students’ performance in math-related situations but also their future career as professionals. Without a doubt, therefore, mathematical anxiety is a recurring problem for many individuals which will negatively affect the academic success and future career of the student.”
Now that you already have both explained the meaning, nature, and dynamics of the term “mathematical anxiety” and articulated the gap of your proposed research, you may now state the main goal of your study. You may say:
“Hence, it is precisely in this context that the researcher aims to determine the lived experiences of those students with mathematical anxiety. In particular, this proposed thesis aims to determine the lived experiences of the junior high school students in New Zealand and identify the factors that caused them to become disinterested in mathematics.”
Please note that you should not end the first paragraph of your background of the study with the articulation of the research goal. You also need to articulate the “thesis statement”, which usually comes after the research goal. As is well known, the thesis statement is the statement of your argument or contention in the study. It is more of a personal argument or claim of the researcher, which specifically highlights the possible contribution of the study. For example, you may say:
“The researcher argues that there is a need to determine the lived experiences of these students with mathematical anxiety because knowing and understanding the difficulties and challenges that they have encountered will put the researcher in the best position to offer some alternatives to the problem. Indeed, it is only when we have performed some kind of a ‘diagnosis’ that we can offer practicable solutions to the problem. And in the case of the junior high school students in New Zealand who are having mathematical anxiety, determining their lived experiences as well as identifying the factors that caused them to become disinterested in mathematics are the very first steps in addressing the problem.”
If we combine the bits and pieces that we have written above, we can now come up with the opening paragraphs of your background of the study, which reads:
As we can see, we can find in the first paragraph 5 essential elements that must be articulated in the background of the study, namely:
1) A brief discussion on what is known about the topic under investigation; 2) An articulation of the research gap or problem that needs to be addressed; 3) What the researcher would like to do or aim to achieve in the study (research goal); 4) The thesis statement , that is, the main argument or claim of the paper; and 5) The major significance or contribution of the study to a particular discipline. So, that’s how you write the opening paragraphs of your background of the study. The next lesson will talk about writing the body of the background of the study.
How to Write the Body of the Background of the Study?
If we liken the background of the study to a sitting cat, then the opening paragraphs that we have completed in the previous lesson would just represent the head of the cat.
This means we still have to write the body (body of the cat) and the conclusion (tail). But how do we write the body of the background of the study? What should be its content?
Truly, this is one of the most difficult challenges that fledgling scholars faced. Because they are inexperienced researchers and didn’t know what to do next, they just wrote whatever they wished to write. Fortunately, this is relatively easy if they know the technique.
One of the best ways to write the body of the background of the study is to attack it from the vantage point of the research gap. If you recall, when we articulated the research gap in the opening paragraphs, we made a bold claim there, that is, there are junior high school students in New Zealand who are experiencing mathematical anxiety. Now, you have to remember that a “statement” remains an assumption until you can provide concrete proofs to it. This is what we call the “epistemological” aspect of research. As we may already know, epistemology is a specific branch of philosophy that deals with the validity of knowledge. And to validate knowledge is to provide concrete proofs to our statements. Hence, the reason why we need to provide proofs to our claim that there are indeed junior high school students in New Zealand who are experiencing mathematical anxiety is the obvious fact that if there are none, then we cannot proceed with our study. We have no one to interview with in the first. In short, we don’t have respondents.
The body of the background of the study, therefore, should be a presentation and articulation of the proofs to our claim that indeed there are junior high school students in New Zealand who are experiencing mathematical anxiety. Please note, however, that this idea is true only if you follow the style of writing the background of the study that I introduced in this course.
So, how do we do this?
One of the best ways to do this is to look for literature on mathematical anxiety among junior high school students in New Zealand and cite them here. However, if there are not enough literature on this topic in New Zealand, then we need to conduct initial interviews with these students or make actual classroom observations and record instances of mathematical anxiety among these students. But it is always a good idea if we combine literature review with interviews and actual observations.
Assuming you already have the data, then you may now proceed with the writing of the body of your background of the study. For example, you may say:
“According to records and based on the researcher’s firsthand experience with students in some junior high schools in New Zealand, indeed, there are students who lost interest in mathematics. For one, while checking the daily attendance and monitoring of the students, it was observed that some of them are not always attending classes in mathematics but are regularly attending the rest of the required subjects.”
After this sentence, you may insert some literature that will support this position. For example, you may say:
“As a matter of fact, this phenomenon is also observed in the work of Estonanto. In his study titled ‘Impact of Math Anxiety on Academic Performance in Pre-Calculus of Senior High School’, Estonanto (2019) found out that, inter alia, students with mathematical anxiety have the tendency to intentionally prioritize other subjects and commit habitual tardiness and absences.”
Then you may proceed saying:
“With this initial knowledge in mind, the researcher conducted initial interviews with some of these students. The researcher learned that one student did not regularly attend his math subject because he believed that he is not good in math and no matter how he listens to the topic he will not learn.”
Then you may say:
“Another student also mentioned that she was influenced by her friends’ perception that mathematics is hard; hence, she avoids the subject. Indeed, these are concrete proofs that there are some junior high school students in New Zealand who have mathematical anxiety. As already hinted, “disinterest” or the loss of interest in mathematics is one of the manifestations of a mathematical anxiety.”
If we combine what we have just written above, then we can have the first two paragraphs of the body of our background of the study. It reads:
“According to records and based on the researcher’s firsthand experience with students in some junior high schools in New Zealand, indeed there are students who lost interest in mathematics. For one, while checking the daily attendance and monitoring of the students, it was observed that some of them are not always attending classes in mathematics but are regularly attending the rest of the required subjects. As a matter of fact, this phenomenon is also observed in the work of Estonanto. In his study titled ‘Impact of Math Anxiety on Academic Performance in Pre-Calculus of Senior High School’, Estonanto (2019) found out that, inter alia, students with mathematical anxiety have the tendency to intentionally prioritize other subjects and commit habitual tardiness and absences.
With this initial knowledge in mind, the researcher conducted initial interviews with some of these students. The researcher learned that one student did not regularly attend his math subject because he believed that he is not good in math and no matter how he listens to the topic he will not learn. Another student also mentioned that she was influenced by her friends’ perception that mathematics is hard; hence, she avoids the subject. Indeed, these are concrete proofs that there are some junior high school students in New Zealand who have mathematical anxiety. As already hinted, “disinterest” or the loss of interest in mathematics is one of the manifestations of a mathematical anxiety.”
And then you need validate this observation by conducting another round of interview and observation in other schools. So, you may continue writing the body of the background of the study with this:
“To validate the information gathered from the initial interviews and observations, the researcher conducted another round of interview and observation with other junior high school students in New Zealand.”
“On the one hand, the researcher found out that during mathematics time some students felt uneasy; in fact, they showed a feeling of being tensed or anxious while working with numbers and mathematical problems. Some were even afraid to seat in front, while some students at the back were secretly playing with their mobile phones. These students also show remarkable apprehension during board works like trembling hands, nervous laughter, and the like.”
Then provide some literature that will support your position. You may say:
“As Finlayson (2017) corroborates, emotional symptoms of mathematical anxiety involve feeling of helplessness, lack of confidence, and being nervous for being put on the spot. It must be noted that these occasionally extreme emotional reactions are not triggered by provocative procedures. As a matter of fact, there are no personally sensitive questions or intentional manipulations of stress. The teacher simply asked a very simple question, like identifying the parts of a circle. Certainly, this observation also conforms with the study of Ashcraft (2016) when he mentions that students with mathematical anxiety show a negative attitude towards math and hold self-perceptions about their mathematical abilities.”
And then you proceed:
“On the other hand, when the class had their other subjects, the students show a feeling of excitement. They even hurried to seat in front and attentively participating in the class discussion without hesitation and without the feeling of being tensed or anxious. For sure, this is another concrete proof that there are junior high school students in New Zealand who have mathematical anxiety.”
To further prove the point that there indeed junior high school students in New Zealand who have mathematical anxiety, you may solicit observations from other math teachers. For instance, you may say:
“The researcher further verified if the problem is also happening in other sections and whether other mathematics teachers experienced the same observation that the researcher had. This validation or verification is important in establishing credibility of the claim (Buchbinder, 2016) and ensuring reliability and validity of the assertion (Morse et al., 2002). In this regard, the researcher attempted to open up the issue of math anxiety during the Departmentalized Learning Action Cell (LAC), a group discussion of educators per quarter, with the objective of ‘Teaching Strategies to Develop Critical Thinking of the Students’. During the session, one teacher corroborates the researcher’s observation that there are indeed junior high school students in New Zealand who have mathematical anxiety. The teacher pointed out that truly there were students who showed no extra effort in mathematics class in addition to the fact that some students really avoided the subject. In addition, another math teacher expressed her frustrations about these students who have mathematical anxiety. She quipped: “How can a teacher develop the critical thinking skills or ability of the students if in the first place these students show avoidance and disinterest in the subject?’.”
Again, if we combine what we have just written above, then we can now have the remaining parts of the body of the background of the study. It reads:
So, that’s how we write the body of the background of the study in research . Of course, you may add any relevant points which you think might amplify your content. What is important at this point is that you now have a clear idea of how to write the body of the background of the study.
How to Write the Concluding Part of the Background of the Study?
Since we have already completed the body of our background of the study in the previous lesson, we may now write the concluding paragraph (the tail of the cat). This is important because one of the rules of thumb in writing is that we always put a close to what we have started.
It is important to note that the conclusion of the background of the study is just a rehashing of the research gap and main goal of the study stated in the introductory paragraph, but framed differently. The purpose of this is just to emphasize, after presenting the justifications, what the study aims to attain and why it wants to do it. The conclusion, therefore, will look just like this:
“Given the above discussion, it is evident that there are indeed junior high school students in New Zealand who are experiencing mathematical anxiety. And as we can see, mathematical anxiety can negatively affect not just the academic achievement of the students but also their future career plans and total well-being. Again, it is for this reason that the researcher attempts to determine the lived experiences of those junior high school students in New Zealand who are experiencing a mathematical anxiety.”
If we combine all that we have written from the very beginning, the entire background of the study would now read:
If we analyze the background of the study that we have just completed, we can observe that in addition to the important elements that it should contain, it has also addressed other important elements that readers or thesis committee members expect from it.
On the one hand, it provides the researcher with a clear direction in the conduct of the study. As we can see, the background of the study that we have just completed enables us to move in the right direction with a strong focus as it has set clear goals and the reasons why we want to do it. Indeed, we now exactly know what to do next and how to write the rest of the research paper or thesis.
On the other hand, most researchers start their research with scattered ideas and usually get stuck with how to proceed further. But with a well-written background of the study, just as the one above, we have decluttered and organized our thoughts. We have also become aware of what have and have not been done in our area of study, as well as what we can significantly contribute in the already existing body of knowledge in this area of study.
Please note, however, as I already mentioned previously, that the model that I have just presented is only one of the many models available in textbooks and other sources. You are, of course, free to choose your own style of writing the background of the study. You may also consult your thesis supervisor for some guidance on how to attack the writing of your background of the study.
Lastly, and as you may already know, universities around the world have their own thesis formats. Hence, you should follow your university’s rules on the format and style in writing your research or thesis. What is important is that with the lessons that you learned in this course, you can now easily write the introductory part of your thesis, such as the background of the study.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.23(2); 2008 Apr

How to prepare a Research Proposal
Health research, medical education and clinical practice form the three pillars of modern day medical practice. As one authority rightly put it: ‘Health research is not a luxury, but an essential need that no nation can afford to ignore’. Health research can and should be pursued by a broad range of people. Even if they do not conduct research themselves, they need to grasp the principles of the scientific method to understand the value and limitations of science and to be able to assess and evaluate results of research before applying them. This review paper aims to highlight the essential concepts to the students and beginning researchers and sensitize and motivate the readers to access the vast literature available on research methodologies.
Most students and beginning researchers do not fully understand what a research proposal means, nor do they understand its importance. 1 A research proposal is a detailed description of a proposed study designed to investigate a given problem. 2
A research proposal is intended to convince others that you have a worthwhile research project and that you have the competence and the work-plan to complete it. Broadly the research proposal must address the following questions regardless of your research area and the methodology you choose: What you plan to accomplish, why do you want to do it and how are you going to do it. 1 The aim of this article is to highlight the essential concepts and not to provide extensive details about this topic.
The elements of a research proposal are highlighted below:
1. Title: It should be concise and descriptive. It must be informative and catchy. An effective title not only prick’s the readers interest, but also predisposes him/her favorably towards the proposal. Often titles are stated in terms of a functional relationship, because such titles clearly indicate the independent and dependent variables. 1 The title may need to be revised after completion of writing of the protocol to reflect more closely the sense of the study. 3
2. Abstract: It is a brief summary of approximately 300 words. It should include the main research question, the rationale for the study, the hypothesis (if any) and the method. Descriptions of the method may include the design, procedures, the sample and any instruments that will be used. 1 It should stand on its own, and not refer the reader to points in the project description. 3
3. Introduction: The introduction provides the readers with the background information. Its purpose is to establish a framework for the research, so that readers can understand how it relates to other research. 4 It should answer the question of why the research needs to be done and what will be its relevance. It puts the proposal in context. 3
The introduction typically begins with a statement of the research problem in precise and clear terms. 1
The importance of the statement of the research problem 5 : The statement of the problem is the essential basis for the construction of a research proposal (research objectives, hypotheses, methodology, work plan and budget etc). It is an integral part of selecting a research topic. It will guide and put into sharper focus the research design being considered for solving the problem. It allows the investigator to describe the problem systematically, to reflect on its importance, its priority in the country and region and to point out why the proposed research on the problem should be undertaken. It also facilitates peer review of the research proposal by the funding agencies.
Then it is necessary to provide the context and set the stage for the research question in such a way as to show its necessity and importance. 1 This step is necessary for the investigators to familiarize themselves with existing knowledge about the research problem and to find out whether or not others have investigated the same or similar problems. This step is accomplished by a thorough and critical review of the literature and by personal communication with experts. 5 It helps further understanding of the problem proposed for research and may lead to refining the statement of the problem, to identify the study variables and conceptualize their relationships, and in formulation and selection of a research hypothesis. 5 It ensures that you are not "re-inventing the wheel" and demonstrates your understanding of the research problem. It gives due credit to those who have laid the groundwork for your proposed research. 1 In a proposal, the literature review is generally brief and to the point. The literature selected should be pertinent and relevant. 6
Against this background, you then present the rationale of the proposed study and clearly indicate why it is worth doing.
4. Objectives: Research objectives are the goals to be achieved by conducting the research. 5 They may be stated as ‘general’ and ‘specific’.
The general objective of the research is what is to be accomplished by the research project, for example, to determine whether or not a new vaccine should be incorporated in a public health program.
The specific objectives relate to the specific research questions the investigator wants to answer through the proposed study and may be presented as primary and secondary objectives, for example, primary: To determine the degree of protection that is attributable to the new vaccine in a study population by comparing the vaccinated and unvaccinated groups. 5 Secondary: To study the cost-effectiveness of this programme.
Young investigators are advised to resist the temptation to put too many objectives or over-ambitious objectives that cannot be adequately achieved by the implementation of the protocol. 3
5. Variables: During the planning stage, it is necessary to identify the key variables of the study and their method of measurement and unit of measurement must be clearly indicated. Four types of variables are important in research 5 :
a. Independent variables: variables that are manipulated or treated in a study in order to see what effect differences in them will have on those variables proposed as being dependent on them. The different synonyms for the term ‘independent variable’ which are used in literature are: cause, input, predisposing factor, risk factor, determinant, antecedent, characteristic and attribute.
b. Dependent variables: variables in which changes are results of the level or amount of the independent variable or variables.
Synonyms: effect, outcome, consequence, result, condition, disease.
c. Confounding or intervening variables: variables that should be studied because they may influence or ‘mix’ the effect of the independent variables. For instance, in a study of the effect of measles (independent variable) on child mortality (dependent variable), the nutritional status of the child may play an intervening (confounding) role.
d. Background variables: variables that are so often of relevance in investigations of groups or populations that they should be considered for possible inclusion in the study. For example sex, age, ethnic origin, education, marital status, social status etc.
The objective of research is usually to determine the effect of changes in one or more independent variables on one or more dependent variables. For example, a study may ask "Will alcohol intake (independent variable) have an effect on development of gastric ulcer (dependent variable)?"
Certain variables may not be easy to identify. The characteristics that define these variables must be clearly identified for the purpose of the study.
6. Questions and/ or hypotheses: If you as a researcher know enough to make prediction concerning what you are studying, then the hypothesis may be formulated. A hypothesis can be defined as a tentative prediction or explanation of the relationship between two or more variables. In other words, the hypothesis translates the problem statement into a precise, unambiguous prediction of expected outcomes. Hypotheses are not meant to be haphazard guesses, but should reflect the depth of knowledge, imagination and experience of the investigator. 5 In the process of formulating the hypotheses, all variables relevant to the study must be identified. For example: "Health education involving active participation by mothers will produce more positive changes in child feeding than health education based on lectures". Here the independent variable is types of health education and the dependent variable is changes in child feeding.
A research question poses a relationship between two or more variables but phrases the relationship as a question; a hypothesis represents a declarative statement of the relations between two or more variables. 7
For exploratory or phenomenological research, you may not have any hypothesis (please do not confuse the hypothesis with the statistical null hypothesis). 1 Questions are relevant to normative or census type research (How many of them are there? Is there a relationship between them?). Deciding whether to use questions or hypotheses depends on factors such as the purpose of the study, the nature of the design and methodology, and the audience of the research (at times even the outlook and preference of the committee members, particularly the Chair). 6
7. Methodology: The method section is very important because it tells your research Committee how you plan to tackle your research problem. The guiding principle for writing the Methods section is that it should contain sufficient information for the reader to determine whether the methodology is sound. Some even argue that a good proposal should contain sufficient details for another qualified researcher to implement the study. 1 Indicate the methodological steps you will take to answer every question or to test every hypothesis illustrated in the Questions/hypotheses section. 6 It is vital that you consult a biostatistician during the planning stage of your study, 8 to resolve the methodological issues before submitting the proposal.
This section should include:
Research design: The selection of the research strategy is the core of research design and is probably the single most important decision the investigator has to make. The choice of the strategy, whether descriptive, analytical, experimental, operational or a combination of these depend on a number of considerations, 5 but this choice must be explained in relation to the study objectives. 3
Research subjects or participants: Depending on the type of your study, the following questions should be answered 3 , 5
- - What are the criteria for inclusion or selection?
- - What are the criteria for exclusion?
- - What is the sampling procedure you will use so as to ensure representativeness and reliability of the sample and to minimize sampling errors? The key reason for being concerned with sampling is the issue of validity-both internal and external of the study results. 9
- - Will there be use of controls in your study? Controls or comparison groups are used in scientific research in order to increase the validity of the conclusions. Control groups are necessary in all analytical epidemiological studies, in experimental studies of drug trials, in research on effects of intervention programmes and disease control measures and in many other investigations. Some descriptive studies (studies of existing data, surveys) may not require control groups.
- - What are the criteria for discontinuation?
Sample size: The proposal should provide information and justification (basis on which the sample size is calculated) about sample size in the methodology section. 3 A larger sample size than needed to test the research hypothesis increases the cost and duration of the study and will be unethical if it exposes human subjects to any potential unnecessary risk without additional benefit. A smaller sample size than needed can also be unethical as it exposes human subjects to risk with no benefit to scientific knowledge. Calculation of sample size has been made easy by computer software programmes, but the principles underlying the estimation should be well understood.
Interventions: If an intervention is introduced, a description must be given of the drugs or devices (proprietary names, manufacturer, chemical composition, dose, frequency of administration) if they are already commercially available. If they are in phases of experimentation or are already commercially available but used for other indications, information must be provided on available pre-clinical investigations in animals and/or results of studies already conducted in humans (in such cases, approval of the drug regulatory agency in the country is needed before the study). 3
Ethical issues 3 : Ethical considerations apply to all types of health research. Before the proposal is submitted to the Ethics Committee for approval, two important documents mentioned below (where appropriate) must be appended to the proposal. In additions, there is another vital issue of Conflict of Interest, wherein the researchers should furnish a statement regarding the same.
The Informed consent form (informed decision-making): A consent form, where appropriate, must be developed and attached to the proposal. It should be written in the prospective subjects’ mother tongue and in simple language which can be easily understood by the subject. The use of medical terminology should be avoided as far as possible. Special care is needed when subjects are illiterate. It should explain why the study is being done and why the subject has been asked to participate. It should describe, in sequence, what will happen in the course of the study, giving enough detail for the subject to gain a clear idea of what to expect. It should clarify whether or not the study procedures offer any benefits to the subject or to others, and explain the nature, likelihood and treatment of anticipated discomfort or adverse effects, including psychological and social risks, if any. Where relevant, a comparison with risks posed by standard drugs or treatment must be included. If the risks are unknown or a comparative risk cannot be given it should be so stated. It should indicate that the subject has the right to withdraw from the study at any time without, in any way, affecting his/her further medical care. It should assure the participant of confidentiality of the findings.
Ethics checklist: The proposal must describe the measures that will be undertaken to ensure that the proposed research is carried out in accordance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical research involving Human Subjects. 10 It must answer the following questions:
- • Is the research design adequate to provide answers to the research question? It is unethical to expose subjects to research that will have no value.
- • Is the method of selection of research subjects justified? The use of vulnerable subjects as research participants needs special justification. Vulnerable subjects include those in prison, minors and persons with mental disability. In international research it is important to mention that the population in which the study is conducted will benefit from any potential outcome of the research and the research is not being conducted solely for the benefit of some other population. Justification is needed for any inducement, financial or otherwise, for the participants to be enrolled in the study.
- • Are the interventions justified, in terms of risk/benefit ratio? Risks are not limited to physical harm. Psychological and social risks must also be considered.
- • For observations made, have measures been taken to ensure confidentiality?
Research setting 5 : The research setting includes all the pertinent facets of the study, such as the population to be studied (sampling frame), the place and time of study.
Study instruments 3 , 5 : Instruments are the tools by which the data are collected. For validated questionnaires/interview schedules, reference to published work should be given and the instrument appended to the proposal. For new a questionnaire which is being designed specifically for your study the details about preparing, precoding and pretesting of questionnaire should be furnished and the document appended to the proposal. Descriptions of other methods of observations like medical examination, laboratory tests and screening procedures is necessary- for established procedures, reference of published work cited but for new or modified procedure, an adequate description is necessary with justification for the same.
Collection of data: A short description of the protocol of data collection. For example, in a study on blood pressure measurement: time of participant arrival, rest for 5p. 10 minutes, which apparatus (standard calibrated) to be used, in which room to take measurement, measurement in sitting or lying down position, how many measurements, measurement in which arm first (whether this is going to be randomized), details of cuff and its placement, who will take the measurement. This minimizes the possibility of confusion, delays and errors.
Data analysis: The description should include the design of the analysis form, plans for processing and coding the data and the choice of the statistical method to be applied to each data. What will be the procedures for accounting for missing, unused or spurious data?
Monitoring, supervision and quality control: Detailed statement about the all logistical issues to satisfy the requirements of Good Clinical Practices (GCP), protocol procedures, responsibilities of each member of the research team, training of study investigators, steps taken to assure quality control (laboratory procedures, equipment calibration etc)
Gantt chart: A Gantt chart is an overview of tasks/proposed activities and a time frame for the same. You put weeks, days or months at one side, and the tasks at the other. You draw fat lines to indicate the period the task will be performed to give a timeline for your research study (take help of tutorial on youtube). 11
Significance of the study: Indicate how your research will refine, revise or extend existing knowledge in the area under investigation. How will it benefit the concerned stakeholders? What could be the larger implications of your research study?
Dissemination of the study results: How do you propose to share the findings of your study with professional peers, practitioners, participants and the funding agency?
Budget: A proposal budget with item wise/activity wise breakdown and justification for the same. Indicate how will the study be financed.
References: The proposal should end with relevant references on the subject. For web based search include the date of access for the cited website, for example: add the sentence "accessed on June 10, 2008".
Appendixes: Include the appropriate appendixes in the proposal. For example: Interview protocols, sample of informed consent forms, cover letters sent to appropriate stakeholders, official letters for permission to conduct research. Regarding original scales or questionnaires, if the instrument is copyrighted then permission in writing to reproduce the instrument from the copyright holder or proof of purchase of the instrument must be submitted.
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- 4. The Introduction
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
The introduction leads the reader from a general subject area to a particular topic of inquiry. It establishes the scope, context, and significance of the research being conducted by summarizing current understanding and background information about the topic, stating the purpose of the work in the form of the research problem supported by a hypothesis or a set of questions, explaining briefly the methodological approach used to examine the research problem, highlighting the potential outcomes your study can reveal, and outlining the remaining structure and organization of the paper.
Key Elements of the Research Proposal. Prepared under the direction of the Superintendent and by the 2010 Curriculum Design and Writing Team. Baltimore County Public Schools.
Importance of a Good Introduction
Think of the introduction as a mental road map that must answer for the reader these four questions:
- What was I studying?
- Why was this topic important to investigate?
- What did we know about this topic before I did this study?
- How will this study advance new knowledge or new ways of understanding?
According to Reyes, there are three overarching goals of a good introduction: 1) ensure that you summarize prior studies about the topic in a manner that lays a foundation for understanding the research problem; 2) explain how your study specifically addresses gaps in the literature, insufficient consideration of the topic, or other deficiency in the literature; and, 3) note the broader theoretical, empirical, and/or policy contributions and implications of your research.
A well-written introduction is important because, quite simply, you never get a second chance to make a good first impression. The opening paragraphs of your paper will provide your readers with their initial impressions about the logic of your argument, your writing style, the overall quality of your research, and, ultimately, the validity of your findings and conclusions. A vague, disorganized, or error-filled introduction will create a negative impression, whereas, a concise, engaging, and well-written introduction will lead your readers to think highly of your analytical skills, your writing style, and your research approach. All introductions should conclude with a brief paragraph that describes the organization of the rest of the paper.
Hirano, Eliana. “Research Article Introductions in English for Specific Purposes: A Comparison between Brazilian, Portuguese, and English.” English for Specific Purposes 28 (October 2009): 240-250; Samraj, B. “Introductions in Research Articles: Variations Across Disciplines.” English for Specific Purposes 21 (2002): 1–17; Introductions. The Writing Center. University of North Carolina; “Writing Introductions.” In Good Essay Writing: A Social Sciences Guide. Peter Redman. 4th edition. (London: Sage, 2011), pp. 63-70; Reyes, Victoria. Demystifying the Journal Article. Inside Higher Education.
Structure and Writing Style
I. Structure and Approach
The introduction is the broad beginning of the paper that answers three important questions for the reader:
- What is this?
- Why should I read it?
- What do you want me to think about / consider doing / react to?
Think of the structure of the introduction as an inverted triangle of information that lays a foundation for understanding the research problem. Organize the information so as to present the more general aspects of the topic early in the introduction, then narrow your analysis to more specific topical information that provides context, finally arriving at your research problem and the rationale for studying it [often written as a series of key questions to be addressed or framed as a hypothesis or set of assumptions to be tested] and, whenever possible, a description of the potential outcomes your study can reveal.
These are general phases associated with writing an introduction: 1. Establish an area to research by:
- Highlighting the importance of the topic, and/or
- Making general statements about the topic, and/or
- Presenting an overview on current research on the subject.
2. Identify a research niche by:
- Opposing an existing assumption, and/or
- Revealing a gap in existing research, and/or
- Formulating a research question or problem, and/or
- Continuing a disciplinary tradition.
3. Place your research within the research niche by:
- Stating the intent of your study,
- Outlining the key characteristics of your study,
- Describing important results, and
- Giving a brief overview of the structure of the paper.
NOTE: It is often useful to review the introduction late in the writing process. This is appropriate because outcomes are unknown until you've completed the study. After you complete writing the body of the paper, go back and review introductory descriptions of the structure of the paper, the method of data gathering, the reporting and analysis of results, and the conclusion. Reviewing and, if necessary, rewriting the introduction ensures that it correctly matches the overall structure of your final paper.
II. Delimitations of the Study
Delimitations refer to those characteristics that limit the scope and define the conceptual boundaries of your research . This is determined by the conscious exclusionary and inclusionary decisions you make about how to investigate the research problem. In other words, not only should you tell the reader what it is you are studying and why, but you must also acknowledge why you rejected alternative approaches that could have been used to examine the topic.
Obviously, the first limiting step was the choice of research problem itself. However, implicit are other, related problems that could have been chosen but were rejected. These should be noted in the conclusion of your introduction. For example, a delimitating statement could read, "Although many factors can be understood to impact the likelihood young people will vote, this study will focus on socioeconomic factors related to the need to work full-time while in school." The point is not to document every possible delimiting factor, but to highlight why previously researched issues related to the topic were not addressed.
Examples of delimitating choices would be:
- The key aims and objectives of your study,
- The research questions that you address,
- The variables of interest [i.e., the various factors and features of the phenomenon being studied],
- The method(s) of investigation,
- The time period your study covers, and
- Any relevant alternative theoretical frameworks that could have been adopted.
Review each of these decisions. Not only do you clearly establish what you intend to accomplish in your research, but you should also include a declaration of what the study does not intend to cover. In the latter case, your exclusionary decisions should be based upon criteria understood as, "not interesting"; "not directly relevant"; “too problematic because..."; "not feasible," and the like. Make this reasoning explicit!
NOTE: Delimitations refer to the initial choices made about the broader, overall design of your study and should not be confused with documenting the limitations of your study discovered after the research has been completed.
ANOTHER NOTE: Do not view delimitating statements as admitting to an inherent failing or shortcoming in your research. They are an accepted element of academic writing intended to keep the reader focused on the research problem by explicitly defining the conceptual boundaries and scope of your study. It addresses any critical questions in the reader's mind of, "Why the hell didn't the author examine this?"
III. The Narrative Flow
Issues to keep in mind that will help the narrative flow in your introduction :
- Your introduction should clearly identify the subject area of interest . A simple strategy to follow is to use key words from your title in the first few sentences of the introduction. This will help focus the introduction on the topic at the appropriate level and ensures that you get to the subject matter quickly without losing focus, or discussing information that is too general.
- Establish context by providing a brief and balanced review of the pertinent published literature that is available on the subject. The key is to summarize for the reader what is known about the specific research problem before you did your analysis. This part of your introduction should not represent a comprehensive literature review--that comes next. It consists of a general review of the important, foundational research literature [with citations] that establishes a foundation for understanding key elements of the research problem. See the drop-down menu under this tab for " Background Information " regarding types of contexts.
- Clearly state the hypothesis that you investigated . When you are first learning to write in this format it is okay, and actually preferable, to use a past statement like, "The purpose of this study was to...." or "We investigated three possible mechanisms to explain the...."
- Why did you choose this kind of research study or design? Provide a clear statement of the rationale for your approach to the problem studied. This will usually follow your statement of purpose in the last paragraph of the introduction.
IV. Engaging the Reader
A research problem in the social sciences can come across as dry and uninteresting to anyone unfamiliar with the topic . Therefore, one of the goals of your introduction is to make readers want to read your paper. Here are several strategies you can use to grab the reader's attention:
- Open with a compelling story . Almost all research problems in the social sciences, no matter how obscure or esoteric , are really about the lives of people. Telling a story that humanizes an issue can help illuminate the significance of the problem and help the reader empathize with those affected by the condition being studied.
- Include a strong quotation or a vivid, perhaps unexpected, anecdote . During your review of the literature, make note of any quotes or anecdotes that grab your attention because they can used in your introduction to highlight the research problem in a captivating way.
- Pose a provocative or thought-provoking question . Your research problem should be framed by a set of questions to be addressed or hypotheses to be tested. However, a provocative question can be presented in the beginning of your introduction that challenges an existing assumption or compels the reader to consider an alternative viewpoint that helps establish the significance of your study.
- Describe a puzzling scenario or incongruity . This involves highlighting an interesting quandary concerning the research problem or describing contradictory findings from prior studies about a topic. Posing what is essentially an unresolved intellectual riddle about the problem can engage the reader's interest in the study.
- Cite a stirring example or case study that illustrates why the research problem is important . Draw upon the findings of others to demonstrate the significance of the problem and to describe how your study builds upon or offers alternatives ways of investigating this prior research.
NOTE: It is important that you choose only one of the suggested strategies for engaging your readers. This avoids giving an impression that your paper is more flash than substance and does not distract from the substance of your study.
Freedman, Leora and Jerry Plotnick. Introductions and Conclusions. University College Writing Centre. University of Toronto; Introduction. The Structure, Format, Content, and Style of a Journal-Style Scientific Paper. Department of Biology. Bates College; Introductions. The Writing Center. University of North Carolina; Introductions. The Writer’s Handbook. Writing Center. University of Wisconsin, Madison; Introductions, Body Paragraphs, and Conclusions for an Argument Paper. The Writing Lab and The OWL. Purdue University; “Writing Introductions.” In Good Essay Writing: A Social Sciences Guide . Peter Redman. 4th edition. (London: Sage, 2011), pp. 63-70; Resources for Writers: Introduction Strategies. Program in Writing and Humanistic Studies. Massachusetts Institute of Technology; Sharpling, Gerald. Writing an Introduction. Centre for Applied Linguistics, University of Warwick; Samraj, B. “Introductions in Research Articles: Variations Across Disciplines.” English for Specific Purposes 21 (2002): 1–17; Swales, John and Christine B. Feak. Academic Writing for Graduate Students: Essential Skills and Tasks . 2nd edition. Ann Arbor, MI: University of Michigan Press, 2004 ; Writing Your Introduction. Department of English Writing Guide. George Mason University.
Writing Tip
Avoid the "Dictionary" Introduction
Giving the dictionary definition of words related to the research problem may appear appropriate because it is important to define specific terminology that readers may be unfamiliar with. However, anyone can look a word up in the dictionary and a general dictionary is not a particularly authoritative source because it doesn't take into account the context of your topic and doesn't offer particularly detailed information. Also, placed in the context of a particular discipline, a term or concept may have a different meaning than what is found in a general dictionary. If you feel that you must seek out an authoritative definition, use a subject specific dictionary or encyclopedia [e.g., if you are a sociology student, search for dictionaries of sociology]. A good database for obtaining definitive definitions of concepts or terms is Credo Reference .
Saba, Robert. The College Research Paper. Florida International University; Introductions. The Writing Center. University of North Carolina.
Another Writing Tip
When Do I Begin?
A common question asked at the start of any paper is, "Where should I begin?" An equally important question to ask yourself is, "When do I begin?" Research problems in the social sciences rarely rest in isolation from history. Therefore, it is important to lay a foundation for understanding the historical context underpinning the research problem. However, this information should be brief and succinct and begin at a point in time that illustrates the study's overall importance. For example, a study that investigates coffee cultivation and export in West Africa as a key stimulus for local economic growth needs to describe the beginning of exporting coffee in the region and establishing why economic growth is important. You do not need to give a long historical explanation about coffee exports in Africa. If a research problem requires a substantial exploration of the historical context, do this in the literature review section. In your introduction, make note of this as part of the "roadmap" [see below] that you use to describe the organization of your paper.
Introductions. The Writing Center. University of North Carolina; “Writing Introductions.” In Good Essay Writing: A Social Sciences Guide . Peter Redman. 4th edition. (London: Sage, 2011), pp. 63-70.
Yet Another Writing Tip
Always End with a Roadmap
The final paragraph or sentences of your introduction should forecast your main arguments and conclusions and provide a brief description of the rest of the paper [the "roadmap"] that let's the reader know where you are going and what to expect. A roadmap is important because it helps the reader place the research problem within the context of their own perspectives about the topic. In addition, concluding your introduction with an explicit roadmap tells the reader that you have a clear understanding of the structural purpose of your paper. In this way, the roadmap acts as a type of promise to yourself and to your readers that you will follow a consistent and coherent approach to addressing the topic of inquiry. Refer to it often to help keep your writing focused and organized.
Cassuto, Leonard. “On the Dissertation: How to Write the Introduction.” The Chronicle of Higher Education , May 28, 2018; Radich, Michael. A Student's Guide to Writing in East Asian Studies . (Cambridge, MA: Harvard University Writing n. d.), pp. 35-37.
- << Previous: Executive Summary
- Next: The C.A.R.S. Model >>
- Last Updated: Jun 18, 2024 10:45 AM
- URL: https://libguides.usc.edu/writingguide
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
The Federal Register
The daily journal of the united states government.
- Legal Status
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
21st Century Cures Act: Establishment of Disincentives for Health Care Providers That Have Committed Information Blocking
A Rule by the Centers for Medicare & Medicaid Services on 07/01/2024
Document Details
Information about this document as published in the Federal Register .
Document Statistics
Published document.
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
Enhanced Content - Table of Contents
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
FOR FURTHER INFORMATION CONTACT:
Supplementary information:, table of contents, i. executive summary, a. purpose of regulatory action, b. summary of major provisions, c. costs and benefits, d. severability, ii. background, a. statutory basis, b. regulatory history, 1. onc cures act final rule, 2. office of inspector general (oig) civil money penalties (cmp) final rule, 3. establishment of disincentives for health care providers that have committed information blocking proposed rule, c. general comments on the disincentives proposed rule, iii. provisions of the regulation and anticipated approach to investigations and referrals, a. relevant statutory terms and provisions, 1. appropriate agency, 2. authorities under applicable federal law, 3. appropriate disincentives, b. approach to determination of information blocking and application of disincentives, 1. oig investigation and referral, a. anticipated priorities, b. coordination with other agencies, c. anticipated approach to referral, 2. general provisions for application of disincentives, 3. transparency for information blocking determinations, disincentives, and penalties, c. appropriate disincentives for health care providers, 1. background, a. impacted health care providers, b. impact of disincentives, 2. medicare promoting interoperability program for eligible hospitals and critical access hospitals (cahs), a. background, b. the medicare promoting interoperability program as an appropriate disincentive for information blocking under the phsa, c. provisions, d. notification and application of the disincentive, 3. promoting interoperability performance category of the medicare merit-based incentive payment system (mips), (1) mips overview—scoring and payment calculations, (2) mips promoting interoperability performance category, b. the mips promoting interoperability performance category requirements as an appropriate disincentive for information blocking under the phsa, (1) alignment of definitions of mips eligible clinician and health care provider under the phsa, (2) information blocking conduct undermines the goals and purpose of the mips promoting interoperability performance category, (1) groups and virtual groups, (2) reweighting policies, d. notification of the disincentive, 4. medicare shared savings program, (1) statutory authority for disincentive, (2) shared savings program regulations, b. provisions, iv. request for information, v. collection of information requirements, vi. regulatory impact statement, a. executive order 12866, b. regulatory flexibility act, c. unfunded mandates reform act, d. executive order 13132, list of subjects, 42 cfr part 414, 42 cfr part 425, 42 cfr part 495, 45 cfr part 171, 42 cfr chapter iv, part 414—payment for part b medical and other health services, part 425—medicare shared savings program, part 495—standards for the electronic health record technology incentive program, 45 cfr subtitle a, part 171—information blocking, subparts e through i [added and reserved], subpart j—disincentives for information blocking by health care providers, subpart k—transparency for information blocking determinations, disincentives, and penalties, enhanced content - submit public comment.
- This feature is not available for this document.
Enhanced Content - Read Public Comments
Enhanced content - sharing.
- Email this document to a friend
Enhanced Content - Document Print View
- Print this document
Enhanced Content - Document Tools
These tools are designed to help you understand the official document better and aid in comparing the online edition to the print edition.
These markup elements allow the user to see how the document follows the Document Drafting Handbook that agencies use to create their documents. These can be useful for better understanding how a document is structured but are not part of the published document itself.
Enhanced Content - Developer Tools
This document is available in the following developer friendly formats:.
- JSON: Normalized attributes and metadata
- XML: Original full text XML
- MODS: Government Publishing Office metadata
More information and documentation can be found in our developer tools pages .
Official Content
- View printed version (PDF)
This PDF is the current document as it appeared on Public Inspection on 06/26/2024 at 4:15 pm. It was viewed 0 times while on Public Inspection.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Centers for Medicare & Medicaid Services (CMS) and Office of the National Coordinator for Health Information Technology (ONC), Department of Health and Human Services (HHS).
Final rule.
This final rule implements the provision of the 21st Century Cures Act specifying that a health care provider determined by the HHS Inspector General to have committed information blocking shall be referred to the appropriate agency to be subject to appropriate disincentives set forth through notice and comment rulemaking. This rulemaking establishes, for certain health care providers, a set of appropriate disincentives using authorities under applicable Federal law.
This rule is effective as of July 31, 2024.
Alexander Baker, Office of Policy, Office of the National Coordinator for Health Information Technology (ONC), (202) 690-7151, for general issues.
Elizabeth Holland, Centers for Medicare & Medicaid Services (CMS), (443) 934-2532, for issues related to the Promoting Interoperability Program and the Promoting Interoperability performance category of the Merit-Based Incentive Payment System.
Aryanna Abouzari, Centers for Medicare & Medicaid Services (CMS), (415) 744-3668 or [email protected] , for issues related to the Medicare Shared Savings Program.
This final rule implements the 21st Century Cures Act (Cures Act) provision for referral of a health care provider (individual or entity), determined by the HHS Office of Inspector General (OIG) to have committed information blocking, “to the appropriate agency to be subject to appropriate disincentives using authorities under applicable Federal law, as the Secretary sets forth through notice and comment rulemaking” (section 3022(b)(2)(B) of the Public Health Service Act (PHSA) ( 42 U.S.C. 300jj-52(b)(2)(B) ), as added by section 4004 of the Cures Act ( Pub. L. 114-255 , Dec. 13, 2016)). This final rule establishes disincentives for certain health care providers (as defined in 45 CFR 171.102 ) that are also Medicare-enrolled providers or suppliers.
This final rule establishes disincentives applicable to certain health care providers (as defined in 45 CFR 171.102 ), determined by OIG to have committed information blocking (as defined in 45 CFR 171.103 ), that also are Medicare-enrolled providers or suppliers. This final rule also provides information related to OIG's investigation of claims of information blocking and referral of a health care provider to an appropriate agency to be subject to appropriate disincentives. Finally, this final rule establishes a process by which information will be shared with the public about health care providers and other actors (health IT developers or other entities offering certified health IT, health information exchanges, and health information networks) that OIG determines have committed information blocking.
Although this final rule does not establish disincentives for all of the health care providers included in the 45 CFR 171.102 definition, the health care providers to whom the disincentives finalized in this rule apply furnish a broad array of services to a significant number of both Medicare beneficiaries and other patients. Thus, this set of disincentives directly advances HHS priorities for deterring information blocking, while also advancing appropriate sharing of electronic health information (EHI) by health care providers [ 1 ] to support safer, more coordinated care for all patients.
We believe it is important to establish appropriate disincentives that account for all health care providers that fall within the definition of health care provider at 45 CFR 171.102 . While effective deterrence of information blocking can benefit patients by reducing the degree to which health care providers engage in this practice, fewer patients will benefit from these deterrent effects if disincentives have not been established for all health care providers within the definition of health care provider at 45 CFR 171.102 . In section IV of the 21st Century Cures Act: Establishment of Disincentives for Health Care Providers That Have Committed Information Blocking proposed rule (Disincentives Proposed Rule), we requested information on how we could establish disincentives for other health care providers, particularly those health care providers not implicated under the CMS authorities Start Printed Page 54663 we proposed to use to establish disincentives in the proposed rule ( 88 FR 74966 and 74967 ).
Consistent with PHSA section 3022(b)(2)(B), in section III.C. of this final rule, CMS has finalized the following disincentives using authorities under applicable Federal law, as follows:
- Under the authority for the Medicare Promoting Interoperability Program in the Social Security Act (SSA), at sections 1886(b)(3)(B)(ix) and 1886(n) for eligible hospitals, and at section 1814(l)(4) for critical access hospitals (CAHs), CMS has finalized that an eligible hospital or CAH is not a meaningful electronic health record (EHR) user in an EHR reporting period if OIG refers, during the calendar year of the reporting period, a determination that the eligible hospital or CAH committed information blocking as defined at 45 CFR 171.103 . As a result, an eligible hospital subject to this disincentive will not be able to earn the three quarters of the annual market basket increase associated with qualifying as a meaningful EHR user, and a CAH subject to this disincentive will have its payment reduced to 100 percent of reasonable costs, from the 101 percent of reasonable costs it might have otherwise earned, in an applicable year.
- Under the authority in SSA sections 1848(o)(2)(A) and (D) and 1848(q)(2)(A)(iv) and (B)(iv), for the Promoting Interoperability performance category of the Merit-based Incentive Payment System (MIPS), CMS has finalized that a health care provider defined in 45 CFR 171.102 that is a MIPS eligible clinician (as defined in 42 CFR 414.1305 and including groups) is not a meaningful EHR user in a performance period if OIG refers, during the calendar year of the reporting period, a determination that the MIPS eligible clinician committed information blocking as defined at 45 CFR 171.103 . CMS also has finalized that the determination by OIG that a MIPS eligible clinician committed information blocking will result in the MIPS eligible clinician, if required to report on the Promoting Interoperability performance category of MIPS, not earning a score in the performance category (a zero score), which is typically a quarter of the total final composite performance score (a “final score” as defined at 42 CFR 414.1305 ). CMS has codified this proposal under the definition of meaningful EHR user for MIPS at 42 CFR 414.1305 and added it to the requirements for earning a score for the MIPS Promoting Interoperability performance category at 42 CFR 414.1375(b) .
- Under the authority in SSA section 1899(b)(2)(G) for the Medicare Shared Savings Program (Shared Savings Program), CMS has finalized that a health care provider as defined in 45 CFR 171.102 that is an accountable care organization (ACO), ACO participant, or ACO provider/supplier, if determined by OIG to have committed information blocking as defined at 45 CFR 171.103 , may be barred from participating in the Shared Savings Program for at least 1 year ( 88 FR 74964 and 74965 ). In this final rule, in consideration of the comments received, CMS has finalized incorporation of an alternative policy discussed in the proposed rule, under which CMS will consider an OIG information blocking determination in light of relevant facts and circumstances before applying a disincentive under the Shared Savings Program, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken, or terminating an ACO's participation agreement with CMS. This will result in a health care provider being removed from an ACO or prevented from joining an ACO; and in the instance where a health care provider is an ACO, this will prevent the ACO's participation in the Shared Savings Program. The relevant facts and circumstances include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether the provider was previously subject to a disincentive in another program, and other factors.
Executive Order 12866 on Regulatory Planning and Review and Executive Order 13563 on Improving Regulation and Regulatory Review direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Section 3(f) of Executive Order 12866 , as amended by Executive Order 14094 , defines a “significant regulatory action” as an action that is likely to result in a rule that may: (1) have an annual effect on the economy of $200 million or more (adjusted every 3 years by the Administrator of the Office of Information and Regulatory Affairs (OIRA) for changes in gross domestic product), or adversely affect in a material way the economy, a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or State, local, territorial, or tribal governments or communities; (2) create a serious inconsistency or otherwise interfere with an action taken or planned by another agency; (3) materially alter the budgetary impact of entitlements, grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raise legal or policy issues for which centralized review would meaningfully further the President's priorities or the principles set forth in the Executive Order, as specifically authorized in a timely manner by the Administrator of OIRA in each case. The Office of Management and Budget (OMB) has determined that this final rule is not a significant regulatory action, as the potential costs associated with this final rule would not be greater than $200 million per year, and it does not meet any of the other requirements to be a significant regulatory action.
We are clarifying and emphasizing our intent that if any provision of this final rule is held to be invalid or unenforceable by its terms, or as applied to any person or circumstance, or stayed pending further action, it shall be severable from this final rule, and from rules and regulations currently in effect, and not affect the remainder thereof or the application of the provision to other persons not similarly situated or to other, dissimilar circumstances. If any provision is held to be invalid or unenforceable, the remaining provisions which could function independently, should take effect and be given the maximum effect permitted by law.
Through this rule, we adopt provisions that are intended to and will operate independently of each other, even if each serves the same general purpose or policy goal. Where a provision is necessarily dependent on another, the context generally makes that clear (such as by cross-reference to a particular standard, requirement, condition, or pre-requisite). Where a provision that is dependent on one that is stayed or held invalid or unenforceable, as described in the preceding paragraph, is included in a subparagraph, paragraph, or section within part 171 of 45 CFR or part 414, 425, or 495 of 42 CFR , we intend that Start Printed Page 54664 other provisions of such subparagraph(s), paragraph(s), or section(s) that operate independently of the provision stayed or held invalid or unenforceable would remain in effect.
The Cures Act was enacted on December 13, 2016, “[t]o accelerate the discovery, development, and delivery of 21st century cures, and for other purposes” ( Pub. L. 114-255 , December 16, 2016). Section 4004 of the Cures Act added section 3022 to the PHSA. Section 3022(a)(1) of the PHSA defines information blocking as a practice that, except as required by law or specified by the Secretary pursuant to rulemaking, is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information, and: (1) if the practice is conducted by a health information technology developer, exchange, or network, such developer, exchange, or network knows, or should know, that such practice is likely to interfere with, prevent, or materially discourage the access, exchange, or use of electronic health information; or (2) if the practice is conducted by a health care provider, such health care provider knows that such practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information. Section 3022(a)(3) of the PHSA further provides that the Secretary shall, through rulemaking, identify reasonable and necessary activities that do not constitute information blocking. Section 3022(a)(4) of the PHSA states that the term “information blocking” does not include any practice or conduct occurring prior to the date that is 30 days after December 13, 2016 (the date of the enactment of the Cures Act). [ 2 ] Section 3022(a)(2) of the PHSA describes certain practices that may constitute information blocking.
Section 3022(b)(1) of the PHSA authorizes OIG to investigate information blocking claims. Section 3022(b)(1)(B) of the PHSA authorizes OIG to investigate claims that “a health care provider engaged in information blocking.” Section 3022(b)(2)(B) of the PHSA provides that any health care provider OIG determines to have committed information blocking shall be referred to the appropriate agency to be subject to appropriate disincentives using authorities under applicable Federal law, as the Secretary sets forth through notice and comment rulemaking. Sections 3022(b)(1)(A) and (C) of the PHSA authorize OIG to investigate health information technology (IT) developers of certified health IT or other entities offering certified health IT, health information exchanges, and health information networks. Section 3022(b)(2)(A) of the PHSA authorizes the imposition of civil money penalties (CMPs) [ 3 ] not to exceed $1 million per violation on those individuals and entities set forth in sections 3022(b)(1)(A) and (C) of the PHSA.
PHSA section 3022 also authorizes ONC, the HHS Office for Civil Rights (OCR), and OIG to consult, refer, and coordinate to resolve claims of information blocking. PHSA section 3022(b)(3)(A) authorizes OIG to refer claims of information blocking to OCR if OIG determines a consultation regarding the health privacy and security rules promulgated under section 264(c) of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) ( Pub. L. 104-191 , Aug. 21, 1996) ( 42 U.S.C. 1320d-2 note ) will resolve such claims. PHSA section 3022(d)(1) specifies that the National Coordinator may serve as a technical consultant to OIG and the Federal Trade Commission (FTC) for purposes of carrying out section 3022 and may share information related to claims or investigations of information blocking with the FTC for purposes of such investigations, in addition to requiring the National Coordinator to share information with OIG, as required by law.
PHSA section 3022(d)(4) requires the Secretary, in carrying out section 3022 and to the extent possible, to ensure that information blocking penalties do not duplicate penalty structures that would otherwise apply with respect to information blocking and the type of individual or entity involved as of the day before the date of enactment of the Cures Act. Section 3022(a)(7) of the PHSA states that, in carrying out section 3022, the Secretary shall ensure that health care providers are not penalized for the failure of developers of health information technology or other entities offering health information technology to such providers to ensure that such technology meets the requirements to be certified under Title XXX of the PHSA.
We address the statutory basis for each disincentive in greater detail in section III.C. of this final rule.
On March 4, 2019, a proposed rule titled 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program (ONC Cures Act Proposed Rule) appeared in the Federal Register ( 84 FR 7424 ). The rule proposed to implement certain provisions of the Cures Act to advance interoperability and support the access, exchange, and use of electronic health information. The ONC Cures Act Proposed Rule included a request for information regarding potential disincentives for health care providers that have committed information blocking and asked whether modifying disincentives already available under existing Department programs and regulations would provide for more effective deterrence ( 84 FR 7553 ).
On May 1, 2020, a final rule titled 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program (ONC Cures Act Final Rule) appeared in the Federal Register ( 85 FR 25642 ). The final rule identified eight reasonable and necessary activities that do not constitute information blocking, consistent with the requirement in PHSA section 3022(a)(3). Such reasonable and necessary activities are often referred to as “exceptions” to the definition of information blocking, or “information blocking exceptions,” as specified in 45 CFR part 171 .
The ONC Cures Act Final Rule finalized definitions that are necessary to implement the statutory information blocking provision in PHSA section 3022, including definitions related to the four classes of individuals and entities covered by the statutory information blocking provision: health care providers, health IT developers, health IT networks, and health IT exchanges.
As the term “health care provider” is not explicitly defined in section 3022 of the PHSA, as added by section 4004 of the Cures Act, the ONC Cures Act Final Rule adopted in 45 CFR 171.102 the definition of health care provider in section 3000(3) of the PHSA [ 4 ] for Start Printed Page 54665 purposes of the information blocking regulations in 45 CFR part 171 . The definitions listed in section 3000 of the PHSA apply “[i]n this title,” which refers to Title XXX of the PHSA ( 85 FR 25795 ). Section 3022 of the PHSA is included in Title XXX.
The ONC Cures Act Final Rule also established in 45 CFR 171.102 regulatory definitions for “health information network or health information exchange” and “health IT developer of certified health IT,” [ 5 ] among other terms. [ 6 ] The preamble text of the ONC Cures Act Final Rule makes clear that an individual or entity could meet both the definition of a health care provider and the definition of a health IT developer of certified health IT ( 85 FR 25798 and 25799 ), or could meet both the definition of a health care provider and a health information exchange or network ( 85 FR 25801 ). We mention these potential scenarios so that health care providers are aware that they would not necessarily only be subject to the disincentives finalized in this rule, but depending on the specific facts and circumstances, they could meet the definition of a health information network, health information exchange, or health IT developer of certified health IT—and therefore be subject to civil money penalties, if found by OIG to have committed information blocking.
On November 4, 2020, an interim final rule with comment period titled Information Blocking and the ONC Health IT Certification Program: Extension of Compliance Dates and Timeframes in Response to the COVID-19 Public Health Emergency (ONC Cures Act Interim Final Rule) appeared in the Federal Register ( 85 FR 70064 ). The ONC Cures Act Interim Final Rule extended certain compliance dates and timeframes adopted in the ONC Cures Act Final Rule to offer the healthcare system additional flexibilities in furnishing services to combat the COVID-19 pandemic, including extending the applicability date for the information blocking provisions to April 5, 2021 ( 85 FR 70068 ). The ONC Cures Act Interim Final Rule also extended from May 2, 2022, to October 6, 2022, the date on which electronic health information as defined in 45 CFR 171.102 for purposes of the information blocking definition in 45 CFR 171.103 would no longer be limited to the subset of EHI that is identified by data elements represented in the United States Core Data for Interoperability (USCDI) standard adopted in 45 CFR 170.213 ( 85 FR 70069 ). [ 7 ] On and after October 6, 2022, practices likely to interfere with access, exchange, or use of any information falling within the definition of EHI in 45 CFR 171.102 may constitute information blocking as defined in 45 CFR 171.103 .
On April 24, 2020, a proposed rule titled Grants, Contracts, and Other Agreements: Fraud and Abuse; Information Blocking; Revisions to the Office of Inspector General's Civil Money Penalty Rules (OIG CMP Proposed Rule) appeared in the Federal Register ( 85 FR 22979 ). The OIG CMP Proposed Rule set forth proposed regulations to incorporate new CMP authority for information blocking and related procedures in PHSA section 3022 ( 85 FR 22982 ). Specific to information blocking, OIG also provided information on—but did not propose regulations for—expected enforcement priorities, the investigation process, and OIG's experience with investigating conduct that includes an intent element ( 85 FR 22984 ).
A final rule titled Grants, Contracts, and Other Agreements: Fraud and Abuse; Information Blocking; Office of Inspector General's Civil Money Penalty Rules appeared in the Federal Register on July 3, 2023 (OIG CMP Final Rule) ( 88 FR 42820 ). This rulemaking addressed imposition of CMPs for information blocking by health IT developers or other entities offering certified health IT, and health information exchanges and health information networks (HIEs/HINs). The OIG CMP Final Rule did not establish appropriate disincentives for health care providers that OIG has determined to have committed information blocking.
In the OIG CMP Final Rule, OIG stated that a health care provider that also meets the definition of a health IT developer of certified health IT, or HIE/HIN, or both, under 45 CFR 171.102 , may be subject to information blocking CMPs ( 88 FR 42829 ). OIG further stated that as part of its assessment of whether a health care provider is a HIN/HIE that could be subject to CMPs for information blocking, OIG anticipates engaging with the health care provider to better understand its functions and to offer the health care provider an opportunity to explain why it is not a HIN/HIE ( 88 FR 42828 ).
On November 1, 2023, a proposed rule titled 21st Century Cures Act: Establishment of Disincentives for Health Care Providers That Have Committed Information Blocking appeared in the Federal Register ( 88 FR 74947 ) (Disincentives Proposed Rule). The Disincentives Proposed Rule proposed to establish a set of appropriate disincentives for information blocking by health care providers using authorities under applicable Federal law, consistent with PHSA section 3022(b)(2)(B). The Disincentives Proposed Rule also proposed to define certain statutory terms and proposed to establish elements of a process for the imposition of appropriate disincentives by an appropriate agency. The Disincentives Proposed Rule further proposed to publicly post information on ONC's website about health care providers that have been determined by OIG to have committed information blocking and subsequently referred by OIG to an appropriate agency to be subject to appropriate disincentives, as well about health IT developers of certified health Start Printed Page 54666 IT and HIEs/HINs and that have been determined by OIG to have committed information blocking. Finally, the Disincentives Proposed Rule requested public comment on establishing disincentives for other health care providers included in the definition of health care provider in 45 CFR 171.102 that are subject to the information blocking regulations, but were not implicated by the disincentives proposed in the Disincentives Proposed Rule.
We received a number of general comments on the Disincentives Proposed Rule. A discussion of those comments and responses can be found below.
Comments. Several commenters expressed general support for the proposed disincentives for health care providers who have been found to have committed information blocking. A few commenters stated that the disincentives will lead to better patient outcomes, improved information sharing, increased transparency, a reduction in systemic inefficiency and waste, and improved accountability and compliance. A few commenters expressed general support for the establishment of the disincentives proposed because the disincentives are necessary and appropriate and would discourage information blocking by health care providers. Commenters also asserted that the proposed disincentives would encourage data exchange and enhance interoperability.
Response. We thank the commenters for their support.
Comments. Many commenters recommended that HHS delay implementation or enforcement of information blocking disincentives against health care providers. Commenters recommended this delay in order for HHS to provide education regarding issues such as: what constitutes information blocking; the investigation process; the application of disincentives; and information about exceptions. Commenters stated that a delay was warranted because the information blocking regulations, including the disincentives discussed in this final rule, are new and complicated, requirements change frequently, and health care providers need time to implement information sharing processes and identify best practices. Commenter recommendations for how long to delay enforcement following the publication of the final rule ranged from 1 to 2 years.
Response. We disagree with commenters that further delay in establishing disincentives for health care providers that commit information blocking is necessary. We note that the information blocking regulations in the ONC Cures Act Final Rule went into effect April 5, 2021 ( 85 FR 70068 ), and several years will have already passed between the date when these regulations went into effect for health care providers and the effective date of this final rule. In addition, the disincentives CMS has finalized in this final rule are established under authorities for existing programs with which health care providers are already familiar. Thus, we do not believe it is necessary to further delay establishment of disincentives. We refer readers to section III.C. of this final rule, in which CMS describes how each of the disincentives it has finalized will be effective upon the effective date of this final rule.
We also note that section III.B.1. of this final rule states that OIG will not begin investigating possible information blocking committed by health care providers until after the effective date of this rule, and that OIG will exercise its enforcement discretion not to make any determinations regarding conduct occurring prior to the effective date of this rule for information blocking disincentives. As OIG will not make a determination on conduct occurring prior to the effective date, OIG will not refer any health care providers based on a determination of conduct occurring prior to the effective date of this rule for information blocking disincentives. This means that no disincentives finalized in this final rule will be applied to conduct occurring before the effective date of this final rule.
We appreciate the recommendations regarding offering educational opportunities that would be helpful to health care providers and will consider these recommendations.
Comments. A few commenters requested that HHS set a maximum period from the date the alleged information blocking complaint was referred, after which HHS would not impose any disincentives, such as a 6-year period to align with the time period for imposing CMPs for other actors. Some commenters expressed concern that the proposed process for investigating claims of information blocking and referring findings to appropriate agencies for disincentives could cause a long delay between the information blocking complaint being filed and the application of a disincentive.
Response. For the disincentives finalized in section III.C. of this final rule, CMS did not propose and has not finalized a maximum period from the date the alleged information blocking complaint was referred, after which CMS would not impose the disincentives it has finalized. Because the authorities used to establish disincentives may include requirements related to timing around the imposition of disincentives, we believe it is more appropriate to allow appropriate agencies to establish any such limits instead of setting a uniform limit for any disincentive established to deter information blocking by health care providers. We note that for the disincentive policy finalized under the Shared Savings Program in section III.C.4., CMS will consider relevant facts and circumstances before imposing a disincentive under the Shared Savings Program, and CMS has stated that one of the relevant factors CMS will consider is the time elapsed since a referral of information blocking has taken place. For the disincentives finalized for the Medicare Promoting Interoperability Program and the MIPS Promoting Interoperability performance category in sections III.C.2. and III.C.3., respectively, CMS has stated that it will impose the applicable disincentive in the EHR reporting period or performance period of the calendar year that a referral of a determination of information blocking is received from OIG. We further recognize that there may be a delay between the initial submission of an information blocking claim and the eventual application of a disincentive if OIG determines that the practices identified in the claim were information blocking and refers the determination to an appropriate agency. However, we are unable to estimate the time necessary to complete investigations of these practices.
As commenters mentioned, section 3022(b)(2)(C) of the PHSA, which applies to actors subject to the information blocking regulations that are not health care providers (health IT developers or other entities offering certified health IT, and HINs/HIEs), requires the imposition of CMPs to follow the procedures set forth in section 1128A of the Social Security Act (SSA). Section 1128A(c)(1) requires that an action for CMPs must be initiated within 6 years from the date of the occurrence. In the OIG CMP Final Rule, OIG stated that this would be 6 years from the date of the violation ( 88 FR 42826 ).
Comments. A few commenters recommended the creation of a centralized HHS coordinating entity to Start Printed Page 54667 implement and oversee information blocking disincentives, provide a main point of contact for health care providers to learn about the process and resolve discrepancies, ensure coordination among agencies, and minimize confusion and potential errors that could cause burden for providers. One commenter stated that supplemental rulemaking would be needed to create this centralized HHS coordinating entity and that HHS should engage in this supplemental rulemaking before finalizing the Disincentives Proposed Rule. A few commenters specifically suggested the creation of a clearinghouse process to ensure disincentives applied are not duplicative, arbitrary, and unduly punitive.
Response. We appreciate this recommendation. While we did not propose to create an entity as recommended by the commenters, we may consider this recommendation in future rulemaking. However, we do not believe that establishing such an entity is necessary to finalize the policies in this final rule, as many of these issues are addressed through existing policies. For instance, regarding coordination between agencies, we refer readers to the discussion in section III.B.1.b. of this final rule where we discuss the ways ONC, OCR, and OIG will consult, refer, and coordinate on information blocking claims as permitted by the Cures Act (see also, 88 FR 42823 and 42824 ). We also refer readers to the information provided about OIG's investigation process in section III.B.1. of this final rule, which includes a discussion of how OIG may engage with health care providers as part of its investigation, as necessary, to understand specific facts and circumstances related to an information blocking claim. The commenters did not provide further information about how such an entity would address issues related to ensuring disincentives are not duplicative, arbitrary, and unduly punitive.
Comments. A few commenters recommended that ONC allow for `complaint clearinghouses,' where health care providers or payers can send their complaints alleging information blocking violations to an independent, private sector third party who would aggregate those complaints over time and submit them as a group to HHS to ensure complaints are unattributed to specific complainants. Commenters suggested this approach could mitigate concerns over retaliation, retribution, or harm to business relationships associated with alleging information blocking violations. A few commenters also recommended ONC, OIG, and CMS be more transparent in providing specifics to the public on how complaints will be handled to ensure interested parties have transparency in knowing the status of their complaints, and when a final decision can be expected.
Response. We thank commenters for their suggestions. As authorized under PHSA section 3022(d)(3)(A), ONC has already established a “a standardized process for the public to submit reports on claims” related to information blocking. We refer readers to the discussion of the complaint process in the ONC Cures Act Final Rule ( 85 FR 25899 and 25900 ), as well as the Information Blocking Portal on ONC's website for more information. [ 8 ] Regarding commenters' concerns around harm resulting from attribution of complaints to specific organizations, we note that PHSA section 3022(d)(2) prohibits the National Coordinator from disclosing “[a]ny information that is received by the National Coordinator in connection with a claim or suggestion of possible information blocking and that could reasonably be expected to facilitate identification of the source of the information” except as may be necessary to carry out the purpose of PHSA section 3022 (PHSA section 3022(d)(2)(A)). As stated in the ONC Cures Act Final Rule, we believe the publishing of complaints could lead to the identification of the source of the information or reasonably facilitate identification of the source; therefore, we do not intend to make complaints publicly available ( 85 FR 25900 ). While the complaint process is not required by statute to be established through rulemaking, we will take commenters' input into consideration as we continue to receive complaints related to information blocking.
Comments. Commenters stated that health care providers are still unclear about what practices are prohibited under the information blocking regulations. Commenters also recommended that supplemental rulemaking or sub-regulatory guidance be provided on certain topics prior to implementation or enforcement of health care provider information blocking disincentives, including: further describing investigative processes and the application of disincentives; the establishment of an appeals process; and describing how the disincentives implemented under this final rule interact with existing quality reporting program rules.
Response. We appreciate commenters' concerns and recognize that many health care providers are still gaining awareness and understanding of the information blocking regulations. We encourage health care providers to review the resources available on ONC's website to learn more about practices that may be information blocking. [ 9 ]
We appreciate commenters' recommendations for topics HHS should consider addressing through notice and comment rulemaking. However, we note this final rule addresses many of these issues, including: the OIG investigative process (section III.B.1.), application of disincentives (section III.B.2.), and appeals processes (section III.B.2.). The discussion of the disincentives finalized in sections III.C.2. through III.C.4. does not identify any interactions with quality reporting program rules. Quality reporting programs are entirely separate authorities from those under which we proposed appropriate disincentives (which we have finalized in section III.C. of this rule); therefore, we are unclear what commenters' concerns are with respect to information blocking disincentives and quality reporting programs.
The discussion of these issues provides additional information regarding the policies we have finalized in this rule and further notice and comment rulemaking on these topics is not necessary before finalizing these policies, due to the completeness of the policies described in this final rule.
Comments. A few commenters recommended that before implementing health care provider information blocking disincentives the agencies should work to advance EHR adoption and interoperability. Commenters recommended that HHS further define and clarify interoperability standards, and recognize that not all health care providers utilize EHRs.
Response. We do not agree that the need for further advances with respect to EHR adoption and interoperability should delay establishing the disincentives for health care providers that have been found to commit information blocking that we finalize in this rule. While we recognize that additional progress can be made to improve interoperability and advance adoption of EHRs, many health care providers are using electronic health information today and could engage in practices that are considered information blocking under PHSA section 3022. Therefore, it is important Start Printed Page 54668 that appropriate disincentives exist to deter information blocking by those health care providers that are currently using electronic health information. We note that HHS has pursued activities to advance interoperability in EHRs and other health IT systems through a variety of initiatives, including the ONC Health IT Certification Program. For more information about initiatives to advance interoperability, we refer readers to resources on ONC's website. [ 10 ]
Comments. Several commenters provided recommendations about specific scenarios that should not be considered information blocking, including: a delay in the release of sensitive and distressing health information and test results, such as for severe or complex diagnoses, to allow for provider review; a delay in the release of information in the interest of patient safety; a delay in the release of information if a patient states that they will harm themselves if they receive a diagnosis from their provider; or instances where a provider attempts in good faith to comply with an exception or not engage in information blocking.
Response. We thank commenters for their recommendations regarding information blocking exceptions, however, we did not propose any exceptions to information blocking in the Disincentives Proposed Rule and these issues are out of scope for this final rule. In the ONC Cures Act Final Rule ( 85 FR 25820 ), ONC established exceptions to information blocking consistent with PHSA section 3022(a)(3), and subsequently made revisions to these exceptions in the HTI-1 Final Rule ( 89 FR 1373 ). We invite readers to review the information blocking exceptions to better understand how various scenarios may be addressed by these exceptions. [ 11 ] We may also consider this input for future rulemaking related to exceptions to information blocking.
Comments. A few commenters recommended delaying the implementation or enforcement of provider information blocking disincentives until issues related to reproductive health data and privacy are resolved. A few commenters expressed concern that the proposals described in section III.C. of the Disincentives Proposed Rule could negatively impact patient-provider relationships, risk patient and provider criminalization, and lead to patients delaying seeking healthcare due to reproductive health data and privacy issues. A few commenters recommended considering the context of the healthcare landscape following the overturning of the Federal constitutional right to an abortion and subsequent legislation in certain states to criminalize people who seek reproductive health care before finalizing the proposals in section III.C. of the Disincentives Proposed Rule. Commenters expressed concern that because of the financial impact the proposed appropriate disincentives may carry, health care providers may disclose sensitive health information, including reproductive health information, to the detriment of people seeking reproductive care. Commenters similarly expressed concern that the Disincentives Proposed Rule could result in the disclosure of other forms of sensitive health information, including information related to contraceptive access, in vitro fertilization (IVF), gender-affirming healthcare, sexually transmitted infections (STIs), intimate partner violence, and sexual assault. A few commenters recommended providers be exempt from information blocking requirements if they do not disclose patient information to protect patient privacy related to sexual and reproductive health and to protect the patient or themselves from criminalization or harassment. The commenter also recommended that a new “good faith” exception to information blocking should be established under which providers acting in “good faith” to withhold sensitive health information are presumed to be acting reasonably and in the best interest of their patients. One commenter recommended that implementation of disincentives should not occur until EHRs can ensure sensitive health data can be protected, clear concise exceptions are created, and consent management software is widely available. Commenters stated that EHR vendors cannot currently meet data segmentation standards for sensitive health information, such as reproductive healthcare data. One commenter recommended delaying implementation for 2 years to allow providers to comply with the anticipated “HIPAA Privacy Rule to Support Reproductive Health Care Privacy” final rule [ 12 ] and ONC's “Health Data, Technology, and Interoperability: Certification Program Updates, Algorithm Transparency, and Information Sharing” proposed rule ( 88 FR 23746 ). [ 13 ]
Response. We acknowledge the concerns commenters may have regarding the sensitivity of health data relating to reproductive health care and will take these comments under consideration. We further acknowledge commenters' concerns that disincentives could lead to health care providers disclosing sensitive health information, including reproductive health information, and welcome commenters' recommendations regarding an exception to information blocking when a health care provider withholds sensitive information to protect the patient or themselves from criminalization or harassment. However, we did not propose exceptions to information blocking in the Disincentives Proposed Rule and believe that such policies are out of scope for this final rule. Instead, we will take these comments under consideration for other rulemaking activities in which we focus on revising and expanding the exceptions to information blocking.
Section 4004 of the Cures Act, which added section 3022 to the PHSA, does not amend existing laws governing the confidentiality, privacy, and security of health information, such as HIPAA, its implementing regulations at 45 CFR parts 160 , and 164 , or other applicable Federal or state laws or regulations. Health care providers are responsible for ensuring their compliance with applicable laws and regulations governing confidentiality, privacy, and security of their patients' health information.
Regarding commenters' statement that implementation of disincentives should not occur until improvements to technical approaches to data segmentation are achieved, we agree that this is an important area for advancement. However, we believe that this work can continue in parallel with the finalization of this rule and establishment of information blocking disincentives for health care providers.
Finally, we acknowledge that health care providers are also focused on meeting other regulatory provisions. However, we reiterate that the information blocking regulations in 45 CFR part 171 have been effective since April 5, 2021, and that this final rule is focused on establishing disincentives for practices that are inconsistent with Start Printed Page 54669 the existing regulations defining information blocking. It does not create new affirmative obligations for health care providers.
Comments. One commenter expressed concerns that the changes to the information blocking regulations have occurred too frequently, thereby creating burden and confusion for health care providers. One commenter expressed concern about the impact this new proposed disincentive structure will have on health care providers, given that they are also navigating other requirements related to EHI, such as surprise billing, electronic prescription, and electronic clinical quality measures. The commenter recommended that CMS remain cognizant of the many regulations that govern the flow of EHI and the differences in health IT use between provider types and sites of service.
Response. We appreciate commenters' concerns. We understand that health care providers are continuing to gain experience and understanding of the information blocking regulations, and that health care providers have numerous compliance obligations with respect to Federal laws and regulations. We will continue to collaborate closely within the Department to consider other requirements that impact health care providers and seek to reduce burden.
Comments. One commenter requested we provide lessons learned from cases of information blocking on the website to help educate actors on what does and does not qualify as information blocking. One commenter recommended a nation-wide marketing campaign to educate patients about information blocking practices and promote awareness of the information blocking website.
Response. We appreciate the commenters' recommendations and will take them into consideration as we develop educational materials in the future. We note that there are resources available on ONC's website [ 14 ] about information blocking, which can help health care providers learn about what practices constitute information blocking and how health care providers can avoid these practices.
In this section, we discuss certain statutory terms and provisions in PHSA sections 3022(a) and (b) related to the establishment of appropriate disincentives for health care providers as defined in 45 CFR 171.102 . For brevity, we refer to PHSA section 3022(b)(2)(B), which states that health care providers that OIG has determined to have committed information blocking “shall be referred to the appropriate agency to be subject to appropriate disincentives using authorities under applicable Federal law, as the Secretary sets forth through notice and comment rulemaking,” as the “disincentives provision” throughout this section.
The disincentives provision states that an individual or entity that is a health care provider determined by OIG to have committed information blocking shall be referred to the “appropriate agency” to be subject to appropriate disincentives. In the Disincentives Proposed Rule, we proposed to define “appropriate agency” in 45 CFR 171.102 to mean a government agency that has established disincentives for health care providers that OIG determines have committed information blocking ( 88 FR 74951 ). An “agency” may be any component of HHS that has established a disincentive or disincentives on behalf of the Secretary of HHS, including any of the Staff or Operating Divisions of HHS. For example, the disincentives finalized in section III.C. of this final rule are established using authorities held by CMS, which is an Operating Division of HHS. Under the disincentives finalized in this final rule, CMS is the “appropriate agency” to which OIG will refer a health care provider to be subject to disincentives.
We invited public comments on our proposed definition of “appropriate agency.” The following is a summary of the comments we received and our responses.
Comments. One commenter agreed that CMS would be the appropriate agency for OIG referrals for enforcement because of the large percentage of health care providers participating in the programs discussed in section III.C. of the Disincentives Proposed Rule and the fact that CMS administers those programs.
Response. We thank the commenter for their support. We wish to clarify that an appropriate agency could include any of the Staff or Operating Divisions of HHS. However, all of the disincentives finalized in this rule were established using authorities for programs administered by CMS.
Comments. One commenter contended that the proposed definition of “appropriate agency” is very broad and requested that the specific agencies that may receive a referral and assess provider disincentives be clarified and listed in the rule.
Response. We appreciate the comment but decline to change the definition of “appropriate agency” to list all of the specific agencies that may receive a referral and impose disincentives. We note that, as of the effective date of this final rule, the only agency that has established disincentives for health care providers is CMS. While other disincentives could be established under other agencies through future notice and comment rulemaking, we cannot preemptively identify the agencies that may establish disincentives at this time. Therefore, we believe maintaining the broad definition of appropriate agency is appropriate as it allows for the potential addition of disincentives established under other agencies in the future.
After consideration of the public comments, we have finalized our definition of “appropriate agency” in 45 CFR 171.102 as proposed to mean a government agency that has established disincentives for health care providers that OIG determines have committed information blocking.
In the Disincentives Proposed Rule we proposed to interpret the phrase “authorities under applicable Federal law” in the disincentives provision to mean that an appropriate agency may only subject a health care provider to a disincentive established using authorities that could apply to information blocking by a health care provider subject to the authority, such as health care providers participating in a program supported by the authority ( 88 FR 74951 ). In section III.C. of this final rule, CMS identifies the authority under which each disincentive has been finalized.
The following is a summary of the comments we received and our responses.
Comments. One commenter expressed concern that the proposed interpretation of “authorities under applicable Federal law” limits the agency's ability to put in place an effective and fair enforcement structure for information blocking by limiting the applicable authority only to those with already existing penalty structures that exist to serve other policy goals. The commenter recommended that HHS revisit its interpretation of “authorities under applicable Federal law” to allow appropriate agencies to promulgate specific disincentives for information blocking conduct that: permit Start Printed Page 54670 consideration of mitigation and aggravating factors; allow for a broader range of disincentives (including technical assistance and corrective action plans); and preserve a health care provider's due process rights.
Response. We appreciate the commenter's recommendations. However, we note that PHSA section 3022(b)(2)(B) specifies that disincentives must be established “using authorities under applicable Federal law.” As a result, disincentives established by an appropriate agency must be consistent with the authority under which the appropriate agency establishes the disincentive through notice and comment rulemaking. Furthermore, under the definition of “disincentive” that we have finalized in 45 CFR 171.102 , a disincentive is imposed for the purposes of deterring information blocking. By finalizing this definition, we intend to limit disincentives to only include the conditions established by an appropriate agency that are intended to have a deterrent effect on information blocking practices. The disincentives provision in PHSA section 3022(b)(2)(B) and the definition of disincentive that we have finalized in 45 CFR 171.102 do not limit an appropriate agency from proposing, via notice and comment rulemaking, to establish other programmatic elements mentioned by the commenters, if such elements are within the scope of the appropriate agency's authority.
Comments. One commenter stated that the interpretation of “authorities under applicable Federal law” described in the Disincentives Proposed Rule limits HHS to promulgating disincentives that are duplicative of existing penalty structures that might otherwise apply to information blocking conduct committed by certain health care providers. The commenter stated that this may conflict with the statutory requirement in PHSA section 3022(d)(4). The commenter stated that Congress' intent with the provision in PHSA section 3022(d)(4) was that HHS, in establishing disincentives, should take all measures possible to not use existing authorities that could apply to information blocking by a health care provider. The commenter further stated that existing authorities under which we proposed to establish disincentives in the Disincentives Proposed Rule, such as the Medicare Promoting Interoperability Program as well as the Medicare Shared Savings Program, exist to serve other policy goals and regulatory requirements, and disincentives established under these authorities should not qualify as an appropriate enforcement structure to target information blocking specifically.
Response. We disagree that the disincentives CMS has finalized in this final rule conflict with the statutory provision in PHSA section 3022(d)(4). Section 3022(d)(4) of the PHSA requires the Secretary, in carrying out section 3022 and to the extent possible, to ensure that information blocking penalties do not duplicate penalty structures that would otherwise apply with respect to information blocking and the type of individual or entity involved as of the day before the date of enactment of the Cures Act. However, the disincentives that CMS has finalized in section III.C. of this final rule create new policies to deter information blocking that are based on a referral of a determination by OIG that a health care provider has committed information blocking as defined in PHSA section 3022(a).
After consideration of the public comments, we continue to view the disincentives provision in PHSA section 3022(b)(2)(B) to require that an appropriate agency may only subject a health care provider to a disincentive established using authorities that could apply to information blocking by a health care provider subject to the authority, such as health care providers participating in a program supported by the authority.
We stated in the Disincentives Proposed Rule that the Cures Act does not specify or provide illustrations for the types of disincentives that should be established ( 88 FR 74951 ). As such, we proposed to define the term “disincentive” in 45 CFR 171.102 to mean a condition specified in 45 CFR 171.1001(a) that may be imposed by an appropriate agency on a health care provider that OIG determines has committed information blocking for the purpose of deterring information blocking practices. In section III.B.2. of the Disincentives Proposed Rule, we proposed to identify in 45 CFR 171.1001(a) those disincentives that have been established pursuant to the statute for the express purpose of deterring information blocking practices ( 88 FR 74952 and 74953 ).
We also noted that the term “appropriate” for disincentives is likewise not defined in PHSA section 3022, nor are illustrations provided. In the Disincentives Proposed Rule, we stated that a disincentive for a health care provider that OIG has determined to have committed information blocking may be any condition, established through notice and comment rulemaking, that would, in our estimation, deter information blocking practices among health care providers subject to the information blocking regulations ( 88 FR 74951 ). In section III.C. of the Disincentives Proposed Rule, CMS described the potential impact that each proposed disincentive would have on a health care provider ( 88 FR 74954 through 74966 ).
Finally, in the Disincentives Proposed Rule we noted that the disincentives provision does not limit the number of disincentives that an appropriate agency can impose on a health care provider ( 88 FR 74951 ). Accordingly, we proposed that a health care provider would be subject to each appropriate disincentive that an agency has established through notice and comment rulemaking and is applicable to the health care provider. We stated that imposing cumulative disincentives, where applicable, would further deter health care providers from engaging in information blocking.
We invited public comments on our proposals to establish disincentives in section III.C. of the Disincentives Proposed Rule ( 88 FR 74954 through 74966 ). The following is a summary of the comments we received and our responses on the definition of the term “disincentive” and related proposals.
Comments. One commenter agreed that a health care provider should be subject to appropriate and applicable disincentives established through notice and comment rulemaking. Some commenters agreed that subjecting health care providers to cumulative disincentives, where applicable, may deter providers from engaging in information blocking.
Comments. A few commenters expressed concern that the proposed definition of “appropriate disincentives” is too broad and unclear. The commenters requested that ONC narrow its definition of “appropriate disincentives” so that it is reflective of the underlying statute's requirement that disincentives be appropriate. Another commenter expressed concern that the definition does not impose limits on what may be deemed “appropriate,” therefore any disincentive proposed by an appropriate agency could theoretically meet this broad standard. Commenters expressed that a disincentive structure that does not consider the severity of the underlying misconduct cannot be considered “appropriate.”
Response. We thank the commenters for their input. We note that we did not propose to define the term “appropriate Start Printed Page 54671 disincentives.” Instead, we proposed to define the term “disincentive,” to mean a condition specified in § 171.1001(a) that may be imposed by an appropriate agency on a health care provider that OIG determines has committed information blocking for the purpose of deterring information blocking practices ( 88 FR 74951 ). We have finalized this proposed definition at 45 CFR 171.102 with a modification to replace the phrase “may be imposed” with “is imposed” to clarify that a disincentive is the completed action by an appropriate agency to impose a condition on a health care provider that OIG determines has committed information blocking.
Regarding commenter concerns that we did not propose to impose limits on what may be deemed “appropriate,” and that a disincentive which does not consider the severity of the underlying misconduct should not be deemed “appropriate,” we reiterate that the term “appropriate” is not defined in PHSA section 3022, nor are illustrations provided. We believe that term “appropriate” is capacious and is best read to give the Secretary significant discretion to craft disincentives using existing authorities. As we noted in the Disincentives Proposed Rule, the key feature of appropriate disincentives is that the agency believes that they will deter information blocking ( 88 FR 74951 ). We have carefully considered each disincentive we have finalized for appropriateness, as it relates to deterring information blocking; in section III.C.2.-III.C.4., CMS describes the potential impact of each proposed disincentive on a health care provider which would result in deterring information blocking practices.
However, we believe the disincentives finalized in section III.C. also align with the use of the term “appropriate” in PHSA section 3022 by including certain limits on the impact of each disincentive. For instance, under the Medicare Promoting Interoperability Program and the MIPS Promoting Interoperability performance category, CMS has finalized disincentives that affect otherwise applicable payment adjustments based on a health care provider failing to meet the requirements of each program by committing information blocking. In sections III.C.2.c. and III.C.3.c., CMS has finalized that the disincentive under each program would only be applied for the EHR reporting period or performance period of the calendar year in which OIG refers a determination of information blocking to CMS. Barring a subsequent referral of a determination of information blocking, the health care provider would be eligible to successfully meet the program's requirements in the following calendar year's EHR reporting period or performance period. As discussed in section III.C.4., the disincentive finalized under the Medicare Shared Savings Program to deter information blocking through potential denial of approval to participate in or removal from the Shared Savings Program, limits the duration of the disincentive to a year to ensure that health care providers who have committed information blocking and corrected their actions are not permanently barred from participating in the Shared Savings Program. By balancing deterrent impact with these limits, CMS has finalized disincentives consistent with the general direction in PHSA section 3022 to establish disincentives that are “appropriate.”
We disagree with the commenter that a disincentive that cannot be adjusted to reflect the severity of the underlying misconduct cannot be considered “appropriate.” To be sure, the agency imposing an appropriate disincentive on a health care provider may not have the flexibility to determine the value of the disincentive for each individual or entity based on their conduct, as authorized for developers, networks, and exchanges that engage in information blocking under PHSA section 3022(b)(2)(A) (through CMPs). But lingering inflexibility is a function of the statute's authorization only to use “existing authorities” to subject health care providers to disincentives rather than CMPs.
Finally, we did not propose to define disincentive in the manner commenters have suggested in order to preserve flexibility for agencies to establish disincentives for information blocking. Since disincentives must be established using authorities under applicable Federal law (in accordance with PHSA section 3022(b)(2)(B)), there may be a limited set of statutory provisions that could be used to establish disincentives. Thus, we proposed and have finalized a definition of disincentive that would not unduly limit our ability to use available authorities to establish disincentives and have not proposed to further limit disincentives through proposing a definition for the term “appropriate.”
Comments. One commenter recommended that ONC revise its proposed definition of “appropriate disincentives” to explicitly incorporate technical assistance or a corrective action plan. The commenter further contended that this adjustment would be more consistent with HHS' enforcement of other regulations, such as the HIPAA Privacy and Security Rules.
Response. We appreciate the commenter's recommendation. We note that we did not propose to define the term “appropriate disincentives.” Instead, we proposed to define the term “disincentive” to mean a condition specified in § 171.1001(a) that may be imposed by an appropriate agency on a health care provider that OIG determines has committed information blocking, for the purpose of deterring information blocking practices. Activities such as the provision of technical assistance or the provision of a corrective action plan may not adequately deter information blocking practices, and we decline to include such activities in the definition of a disincentive at this time. We further refer readers to resources on ONC's website [ 15 ] about information blocking, which can help health care providers learn about what practices constitute information blocking and how health care providers can avoid these practices.
Comments. Many commenters did not support our proposal for a health care provider to be subject to each appropriate disincentive established by an appropriate agency applicable to such health care provider, without limit to the number of disincentives, and disagreed that this policy would deter providers from engaging in information blocking. One commenter contended that unlimited cumulative disincentives should not be considered appropriate.
Several commenters expressed that subjecting health care providers to multiple disincentives for the same misconduct, simply based on their participation in multiple programs rather than the severity of the conduct, is duplicative, overly punitive, and heightens the risk for providers who participate in multiple CMS programs. A few commenters recommended that HHS establish a clear process to reconcile multiple disincentives and ensure fair and non-duplicative or punitive enforcement for providers participating in multiple programs. A few commenters suggested limiting the number of disincentives that could be applied or clarifying under which program the disincentive would be applied. A few commenters expressed concern that the allowance of cumulative disincentives will create confusion and complexity.
Response. We thank commenters for their input. We disagree with commenters that multiple disincentives will not deter information blocking, as Start Printed Page 54672 the increased impact on a health care provider of receiving cumulative disincentives is likely to be a stronger deterrent due to potentially imposing greater adverse consequences on the health care provider that commits information blocking. Moreover, health care providers who participate in multiple programs may be larger than health care providers who do not participate in multiple programs, or may have a greater ability to influence health information exchange than other health care providers, and so may need greater disincentive exposure to deter information blocking practices.
Finally, we believe that the possibility of receiving cumulative disincentives will have a greater deterrent effect on health care providers that are determined to have committed information blocking, since individual disincentives are likely to have variable impacts depending on the circumstances of a given health care provider, as further discussed in section III.C.1. of this final rule. If a health care provider expects to only be subject to one disincentive, and the health care provider expects the disincentive to have a small impact, for instance, through minimal exposure under a certain program, the value of that disincentive to deter information blocking practices for that health care provider will be minimized. The availability of disincentives under more than one authority can mitigate this issue, as under our policy a health care provider may expect that they could be subject to cumulative disincentives established under different authorities, increasing the likelihood that there is an available disincentive that will have a meaningful deterrent effect for that specific health care provider.
We also disagree with the commenter that the term “appropriate” should be interpreted to prohibit applying multiple disincentives on a health care provider that has committed information blocking. PHSA section 3022(b)(2)(B) specifically contemplates that a health care provider may be subject to “appropriate disincentives”—plural. The plain language of the statute therefore suggests that multiple “disincentives” would be “appropriate.”
We further disagree that subjecting a health care provider to multiple disincentives is unfair and overly punitive. The disincentives that CMS has finalized in this final rule are established under authorities which provide for specific requirements for programs authorized under those authorities. CMS describes in section III.C. how information blocking committed by a health care provider would conflict with the requirements under each of the programs through which a disincentive has been established. Accordingly, we believe it is reasonable that a health care provider that has acted in a manner inconsistent with these programs by committing information blocking could be subject to a disincentive under that authority, regardless of whether the health care provider has also been subject to a disincentive established under another authority.
However, we believe it is necessary to provide further clarification around our proposed policy with respect to cumulative disincentives. Specifically, we believe that our proposed policy may not have accounted for scenarios under which an appropriate agency may choose to exercise discretion when imposing a disincentive. For example, in section III.C.4. of this final rule, CMS has finalized a policy under the authority for the Shared Savings Program, which CMS originally discussed as an alternative policy in the Disincentives Proposed Rule ( 88 FR 74966 ). This finalized policy will permit CMS, as the appropriate agency, to consider relevant facts and circumstances when deciding whether to apply a disincentive to an ACO, ACO participant, or ACO provider/supplier in the Shared Savings Program.
We note that CMS has finalized this alternative policy for the Medicare Shared Savings Program only, as this policy is consistent with existing practices under the Shared Savings Program for addressing program integrity issues among ACOs, ACO participants, or ACO providers/suppliers. In addition, this policy addresses scenarios specific to imposing a disincentive under the Shared Savings Program, for instance, where removal of one entity from participation in an ACO could result in the ACO not meeting program requirements such as falling below the 5,000 assigned beneficiary threshold required by 42 CFR 425.110(a)(1) , thereby interrupting care coordination benefits of beneficiaries receiving care from ACO participants and ACO providers/suppliers that did not commit information blocking. Under the finalized alternative policy, CMS will consider relevant facts and circumstances before imposing a disincentive under the Shared Savings Program. The relevant facts and circumstances include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether the provider was previously subject to a disincentive in another program, and other factors. We refer readers to section III.C.4. for a complete discussion of the alternative policy finalized under the Shared Savings Program. As discussed in sections III.C.2. and III.C.3. of this final rule, the Medicare Promoting Interoperability Program and the MIPS Promoting Interoperability performance category will impose a disincentive on an applicable health care provider following a referral of an information blocking determination by OIG.
Thus, we are revising our proposed policy for consistency with the policies finalized in this rule. Where we stated in the proposed rule ( 88 FR 74951 ) that a health care provider “would” be subject to each appropriate disincentive that an agency has established through notice and comment rulemaking and is applicable to the health care provider, in this final rule we clarify that, under the disincentives provision, a health care provider “may” be subject to each appropriate disincentive that an agency has established through notice and comment rulemaking and is applicable to the health care provider. Under this revised policy, we acknowledge that an appropriate agency could establish a policy that allows for discretion in imposing a disincentive, consistent with the agency's authority and implementing regulations.
Finally, we disagree with the commenters that a cumulative disincentives approach will introduce more confusion and complexity. We believe this final rule provides clarity about the disincentives established under each of the relevant programs to ensure health care providers understand the consequences they may face for committing information blocking with respect to the requirements of each program.
Comments. A few commenters expressed concern about the negative and disproportionate impact of potentially imposing cumulative disincentives on small and less resourced practices. One commenter recommended considering the relative impact of cumulative disincentives on the health care provider, such as the size and resources of the provider.
Response. We appreciate the commenters' concerns about the impact of cumulative disincentives on small and less resourced practices. However, we disagree with commenters that we should revise our policy with respect to cumulative disincentives to be based on the size and resources of the health care provider subject to the disincentive, as we believe this policy should be consistent across health care providers, Start Printed Page 54673 regardless of their size, and that any considerations with respect to how an appropriate disincentive should impact health care providers should be addressed by the appropriate agency establishing the disincentive.
An appropriate agency, in establishing a disincentive and related policies, could retain or implement policies based on the type of health care provider subject to the disincentive, including small practices, consistent with the agency's authority. For instance, CMS automatically reweights the MIPS Promoting Interoperability performance category to zero percent of a MIPS eligible clinician's final score if the MIPS eligible clinician is in a small practice as defined in 42 CFR 414.1305 and does not submit data for the MIPS Promoting Interoperability performance category for the applicable performance period ( 42 CFR 414.1380(c)(2)(i)(C) ( 9 )). In other words, if the MIPS eligible clinician meets this criterion for automatic reweighting at 42 CFR 414.1380(c)(2)(i)(C) ( 9 ), the MIPS eligible clinician is not required to complete the requirements for earning a score for the Promoting Interoperability performance category as set forth in 42 CFR 414.1375 . In such event, CMS does not assign a score for the MIPS eligible clinician for the Promoting Interoperability performance category and redistributes the weight of the performance category (typically 25 percent of the final score) to the remaining performance categories on which the MIPS eligible clinician is scored in accordance with 42 CFR 414.1380(c)(2)(ii) . In section III.C. of the proposed rule and section III.C. of this final rule, CMS has neither proposed nor finalized any policy that would amend this automatic reweighting policy at 42 CFR 414.1380(c)(2)(i)(C) ( 9 ) for MIPS eligible clinicians in a small practice if such practice were subject to the disincentive being finalized as discussed section III.C.3. of this final rule.
After consideration of the public comments, we have finalized our definition of the term “disincentive” in 45 CFR 171.102 as proposed to mean a condition specified in 45 CFR 171.1001(a) that is imposed by an appropriate agency on a health care provider that OIG determines has committed information blocking for the purpose of deterring information blocking practices. We have also finalized our policy, with modification, that a health care provider may be subject to each appropriate disincentive that an agency has established through notice and comment rulemaking and is applicable to the health care provider.
In this section we provide additional detail about the process by which a health care provider that has committed information blocking may be subject to appropriate disincentives for information blocking. This section begins with a discussion, provided for informational purposes and not including any final policies, of an OIG investigation of a claim of information blocking and how OIG intends to refer a health care provider it determines has committed information blocking to an appropriate agency. Next, we discuss finalized proposals related to the application of a disincentive by an appropriate agency. Finally, we discuss our finalized approach to provide transparency into the nationwide health IT infrastructure by making information available to the public about health care providers that have been determined by OIG to have committed information blocking and have been subject to an appropriate disincentive for information blocking, and about health IT developers of certified health IT and HIEs/HINs and that have been determined by OIG to have committed information blocking.
In the Disincentives Proposed Rule, we provided information regarding OIG's anticipated approach to information blocking investigations of health care providers ( 88 FR 74951 and 74952 ). We noted that this information was not a regulatory proposal and was provided for information purposes only. Preamble discussion of investigation priorities for health care provider information blocking claims included in the Disincentives Proposed Rule, and restated below, is not binding on OIG and HHS. It does not impose any legal restrictions related to OIG's discretion to choose which health care provider information blocking complaints to investigate. As the discussion in the Disincentives Proposed Rule was not a regulatory proposal, we have not included direct responses to comments provided on this section (III.B.1.). However, to improve public understanding of how OIG anticipates it will approach information blocking investigations of health care providers, this section (III.B.1.) of the preamble provides an informational statement to supplement the discussion set forth in the Disincentives Proposed Rule.
We clarify here that OIG's investigation will depend on the specific facts and circumstances presented in the allegation. OIG will evaluate each allegation based on the facts and circumstances presented in the allegation. As OIG investigates the allegations, though, the scope of the investigation may change, and OIG may change the individual(s) or entity(ies) under investigation depending on the specific facts and circumstances it has found. Indeed, through conducting an investigation, OIG will collect evidence which it will use to evaluate the individual(s) or entity(ies) with potential information blocking liability and potential information blocking conduct. The vast bulk of material and relevant evidence (that is, evidence relating to whether the actor committed information blocking) will come from the actor whose conduct is at issue.
As part of OIG's investigation, OIG will need to evaluate whether an individual or entity meets the definition of an actor under ONC's regulations. OIG has previously stated that it will look to ONC's regulations and any related guidance in evaluating whether an individual or entity meets a specific actor definition, and OIG will continue to do so for health care provider investigations ( 88 FR 42828 ). OIG will look to the regulations in effect at the time the conduct occurred. Based on the definitions ONC has finalized for health IT developer of certified health IT and HIN/HIE, a health care provider, as set forth in 45 CFR 171.102 , may meet the definition of a health care provider and one of those definitions as well ( 88 FR 42829 ). OIG anticipates being in contact with health care providers as part of its investigation, as necessary, to understand the specific facts and circumstances. For example, OIG may need to engage with the health care provider to understand whether the health care provider is a HIN/HIE or a health IT developer of certified health IT. And as mentioned above, much of the evidence gathered by OIG will likely come from the individual(s) or entity(ties) under investigation.
As part of an investigation, OIG will evaluate whether information blocking has occurred. OIG has previously stated that it will look to ONC's regulations and any related guidance in evaluating whether conduct constitutes information blocking, and OIG will continue to do so with respect to health care providers ( 88 FR 42827 ). OIG will look to ONC's information blocking regulations in 45 CFR part 171 in effect at the time the conduct occurred. Through conducting an investigation, OIG will collect evidence, which it will use to evaluate whether conduct constitutes information blocking and whether an actor had the requisite Start Printed Page 54674 intent. As mentioned above, OIG anticipates engaging with health care providers during this process as it learns the facts and circumstances of the allegation under investigation.
Regarding the timing of investigations, OIG will not begin investigating health care providers until after the effective date of this rule, and will exercise its enforcement discretion not to make any determinations regarding conduct occurring prior to the effective date of this rule for information blocking disincentives. As OIG will not make a determination on conduct occurring prior to the effective date, OIG will not refer any health care providers based on a determination of conduct occurring prior to the effective date of this rule for information blocking disincentives. This means that no disincentives finalized in this final rule will be applied to conduct occurring before the effective date of this final rule.
As with other conduct that OIG has authority to investigate, OIG has discretion to choose which information blocking complaints to investigate. To maximize efficient use of resources, OIG generally focuses on selecting cases for investigation that are consistent with its enforcement priorities and intends to apply that rationale to its approach for selecting information blocking complaints for investigation.
For investigations of health care providers, the Disincentives Proposed Rule stated that OIG expects to use four priorities: (i) resulted in, are causing, or have the potential to cause patient harm; (ii) significantly impacted a provider's ability to care for patients; (iii) were of long duration; and (iv) caused financial loss to Federal health care programs, or other government or private entities ( 88 FR 74951 ). As mentioned in the above section concerning OIG investigations, OIG's expected priorities are informational only and are not binding on OIG decision making.
OIG's priorities for health care provider investigations differ from the priorities set out in the OIG CMP Final Rule, due to the differences in intent. In the OIG CMP Final Rule, OIG stated that it would prioritize actors who had actual knowledge, as actual knowledge is more egregious, when a lower intent is required (that is, when the standard is “knows, or should know”) ( 88 FR 42823 ). However, under PHSA section 3022(a), the intent requirement for health care providers is that the health care provider “knows” that a practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information. Because the Cures Act only provides a single intent standard (“knows”), OIG will not consider actual knowledge as part of its priorities for health care provider actors.
Consistent with the OIG CMP Final Rule ( 88 FR 42822 ), OIG's enforcement priorities are a tool OIG uses to triage allegations and allocate resources. OIG provides information about its enforcement priorities so the public and stakeholders have a better understanding of how OIG anticipates allocating resources for enforcement. OIG's enforcement priorities will inform decisions about which information blocking allegations to pursue, but these priorities are not dispositive. Each allegation will be assessed to determine whether it implicates one or more of the enforcement priorities, or otherwise merits further investigation and potential enforcement action. There is no specific formula OIG can apply to every allegation that allows OIG to effectively evaluate and prioritize which claims merit investigation.
Although OIG's anticipated priorities are framed around individual allegations, OIG may evaluate allegations and prioritize investigations based in part on the volume of claims relating to the same (or similar) practices by the same entity or individual.
This section summarizes the discussion in the OIG CMP Final Rule of the ways ONC, OCR, and OIG will consult, refer, and coordinate on information blocking claims as permitted by the Cures Act ( 88 FR 42823 ).
PHSA section 3022(d)(1) states that the National Coordinator may serve as a technical consultant to the Inspector General. OIG will accordingly consult with ONC throughout the investigative process. Additionally, PHSA section 3022(b)(3)(A) provides the option for OIG to refer claims of information blocking to OCR when a consultation regarding the health privacy and security rules promulgated under section 264(c) of HIPAA will resolve such claims. Depending on the facts and circumstances of the claim, OIG will exercise this statutory discretion as appropriate to refer information blocking claims to OCR for resolution. There is no set of facts or circumstances that will always be referred to OCR. OIG will work with OCR to determine which claims should be referred to OCR under the authority provided in PHSA section 3022(b)(3)(A). It is important to note that while section 3022(b)(3)(A) of the PHSA specifically provides OIG with the authority to refer information blocking claims to OCR, OIG's statutory authority to refer to OCR allegations of violations of the HIPAA Privacy, Security, or Breach Notification Rules [ 16 ] is not solely based on PHSA section 3022(b)(3)(A). Thus, OIG's authority to refer to OCR such allegations against health care providers is not limited to claims of information blocking.
Finally, OIG anticipates coordinating with other HHS agencies to avoid duplicate penalties as identified in section 3022(d)(4) of the PHSA. Depending on the facts and circumstances, OIG may also consult or coordinate with a range of other government agencies, including CMS, FTC, or others ( 88 FR 42823 and 42824 ).
During an investigation of information blocking by a health care provider, but prior to making a referral, OIG will coordinate with the appropriate agency to which OIG plans to refer its determination of information blocking. This coordination will ensure that the appropriate agency is aware of a potential referral and that OIG provides the information the agency needs to take appropriate action. OIG's referral to the appropriate agency will explain its determination that a health care provider committed information blocking, including meeting the requirements of the intent element of PHSA section 3022(a)(1)(B)(ii).
We note that PHSA section 3022 authorizes OIG to investigate claims of information blocking and requires OIG to refer health care providers to an appropriate agency when it determines a health care provider has committed information blocking, to be subject to appropriate disincentives. Once OIG has concluded its investigation and is prepared to make a referral, it will send information to the appropriate agency indicating that the referral is made pursuant to the statutory requirement in PHSA section 3022(b)(2)(B). As part of the referral, OIG will provide information to explain its determination, which may include: the dates when OIG has determined the information blocking violation(s) occurred; analysis to explain how the evidence demonstrates the health care provider committed information blocking (for instance, that the health care provider's “practice” [ 17 ] meets each Start Printed Page 54675 element of the information blocking definition); copies of evidence collected during the investigation (regardless of whether it was collected by subpoena or voluntarily provided to OIG); copies of transcripts and video recordings (if applicable) of any witness and affected party testimony; and copies of documents OIG relied upon to make its determination that information blocking occurred. OIG may provide additional information as part of its referral based on consultation with the appropriate agency, to the extent permitted by applicable law.
Following an investigation through which OIG determines a health care provider has committed information blocking, and OIG's referral of this determination to an appropriate agency, the health care provider may be subject to disincentives that have been established under applicable Federal law through notice and comment rulemaking. In this section, we include general provisions and information related to the application of disincentives. For information on the specific disincentives and further discussion about how each disincentive will be applied, we refer readers to section III.C. of this final rule.
In the Disincentives Proposed Rule, we proposed to add a new subpart J to 45 CFR part 171 , entitled “Disincentives for Information Blocking by Health Care Providers” ( 88 FR 74952 and 74953 ). We proposed in 45 CFR 171.1000 that this subpart sets forth disincentives that an appropriate agency may impose on a health care provider based on a determination of information blocking referred to that agency by OIG, and certain procedures related to those disincentives. We proposed in 45 CFR 171.1001(a) that health care providers that commit information blocking would be subject to the following disincentives from an appropriate agency based on a determination of information blocking referred by OIG, where applicable. The disincentives proposed for inclusion in 45 CFR 171.1001(a)(1) through (3) corresponded to the appropriate disincentives proposed in section III.C. of the Disincentives Proposed Rule:
- An eligible hospital or CAH as defined in 42 CFR 495.4 is not a meaningful EHR user as also defined in that section;
- A MIPS eligible clinician as defined in 42 CFR 414.1305 , who is also a health care provider as defined in 45 CFR 171.102 , is not a meaningful EHR user for MIPS as also defined in 42 CFR 414.1305 ; and
- ACOs who are health care providers as defined in 45 CFR 171.102 , ACO participants, and ACO providers/suppliers will be removed from, or denied approval to participate, in the Medicare Shared Savings Program as defined in 42 CFR part 425 for at least 1 year.
We noted that in the future, if we propose to establish additional disincentives, we intend to add such disincentives to the disincentives listed in 45 CFR 171.1001 ( 88 FR 74953 ).
We did not receive any comments on these proposals. However, we have modified the regulation text in several ways to increase clarity. First, we have made minor modifications to the language of the proposed “scope” section, in 45 CFR 171.1000 , to better reflect language used in this final rule. Second, we have replaced the proposed paragraph (a) from 45 CFR 171.1001 , which was redundant with the proposed “scope” section ( 45 CFR 171.1000 ), and reorganized the section to clearly reflect that the disincentives finalized in this final rule, and that a health care provider may be subject to, were established by CMS ( 45 CFR 171.1001(a) as finalized). If we finalize additional disincentives in the future, we will add them to a paragraph under 45 CFR 171.1001 reflecting the appropriate agency that has established the disincentive.
We have finalized, as proposed, the following disincentives in 45 CFR 171.1001(a)(1)-(3) :
- ACOs who are health care providers as defined in 45 CFR 171.102 , ACO participants, and ACO providers/supplies will be removed from, or denied approval to participate, in the Medicare Shared Savings Program as defined in 42 CFR part 425 for at least 1 year.
In the Disincentives Proposed Rule, we proposed in 45 CFR 171.1002(a) through (d) that an appropriate agency that imposes a disincentive or disincentives in § 171.1001(a) would send a notice (using usual methods of communication for the program or payment system) to the health care provider subject to the disincentive or disincentives ( 88 FR 74953 ). We proposed that this notice includes:
- A description of the practice or practices that formed the basis for the determination of information blocking referred by OIG;
- The basis for the application of the disincentive or disincentives being imposed;
- The effect of each disincentive; and
- Any other information necessary for a health care provider to understand how each disincentive will be implemented.
In the Disincentives Proposed Rule we stated that the information in this notice would be based upon the authority used to establish the disincentive and policy finalized by the agency establishing the disincentive ( 88 FR 74953 ). For instance, the notice may contain specific information regarding when a disincentive would be imposed, which may be contingent on both the authority used to establish the disincentive and the specific policy under which the disincentive is established. We noted that, where a health care provider that has been determined to have committed information blocking is subject to multiple disincentives established by an appropriate agency, nothing in this proposal would prevent the appropriate agency from combining these notices into a single communication.
Comments. One commenter requested clarification regarding whether the proposal to send a notice to the health care provider subject to the disincentive implies that all health care providers who have been identified as alleged information blockers will receive a disincentive.
Response. A health care provider would only be subject to a disincentive or disincentives and receive the notification described in this section after a determination has been made by OIG that the health care provider committed information blocking and OIG has referred that determination to the appropriate agency, which is CMS for the purposes of the disincentives finalized in this rule. A health care provider that is merely alleged to have committed information blocking but has not been investigated and determined by OIG to have committed information blocking, would not receive a notification described in this section.
Comments. One commenter expressed support for the proposed notification policies and stated that these policies would improve transparency. Start Printed Page 54676
Response. We thank the commenter for their support.
Comments. A few commenters recommended adding information or a communications channel so that health care providers who have been notified of a disincentive can respond to or communicate with OIG and the agency issuing the disincentive regarding the finding, possible mitigating circumstances, or establish a process to deter further cases of information blocking. One commenter observed that this would increase transparency, avoid patient confusion, and mitigate potential unnecessary reputational damage. One commenter expressed concern that the proposed notifications only inform health care providers of a disincentive after they have been found to have committed information blocking. This commenter expressed concern that a health care provider found to have committed information blocking may have additional practices being investigated or practices that could lead to another finding of information blocking and that these practices would not be included in the notification, for example, for them to fix potential issues. This commenter recommended creating a form notification that would inform health care providers of the information blocking issues that have led to the disincentive so they could be fixed.
Response. We appreciate commenters' concerns and wish to clarify that the notifications proposed in this section would be issued by an appropriate agency following a referral of a determination of information blocking by OIG that leads to the imposition of a disincentive. For discussion of when communication between OIG and a health care provider about alleged information blocking practices may occur as part of an investigation (that is, prior to a determination of information blocking), we refer readers to section III.B.1. of this final rule.
After consideration of the public comments, we have finalized our proposal with modification. In the Disincentives Proposed Rule, we proposed in § 171.1002 that an appropriate agency “would send a notice to the health care provider subject to the disincentive or disincentives.” However, we believe that the use of the affirmative “shall,” which we have finalized in the text of § 171.1002, to describe the action of an appropriate agency will provide greater clarity to health care providers and better conveys the intent of the policy, which is that an appropriate agency will send this notice in all cases in which disincentives have been imposed on a health care provider. For the finalized text of § 171.1002, we also revise our proposed reference to disincentives “specified in § 171.1001(a)” to refer to disincentives “specified in § 171.1001” instead, since we have finalized that disincentives may be listed throughout § 171.1001 and not only under paragraph (a), which specifically lists disincentives established by CMS. Therefore, we have finalized in 45 CFR 171.1002 that an appropriate agency that imposes a disincentive or disincentives in § 171.1001 shall send a notice (using usual methods of communication for the program or payment system) to the health care provider subject to the disincentive or disincentives. We have finalized in 45 CFR 171.1002(a) through (d) the elements of the notice as proposed.
In the Disincentives Proposed Rule, we noted that, following the application of a disincentive, a health care provider, as defined in 45 CFR 171.102 , may have the right to appeal administratively a disincentive if the authority used to establish the disincentive provides for such an appeal ( 88 FR 74953 ). We noted that PHSA section 3022(b)(2)(C) requires that the imposition of CMPs that apply to health IT developers of certified health IT, and HINs/HIEs, that have committed information blocking, follow the procedures of SSA section 1128A, which includes procedures for appeals. However, the Cures Act did not provide similar instruction regarding administrative appeals of disincentives for health care providers established under PHSA section 3022(b)(2)(B), and we did not propose a specific administrative appeals process for health care provider appeals. Therefore, any right to appeal administratively a disincentive, if available, would be provided under the authorities used by the Secretary to establish the disincentive through notice and comment rulemaking.
To provide additional information on these issues to the public, we summarize and respond to comments on our statement regarding appeals.
Comments. Many commenters expressed concern that there is not a clearly defined appeals process that would apply across all provider types. Many of these commenters recommended that HHS adopt a single appeals process through notice and comment rulemaking. Many commenters expressed concern that relying on each program's appeals process creates an unfair structure in which providers do not have equal appeal rights. Some of these commenters further stated that this could require some providers to appeal multiple times and that other providers may not be able to appeal at all. Some commenters stated that the differing appeals processes could create undue administrative burden, with some requesting a single or streamlined process. A few commenters recommended that HHS ensure that any future disincentives for other provider types also allow for a clear and straightforward appeals process.
Response. As noted in the Disincentives Proposed Rule, PHSA section 3022(b)(2)(C) requires that the imposition of CMPs that apply to health IT developers of certified health IT, and HINs/HIEs, that have committed information blocking, follow the procedures of SSA section 1128A, which includes procedures for administrative appeals ( 88 FR 74953 ). The Cures Act did not provide similar instruction regarding administrative appeals of appropriate disincentives for health care providers established under PHSA section 3022(b)(2)(B), and we did not propose and have not finalized any regulations relating to administrative appeals of the imposition of disincentives. Instead, we reiterate that any right to appeal administratively a disincentive, if available, would be provided under the authority used by the Secretary to establish a disincentive.
Section 3022(b)(2)(B) of the PHSA requires that an OIG determination be referred to the appropriate agency to “be subject to appropriate disincentives using authorities under applicable Federal law.” In establishing disincentives using authorities under applicable Federal law, any administrative appeals processes required under those existing authorities would also apply to the disincentives established by an appropriate agency under that authority. We recognize that reliance on any administrative appeals processes under the authority used to establish a disincentive may result in variability in the appeals processes available to health care providers, and that in some cases, administrative appeals processes may be limited or unavailable. However, we disagree that establishing a new single process for administrative appeals would effectively address this variability, as such a process may conflict with, or duplicate, administrative review or appeals processes available under existing authorities. Accordingly, we did not propose such a process in the Disincentives Proposed Rule.
If we establish additional disincentives in the future, we will evaluate any administrative review or appeals process available under the Start Printed Page 54677 authority used to establish the disincentive and how a disincentive would be treated under such a process. However, we decline to limit future disincentives to those which provide for administrative appeals processes meeting certain standards, as we must balance these considerations with our goal of identifying disincentives for all health care providers subject to the information blocking regulations, as defined in 45 CFR 171.102 .
Comments. Many commenters provided recommendations for elements that should be incorporated into an appeals process. Commenters recommended that all health care providers should have the ability to appeal an information blocking determination by OIG before referral or application of a disincentive, as well as the ability to appeal the application and calculation of the disincentive. Other commenters recommended that HHS include evaluation criteria and definitions of intent within the appeals process to ensure transparency. A few commenters suggested that health care providers have the ability to provide further information that may impact a determination. Some commenters recommended entities that the commenters asserted would be appropriate to handle the appeals; the specific entities that commenters recommended were OIG, CMS, ONC, HHS, an Administrative Law Judge, or an impartial agency not involved in the finding or disincentive. Some commenters recommended that HHS ensure that the entity reviewing appeals have sufficient technical expertise to review the OIG finding.
Some commenters recommended potential models for the appeals process, including the process described for ACOs in the Disincentives Proposed Rule, the process established for health IT developers of certified health IT, HINs/HIEs, Medicare programs, and the process for appealing enforcement of the rules promulgated under the Administrative Simplification provisions of HIPAA. Some commenters recommended that HHS clearly define the timelines for the appeals process and build these into the timeline for applying disincentives.
Response. We appreciate commenters' recommendations regarding elements that should be included in an administrative appeals process, as well as recommendations regarding existing appeals processes that would be an appropriate model for review and appeal of disincentives. However, we did not propose to establish a single process for the administrative appeal of either a determination by OIG of information blocking or a disincentive imposed by an appropriate agency based on a referral of a determination of information blocking. Instead, the ability of a health care provider subject to a disincentive to appeal administratively the specific items identified by commenters, including the information blocking determination by OIG, the determination that information blocking conduct met the required intent standard, the application of a disincentive, and the calculation of the disincentive, would be based on the scope of any administrative appeal rights provided under the authority used to establish an appropriate disincentive. Likewise, any timelines for an administrative appeals process may depend upon timelines already established related to administrative appeal rights under the authority used to establish a disincentive.
We appreciate the comment regarding technical expertise in review of any administrative appeals of a disincentive. While the responsibility for reviewing an appeal administratively would be determined by the authority under which the disincentive has been established and could vary across disincentives, we expect that other agencies, such as ONC, could potentially provide technical assistance to an appropriate agency as part of any administrative appeals process that is available and exercised by a health care provider. We encourage readers to review the information in section III.C. of this final rule where CMS provides further discussion of relevant policies related to administrative appeal, review, and reconsideration under authorities used to establish disincentives.
Comments. One commenter requested clarification about the impact an appeal would have on the application of a disincentive and the proposed posting of information on the ONC website.
Response. Regarding the impact an appeal would have on the application of a disincentive, we reiterate that any right to appeal administratively a disincentive, if available, would be provided under the authorities used by the Secretary to establish the disincentive. Therefore, the impact of any appeal rights provided for by a specific authority would depend on that authority. We encourage readers to review the information in section III.C. of this final rule where CMS provides further discussion of relevant policies related to appeal, review, and reconsideration under authorities used to establish disincentives.
As discussed further in section III.B.3. of this final rule regarding our proposal for posting of information on ONC's website, we have finalized our proposal regarding information that will be publicly posted on ONC's website about actors that have been determined by OIG to have committed information blocking (specifically, where the actor is a health care provider, the health care provider's name, business address (to ensure accurate provider identification), the practice found to have been information blocking, including when the practice occurred, the disincentive(s) applied, and where to find additional information, where available, about the determination of information blocking that is publicly available via HHS or another part of the U.S. Government). Further, we have finalized at 45 CFR 171.1101(a)(2) that the information specified in 45 CFR 171.1101(a)(1) will not be posted prior to a disincentive being imposed and will not include information about a disincentive that has not been applied. As noted in section III.B.3., we have modified our finalized policy to provide further clarification that posting of information about a disincentive will not occur until after any available administrative appeals process has been completed.
Comments. One commenter recommended not applying disincentives in any program that does not have an appeals process that would allow health care providers to appeal the finding and the disincentive.
Response. We appreciate the commenter's recommendation. However, we decline to limit the establishment of disincentives to those disincentives which can be established using authorities that provide for administrative appeal rights meeting certain standards. Since we must establish disincentives using authorities under applicable Federal law as required under PHSA section 3022(b)(2)(B), we must balance our interest in providing for administrative appeal rights with a limited set of available authorities which can be used to establish appropriate disincentives. We believe that focusing only on those authorities which provide for a specific set of administrative appeal rights would limit our ability to meet our goal of establishing appropriate disincentives for the health care providers subject to the information blocking regulations, as defined in 45 CFR 171.102 .
We did not propose to establish a single administrative appeals process for health care providers to appeal the application of disincentives being finalized in this rule. We reiterate that any right to appeal administratively a disincentive, if available, would be provided under the authorities used by Start Printed Page 54678 the Secretary to establish the disincentive.
In the Disincentives Proposed Rule, we stated that it is important to promote transparency about how and where information blocking is impacting the nationwide health information technology infrastructure ( 88 FR 74953 ). We further stated that publicly releasing information, including applicable public settlements, penalties, and disincentives, about actors that have been determined by OIG to have committed information blocking can inform the public about how and where information blocking is occurring within the broader health information technology infrastructure.
PHSA section 3001(c)(4) ( 42 U.S.C. 300jj-11(c)(4) ) requires that the National Coordinator maintain an internet website “to ensure transparency in promotion of a nationwide health information technology infrastructure.” We believe this provision provides the National Coordinator with the authority to post information on ONC's website if that information has an impact on issues relating to transparency in the promotion of a nationwide health information technology infrastructure. In the Disincentives Proposed Rule, we proposed to add a new subpart K to 45 CFR part 171 , entitled “Transparency for Information Blocking Determinations, Disincentives, and Penalties” ( 88 FR 74953 ). As proposed in 45 CFR 171.1100 , this subpart would set forth the information that would be publicly posted on ONC's website about actors that have been determined by OIG to have committed information blocking.
We proposed in 45 CFR 171.1101 that, in order to provide insight into how and where information blocking conduct is impacting the broader nationwide health information technology infrastructure, ONC would post on its public website information about actors that have been determined by OIG to have committed information blocking ( 88 FR 74953 ). For health care providers that are subject to a disincentive, we proposed in 45 CFR 171.1101(a)(1) that the following information would be posted: health care provider's name, business address (to ensure accurate provider identification), the practice found to have been information blocking, the disincentive(s) applied, and where to find additional information, where available, about the determination of information blocking that is publicly available via HHS or another part of the U.S. Government. We proposed in 45 CFR 171.1101(a)(2) that the information specified in 45 CFR 171.1101(a)(1) would not be posted prior to a disincentive being imposed and would not include information about a disincentive that has not been applied.
We recognized that under the authorities used to establish the disincentives proposed in section III.C. of the Disincentives Proposed Rule, an appropriate agency may have other obligations related to the release of information about a participant that is a health care provider (as defined in 45 CFR 171.102 ) in programs under that authority ( 88 FR 74953 and 74954 ). For instance, under SSA section 1848(q)(9)(C), MIPS eligible clinicians have a right to review information about their performance in MIPS prior to having this information publicly posted on the Compare Tool in accordance with 42 CFR 414.1395 . Therefore, we proposed in 45 CFR 171.1101(a)(3) that posting of the information about health care providers that have been determined to have committed information blocking and have been subject to a disincentive would be conducted in accordance with existing rights to review information that may be associated with a disincentive specified in 45 CFR 171.1001 . For instance, where a health care provider, as defined in 45 CFR 171.102 , has a statutory right to review performance information, this existing right would be exercised prior to public posting of information regarding information blocking on the website described above.
In order to provide insight into how and where information blocking conduct is impacting the broader nationwide health information technology infrastructure, we also proposed in 45 CFR 171.1101(b)(1) to post on ONC's public website information specified in 45 CFR 171.1101(b)(1) about health IT developers of certified health IT and HINs/HIEs that have been determined by OIG to have committed information blocking and have either resolved their CMP liability with OIG or had a CMP imposed by OIG for information blocking under subpart N of 42 CFR part 1003 ( 88 FR 74954 ). To ensure accurate identification of actors, we proposed in 45 CFR 171.1101(b)(1) to post the type of actor (for example, HINs/HIEs or health IT developers of certified health IT) and the actor's legal name, including any alternative or additional trade name(s) under which the actor operates.
The last information we proposed to post on our public website, for all actors, would be the two types of information mentioned above regarding health care providers ( 88 FR 74954 ). First, in 45 CFR 171.1101(a)(1)(iii) and (b)(1)(iii) , we proposed to post a description of the practice, as the term is defined in 45 CFR 171.102 and referenced in 45 CFR 171.103 , found to have been information blocking. In the case of a resolved CMP liability, we would post the practice alleged to be information blocking. This information will help provide transparency into how information blocking conduct is impacting the nationwide health information technology infrastructure, and in particular, specific practices that are impacting the infrastructure. Second, in 45 CFR 171.1101(a)(1)(v) and (b)(1)(iv) , we proposed to post where to find additional information about the determination (or resolution of CMP liability) of information blocking that is publicly available via HHS or, where applicable, another part of the U.S. Government. This information could include hyperlinks and other information, to help interested persons find any additional information about the determination, settlement, penalty, or disincentive that has been made publicly available by the U.S. Government. Such publicly available information would include any summaries or media releases that may be posted by OIG, or another part of HHS, on their internet website(s). It could also include additional information that may be made publicly available about the determination by or other parts of the U.S. Government. For example, if an actor who has exhausted applicable administrative appeal procedures and brought action in a Federal court for review of the decision that has become final, we could post information on our website about the existence of the court action and where or how to access information about the determination, or resulting court action, that has been made publicly available by the court. This information would provide additional context for how information blocking conduct is impacting the nationwide health information technology infrastructure.
In the Disincentives Proposed Rule, we stated that publicly posting information about actors that have been determined by OIG to have committed information blocking is important for providing transparency into how and where information blocking conduct is occurring within and impacting the broader nationwide health information technology infrastructure ( 88 FR 74954 ). Between April 5, 2021, and September 30, 2023, we received over 800 claims of information blocking through the Start Printed Page 54679 Report Information Blocking Portal. [ 18 ] We publicly post information about these claims, which we update monthly. Beyond posting the number of claims, the posted information includes claim counts by type of claimant and claim counts by potential actor. [ 19 ] While OIG has not necessarily evaluated whether these claims qualify as information blocking, this information provides transparency about how participants in the nationwide health IT infrastructure perceive actions by actors that are part of the same infrastructure, which is intended to support the access, exchange, and use of EHI. A natural progression of the posting of such information is the posting of information about actual information blocking determinations by OIG, including any disincentives applied to health care providers. This information can help the public understand how the information blocking regulations, which seek to prevent and address practices that unreasonably or unnecessarily interfere with lawful access, exchange, or use of EHI through the nationwide health IT infrastructure, are being enforced. It would also provide clarity regarding how and where actors are engaging in information blocking practices within the nationwide health IT infrastructure. Based on this information, participants in the nationwide health IT infrastructure, as well as members of the general public, can confirm or dispel perceptions of information blocking within that infrastructure. Additionally, the combined transparency into the processes Congress authorized and instructed HHS to implement (that is, ONC implementing a claims reporting process, disincentives for applicable actors found to have committed information blocking by OIG) would foster public confidence in the information blocking enforcement framework and potentially encourage public participation in that framework, whether by submitting a claim of information blocking or participating in an OIG information blocking investigation. We invited public comments on these proposals, including comments on whether we should publicly post additional information (and why) about health care providers, health IT developers of certified health IT, or HINs/HIEs that have been determined by OIG to have committed information blocking.
Comments. Many commenters supported the proposal to publicly post information about actors that have been determined to have committed information blocking. Several commenters expressed that the proposal would increase transparency by: providing a better understanding for the healthcare community, including patients, about information blocking practices and how they are assessed by HHS; providing greater visibility to regulators and other health system stakeholders on the gaps and barriers to information sharing; showing the degree to which healthcare data is currently being blocked; supporting patients in making informed decisions about future care; and providing health care providers with information about health IT developers of certified health IT and HINs/HIEs. Several commenters expressed that public posting will further help prevent information blocking violations, enhance accountability, and drive improvements.
Response. We thank commenters for the support of our proposal to publicly post information about actors that have been determined to have committed information blocking and, in the case of health care providers, have been subject to a disincentive.
Comments. A few commenters supported the proposal, in 45 CFR 171.1101(a)(3) , that posting of the information specified in 45 CFR 1101(a)(1) about health care providers that have been determined to have committed information blocking and have been subject to a disincentive would be conducted in accordance with existing rights to review information that may be associated with the applied disincentive. Other commenters expressed concern over not having the ability to review what information is posted prior to the information being publicly posted and recommended being able to review the information for accuracy before posting. One commenter expressed concern that health care provider information could be erroneously posted and the burden to correct any inaccurate postings would fall upon the provider after the fact.
Response. We thank commenters for their comments. We did not propose a unique process by which health care providers would be provided an opportunity to review information prior to posting on ONC's website. The information that would be posted is basic information about the health care provider and the information blocking determination (for example, provider name and address, practice found to be information blocking, disincentive(s) applied, and where to find additional information about the determination of information blocking that is publicly available via HHS or, where applicable, another part of the U.S. Government) that would be derived and confirmed through the OIG investigation and referral to CMS. HHS will work with healthcare providers to correct any clerical errors in these information elements to be posted prior to the information being posted on ONC's website or to correct such information after posting.
Further, in the Disincentives Proposed Rule, we recognized that an appropriate agency may have other program obligations related to release of information about a participant that is a health care provider (as defined in 45 CFR 171.102 ) in such programs ( 88 FR 74953 and 74954 ). On this basis, we proposed at 45 CFR 171.1101(a)(3) that posting of the information about health care providers that have been determined to have committed information blocking and have been subject to a disincentive would be conducted in accordance with existing rights to review information that may be associated with a disincentive specified in 45 CFR 171.1001 . For instance, where a health care provider, as defined in 45 CFR 171.102 , has a statutory right to review performance information, this existing right would be exercised prior to public posting of information regarding information blocking on the website described above. We believe that establishing an additional review process could potentially conflict with or duplicate these existing statutory review rights, such as review rights provided under MIPS at SSA section 1848(q)(9)(C).
Comments. Many comments recommended against public posting until after a health care provider has completed an appeals process. Many commenters also recommended not publicly posting information on the ONC website if the actor(s) are conducting or have completed educational or corrective steps, including providing a period of one or more years for actors to complete corrective actions or come into compliance before public posting.
Response. We did not propose a single administrative appeals process for information blocking disincentives. Instead, as described in section III.B.2. of this final rule, any right to appeal administratively a disincentive, if available, would be provided under the authorities used by the Secretary to establish a disincentive through notice Start Printed Page 54680 and comment rulemaking. In proposing at 45 CFR 171.1101(a)(2) that information will not be posted prior to a disincentive being imposed and will not include information about a disincentive that has not been applied, we intended to capture scenarios where a health care provider may have a right to administratively appeal under the authority used to establish the disincentive. Our intent was to be consistent with our proposal for health IT developers of certified health IT and HIN/HIEs in 45 CFR 171.1101(b)(2) , which states that information will not be posted on ONC's website until a CMP has become final consistent with the procedures in subpart O of 42 CFR part 1003 , which include procedures for an appeal of a CMP. However, we believe that additional clarity regarding the issue of appeals highlighted by the commenters is necessary to ensure the language reflects our intended policy. Therefore, we have finalized a modification to the provision in 45 CFR 171.1101(a)(2) to add that information will not be posted prior to the completion of any administrative appeals process pursued by the health care provider, for example, an appeals process provided for under the authority used to establish the disincentive.
For health care providers, we note that we did not propose, and have not finalized, corrective action options for those health care providers that OIG has determined to have committed information blocking, including remedial actions, to avoid public posting. Regarding corrective action plans for health IT developers of certified health IT or HINs/HIEs, we refer readers to the discussion in the OIG CMP Final Rule, in which OIG states that it does not anticipate using alternatives to CMPs such as corrective action plans at the time of the final rule but may consider such approaches in the future ( 88 FR 42824 ).
Comments. One commenter stated that public posting should not be implemented until all health care providers are equally disincentivized for information blocking. Another commenter urged ONC to delay the launch of this website until regulated health care providers and the relevant Federal agencies have had experience with investigations and referrals for disincentives and actors have received clearer guidance.
Response. We acknowledge commenters' concerns that this final rule does not finalize disincentives that apply to all the types of health care providers included in the health care provider definition at 45 CFR 171.102 . However, it is important to begin providing transparency about those health care providers to whom the disincentive(s) finalized in this rule are applied in order to begin providing the public with transparency about how and where information blocking is impacting the nationwide health information technology infrastructure.
PHSA section 3001(c)(4) requires that the National Coordinator maintain an internet website “to ensure transparency in promotion of a nationwide health information technology infrastructure.” The website where the information would appear is not a new website but rather the current ONC website. [ 20 ] We disagree that posting on the website should be delayed until regulated health care providers and Federal agencies have had experience with investigations and referrals for disincentives. Federal agencies have experience with investigations and referrals, and health care provider information already appears on several websites throughout the Federal government. We also provide data on the ONC website about claims or suggestions of possible information blocking collected through the Report Information Blocking Portal [ 21 ] and education resources and guidance on the information blocking regulations on the ONC website. [ 22 ]
Comments. Some commenters stated that posting health care provider information is a second penalty on top of the monetary disincentive. One commenter asked if public posting is considered a disincentive and recommended it be classified as such. Several commenters expressed concerns about the proposal to list the names of actors determined to have engaged in information blocking on ONC's website, stating that this provision will do little to advance transparency regarding the impact of information blocking on the nationwide health information technology infrastructure but will result in public shaming of actors who have already been penalized for their conduct.
Response. We do not agree with commenters that publicly posting health care provider names constitutes a disincentive. We also disagree with commenters that the posting of health care provider names would be sufficient to deter information blocking, consistent with our discussion of appropriate disincentives in section III.A.3. of this final rule. We note that the disincentives CMS proposed and has finalized in this final rule would each potentially result in a consequence for a health care provider that has been determined by OIG to have committed information blocking, which CMS has stated would deter information blocking practices. The posting of information about health care providers that have committed blocking and been subject to a disincentive does not reflect a consequence commensurate with an OIG determination that the health care provider committed information blocking or the disincentives CMS has finalized.
Last, we disagree that the posting of health care provider names following the imposition of a disincentive as part of the information publicly posted on ONC's website will not advance transparency about information blocking practices. As we have stated, the purpose of posting health care provider names is to ensure transparency in promotion of a nationwide health information technology infrastructure, as we explain elsewhere in this final rule.
Comments. A few commenters did not support public posting due to the delay from when the information blocking practice may have occurred and when the information would be publicly posted, stating that public posting after an actor has completed corrective action would unfairly label them information blockers and impose reputational harm after they have already come into compliance. One commenter specifically expressed concern with the delay in timing from when the information blocking act may have occurred to when the information would be publicly posted, because it may result in current health care providers operating under an organizational TIN being punished for conduct committed by persons who no longer operate under that TIN and that this could steer patients away from these health care providers to the patient's detriment. A few commenters expressed concern that a group of health care providers could suffer reputational harm from public posting based on a single actor, for instance, commenters expressed concerns about potential harm from public posting information about health care providers who are not involved in the information blocking or commit inadvertent acts.
Response. We appreciate commenters' concerns regarding the period of time which may exist between the occurrence of the information blocking conduct and the posting of information following the imposition of a disincentive. We note that we did not Start Printed Page 54681 propose to establish a corrective action plan or other process to allow any health care provider to demonstrate compliance with the information blocking regulations following a determination by OIG that a practice is information blocking. We also remind readers that the definition of information blocking for health care providers requires that the health care provider “knows” that a practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information. This means there would not be posting of unintentional, inadvertent acts of health care providers. While a health care provider may subsequently pursue efforts to cease the information blocking practice which resulted in the imposition of a disincentive, it is still beneficial for the public to be able to understand how and where information blocking is impacting the nationwide health information technology infrastructure, including what aspects of that infrastructure are being impacted by health care providers.
Regarding health care providers operating under or employed by a larger entity, we note that under the regulations we have finalized, the information posted on ONC's website will be specific to the health care provider that OIG has determined has committed information blocking and that has been subject to a disincentive. If OIG determines that a health care provider who is an individual has committed information blocking and refers that individual to an appropriate agency, and the individual is subject to a disincentive, ONC would post only information regarding the individual, not any other entities with which the individual is associated. If OIG determines that a health care provider that is an entity, such as a group practice, has committed information blocking, and the entity is subject to a disincentive, ONC would post information about the entity.
Comments. Some commenters recommended ONC use certain criteria or thresholds in order to decide whether to publicly post information about a health care provider for information blocking. Commenters recommended that ONC consider the following factors before determining whether to publicly post information, including: whether there is frequent, repeat, or significant information blocking, as opposed to minor conduct undertaken in good faith; whether the public would benefit from the information; whether the actor has corrected the information blocking; and time since the information blocking occurred. Other commenters recommended drawing greater attention to repeat offenders and actors who continue to perform the same type of information blocking for an extended period of time over actors who had a single violation that they remediated quickly.
Response. We appreciate commenters' suggestions, but we did not propose to utilize criteria to determine whether to publicly post information about a health care provider and decline to adopt them in this final rule. We believe it is important to provide transparency with respect to any determination of information blocking that has resulted in a health care provider being subject to a disincentive in order to increase understanding about how and where information blocking is impacting the nationwide health information technology infrastructure, including the scope of information blocking practices that have resulted in disincentives.
Regarding the suggested factor which referenced “minor conduct undertaken in good faith,” we remind readers, as we did in a prior response, that information blocking has an element of intent. For health care providers, that intent is that the health care provider knows that a practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information.
We also remind readers that, as discussed in III.B.1.a. of this final rule, OIG expects to use four priorities to inform decisions about which information blocking allegations to pursue: (i) resulted in, are causing, or have the potential to cause patient harm; (ii) significantly impacted a provider's ability to care for patients; (iii) were of long duration; and (iv) caused financial loss to Federal health care programs, or other government or private entities. However, these priorities are not dispositive. OIG will assess each allegation to determine whether it implicates one or more of the enforcement priorities, or otherwise merits further investigation and potential enforcement action, and OIG may evaluate allegations and prioritize investigations based in part on the volume of claims relating to the same (or similar) practices by the same entity or individual.
Comments. A few commenters recommended that only deidentified actor information be posted, at least initially. One commenter did not support public posting of obstetrician-gynecologists' names, practice information and information blocking determination, expressing concern about criminalization and scrutiny of reproductive healthcare data. One commenter recommended that rather than listing the details of information blockers, ONC list all health care providers who are successfully exchanging information.
Response. We disagree with the commenter that only deidentified actor information should be publicly posted. The identification of health care providers that have committed information blocking is important for the public to be aware of the particular circumstances in which information blocking is occurring and, therefore, to understand which aspects of the nationwide health information technology infrastructure are being impacted. We also disagree with the commenter that we should not publicly post information regarding obstetrician-gynecologists. If a health care provider has been determined by OIG to have committed information blocking and is subject to a disincentive, we will post information regardless of their specialty or practice. To promote transparency, we believe it is important to release information about actors that have been determined by OIG to have committed information blocking to inform the public about how and where information blocking is occurring within the broader health information technology infrastructure. For more information about concerns regarding withholding electronic health information related to reproductive health care, we refer readers to a May 13, 2024, blog post on ONC's website [ 23 ] that explains how the “HIPAA Privacy Rule to Support Reproductive Health Care Privacy” final rule ( 89 FR 32976 ), which appeared in the Federal Register on April 26, 2024, and the information blocking regulations work together to protect the privacy of such health information.
Regarding the recommendation to post information about those health care providers that are successfully exchanging information, we note that we did not propose to do so in the Disincentives Proposed Rule and decline to finalize such a policy in this final rule. We also note that some of this type of information may be made available through existing mechanisms. For instance, the Medicare Promoting Interoperability Program and MIPS Promoting Interoperability performance category involve public reporting components about health care provider performance in these programs, which Start Printed Page 54682 can reflect successful performance on measures of health information exchange that contribute to performance under these programs.
Comments. A few commenters recommended posting the year or timeframe in which the information blocking violation occurred. Several commenters recommended establishing a time limit on posting the information to the website by which to remove health care providers from the information blocking list on the website.
Response. We thank commenters for the recommendation to include the timeframe during which the information blocking occurred as part of the information we are publicly posting. We stated in the Disincentive Proposed Rule that we sought to help the public understand “how” and “where” information blocking is occurring within the broader health information technology infrastructure ( 88 FR 74953 ). We agree that information about when information blocking occurred is a critical piece of information and that this concept is implicit in our interest in providing transparency regarding “how” and “where” information blocking occurred to support understanding of the scope of information blocking practices over time that impact the nationwide health information technology infrastructure. To this point, knowing when information blocking occurred is part of knowing how it occurred. Without this information, the public, including other health care providers, would not know whether a particular practice determined to be information blocking was a recent occurrence that may have implications for their own recent or current interactions with the health care provider that was found to have committed information blocking; or whether the practice occurred at a time when such health care providers had no interactions with the health care provider found to have committed information blocking. Therefore, we agree with commenters that it would be appropriate to explicitly identify the timeframe as part of the “description of the [information blocking] practice” that we proposed to include in the information for posting in 45 CFR 171.1101(a)(1)(iii) for health care providers and 45 CFR 171.1101(b)(1)(iii) ( 88 FR 74954 ). Accordingly, we have modified the language in 45 CFR 171.1101(a)(1)(iii) and 45 CFR 171.1101(b)(1)(iii) to clarify that the description of the practice includes when the practice occurred.
We did not propose to put a time limit on how long the information would be posted on ONC's website, and we are not adopting the commenter's recommendation. We may consider this recommendation in future rulemaking.
Comments. One commenter expressed concern that HHS could further use the posted information to apply additional disincentives or bar a physician from participation in other programs and that additional rulemaking would be needed for such uses.
Response. We appreciate the commenter's concern; however, we note that the imposition of a disincentive would be based on a referral from OIG of its determination that a health care provider committed information blocking, rather than the public posting of information on ONC's website. Moreover, we note that we have finalized that the disincentives established for health care providers pursuant to PHSA section 3022(b)(2)(B) are listed in 45 CFR 171.1001 . Other actions not listed in 45 CFR 171.1001 taken by Federal programs based on the information publicly posted on ONC's website would not be a disincentive and are outside the scope of this final rule.
Comments. One commenter stated that public posting of information would lead to unintended consequences such as distrust or an adversarial relationship between actors subject to the information blocking regulations and HHS. Another commenter expressed concern that public posting, combined with the potential for significant disincentives, would deter information blocking complaints. The commenter stated that the health data interoperability community is dependent upon good working relationships between individuals and organizations that operate in the space and that a complainant may refrain from submitting information blocking claims in order to maintain a good relationship with the individual or entity alleged to have committed information blocking.
Response. We appreciate commenters' input but believe that the value of publicly posting this information outweighs any concerns about increasing distrust between health care providers and HHS or between health care providers and other entities supporting health information exchange. We note that information blocking negatively impacts health care providers by limiting access to electronic health information that may be necessary for effective care delivery and suggest that all parties committed to increasing the exchange of electronic health information should support the public availability of information about how and where information blocking is impacting the nationwide health information technology infrastructure.
After consideration of the public comments, we have finalized these proposals with the modifications discussed above.
In the Disincentives Proposed Rule, we proposed to establish a set of disincentives for health care providers that have committed information blocking ( 88 FR 74954 through 74966 ). We noted that each of the proposed disincentives would be imposed by CMS following a referral of a determination of information blocking by OIG. We stated that each of the disincentives was being proposed using authorities under applicable Federal law, consistent with PHSA section 3022(b)(2)(B).
In the Disincentives Proposed Rule, we stated that the proposed disincentives would apply to a subset of the individuals and entities meeting the information blocking regulations' definition of health care provider at 45 CFR 171.102 ( 88 FR 74954 and 74955 ). As discussed hereafter, this rule establishes disincentives for health care providers (as defined in 45 CFR 171.102 ) that are also eligible to participate in certain Federal programs: the Medicare Promoting Interoperability Program and the MIPS Promoting Interoperability performance category (previously the EHR Incentive Programs); and the Medicare Shared Savings Program.
In the Disincentives Proposed Rule, we recognized that the disincentives proposed would only apply to certain health care providers and that the information blocking regulations are also applicable to health care providers that are not eligible to participate in these programs ( 88 FR 74955 ). However, the policies we have finalized in this rule are a first step that focuses on authorities that pertain to certain health care providers that furnish a broad array of healthcare services to large numbers of Medicare beneficiaries and other patients. We believe optimal deterrence of information blocking calls for imposing appropriate disincentives on all health care providers (as defined at 45 CFR 171.102 ) determined by OIG to have committed information blocking. In section IV. of this final rule, we acknowledge public comments received in response to a request for information on establishing disincentives, using applicable Federal law, that could be Start Printed Page 54683 imposed on a broader range of health care providers.
In the Disincentives Proposed Rule, we stated that we believe the proposed disincentives would deter information blocking by health care providers. However, we recognized that the actual monetary impact resulting from the application of the disincentives may vary across health care providers subject to the disincentive ( 88 FR 74955 ). For example, the disincentive proposed in section III.C.3. of the Disincentives Proposed Rule, for the MIPS Promoting Interoperability performance category, would result in an adjustment to payments under Medicare Part B to MIPS eligible clinicians (as defined in 42 CFR 414.1305 ). This disincentive would reduce to zero the Promoting Interoperability performance category score of any MIPS eligible clinician that has been determined by OIG to have committed information blocking (as defined at 45 CFR 171.103 ) during the calendar year (CY) of the referral of a determination from OIG. However, the actual financial impact experienced by a health care provider because of this proposed disincentive being applied in MIPS would vary. For example, Part B payments to the MIPS eligible clinician are subject to a MIPS payment adjustment factor, which CMS determines based on the MIPS eligible clinician's final score. We noted that, in determining each MIPS eligible clinician's final score, CMS considers the assigned weight of, and the MIPS eligible clinician's performance in, the four MIPS performance categories, including the Promoting Interoperability performance category. The MIPS eligible clinician's final score then determines whether the eligible clinician earns a negative, neutral, or positive payment adjustment factor that will be applied to the amounts otherwise paid to the MIPS eligible clinician under Medicare Part B for covered professional services during the applicable MIPS payment year ( 88 FR 74955 ).
In the interest of addressing this variability, we discussed in the Disincentives Proposed Rule that we had considered whether we could propose an alternative approach under which we would tailor the monetary impact of a disincentive imposed on a health care provider to the severity of the conduct in which the health care provider engaged ( 88 FR 74955 ). However, we stated that we did not believe it would be feasible to develop such an approach for the disincentives proposed for health care providers. We noted that, because disincentives must be established using authorities under applicable Federal law, the statute under which a disincentive is being established would need to specifically authorize or provide sufficient discretion for an appropriate agency to be able to adjust the monetary impact of the disincentive to fit the gravity or severity of the information blocking the health care provider has been determined to have committed. We noted that, based on our review of potential authorities under which to establish disincentives, we believed many authorities do not provide discretion to adjust the monetary impact of a potential disincentive in this fashion. For instance, in the Disincentives Proposed Rule, CMS proposed to establish a disincentive through the Medicare Promoting Interoperability Program utilizing authority in SSA section 1886 ( 88 FR 74955 ). Under this authority, CMS, as specified in section 1886(b)(3)(B)(ix)(I) of the SSA, adjusts payments for eligible hospitals by a fixed proportion, based on whether or not an eligible hospital (as defined in section 1886(n)(6)(B) of the SSA) is a meaningful EHR user.
We did not make any proposals in this section of the Disincentives Proposed Rule; however, we summarize and respond below to general comments that we received on this discussion.
Comments. Some commenters expressed support for disincentives for health care providers who have been found to have committed information blocking. These commenters expressed that these disincentives will lead to better patient outcomes, improved information sharing, increased transparency, a reduction in systemic inefficiency and waste, and improved accountability and compliance. Some commenters agreed that the three programs described in the Disincentives Proposed Rule (that is, the Medicare Promoting Interoperability Program for eligible hospitals and CAHs, the Promoting Interoperability performance category of MIPS, and the Medicare Shared Savings Program) are appropriate programs under which to establish disincentives.
Response. We thank commenters for their support of the proposed disincentives.
Comments. One commenter expressed that the proposed disincentives impose substantial punishments on health care providers found to have engaged in information blocking and thereby exceed the regulatory authorities delegated to HHS agencies by Congress. The commenter stated that the term “disincentivize” means the act of creating a disincentive or withdrawing a previously existing incentive. However, the commenter stated that the Disincentives Proposed Rule proposed penalties that would impose significant punishments on health care providers found to have engaged in information blocking. The commenter cited West Virginia v. EPA, [ 24 ] to suggest that the rule “may” have the type of significant impact that requires Congress explicitly to grant regulatory power to the agency.
Response. We disagree that the disincentives that CMS has finalized in section III.C. of this final rule exceed the regulatory authority Congress granted to the Secretary in the Cures Act. Section 4004 of the Cures Act amended the PHSA to create section 3022(b)(2)(B), which states that a health care provider “shall be referred to the appropriate agency to be subject to appropriate disincentives using authorities under applicable Federal law, as the Secretary sets forth through notice and comment rulemaking.” The commenter does not dispute that each of the disincentives CMS proposed and has finalized in this section (III.C.) use authorities under applicable Federal law, and we are adopting each disincentive through this notice-and-comment rulemaking. The agency is applying existing authorities to individuals and entities that are already subject to them, to disincentivize one set of prohibited behaviors. This is not one of the “extraordinary cases” in which the “history and the breadth of the authority that the agency has asserted, and the economic and political significance of that assertion” merits increased scrutiny. [ 25 ] Even if it did, the statute has specifically delegated responsibility for establishing appropriate disincentives to the Secretary of HHS, through notice and comment rulemaking, and so provides all express authorization that might be needed.
The commenter reads the term “disincentive” to exclude penalties or punishment. We agree that we should account for statute's use of the term “disincentives.” We do so by adopting a definition of “disincentive” in 45 CFR 171.102 that includes conditions imposed by an appropriate agency on a health care provider that OIG determines has committed information blocking, for the purpose of deterring information blocking. A disincentive could be any condition that would have a deterrent effect on information blocking, as explained in section III.A.3. of this final rule. But we reject the commenter's effort to draw a strict line between deterrence and punishment. Start Printed Page 54684 Those two concepts are often interrelated. [ 26 ]
Finally, CMS has finalized disincentives that are designed to deter information blocking; they are not impermissibly punitive. As discussed in section III.C.2. of this final rule, a reduction of three quarters of the annual market basket update deters eligible hospitals from engaging in information blocking because it would reduce the inpatient prospective payment system (IPPS) payment that an eligible hospital could have earned had it met other requirements under the Medicare Promoting Interoperability Program. For CAHs, receiving 100 percent of reasonable costs instead of the 101 percent of reasonable costs that a CAH may have earned for successful participation in the Medicare Promoting Interoperability Program deters information blocking by CAHs because it reduces the reimbursement a CAH could have received had it met other requirements under the Medicare Promoting Interoperability Program. For MIPS eligible clinicians, the disincentive under the MIPS Promoting Interoperability performance category deters information blocking by other MIPS eligible clinicians because a MIPS eligible clinician who receives a score of zero in the MIPS Promoting Interoperability performance category under the disincentive cannot earn a positive MIPS payment adjustment factor that they otherwise could have earned for their performance in MIPS ( 88 FR 74960 ). Finally, the disincentive CMS has finalized under the Shared Savings Program deters information blocking by potentially withholding revenue which an ACO or participant in an ACO might otherwise have earned through participation in the Shared Savings Program.
Comments. Many commenters expressed concern that the proposed disincentives will have a differential impact, are variable and confusing, and are not equitable across programs, circumstances of individual health care providers, and years. A few commenters expressed concern that there would be a much greater burden for clinicians in the Shared Savings Program compared to clinicians who are only subject to disincentives under the MIPS Promoting Interoperability performance category, because the monetary disincentive would be much greater for Shared Savings Program clinicians and would potentially interrupt care coordination and harm Medicare beneficiaries' care. Others stated that hospitals could be disproportionately impacted, citing concerns about the high variability of disincentive amounts that could be imposed on hospitals based on the market basket increase in a given year and the proportion of Medicare patients served. Commenters also noted that hospitals face unique financial and operational challenges, such as narrow operating margins and minimal reserves. Several commenters expressed concern that disincentives would lead to a larger burden and impact for health care providers with a larger proportion of Medicare claims, patients, and reimbursement. Several commenters expressed concern that disincentives would vary from year to year based on the value of the market basket adjustment and certain performance incentives in a given year. A few commenters specifically expressed concern that variation in disincentives between referral years could be based on how quickly OIG processes the case and refers it to CMS for action.
Response. We understand commenters' concerns about the potential for the disincentives CMS has finalized in this rule to vary based on factors related to the circumstances of the health care provider, such as the amount of Medicare reimbursement received. However, under PHSA section 3022(b)(2)(B), we must establish disincentives “using authorities under applicable Federal law.” As discussed in section III.A.2. of this final rule, we may therefore only establish, through notice and comment rulemaking, a disincentive for health care providers using an authority Congress has previously granted to an appropriate agency. Where these authorities result in differential treatment of a health care provider based on the health care provider's circumstances or based on changes to the regulations promulgated under that authority over time, these elements will ultimately impact the value of the disincentive established under that authority. We acknowledged this variability, providing a specific example with respect to the MIPS Promoting Interoperability performance category, in the Disincentives Proposed Rule ( 88 FR 74955 ).
However, we disagree that this variability is a compelling reason to not establish a certain disincentive. Such variability already exists as part of these programs. For instance, the monetary impact on an eligible hospital that is not a meaningful EHR user because it fails to meet the objectives and measures associated with the Medicare Promoting Interoperability Program will be higher for an eligible hospital that receives a greater volume of Medicare payment than an eligible hospital that receives a lower volume of Medicare payment. Under section 1886(b)(3)(B)(ix) of the SSA, if an eligible hospital does not demonstrate that it has met the requirements to be a meaningful EHR user under section 1886(n)(3)(A), CMS reduces the eligible hospital's payment by three quarters of the applicable percentage increase in the market basket update or rate-of-increase for hospitals. Under SSA 1886(b)(3)(B), the market basket update is a percentage applied to a hospital's base operating cost, meaning that the monetary value of the market basket update depends on the hospitals' base operating cost. This variability is integrated into the authority Congress established for the program, and Congress has required the Secretary to establish appropriate disincentives using authorities under Federal law.
We further disagree with the commenters that ensuring equitable treatment across programs is necessary to finalize the disincentives we are establishing in this final rule. The authorities under which we have finalized disincentives require health care providers to satisfy certain requirements in order to participate in a program that may provide incentives or other benefits. In the case of the MIPS Promoting Interoperability performance category and the Medicare Promoting Interoperability Program, eligible clinicians, and eligible hospitals and CAHs, have the opportunity earn positive Medicare payment adjustments as specified under each authority. Under the Shared Savings Program, ACOs, ACO providers/suppliers, and participants have the opportunity to earn additional revenue through participation in an ACO if the ACO meets the requirements to earn shared savings payments.
As discussed in section III.C., by committing information blocking, a health care provider is engaging in behavior that conflicts with core requirements of each of these programs. Health care providers that participate in CMS programs offering opportunities to receive positive payment adjustments or additional revenue take on increased responsibilities associated with these programs. To deter information blocking, we believe that where a health care provider commits information blocking, it should not receive these benefits, consistent with the increased responsibilities that these programs impose. Thus, as discussed by CMS under each part of this section (III.C.) in which it has finalized a disincentive, Start Printed Page 54685 each of these disincentives is warranted under the authorities that CMS has used to establish the disincentive.
Comments. Many commenters expressed concern that the proposed disincentives could be extreme or harsh for health care providers. A few commenters expressed concern that the burden of health care provider information blocking disincentives would be greater for smaller, safety net, and less resourced health care providers. One commenter expressed concern that the disincentives would create extra burden for health care providers or sites of service that lack experience with electronic health records.
Response. We appreciate commenters' concerns regarding the potential impact of the proposed disincentives, especially on smaller health care providers. However, we remind readers that, as noted in section III.A.3. of this final rule, we believe that disincentives should have the effect of deterring information blocking practices. We also remind readers that, in order for a practice by a health care provider to be considered information blocking under PHSA section 3022(a), the health care provider must know that “such practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information.” Therefore, we believe that health care providers can avoid the burden of the finalized disincentives by not engaging in information blocking, including conduct that the health care provider knows is unreasonable. Finally, we note that certain authorities used by CMS to finalize disincentives in this final rule include policies which already reflect the size of the health care provider, such as payment adjustments which reflect the volume of payments received by a health care provider under Medicare.
Regarding the comment that disincentives will create additional burden for health care providers that lack experience with electronic health records, we understand that commenters are concerned that a health care provider could be determined to have committed information blocking due to a lack of knowledge or expertise about technology tools used to exchange of electronic health information. However, we wish to emphasize that for a practice committed by a health care provider to meet the definition of information blocking, the health care provider must know that such practice is unreasonable, as discussed above.
Comments. Many commenters expressed concern that the proposed disincentive structure does not provide flexibility for HHS to apply disincentives that are reasonable, appropriate, and proportional for the specific instance of information blocking. Many commenters recommended that disincentives should be tailored to the severity or frequency of conduct, or the degree to which the conduct resulted in patient harm. Other commenters suggested tailoring disincentives based on other factors such as: whether the health care provider participates in an HIE; whether a health care provider made a good faith attempt to not engage in information blocking or comply with an exception; whether the health care provider made proactive efforts to promote access to information; state-specific circumstances affecting the health care provider; and whether the health care provider is engaged in complicated medical areas, such as reproductive and gender-affirming care. Commenters expressed that tailoring disincentives in this way would increase the proposed policy's effectiveness and reduce disproportionate impact. Some commenters recommended including a maximum disincentive amount to ensure health care providers are not unduly penalized. Commenters stated that although the Cures Act requires the disincentives to be made “using authorities under applicable Federal law,” such language could permit different disincentive thresholds, scaling, or other ways to establish and appropriately calibrate financial penalties. A few commenters recommended that the alternative policy discussed in the Disincentives Proposed Rule for the Shared Savings Program, in which CMS would review other facts and circumstances of the case should be applied for all health care provider information blocking disincentives to allow for consideration of frequency, severity, and intent and to allow for remediation.
Response. We acknowledge commenters' recommendations to link the impact of disincentives to different factors, such as the severity or scale of the conduct. As discussed in the Disincentives Proposed Rule, we considered whether we could propose an alternative approach under which we would tailor the monetary impact of a disincentive imposed on a health care provider to the severity of the conduct in which the health care provider engaged ( 88 FR 74955 ). However, we stated that, because disincentives must be established using authorities under applicable Federal law, the statute under which a disincentive is being established would need to specifically authorize or provide sufficient discretion for an appropriate agency to be able to adjust the monetary impact of the disincentive to fit the gravity or severity of the information blocking the health care provider has been determined to have committed. We further noted that, based on our review of potential authorities under which to establish disincentives, many authorities do not provide discretion to adjust the monetary impact of a potential disincentive in this fashion ( 88 FR 74955 ). For instance, as discussed in the Disincentives Proposed Rule, the authority we used in section 1886(b)(3)(B)(ix)(I) of the SSA to establish a disincentive under the Medicare Promoting Interoperability Program does not allow for such discretion ( 88 FR 74955 ).
In the case of the Shared Savings Program, CMS has finalized a policy based on an alternative proposal discussed in the Disincentives Proposed Rule. This policy will allow the Shared Savings Program to exercise discretion about whether or not to impose a disincentive based on certain factors, consistent with existing discretion exercised by the Shared Savings Program when addressing program integrity issues and issues specific to the effects of imposing a disincentive under the Shared Savings Program on other individuals and entities that may participate in an ACO. CMS states in section III.C.4. that these factors include the time since the information blocking conduct occurred and whether the ACO or provider/supplier has taken steps to mitigate this conduct. However, it is important to note that CMS has finalized this as a policy specific to the Shared Savings Program.
For disincentives established under the Medicare Promoting Interoperability Program and the MIPS Promoting Interoperability performance category, which have been established under different authorities in the SSA, CMS did not propose and has not finalized to take such factors into consideration before imposing a disincentive.
Comments. Many commenters recommended that steps such as initial notices or warnings of non-compliance, education, corrective action, and technical assistance be utilized before applying a disincentive for a health care provider found to have committed information blocking. Several commenters recommended that education and technical assistance should be provided before applying a disincentive for specific health care providers, including health care providers that disproportionately serve low-income, minority, underserved, or Start Printed Page 54686 immigrant populations; solo and small practitioners; and other less resourced health care providers. Some commenters recommended these steps should be used until health care providers gain experience with the information blocking requirements or for first time offenders. Commenters recommended these steps for a number of reasons, including: the information blocking requirements are new and complex and many health care providers do not yet fully understand the requirements; most information blocking is inadvertent and should not be subject to significant penalties as an initial step; such steps could avoid potential negative impacts on patient access and Medicare participation; and corrective steps before a disincentive would better promote information sharing and prevent future information blocking violations.
Many commenters expressed that such approaches would be consistent with other HHS and CMS programs and policies that allow for education and corrective steps. A few commenters expressed that the terminology used in PHSA section 3022(b)(2)(B), “appropriate disincentives,” allows HHS to establish disincentives other than financial disincentives. One commenter stated that the programs HHS is proposing to establish disincentives for already have the authority to provide health care providers with opportunities for corrective action, education, and learning periods before imposing disincentives.
Response. We appreciate the commenters' recommendations to provide for corrective action plans, technical assistance, or other activities for health care providers that have been determined by OIG to have committed information blocking. We note that we did not propose, and have not finalized, that elements such as individualized or corrective action would be generally available to any health care provider that OIG has determined to have committed information blocking. Nor did we propose that activities such as corrective action plans or technical assistance would be generally available to certain types of health care providers, such as less-resourced providers or first-time offenders. Commenters did not identify a separate authority under which to establish the general availability of a corrective action plan process for any health care provider that has been determined by OIG to have committed information blocking. We note that in section III.C.4. of this final rule, CMS has finalized that, prior to imposing a disincentive under the Shared Savings Program, it will take into consideration any evidence that indicated whether conduct that resulted in a determination of information blocking had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence.
Regarding commenters' suggestions to provide education and technical assistance before applying a disincentive for health care providers that are smaller, less resourced, or care for specific populations, we note that any considerations with respect to how an appropriate disincentive should impact health care providers with certain attributes would be addressed by the appropriate agency establishing the disincentive. In section III.A.1, an appropriate agency, in establishing a disincentive, could retain or implement policies based on the type of health care provider subject to the disincentive, including small practices, consistent with the agency's authority. For instance, in section III.A.3. of this final rule we discuss existing regulations under the MIPS Promoting Interoperability performance category which pertain to small practices.
Regarding commenters' suggestion to not impose disincentives on “first-time offenders” and instead utilize a corrective action plan, we note that such a policy ignores both the intent standard for a finding of information blocking by a health care provider and how any disincentive may impact a provider's behavior. To engage in information blocking, a health provider must know that the practice was unreasonable and that the practice was likely to interfere with, prevent, or materially discourage the access, exchange, or use of EHI. This intent standard supports establishing disincentives that would appropriately address and deter such culpable actions by health care providers. Further, creating a blanket policy that would give each health care provider a “free offense” could incentivize providers not to refrain from committing information blocking until they are caught. We do not believe that is the type of “disincentive” required by the statute.
Comments. A few commenters recommended considering whether a health care provider has self-disclosed a violation before applying a disincentive. Several commenters recommended offering a self-disclosure protocol (SDP).
Response. We appreciate the commenters' recommendations. The Cures Act did not require, and we did not propose, to establish a self-disclosure protocol for health care providers who have committed information blocking. We note that OIG stated in the OIG CMP Final Rule that it would make a self-disclosure protocol available to those actors seeking to resolve their information blocking CMP liability ( 88 FR 42824 and 42825 ). However, we do not believe a self-disclosure protocol would be feasible with respect to the finalized disincentives for health care providers. An appropriate agency's ability to adjust a disincentive to reflect the severity of the underlying information blocking conduct is dependent on whether the authority under applicable Federal law used to establish the disincentives allows for such an adjustment, consistent with section 3022(b)(2)(B) of the PHSA. For instance, as discussed previously, we are unable to adjust the amount of the reduction in the market basket increase, which is the basis for the disincentive finalized under the Medicare Promoting Interoperability Program in section III.C.2. of this final rule. With respect to this finalized disincentive, a self-disclosure protocol would have limited utility as we would be unable to adjust a health care provider's “liability”.
Comments. One commenter requested clarification of whether the proposed disincentive structure allows CMS to determine whether to apply a disincentive once it receives a referral from OIG, and if so, if CMS can determine to which program or programs a disincentive may apply (for example if a physician works in a hospital).
Response. Under PHSA section 3022(b)(2)(B), as discussed in section III.A.2. of this final rule, disincentives must be established using authorities under applicable Federal law, as the Secretary sets forth through notice and comment rulemaking. As we have finalized in section III.A., a health care provider who has committed information blocking and is referred by OIG to an appropriate agency could be subject to each disincentive established by the appropriate agency that is applicable to the health care provider. CMS has finalized in the Shared Savings Program a policy in this final rule under which it will consider certain factors prior to taking action against an ACO, ACO participant, or ACO provider/supplier, consistent with existing processes in the Shared Savings Program. CMS did not propose and has not finalized a policy to consider additional factors prior to imposing the disincentives being finalized under the Medicare Promoting Interoperability Program and the MIPS Promoting Interoperability performance category.
Comments. Some commenters expressed concern about the unintended consequences of the proposed Start Printed Page 54687 disincentives. Commenters suggested that the proposed disincentives may discourage health care providers from participating in the Medicare programs, including quality and value-based programs. Additionally, commenters expressed that health care providers who receive a greater proportion of their payments from Medicare would be exposed to greater financial risk under the proposed disincentives and would therefore be disincentivized to treat Medicare beneficiaries. Commenters also stated that the financial impacts of the proposed disincentives could: cause hospitals and health systems to disinvest from health IT; reduce the ability to report existing interoperability measures; increase financial risk for already precarious health care providers; impact access to care; increase documentation burden for health care providers to demonstrate they are not information blocking; reduce physician morale; and increase burnout. A few commenters recommended that HHS design disincentives through collaboration with interested parties. Others recommended that if HHS implements the rule as proposed that it monitor for potential unintended consequences and impacts of the disincentives on deterring information blocking.
Response. We appreciate the commenters' concerns, but we disagree that establishing disincentives will discourage participation in these programs. Each of the programs for which CMS has finalized disincentives already requires health care providers to meet certain requirements, which they have been willing to meet in order to potentially earn the incentives or benefits associated with these programs. To avoid the disincentives finalized by CMS in this rule, health care providers do not need to complete any additional program requirements beyond refraining from conduct that meets the definition of information blocking in the information blocking regulations, which have been effective since April 5, 2021 ( 85 FR 70066 ). Due to the lack of significant administrative burden associated with disincentives, we do not believe finalizing these policies will lead to significant numbers of health care providers forgoing the opportunity to earn the incentives or benefits available from the programs under which we have finalized disincentives.
Comments. Several commenters recommended other authorities under which to propose disincentives, or programs that should serve as models for disincentives. These included: the Administrative Simplification provisions of HIPAA; CMS Conditions of Coverage and Conditions of Participation; electronic prescribing of controlled substances (EPCS) disincentives for certain health care provider types; and CMS' Improper Payment Measurements Program's Payment Error Rate Measurement's (PERM). Commenters identified aspects of these programs that they asserted would be desirable as part of the implementation of disincentives, such as: education and corrective action plans to allow actors to resolve liability; non-punitive methods of resolution; a warning and grace period prior to penalties similar to warnings provided for price transparency requirements; and a tiered approach depending on the severity of the violation, which they stated would result in appropriate disincentives and a more just determination.
Response. We thank commenters for their recommendations and may consider them for future rulemaking.
We did not make any proposals and have not finalized any policies in this section.
In the Disincentives Proposed Rule, CMS stated that we intended to use existing Medicare Promoting Interoperability Program authority concerning the meaningful use of certified EHR technology (CEHRT) to impose disincentives on eligible hospitals and CAHs that OIG determines have committed information blocking (defined in 45 CFR 171.103 ) and for which OIG refers a determination to CMS ( 88 FR 74955 ). Under section 1886(n)(3)(A) of the SSA, an eligible hospital or CAH [ 27 ] is treated as a meaningful EHR user for the EHR reporting period for a payment year if it demonstrates to the satisfaction of the Secretary, among other requirements, that during the EHR reporting period: (1) the eligible hospital used CEHRT in a meaningful manner; and (2) the CEHRT is connected in a manner that provides, in accordance with law and standards applicable to the exchange of information, for the electronic exchange of health information. In the Disincentives Proposed Rule, CMS stated that the requirements for an eligible hospital or CAH to be a meaningful EHR user would be substantially undermined and frustrated if the eligible hospital or CAH commits information blocking, such that application of an appropriate disincentive is warranted ( 88 FR 74955 ).
Under section 1886(b)(3)(B)(ix) of the SSA, if an eligible hospital does not demonstrate that it has met the requirements to be a meaningful EHR user under section 1886(n)(3)(A), CMS will reduce the eligible hospital's payment by three quarters of the applicable percentage increase in the market basket update, or rate-of-increase for hospitals. Under section 1814(l)(4) of the SSA, if the Secretary determines that a CAH has not been a meaningful EHR user for a given EHR reporting period, CMS will pay that CAH 100 percent of its reasonable costs, instead of 101 percent of reasonable costs, which is the amount that the CAH would have received as a meaningful EHR user under the Medicare Promoting Interoperability Program.
As discussed in the Disincentives Proposed Rule, HHS has authority to apply disincentives to both eligible hospitals and CAHs ( 88 FR 74955 ). PHSA section 3022(b)(2)(B) authorizes HHS to apply disincentives to health care providers OIG determines have committed information blocking. As discussed in section II.B.1 of the Disincentives Proposed Rule, HHS has adopted, for purposes of the information blocking regulations in 45 CFR part 171 , the definition of health care provider in section 3000(3) of the PHSA, which includes health care providers that are eligible for participation in the Medicare Promoting Interoperability Program ( 88 FR 74949 and 74950 ). The definition of “health care provider” in section 3000(3) of the PHSA includes “hospital” as a health care provider. Section 1886(n)(6)(B) of the SSA defines the term “eligible hospital” for the purposes of the Medicare Promoting Interoperability Program ( 75 FR 44316 and 44317 ) as “a hospital that is a subsection (d) hospital or a subsection (d) Puerto Rico hospital.” Eligible hospitals are in one of the fifty States or the District of Columbia ( 75 FR 44448 ). Hospitals in Puerto Rico became eligible hospitals for the Medicare Promoting Interoperability Program with the passage of the Consolidated Appropriations Act of 2016 ( Pub. L. 114-113 , Dec. 18, 2015). A CAH is defined in section 1861(mm) of the SSA as “a facility that has been certified as a critical access hospital under section 1820(e).” “Hospital” is not further defined under the PHSA definition in section 3000(3). Therefore, CMS interprets the term “hospital” in section 3000(3) of the PHSA to include both eligible hospitals and CAHs that are Start Printed Page 54688 eligible to participate in the Medicare Promoting Interoperability Program.
As discussed in the Disincentives Proposed Rule, the requirements under SSA section 1886(n)(3)(A) that an eligible hospital or CAH must meet to a be meaningful EHR user, particularly the first two requirements under SSA section 1886(n)(3)(A)(i) and (ii), would be substantially undermined and frustrated if the eligible hospital or CAH commits information blocking, such that application of an appropriate disincentive is warranted ( 88 FR 74956 ). To be considered a meaningful EHR user under section 1886(n)(3)(A) of the SSA, an eligible hospital or CAH must, in brief: (1) demonstrate to the satisfaction of the Secretary the use of CEHRT in a meaningful manner, (2) demonstrate to the satisfaction of the Secretary that their CEHRT is connected in a manner that provides for electronic exchange of health information to improve the quality of health care, and (3) use CEHRT to submit information concerning quality measures and other measures as specified. With respect to the electronic exchange of health information requirement in SSA section 1886(n)(3)(A)(ii), an eligible hospital or CAH must demonstrate to the satisfaction of the Secretary that its CEHRT is “connected in a manner that provides, in accordance with law and standards applicable to the exchange of information, for the electronic exchange of health information to improve the quality of health care, such as promoting care coordination, and . . . demonstrates . . . that the hospital has not knowingly and willfully taken action (such as to disable functionality) to limit or restrict the compatibility or interoperability of the certified EHR technology.” Two examples of the CMS requirements for health information exchange include the requirement for eligible hospitals and CAHs to report on the Health Information Exchange Objective and the Provider to Patient Exchange Objective, both of which are part of the requirements for demonstrating the meaningful use of CEHRT, in accordance with SSA section 1886(n)(3).
As discussed in the Disincentives Proposed Rule, by establishing a disincentive for information blocking under the Medicare Promoting Interoperability Program, CMS is using an authority under applicable Federal law as required in section 3022(b)(2)(B) of the PHSA ( 88 FR 74956 ). Eligible hospitals and CAHs that OIG determines to have committed information blocking, and for which OIG refers its determination to CMS, would be subject to a disincentive under applicable law, as they are participating in the Medicare Promoting Interoperability Program authorized by that applicable law. In addition, the Medicare Promoting Interoperability Program requires eligible hospitals and CAHs to engage in practices that encourage the access, exchange, and use of electronic health information to avoid a downward payment adjustment. The requirements an eligible hospital or CAH must meet to be treated as a meaningful EHR user in section 1886(n)(3)(A)(i) and (ii) of the SSA specify that an eligible hospital or CAH must demonstrate that it meets these requirements “to the satisfaction of the Secretary.” As discussed in the Disincentives Proposed Rule, CMS believes these provisions authorize the Secretary to interpret these requirements through rulemaking as necessary to ensure that an eligible hospital or CAH satisfies the requirements to be a meaningful EHR user as defined by the Secretary ( 88 FR 74956 ). Specifically, CMS believes it is appropriate for the Secretary to interpret these requirements through rulemaking to determine that an eligible hospital or CAH that has committed information blocking, and for which OIG refers its determination of information blocking to CMS, has not met the definition of a meaningful EHR user. This proposal is consistent with the goals of the Medicare Promoting Interoperability Program, which include the advancement of CEHRT utilization, focusing on interoperability and data sharing ( 81 FR 79837 ); information blocking by eligible hospitals and CAHs would frustrate both these goals ( 88 FR 74956 ).
In the Disincentives Proposed Rule, CMS also stated that it believes the proposed disincentive under the Medicare Promoting Interoperability Program would be an appropriate disincentive that would deter information blocking by eligible hospitals and CAHs, consistent with the discussion in section III.A.3. of the Disincentives Proposed Rule ( 88 FR 74956 ). While the exact monetary impact of the disincentive would vary based on the specific eligible hospital, CMS believes a reduction of three quarters of the annual market basket update would deter eligible hospitals from engaging in information blocking because it would reduce the inpatient prospective payment system (IPPS) payment that an eligible hospital could have earned had it met other requirements under the Medicare Promoting Interoperability Program. Similarly, though the exact dollar amount would vary based on the specific CAH, CMS believes that receiving 100 percent of reasonable costs instead of the 101 percent of reasonable costs that a CAH may have earned for successful participation in the Medicare Promoting Interoperability Program would deter information blocking by CAHs because it would reduce the reimbursement a CAH could have received had it met other requirements under the Medicare Promoting Interoperability Program ( 88 FR 74956 ).
In the Disincentives Proposed Rule, HHS analyzed the range of potential disincentive amounts an eligible hospital could be subject to if the proposed disincentive was imposed, to illustrate the degree to which this disincentive could deter eligible hospitals from engaging in information blocking. For more information about this analysis, we refer readers to the Disincentive Proposed Rule ( 88 FR 74956 and 74957 ).
In the Disincentives Proposed Rule, CMS proposed to revise the definition of “Meaningful EHR User” in 42 CFR 495.4 to state that an eligible hospital or CAH is not a meaningful EHR user in a calendar year if OIG refers a determination that the eligible hospital or CAH committed information blocking, as defined at 45 CFR 171.103 , during the calendar year of the EHR reporting period ( 88 FR 74957 ). As a result of the proposal, CMS would apply a downward payment adjustment under the Medicare Promoting Interoperability Program to any such eligible hospital or CAH because the eligible hospital or CAH would not be a meaningful EHR user, as required under SSA sections 1886(b)(3)(B)(ix) and 1814(l)(4). For eligible hospitals, CMS would apply the downward adjustment to the payment adjustment year that occurs 2 years after the calendar year when the OIG referral occurs. For CAHs, CMS would apply the downward adjustment to the payment adjustment year that is the same as the calendar year when the OIG referral occurs.
In the Disincentives Proposed Rule, CMS noted that as a result of these proposals, an eligible hospital or CAH that otherwise fulfilled the required objectives and measures to demonstrate that it is a meaningful EHR user for an EHR reporting period would nevertheless not be a meaningful EHR user for that EHR reporting period if Start Printed Page 54689 OIG refers a determination of information blocking to CMS during the calendar year in which the EHR reporting period falls ( 88 FR 74957 ). CMS considered applying this proposed disincentive based on the date that the eligible hospital or CAH committed the information blocking as determined by OIG, instead of the date OIG refers its determination to CMS. However, a significant amount of time could pass between the date when the eligible hospital or CAH is determined to have committed information blocking, and the date when OIG makes a referral to CMS, due to the time required for OIG to fully investigate a claim of information blocking. Such delay between the date the information blocking occurred, and OIG's referral could complicate the application of the disincentive and would likely necessitate reprocessing of a significant number of claims. Therefore, CMS proposed to use the date of the OIG referral instead of the date of the information blocking occurrence to apply the proposed disincentive. Accordingly, CMS would apply the proposed disincentive to the payment adjustment year associated with the calendar year in which the OIG referred its determination to CMS ( 88 FR 74957 ).
CMS further noted in the Disincentives Proposed Rule that if an eligible hospital or CAH received the applicable downward payment adjustment because CMS had already determined the eligible hospital or CAH had otherwise not been a meaningful EHR user during the applicable EHR reporting period due to its performance in the Medicare Promoting Interoperability Program, imposition of the proposed disincentive would result in no additional impact on the eligible hospital or CAH during that payment adjustment year ( 88 FR 74957 ). Finally, even if multiple information blocking violations were identified as part of OIG's determination (including over multiple years) and referred to CMS, each referral of an information blocking determination by OIG would only affect an eligible hospital's or CAH's status as a meaningful EHR user in a single EHR reporting period during the calendar year when the determination of information blocking was referred to CMS by OIG. Unless OIG makes an additional referral of an information blocking determination in the subsequent calendar year, an eligible hospital or CAH would again be able to qualify as a meaningful EHR user starting in the subsequent EHR reporting period ( 88 FR 74957 ).
CMS invited public comment on these proposals, particularly on its approach to the application of a disincentive for OIG determinations that found that information blocking occurred in multiple years and whether there should be multiple disincentives for such instances (for example, disincentives in multiple calendar years/reporting periods compared to only the calendar year/reporting period in which OIG made the referral). The following is a summary of the comments we received and our responses.
Comments. One commenter supported our proposal to apply disincentives to eligible hospitals and CAHs, referred by OIG to CMS, for information blocking. The commenter stated that the approach would not involve additional services or requirements for patients, and that this structure incentivizes the use of health IT and exchange of electronic health information.
Response. We thank this commenter for their support and agree that using an existing program and its existing structure to establish a disincentive, without including additional requirements for eligible hospitals and CAHs, does incentivize the meaningful use of CEHRT. We also agree that this approach continues to promote the interoperable exchange of health information for patients, eligible hospitals, and CAHs.
Comments. One commenter supported the underlying goal of encouraging information exchange but strongly opposed the proposed disincentive. They stated that these disincentives could damage essential eligible hospitals and CAHs and undermine HHS goals by decreasing resources available to otherwise make appropriate investments in their IT infrastructure. Several commenters opposed the disincentive stating that it is excessive, potentially harmful to already fragile eligible hospitals and CAHs, and has the potential to eliminate annual payment updates for offenders. Several commenters stated that this disincentive is unsustainable financially.
Response. We thank commenters for sharing this feedback and expressing their concerns. We disagree that this disincentive is unsustainable, excessive, and potentially harmful. This disincentive utilizes the existing payment adjustments that are currently applied under the Medicare Promoting Interoperability Program (previously the Medicare EHR Incentive Program) and were authorized as part of the American Recovery and Reinvestment Act of 2009, and we have chosen to use that authority for these payment adjustments to establish a disincentive for information blocking determinations by OIG. As described, we consider eligible hospitals and CAHs that commit information blocking as not demonstrating the meaningful use of CEHRT. We are aligning the disincentive we are finalizing with the existing process for those who do not meet the minimum requirements for demonstrating the meaningful use of CEHRT.
In addition, there are eligible hospitals and CAHs that receive the same payment adjustment as would apply under this disincentive due to their failure to participate, or through unsuccessfully demonstrating meaningful use by not meeting the minimum program requirements in the EHR reporting period for a payment adjustment year. These hospitals would not experience an additional impact if OIG refers a determination that they committed information blocking, if such eligible hospitals or CAHs also fail to participate or unsuccessfully demonstrate meaningful use by not meeting the minimum program requirements in a given EHR reporting period. Foundationally, being considered a meaningful user of CEHRT in the Medicare Promoting Interoperability Program reflects that an eligible hospital or CAH is meaningfully using health IT and sharing health information. If an eligible hospital or CAH is not meaningfully using CEHRT, including by engaging in information blocking conduct, they would be subject to the same payment adjustment as would an eligible hospital or CAH that fails to meet our other program requirements.
Comments. Many commenters supported our proposed disincentive policy but have asked for an extension in various forms. Some commenters asked that disincentives start 2 years after the effective date of this final rule, to give eligible hospitals and CAHs two additional years of support and education to understand what is considered information blocking, and to ensure adequate training for their staff. Several commenters asked for an undefined grace period to educate staff and utilize support services from OIG, ONC, and CMS, to fully understand these policies before the disincentives are implemented. A few commenters suggested that we delay the disincentives policy, and instead start with a non-enforcement period before punitive penalties begin. Lastly, some commenters asked that we delay the disincentives policy, and instead start with a corrective action plan, followed by punitive penalties in the future. Start Printed Page 54690
Response. We thank commenters for their feedback. We appreciate the suggestions asking for additional support and education and may consider this feedback. However, we do not agree that the disincentive policy should be delayed for a minimum of 2 years after the release of this final rule. As discussed above, the payment adjustment structure for not meeting the definition of being a meaningful user of CEHRT under the Medicare Promoting Interoperability Program is not new or unique to information blocking. Eligible hospitals and CAHs are already subject to payment adjustments under the Medicare Promoting Interoperability Program if they fail to meet the requirements of being a meaningful user of CEHRT based on not meeting minimum program requirements (sections 1886(b)(3)(B) and 1814(l) of the Act). We have finalized our proposal to update the definition of meaningful EHR user in 42 CFR 495.4 to exclude from that definition eligible hospitals and CAHs that OIG refers to CMS based on a determination of information blocking. Therefore, the only additional requirement for eligible hospitals and CAHs is that OIG did not refer a determination that the eligible hospital or CAH committed information blocking as defined at 45 CFR 171.103 during the calendar year of the EHR reporting period. We further note that the information blocking regulations in the ONC Cures Act Final Rule went into effect April 5, 2021 ( 85 FR 70068 ), and several years will have already passed between the date when these regulations went into effect for health care providers and the effective date of this final rule.
We refer readers to section III.B.1. of this final rule which states that OIG will not begin investigating health care providers until after the effective date of this rule, and that OIG will exercise its enforcement discretion not to make any determinations regarding conduct occurring prior to the effective date of this rule for information blocking disincentives. As OIG will not make a determination on conduct occurring prior to the effective date, OIG will not refer any health care providers based on a determination of conduct occurring prior to the effective date of this rule for information blocking disincentives (see also, 88 FR 42823 and 42824 ). This means that no disincentives finalized in this final rule will be applied to conduct occurring before the effective date of this final rule, which is 30 days after the final rule appears in the Federal Register .
Comments. Many commenters asked that CMS reconsider the disincentives policy to reflect a tiered approach, proportional to severity and frequency, suggesting that as proposed, a singular disincentive conflates egregious claims with minor claims, and one-time offenders with repeat offenders. Several commenters suggested that CMS consider applying a disincentive only to egregious claims rather than all claims.
Response. We thank commenters for this feedback. As discussed previously, the definition of meaningful EHR user is central to the Medicare Promoting Interoperability Program and this policy. While we acknowledge there may be varying levels of severity, frequency, and potential patient harm encompassed in different OIG determinations of information blocking, we will receive all determinations of information blocking that are referred to CMS by OIG. As we have finalized our proposal to revise the definition of “Meaningful EHR User,” the disincentive associated with not being a meaningful EHR user would be applying the existing downward adjustment under the Medicare Promoting Interoperability Program. This downward adjustment was established in the American Recovery and Reinvestment Act of 2009, and CMS does not have the flexibility to adjust the level of the downward adjustment utilizing a tiered approach. For instance, as discussed in the Disincentives Proposed Rule ( 88 FR 74955 ), under section 1886(b)(3)(B)(ix)(I) of the SSA, CMS adjusts payments for eligible hospitals by a fixed proportion, based on whether an eligible hospital (as defined in section 1886(n)(6)(B) of the SSA) is a meaningful EHR user.
We note that while our proposed policy states that each referral of an information blocking determination by OIG would only affect an eligible hospital's or CAH's status as a meaningful EHR user in a single EHR reporting period during the calendar year when the determination of information blocking was referred by OIG, it is possible that repeated subsequent determinations could be referred by OIG in future years. We will address all determinations referred by OIG applicable to eligible hospitals and CAHs within the existing payment adjustment under the Medicare Promoting Interoperability Program, as finalized in this final rule.
As for commenters' concerns that a single disincentive conflates egregious claims with minor claims, we remind readers that prior to the application of the disincentive OIG will investigate an allegation and determine if information blocking has occurred. As discussed in III.B.1. of this final rule, OIG's enforcement priorities inform decisions about which information blocking allegations to pursue, but they are not dispositive. Indeed, OIG will assess each allegation to determine whether it implicates one or more of the enforcement priorities, or otherwise merits further investigation and potential enforcement action, and OIG may evaluate allegations and prioritize investigations based in part on the volume of claims relating to the same (or similar) practices by the same entity or individual. Additionally, we take this opportunity to remind readers that CMS's application of a disincentive will be based on the referral of OIG's determination that information blocking has occurred. Information blocking includes an element of intent, which for health care providers is that the health care provider knows that a practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of EHI.
Comments. A few commenters raised concerns regarding the timing between OIG receiving a referral, the claim being referred to CMS, and the timing of the disincentive. Commenters asked that disincentives be the same for all cases of information blocking, rather than based on hospital size, annual market basket updates, or reasonable costs. Under the proposal, if a large eligible hospital and a CAH are each referred to OIG with a claim of information blocking, the penalties vary based on EHR reporting period, size, and hospital type.
Response. We thank commenters for sharing this feedback. We understand that some commenters believe that the disincentive should be based on the date that the information blocking occurred, but doing so would be administratively difficult, and therefore impractical, to implement because it would likely involve reprocessing past claims. Since we expect the time it takes OIG to fully investigate an information blocking claim and refer a determination to CMS will vary, we decided not to use the date that OIG determines information blocking conduct occurred to determine the application of the payment adjustment. Instead, CMS will use the date of the OIG referral to CMS and specify that the eligible hospital or CAH is not a meaningful user of CEHRT for the EHR reporting period in that calendar year. The payment adjustment will apply to the payment adjustment year 2 years Start Printed Page 54691 later. We agree that with the existing payment adjustment under the Medicare Promoting Interoperability Program, there is variation in the annual market basket updates for eligible hospitals and in reasonable costs for CAHs. As a result of that variability, there would be variability in the amount of any disincentives imposed under the Medicare Promoting Interoperability Program as a result of an OIG referral of a determination of information blocking. While CMS did consider alternative approaches ( 88 FR 74957 ), we have finalized our proposal to revise the definition of meaningful EHR user in 42 CFR 495.4 , and therefore the requirements to be considered a meaningful EHR user. While we are mindful there is variation in the monetary impact of payment adjustments under the Medicare Promoting Interoperability Program based on size, hospital type, and timing of receiving the referral of an OIG determination of information blocking, we respectfully disagree with commenters that the monetary impact of the disincentive should be the same for all eligible hospitals or CAHs, as this could disproportionately impact hospitals with lower Medicare claims volumes.
After consideration of the public comments, CMS has finalized our proposal to revise the definition of “Meaningful EHR User” in 42 CFR 495.4 to state that an eligible hospital or CAH is not a meaningful EHR user in a calendar year if OIG refers a determination that the eligible hospital or CAH committed information blocking, as defined at 45 CFR 171.103 , during the calendar year of the EHR reporting period.
For eligible hospitals, CMS will apply a downward payment adjustment to the payment year that occurs 2 years after the calendar year when an OIG referral occurs. This is a reduction of three quarters of the annual market basket update that an eligible hospital could have earned.
For CAHs, CMS will apply a downward payment adjustment to the payment year that is the same as the calendar year when the OIG referral occurs. This reduction results in a payment of 100 percent of reasonable costs instead of the 101 percent of reasonable costs that a CAH could have earned.
Lastly, CMS has finalized our proposal that if multiple information blocking violations are identified as part of OIG's determination (including over multiple years) and referred to CMS, each referral of an information blocking determination by OIG will only affect an eligible hospital's or CAH's status as a meaningful EHR user in a single EHR reporting period during the calendar year when the determination of information blocking was referred to CMS by OIG.
In the Disincentives Proposed Rule, CMS stated that after OIG has determined that a health care provider has committed information blocking and referred that health care provider to CMS, CMS would notify the eligible hospital or CAH that OIG determined that the eligible hospital or CAH committed information blocking as defined under 45 CFR 171.103 , and thus the eligible hospital or CAH was not a meaningful EHR user for the EHR reporting period in the calendar year when OIG referred its information blocking determination to CMS. This notice would be issued in accordance with the notice requirements proposed at 45 CFR 171.1002 , as discussed in section III.B.2. of the proposed rule.
As a result of our proposal to modify the definition of meaningful EHR user in 42 CFR 495.4 , the application of the disincentive would result in a downward payment adjustment for eligible hospitals 2 years after the OIG referral of a determination of information blocking to CMS. Based upon the existing regulation at 42 CFR 495.4 , the downward payment adjustment would apply 2 years after the year of the referral and the EHR reporting period in which the eligible hospital was not a meaningful EHR user. For CAHs, the downward payment adjustment would apply to the payment adjustment year in which the OIG referral was made.
CMS invited public comment on these proposals. The following is a summary of the comments we received and our responses.
Comments. Commenters asked for ample notification from CMS that a determination has been referred from OIG to CMS regarding information blocking.
Response. We thank commenters for their support on this proposal and agree that ample notification and communication is necessary.
After consideration of the public comments, CMS has finalized our proposal that we will notify an eligible hospital or CAH that OIG has determined that the eligible hospital or CAH committed information blocking as defined under 45 CFR 171.103 , and, as a result, that the eligible hospital or CAH was not a meaningful EHR user for EHR reporting period in the calendar year when OIG referred its information blocking determination to CMS.
MIPS requires that MIPS eligible clinicians use CEHRT, as defined at SSA section 1848(o)(4) and 42 CFR 414.1305 , [ 28 ] in a meaningful manner, in accordance with SSA sections 1848(q)(2)(A)(iv) and (B)(iv) and 1848(o)(2) and 42 CFR 414.1375 , to earn a score for the MIPS Promoting Interoperability performance category. In the Disincentives Proposed Rule, CMS stated that we intend to use this existing authority, requiring the meaningful use of CEHRT, to impose disincentives on MIPS eligible clinicians that OIG determines to have committed information blocking as defined at 45 CFR 171.103 ( 88 FR 74957 and 74958 ).
As authorized by the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) ( Pub. L. 114-10 , April 16, 2015), the Quality Payment Program is a value-based payment program, [ 29 ] by which the Medicare program rewards MIPS eligible clinicians who provide high-value, high-quality services in a cost-efficient manner. The Quality Payment Program includes two participation tracks for clinicians providing services under the Medicare program: MIPS and Advanced Alternative Payment Models (APMs). The statutory requirements for MIPS are set forth in SSA sections 1848(q) and (r).
For the MIPS participation track, MIPS eligible clinicians are subject to a MIPS payment adjustment (positive, negative, or neutral) based on their performance in four performance categories (cost, quality, improvement activities, and Promoting Interoperability) compared to the Start Printed Page 54692 established performance threshold for that performance period/MIPS payment year. CMS assesses each MIPS eligible clinician's total performance according to established performance standards with respect to the applicable measures and activities specified in each of these four performance categories during a performance period to compute a final composite performance score (a “final score” as defined at 42 CFR 414.1305 ) in accordance with our policies set forth in 42 CFR 414.1380 .
In calculating the final score, CMS must apply different weights for the four performance categories, subject to certain exceptions, as set forth in SSA section 1848(q)(5) and at 42 CFR 414.1380 . Unless CMS assigns a different scoring weight pursuant to these exceptions, for the CY 2024 performance period/2026 MIPS payment year and subsequent performance periods/MIPS payment years, [ 30 ] the scoring weights are as follows: 30 percent for the quality performance category; 30 percent for the cost performance category; 15 percent for the improvement activities performance category; and 25 percent for the Promoting Interoperability performance category (SSA section 1848(q)(5)(E); 42 CFR 414.1380(c)(1) ).
To calculate the payment adjustment factor that will be applied to the amounts otherwise paid to MIPS eligible clinicians under Medicare Part B for covered professional services during the applicable MIPS payment year, CMS then compares the final score to the performance threshold CMS has established for that performance period/MIPS payment year at 42 CFR 414.1405(b) . The MIPS payment adjustment factors specified for a year must result in differential payments such that MIPS eligible clinicians with final scores above the performance threshold receive a positive MIPS payment adjustment factor, those with final scores at the performance threshold receive a neutral MIPS payment adjustment factor, and those with final scores below the performance threshold receive a negative MIPS payment adjustment factor. As further specified in SSA section 1848(q)(6)(F) and 42 CFR 414.1405 , CMS also applies a scaling factor to determine the MIPS payment adjustment factor for each MIPS eligible clinician, and CMS must ensure that the estimated aggregate increases and decreases in payments to all MIPS eligible clinicians as a result of MIPS payment adjustment factors are budget neutral for that MIPS payment year. As provided in SSA sections 1848(q)(6)(A) and (B)(iv) and 42 CFR 414.1405(c) , the positive MIPS payment adjustment factor may be up to 9 percent for a final score of 100 and the negative MIPS payment adjustment factor may be up to negative 9 percent for a final score of zero.
For MIPS eligible clinicians, SSA section 1848(q)(2)(A)(iv) includes the meaningful use of CEHRT as one of the four performance categories by which a MIPS eligible clinician is assessed to determine a MIPS payment adjustment factor, as discussed previously. CMS refers to this performance category as the Promoting Interoperability performance category. SSA section 1848(q)(2)(B)(iv) provides that the requirements set forth in SSA section 1848(o)(2) for determining whether a MIPS eligible clinician is a meaningful user of CEHRT also apply to CMS's assessment of MIPS eligible clinicians' performance on measures and activities with respect to the MIPS Promoting Interoperability performance category. Also, SSA section 1848(o)(2)(D) generally provides that the requirements for being a meaningful EHR user under section 1848(o)(2) continue to apply for purposes of MIPS.
A MIPS eligible clinician that is not a meaningful user of CEHRT in accordance with SSA section 1848(o)(2)(A) cannot satisfy the requirements of the MIPS Promoting Interoperability performance category and, therefore, would earn a score of zero for this performance category. Applying the weights for the performance categories under 42 CFR 414.1380(c)(1) , a score of zero for the Promoting Interoperability performance category would mean that the maximum final score a MIPS eligible clinician could achieve, if they performed perfectly in the remaining performance categories, would be 75 points.
To be a meaningful EHR user under SSA section 1848(o)(2)(A) (and therefore meet the requirements of the MIPS Promoting Interoperability performance category under SSA section 1848(q)(2)(B)(iv)), a MIPS eligible clinician must meet three requirements related to the meaningful use of CEHRT during a performance period for a MIPS payment year. In brief, the MIPS eligible clinician must: (1) demonstrate to the satisfaction of the Secretary the use of CEHRT in a meaningful manner; (2) demonstrate to the satisfaction of the Secretary that their CEHRT is connected in a manner that provides for electronic exchange of health information to improve the quality of care; and (3) use CEHRT to submit information concerning quality measures and other measures as specified.
More specifically, for the first requirement under SSA section 1848(o)(2)(A)(i), a MIPS eligible clinician must demonstrate, to the satisfaction of the Secretary, that during the relevant performance period, the MIPS eligible clinician is “using certified EHR technology in a meaningful manner.” For the second requirement under SSA section 1848(o)(2)(A)(ii), a MIPS eligible clinician must demonstrate, to the satisfaction of the Secretary, that during the relevant period CEHRT is “connected in a manner that provides, in accordance with law and standards applicable to the exchange of information, for the electronic exchange of health information to improve the quality of health [ 31 ] care, such as promoting care coordination” and the MIPS eligible clinician demonstrates, through “a process specified by the Secretary, such as the use of an attestation” that the MIPS eligible clinician “has not knowingly and willfully taken action (such as to disable functionality) to limit or restrict the compatibility or interoperability of the certified EHR technology.” For the third requirement under SSA section 1848(o)(2)(A)(iii), a MIPS eligible clinician currently must submit information via their CEHRT on “such clinical quality measures and such other measures as selected by the Secretary” in “a form and manner specified by the Secretary,” including measures focused on providing patients with electronic access to their electronic health information, sending electronic health information to other health care providers, and receiving and incorporating electronic health information from other health care providers.
As discussed further in section III.C.3.b. of the Disincentives Proposed Rule ( 88 FR 74959 and 74960 ) and this final rule, these three requirements for a MIPS eligible clinician to be Start Printed Page 54693 determined to be a meaningful user of CEHRT, particularly the first two requirements under SSA section 1848(o)(2)(A)(i) and (ii), would be substantially undermined and frustrated if the MIPS eligible clinician commits information blocking, such that application of an appropriate disincentive is warranted.
In the Disincentives Proposed Rule, CMS stated it believes that the requirements set forth in SSA sections 1848(q)(2)(B)(iv) and 1848(o)(2)(A) for the MIPS Promoting Interoperability performance category are an applicable Federal law for the purposes of establishing a disincentive for a health care provider that participates in MIPS and has been determined by OIG to have committed information blocking ( 88 FR 74959 ). First, the definitions of MIPS eligible clinician and health care provider under 45 CFR 171.102 and the PHSA generally are aligned. Second, committing information blocking not only violates the law and principles set forth in the Cures Act, but also undermines the goals and purpose of the MIPS Promoting Interoperability performance category. On such basis, CMS proposed an appropriate disincentive for MIPS eligible clinicians that OIG determines have committed information blocking and for whom OIG refers its determination of information blocking to CMS, as discussed further in section III.C.3.c. of the Disincentives Proposed Rule ( 88 FR 74959 through 74962 ).
In the Disincentives Proposed Rule, CMS noted that it believes that the definitions of MIPS eligible clinician under the SSA and 42 CFR 414.1305 and health care provider under PHSA section 3000(3) and 45 CFR 171.102 generally are aligned ( 88 FR 74959 ). CMS believes this alignment will permit application of appropriate disincentives, as required by PHSA section 3022(b)(2)(B), to MIPS eligible clinicians, except for qualified audiologists. CMS proposed to codify this exception in the definition of Meaningful EHR User for MIPS at 42 CFR 414.1305 ( 88 FR 74959 ).
Beginning with the 2024 MIPS payment year, a MIPS eligible clinician is defined in 42 CFR 414.1305 as including: (1) a physician (as defined in SSA section 1861(r)); (2) a physician assistant, nurse practitioner, and clinical nurse specialist (as defined in SSA 1861(aa)(5)); (3) a certified registered nurse anesthetist (defined in SSA section 1861(bb)(2)); (4) a physical therapist or occupational therapist; (5) a qualified speech-language pathologist; (6) a qualified audiologist (as defined in SSA section 1861(ll)(4)(B)); (7) a clinical psychologist (as defined by the Secretary for purposes of SSA section 1861(ii)); (8) a registered dietician or nutrition professional; (9) a clinical social worker (as defined in SSA section 1861(hh)(1)); (10) a certified nurse midwife (as defined in SSA section 1861(gg)(2)); and (11) a group, identified by a unique single taxpayer identification number (TIN), with two or more eligible clinicians, one of which must be a MIPS eligible clinician, identified by their individual national provider identifier (NPI) and who have reassigned their billing rights to the single group TIN. However, for a given performance period/MIPS payment year, a MIPS eligible clinician does not include an eligible clinician who meets one of the exclusions set forth in 42 CFR 414.1310(b) , including being a Qualifying APM participant, Partial Qualifying APM Participant that does not elect to participate in MIPS, or does not exceed the low volume threshold (as these terms are defined in 42 CFR 414.1305 ).
Meanwhile, the definition of “health care provider” under PHSA section 3000(3) as implemented in 45 CFR 171.102 , includes the following which are also considered MIPS eligible clinicians: (1) a “group practice” (which is not defined in the PHSA); (2) a physician (as defined in SSA section 1861(r)); (3) practitioners, as defined in SSA section 1842(b)(18)(C) to include: (a) a physician assistant, nurse practitioner, and clinical nurse specialist (as defined in SSA 1861(aa)(5)); (b) a certified registered nurse anesthetist (defined in SSA section 1861(bb)(2)); (c) a certified nurse-midwife (as defined in SSA section 1861(gg)(2)); (d) a clinical social worker (as defined in SSA section 1861(hh)(1)); (e) a clinical psychologist (as defined by the Secretary for purposes of SSA section 1861(ii)); and (f) a registered dietician or nutrition professional; (4) therapists, as defined in SSA section 1848(k)(3)(B)(iii) to include: (a) a physical therapist; (b) an occupational therapist; and (c) a qualified speech-language pathologist; and (5) “any other category of health care facility, entity, practitioner, or clinician determined appropriate by the Secretary” ( 88 FR 74959 ).
At this time, only a qualified audiologist, included in the definition of MIPS eligible clinician in 42 CFR 414.1305 since the CY 2019 performance period/2021 MIPS payment year, is not identified as a health care provider under 45 CFR 171.102 and PHSA section 3000(3). Because qualified audiologists are not included in the PHSA definition of health care provider, CMS proposed that MIPS eligible clinicians who are qualified audiologists would not be subject to the disincentive proposed for the MIPS Promoting Interoperability performance category ( 88 FR 74959 ).
As discussed previously, groups, and multispecialty groups (as defined in 42 CFR 414.1305 ) also are included in the definition of MIPS eligible clinician and therefore are subject to payment adjustments under MIPS based on the performance of MIPS eligible clinicians that are included in these groups, under different sets of regulations in 42 CFR part 414, subpart O . Meanwhile, as discussed previously, the definition of health care provider in PHSA section 3000(3) includes “group practice,” but does not define what this term means. Accordingly, in the Disincentives Proposed Rule, CMS stated that it also believes that a group may be subject to the disincentive proposed for the MIPS Promoting Interoperability performance category if the group has been determined by OIG to have committed information blocking, or if MIPS eligible clinicians included in the group have committed information blocking ( 88 FR 74959 ).
As discussed in the Disincentives Proposed Rule, health care providers that engage in information blocking undermine and frustrate the purpose for requiring MIPS eligible clinicians to use CEHRT in a meaningful manner ( 88 FR 74960 ). Specifically, requiring MIPS eligible clinicians to use CEHRT is not limited to MIPS eligible clinicians adopting and implementing CEHRT for documenting clinical care in lieu of paper-based medical records. For use of CEHRT to be meaningful, SSA section 1848(o)(2)(A) requires that MIPS eligible clinicians use CEHRT to communicate with other treating health care providers, pharmacies, and oversight authorities regarding the patient's health information, including the MIPS eligible clinician's review and treatment of the patient's health. SSA sections 1848(o)(2)(A)(i) and (ii) require that MIPS eligible clinicians demonstrate Start Printed Page 54694 that they are meaningfully using CEHRT's key functionalities, such as electronically prescribing, and ensuring that CEHRT is “connected in a manner that provides, in accordance with law and standards applicable to the exchange of information, for the electronic exchange of health information to improve the quality of health care,” such as “promoting care coordination.” SSA section 1848(o)(2)(A)(ii) further requires that the MIPS eligible clinician demonstrate that they have not “knowingly and willfully taken action (such as to disable functionality) to limit or restrict the compatibility or interoperability” of CEHRT, which is similar to the directive to investigate and discourage information blocking under PHSA section 3022. In the Disincentives Proposed Rule, CMS noted that establishing an appropriate disincentive for information blocking under the MIPS Promoting Interoperability performance category would not only deter information blocking but would strengthen an existing merit-based incentive payment system that already encourages health care providers to support the access, exchange, and use of electronic health information ( 88 FR 74960 ).
Furthermore, the requirements to be treated as a meaningful EHR user in SSA sections 1848(o)(2)(A)(i) and (ii) specify that a MIPS eligible clinician must demonstrate that they meet these requirements to the satisfaction of the Secretary. In the Disincentives Proposed Rule, CMS stated it believes these provisions authorize the Secretary to interpret these requirements through rulemaking as necessary to ensure that a MIPS eligible clinician satisfies the requirements to be a meaningful user of CEHRT as defined by the Secretary ( 88 FR 74960 ). Specifically, CMS noted that it believes it is appropriate for the Secretary to interpret these requirements through rulemaking to determine that a MIPS eligible clinician that has committed information blocking is not a meaningful EHR user ( 88 FR 74960 ). In the Disincentives Proposed Rule ( 88 FR 74960 ), CMS noted that the proposal was consistent with the goals of the MIPS Promoting Interoperability performance category, which include promoting health care efficiency and encouraging widespread health information exchange ( 81 FR 77200 through 77202 ). CMS stated that information blocking by MIPS eligible clinicians frustrates both these goals ( 88 FR 74960 ).
As noted in the Disincentives Proposed Rule, CMS believes a disincentive for information blocking associated with the MIPS Promoting Interoperability performance category would be an appropriate disincentive that would deter information blocking by other MIPS eligible clinicians, consistent with the discussion in section III.A.3. of the Disincentives Proposed Rule ( 88 FR 74960 ). While the exact monetary impact of the disincentive may vary for each MIPS eligible clinician based on the various factors CMS considers when determining the MIPS payment adjustment factor, CMS believes the proposed disincentive would deter information blocking by other MIPS eligible clinicians. In the Disincentives Proposed Rule, CMS noted that a MIPS eligible clinician who receives a score of zero in the MIPS Promoting Interoperability performance category under the proposed disincentive may not be able to earn a positive or neutral MIPS payment adjustment factor that they otherwise could have earned for their performance in MIPS ( 88 FR 74960 ).
In the Disincentives Proposed Rule, to illustrate the degree to which this disincentive could deter information blocking, HHS analyzed the range of potential disincentive amounts MIPS eligible clinicians could be subject to if the proposed disincentive was imposed, using payment and MIPS data from 2021, the most recent year of publicly available data. For more information about this analysis, we refer readers to the Disincentives Proposed Rule ( 88 FR 74960 ).
Under the authority in SSA sections 1848(o)(2)(A) and (D), and 1848(q)(2)(A)(iv) and (B)(iv), for the MIPS Promoting Interoperability performance category, CMS proposed that a MIPS eligible clinician would not be a meaningful EHR user in a performance period if OIG refers a determination that the MIPS eligible clinician committed information blocking (as defined at 45 CFR 171.103 ) at any time during the calendar year of the performance period ( 88 FR 74960 and 74961 ). [ 32 ] CMS also proposed that the determination by OIG that the MIPS eligible clinician committed information blocking would result in a MIPS eligible clinician that is required to report on the MIPS Promoting Interoperability performance category not earning a score in the performance category (a zero score), which is typically a quarter of the total final score. CMS proposed to codify this proposal under the definition of meaningful EHR user for MIPS at 42 CFR 414.1305 and amend the requirements for earning a score for the MIPS Promoting Interoperability performance category at 42 CFR 414.1375(b) ( 88 FR 74960 and 74961 ).
CMS considered applying the proposed disincentive based on the date that the MIPS eligible clinician committed the information blocking as determined by OIG, instead of the date OIG refers its determination to CMS ( 88 FR 74961 ). However, a significant period could pass between the date when the MIPS eligible clinician is determined to have committed information blocking, and the date when OIG makes a referral to CMS, due to the time required for OIG to fully investigate a claim of information blocking. Such delay between the date the information blocking allegedly occurred and OIG's referral could complicate our application of the disincentive and would likely necessitate reprocessing of a significant number of claims. Therefore, CMS decided to use the date of the OIG referral instead of the date of the information blocking occurrence to apply this proposed disincentive. Accordingly, CMS proposed to apply the proposed disincentive to the MIPS payment year associated with the calendar year in which OIG referred its determination to CMS ( 88 FR 74961 ).
As provided in 42 CFR 414.1320 , the applicable MIPS payment year is 2 calendar years after the performance period. The time period between the performance period and the MIPS payment year permits CMS to review each MIPS eligible clinician's performance to determine their final score and MIPS payment adjustment factor. We noted that, under the Start Printed Page 54695 proposal, if OIG referred its determination that a MIPS eligible clinician committed information blocking in calendar year 2025, then CMS would apply the disincentive proposed herein for the 2027 MIPS payment year ( 88 FR 74961 ).
In the Disincentives Proposed Rule, first, CMS proposed to amend the definition of “meaningful EHR user for MIPS” at 42 CFR 414.1305 ( 88 FR 74961 ). The current definition states that a “meaningful EHR user for MIPS means a MIPS eligible clinician who possesses CEHRT, uses the functionality of CEHRT, reports on applicable objectives and measures specified for the Promoting Interoperability performance category for a performance period in the form and manner specified by CMS, does not knowingly and willfully take action (such as to disable functionality) to limit or restrict the compatibility or interoperability of CEHRT, and engages in activities related to supporting providers with the performance of CEHRT.” CMS proposed to add to this definition that a MIPS eligible clinician is not a meaningful EHR user in a performance period if OIG refers a determination that the clinician committed information blocking (as defined at 45 CFR 171.103 ) during the calendar year of the performance period ( 88 FR 74961 ). CMS also proposed other minor technical changes to the language of the definition. In the Disincentives Proposed Rule, CMS noted that, in tandem with other proposals for MIPS in this section, the proposed amendment to the definition in 42 CFR 414.1305 would result in a MIPS eligible clinician not being able to earn points associated with the Promoting Interoperability performance category they may otherwise have earned, potentially resulting in a negative or neutral payment adjustment. As such, we stated that this potential outcome likely would deter health care providers from engaging in information blocking ( 88 FR 74961 ).
Second, CMS proposed to amend the requirements for earning a score for the MIPS Promoting Interoperability performance category by adding a new requirement at 42 CFR 414.1375(b) ( 88 FR 74961 ). Currently, 42 CFR 414.1375(b) provides that, to earn a score (other than zero) for the Promoting Interoperability performance category, the MIPS eligible clinician must meet certain requirements, including using CEHRT, reporting on the objectives and associated measures as specified by CMS, and attesting to certain statements and activities. CMS proposed to amend 42 CFR 414.1375(b) by adding that the MIPS eligible clinician must be a meaningful EHR user for MIPS as defined at 42 CFR 414.1305 . In conjunction with the proposal to amend the definition of a meaningful EHR user for MIPS at 42 CFR 414.1305 discussed previously, CMS noted the proposal would establish a clear basis to apply a score of zero for the MIPS Promoting Interoperability performance category to a MIPS eligible clinician that fails to meet the definition of meaningful EHR user for MIPS during a performance period, specifically if OIG refers a determination of information blocking during the calendar year of the performance period ( 88 FR 74961 ).
In the Disincentives Proposed Rule, CMS noted that, under these proposals, a MIPS eligible clinician that OIG determines has committed information blocking would not be a meaningful EHR user, and therefore would be unable to earn a score (instead, earning a score of zero) for the MIPS Promoting Interoperability performance category ( 88 FR 74961 ). Because a MIPS eligible clinician that has committed information blocking would not be a meaningful EHR user for a given performance period, they would earn a zero for the Promoting Interoperability performance category for the calendar year of the applicable performance period in which the determination of information blocking was referred by OIG. For example, if OIG refers a determination that a MIPS eligible clinician committed information blocking to CMS in CY 2026, CMS would apply a score of zero for the Promoting Interoperability performance category for the 2028 MIPS payment year to the MIPS eligible clinician.
In the Disincentives Proposed Rule, CMS explained that under this proposed disincentive for information blocking, a score of zero for the MIPS Promoting Interoperability performance category would negatively impact 25 percent of the MIPS eligible clinician's final score such that it would likely result in a negative MIPS payment adjustment for the applicable MIPS payment year ( 88 FR 74961 ). For example, applying the weights for the performance categories under 42 CFR 414.1380(c)(1) , a score of zero for the Promoting Interoperability performance category would mean that the maximum final score a MIPS eligible clinician could achieve, if they performed perfectly in the remaining performance categories, would be 75 points.
Then, as discussed previously, to determine the MIPS payment adjustment factor, CMS compares the MIPS eligible clinician's final score to the established performance threshold for that MIPS payment year. In 42 CFR 414.1405(b)(9)(ii) , CMS established that the performance threshold for the 2025 MIPS payment year is 75 points. If, under this example, a MIPS eligible clinician still achieved 75 points for their final score for the 2025 MIPS payment year matching the established performance threshold of 75 points, then they would receive a neutral MIPS payment adjustment factor.
In the CY 2024 Physician Fee Schedule proposed rule, CMS proposed that the performance threshold for the 2026 MIPS payment year would be 82 points ( 88 FR 52596 through 52601 ). This proposal was not finalized in the CY 2024 Physician Fee Schedule Final Rule; instead, CMS finalized the performance threshold for the 2026 MIPS payment year as 75 points at 42 CFR 414.1405(b)(9)(iii) ( 88 FR 79374 through 79376 ). However, if some other performance threshold higher than 75 points is finalized in a future MIPS payment year, then a MIPS eligible clinician (that OIG determined committed information blocking and received a score of zero in the Promoting Interoperability performance category and therefore, under our example, a final score of 75 points) would receive a negative MIPS payment adjustment factor. If CMS finalizes a performance threshold higher than 75 points in a future MIPS payment year, then the proposed disincentive would likely to result in a MIPS eligible clinician that commits information blocking, as determined by OIG, receiving a negative payment adjustment, up to negative nine percent for a final score of zero as set forth in 42 CFR 414.1405(b)(2) and (c) .
In the Disincentives Proposed Rule, CMS explained that, under these proposals, a MIPS eligible clinician, that otherwise fulfilled other requirements to demonstrate meaningful use of CEHRT for a performance period to earn a score for the Promoting Interoperability performance category, would nevertheless not be a meaningful EHR user for that performance period if OIG refers a determination of information blocking during the calendar year of the performance period ( 88 FR 74962 ). This would result in the MIPS eligible clinician nevertheless earning a score of zero for the Promoting Interoperability performance category. [ 33 ] Furthermore, if a MIPS eligible clinician earned a score of zero for the Promoting Interoperability performance category for a given year because CMS had Start Printed Page 54696 already determined the MIPS eligible clinician had otherwise not been a meaningful EHR user in that performance period due to its performance in the Promoting Interoperability performance category, imposition of the proposed disincentive would result in no additional impact on the MIPS eligible clinician during that MIPS payment year ( 88 FR 74962 ).
CMS clarified in the Disincentives Proposed Rule that, even if multiple information blocking violations were identified as part of OIG's determination (including over multiple years) and referred to CMS, each referral of an information blocking determination by OIG would only affect a MIPS eligible clinician's status as a meaningful EHR user in a single performance period during the calendar year when the determination of information blocking was referred by OIG ( 88 FR 74962 ). Barring an additional referral of an information blocking determination by OIG in the subsequent calendar year, a MIPS eligible clinician could be deemed a meaningful EHR user and earn a score for the Promoting Interoperability performance category in the following calendar year.
CMS invited public comment on these proposals. CMS particularly requested comment on its approach to the application of a disincentive for OIG determinations that found that information blocking occurred in multiple years and whether there should be multiple disincentives for such instances (for example, disincentives in multiple calendar years/performance periods compared to only one disincentive in the calendar year in which a referral from OIG is made).
The following is a summary of the comments received and our responses.
Comments. A few commenters supported the proposed application of disincentives in MIPS generally. One commenter noted that the disincentives will incentivize health IT use. Another commenter expressed that the Promoting Interoperability performance category is an appropriate avenue through which to apply the disincentives. A few commenters expressed general support for CMS' goals and purposes in applying disincentives to the MIPS Promoting Interoperability performance category, noting that the proposal is consistent with the Cures Act and that information blocking undermines the meaningful use of certified EHR technology.
Response. We appreciate the support of these commenters. We believe that information blocking disrupts the meaningful use of CEHRT and exchange of electronic health information, as required by SSA section 1848(o)(2)(A)(i) and (ii), and should be deterred. We believe the disincentive will serve as a deterrent to information blocking practices and reduce the incidence of information blocking.
Comments. One commenter supported the proposal that health care providers determined by OIG to have engaged in information blocking should not be considered a meaningful EHR user within MIPS.
Response. We appreciate the support of this commenter. We agree that information blocking is not consistent with the goals of the MIPS Promoting Interoperability performance category to support meaningful use of CEHRT and exchange of electronic health information, as required by SSA section 1848(o)(2)(A)(i) and (ii).
Comments. A few commenters requested clarification on whether the reductions to the MIPS incentive payment will be deemed Recovered Penalty Funds pursuant to the Cures Act.
Response. We note that “reductions to the MIPS incentive payment” does not accurately reflect how MIPS may affect MIPS eligible clinician's payments for covered professional services under Medicare Part B. We refer readers to our description of MIPS, including how CMS assesses a MIPS eligible clinician's performance and calculates and applies MIPS payment adjustment factors in section III.C.3.a. of the Disincentives Proposed Rule ( 88 FR 74957 through 74959 ) and this final rule.
We reiterate that CMS proposed that OIG's referral of its determination that the MIPS eligible clinician committed information blocking would result in CMS specifying that the MIPS eligible clinician is not a meaningful EHR user and assigning the MIPS eligible clinician a score of zero for the Promoting Interoperability performance category. As we described in the Disincentive Proposed Rule, this may result in CMS calculating a MIPS payment adjustment factor that is neutral or negative ( 88 FR 74961 and 74962 ). When applied, a MIPS payment adjustment factor potentially adjusts Medicare Part B payments upwards or downwards and are not considered recovered penalty funds pursuant to Section 4004 of the Cures Act.
Comments. Commenters sought clarity on whether, if an eligible hospital or CAH was found to have committed information blocking for which CMS imposed a disincentive under the Medicare Promoting Interoperability Program, a MIPS eligible clinician that practices in, or is affiliated with that eligible hospital or CAH (for example, an outpatient clinic) would also receive a disincentive under MIPS. Additionally, commenters sought clarity on whether a MIPS-eligible clinician that commits information blocking in a hospital setting would be assigned disincentives under both MIPS and the Medicare Promoting Interoperability Program based on the same finding.
Response. If OIG refers a determination of information blocking for a health care provider, CMS will apply disincentives established through notice and comment rulemaking that are applicable to that health care provider. Accordingly, if OIG found that an eligible hospital or CAH committed information blocking and referred the determination to CMS, we would only impose the disincentive under the Medicare Promoting Interoperability Program, which is applicable to eligible hospitals and CAHs, to the hospital that has committed information blocking. We would not impose a separate disincentive on individual MIPS eligible clinicians that are affiliated with the hospital, provided that OIG did not similarly find that the individual MIPS eligible clinician(s) also committed information blocking and referred that determination to CMS.
Comments. Several commenters expressed concern about the impact the proposed MIPS disincentive will have on patient access to care. A few commenters expressed that MIPS eligible clinicians may take on fewer Medicare beneficiaries as patients as a result of the potential impact of disincentives, while others may choose not to participate in the Medicare program at all, which may ultimately impact beneficiary access to care. One commenter contended that, because psychiatrists may be impacted, Medicare beneficiaries seeking mental health services could be negatively impacted by the proposed disincentives.
Response. While we understand these concerns, MIPS eligible clinicians already are required to demonstrate they are not knowingly or willfully taking actions to limit or restrict the compatibility or interoperability of the CEHRT they use as set forth in SSA section 1848(o)(2)(A)(ii). One of the current requirements of the MIPS Promoting Interoperability performance category is to attest “yes” to the self-reported attestation statement that they did not knowingly or willfully take action to limit or restrict compatibility or interoperability of CEHRT, which may include actions that are information blocking ( 42 CFR 414.1375(b)(3)(iii) ). Start Printed Page 54697
In addition, we believe that the practice of information blocking could cause potential harm to patients. Information blocking does not promote healthcare efficiency and does not encourage widespread health information exchange. We refer readers to our discussion of how information blocking conduct undermines the goals and purpose of the MIPS Promoting Interoperability performance category in section III.C.3.b.(2). of the Disincentives Proposed Rule ( 88 FR 74960 ) and this final rule.
Comments. Several commenters expressed concern about the impact the proposed MIPS disincentive may have to increase burden and financial distress on health care providers. One commenter did not support the proposed impact of disincentive estimates for MIPS, noting that the penalties are economically significant and may be catastrophic for some practices. One commenter stated that this increasing burden is due to the changing nature of the underlying programs, requiring health care providers to continually monitor changes.
Response. Finalizing the proposed disincentive provisions related to MIPS eligible clinicians should not increase burden on clinicians as it does not require the clinician to do anything additional. The proposed disincentive only applies if the MIPS eligible clinician engages in information blocking contrary to statute, including SSA section 1848(o)(2)(A). As for financial distress, MIPS eligible clinicians can avoid receipt of a disincentive for information blocking by not interfering with, preventing, or materially discouraging the access, exchange, or use of electronic health information.
Comments. Several commenters shared recommendations on how CMS should apply disincentives in MIPS. A few commenters recommended that CMS establish more than one disincentive for MIPS eligible clinicians who are referred by OIG to allow the agency flexibility in determining the disincentive appropriate for each case. One commenter recommended that CMS provide clinicians who are successfully sharing information additional points for their MIPS score.
Response. We thank commenters for their feedback. While we initially considered different approaches, we proposed to amend the definition of meaningful EHR user. We proposed that a MIPS eligible clinician who is referred to CMS by OIG for information blocking would not be considered a meaningful EHR user, thereby resulting in earning a zero for the Promoting Interoperability performance category.
While we acknowledge information blocking conduct may vary in levels of severity, frequency, and potential patient harm, we believe our proposed disincentive for MIPS is most closely aligned with the directive at PHSA section 3022(b)(2)(B) (to apply an appropriate disincentive using authorities under applicable Federal law) and the statutory criteria for being treated as a meaningful EHR user in SSA section 1848(o)(2)(A) for the MIPS Promoting Interoperability performance category under SSA section 1848(q)(2)(B)(iv), as discussed previously. Information blocking inhibits the meaningful use of CEHRT and the electronic exchange of health information as required by SSA section 1848(o)(2)(A). Failure to meet all three criteria to be treated as a meaningful EHR user at SSA section 1848(o)(2)(A) means the MIPS eligible clinician has failed to meet the requirements for the MIPS Promoting Interoperability performance category, which we believe warrants a score of zero. We believe this disincentive is most consistent with these statutory requirements for a MIPS eligible clinician to demonstrate they are a meaningful user of CEHRT because, as discussed previously, information blocking undermines the goals and purposes of these requirements.
Comments. A few commenters supported the proposal to use the date of the OIG referral instead of the date of the information blocking occurrence to apply the disincentive within MIPS, stating that this approach would avoid reprocessing of claims, allow health care providers to plan for a disincentive, and prevent additional administrative burden in the process.
Response. We agree that using the date of the referral is the preferred approach as it allows us to apply the disincentive to the applicable MIPS payment year.
Comments. One commenter recommended that CMS apply the disincentive to the performance period following the year in which OIG makes a determination on information blocking. A commenter expressed concern that the two-year period between when the referral occurs and when the disincentive is applied is too long and may not serve to correct health care provider behavior as a result. Another commenter recommended CMS not apply the disincentive in two MIPS payment years unless the information blocking conduct spanned more than 1 year, and that CMS apply the disincentives according to the length of time over which the conduct occurred.
Response. We considered applying the disincentive to the year following the OIG referral but determined that it was not administratively feasible under CMS's existing MIPS policies and processes. We proposed that the disincentive be applied to the MIPS payment year 2 years after the year of the OIG referral. This aligns with current MIPS policy and processes, as the MIPS payment adjustment is applied to the MIPS payment year 2 years after the performance period.
We did not propose to apply the disincentive to multiple years. Even if a referral from OIG identified information blocking conduct that occurred over multiple years, we would only apply a payment adjustment to the year the OIG referral was made.
Comments. One commenter did not support the proposed amendments to the definition of a “meaningful EHR user for MIPS,” noting that the proposed policy does not consider the severity of the information blocking determination and is inconsistent with OIG's existing policies of considering multiple factors prior to determining the severity of a penalty for HIEs/HINs.
Response. We thank the commenter for their feedback; however, we disagree. We believe that any instance of information blocking should not occur. OIG completes their investigation and then refers the determination to CMS. OIG does not impose the disincentive. We recognize that PHSA section 3022(b)(2)(A) states that, for health IT developers of certified health IT and HINs/HIEs who have committed information blocking that are subject to CMPs, the amount of the CMP shall consider factors such as the nature and extent of the information blocking. However, as discussed previously in this rule, this provision does not apply to health care providers that OIG refers to an appropriate agency to be subject to appropriate disincentives using authorities under applicable Federal law, as stated in PHSA section 3022(b)(2)(B). The proposal we have finalized in this final rule is established under the authority for the MIPS Promoting Interoperability performance category in SSA section 1848(q). This authority is discussed previously in detail and in the Disincentives Proposed Rule ( 88 FR 74958 and 74959 ). As we discuss in a previous response to a comment, this authority does not provide us with the ability to adjust payments under MIPS according to a set of factors related to the severity of information blocking practices. Start Printed Page 54698
Comments. Many commenters did not support the proposal to assign a zero score for the MIPS Promoting Interoperability performance category if a health care provider has committed information blocking. Many commenters expressed that the proposed disincentive is too severe, with some expressing concern that it would prevent eligible clinicians from earning a positive payment adjustment under MIPS and would likely result in a negative payment adjustment, especially if the performance threshold is increased in future years. Based on this, one commenter disagreed that the rule is not economically significant.
Response. We thank commenters for their feedback. We believe that committing information blocking is not only inconsistent with PHSA section 3022 but also undermines the goals and purpose of the MIPS Promoting Interoperability performance category. We refer readers to our discussion in section III.C.3.b.(2). of the Disincentives Proposed Rule ( 88 FR 74960 ) and this final rule.
As we discuss in a previous response to a comment, information blocking inhibits the meaningful use of CEHRT and the electronic exchange of health information as required by SSA section 1848(o)(2)(A). Failure to meet all three criteria to be treated as a meaningful EHR user at SSA section 1848(o)(2)(A) means the MIPS eligible clinician has also failed to meet the requirements for the MIPS Promoting Interoperability performance category, which warrants a score of zero. This disincentive is consistent with the statutory requirements for a MIPS eligible clinician to demonstrate they are a meaningful user of CEHRT because, as discussed previously, information blocking undermines the goals and purposes of these requirements.
We disagree that the disincentive is severe. It is closely aligned with the directive at PHSA section 3022(b)(2)(B) (to apply an appropriate disincentive using authorities under applicable Federal law) and the statutory requirements for MIPS. As discussed in section III.C.3.a.(1). of the Disincentives Proposed Rule ( 88 FR 74957 and 74958 ) and this final rule, a MIPS eligible clinician receiving a final score of zero for all applicable performance categories would result in a negative MIPS adjustment factor of negative 9 percent (sections 1848(q)(6)(A) and (B)(iv); 42 CFR 414.1405(c) ). The MIPS statute at SSA sections 1848(q)(6)(A) and (B) establishes the framework by which CMS calculates MIPS payment adjustment factors based on CMS' assessment of MIPS eligible clinicians' performance in the four performance categories. Nothing in the MIPS disincentive we proposed and have finalized in this rule alters that framework. Instead, this disincentive explicitly relies on that framework, providing that an OIG referral of its determination that a MIPS eligible clinician committed information blocking means the MIPS eligible clinician does not meet the requirements for the Promoting Interoperability performance category, and therefore warrants receiving a zero score for that category.
Further, we note that, after application of the linear scaling factor and budget neutrality, a final score above zero, but below the applicable performance threshold, may result in calculation of a MIPS payment adjustment factor between negative 9 percent and zero percent. [ 34 ] Depending on how the MIPS eligible clinician performs in the other performance categories and the weight assigned to the applicable performance categories for the final score, the potential effect of application of this disincentive (a zero score for the Promoting Interoperability performance category) on calculation of the MIPS payment adjustment factor may be limited.
As we state in section VI. of this final rule, the Office of Management and Budget has determined that the proposed rule is not a significant regulatory action as the potential costs associated with the proposed rule would not be greater than $200 million per year nor would this action meet the other conditions necessary to be deemed significant.
Comments. Some expressed concern that disincentives may have a significant negative financial impact on practices. A few commenters contended that the proposed disincentive was too severe for first time offenders. Other commenters expressed concern about the impact this proposal would have on smaller practices, with some expressing concern that it may cause disproportionate financial distress to smaller practices.
Response. We appreciate the feedback, but health care providers, including MIPS eligible clinicians, should not engage in information blocking practices. The impact associated with the disincentive meets our goal of deterring information blocking, which includes “first-time” conduct by health care providers. We also reiterate that information blocking practices by health care providers include an element of intent, in which the health care provider must know that a practice is unreasonable and likely to interfere with the exchange, access or use of electronic health information. We remind readers that we did not propose to modify our reweighting policies and small practices will continue to be automatically reweighted for the Promoting Interoperability performance category as provided in 42 CFR 414.1380(c)(2)(i)(C) ( 9 ).
Comments. One commenter expressed concern that, upon receipt of notice from CMS regarding OIG's finding that the MIPS eligible clinician committed information blocking and application of the disincentive, individual MIPS eligible clinicians or groups will have less incentive to report additional measures under the MIPS Promoting Interoperability performance category. They recommended deducting 10 points from the category score in a calendar year of the performance period if the OIG refers a determination of information blocking. Several commenters recommended that CMS instead implement a scalable system that would impose different disincentives depending on the severity or mitigating factors of the information blocking violation. A few commenters recommended a percentage or point deduction rather than failing the entire Promoting Interoperability performance category and scaling it to severity.
Response. We thank commenters for their input. While we did initially consider some of these alternatives, we ultimately decided not to propose them. As we discuss in a previous response to a comment, the disincentive we proposed and have finalized closely aligns with the directive at PHSA section 3022(b)(2)(B) (to apply an appropriate disincentive using authorities under applicable Federal law) and the statutory requirements MIPS. Information blocking inhibits the meaningful use of CEHRT and the electronic exchange of health information as required by SSA section 1848(o)(2)(A). Failure to meet all three criteria to be treated as a meaningful EHR user at SSA section 1848(o)(2)(A) means the MIPS eligible clinician has also failed to meet the requirements for the MIPS Promoting Interoperability performance category, which warrants a Start Printed Page 54699 score of zero. We believe any other disincentive option would be contrary to these statutory requirements for a MIPS eligible clinician to demonstrate they are a meaningful user of CEHRT because, as discussed previously, information blocking undermines the goals and purposes of these requirements.
The policies that we proposed and have finalized, including modification to the definition of meaningful EHR user for MIPS ( 42 CFR 414.1305 ), will result in a MIPS eligible clinician not being able to earn points associated with the Promoting Interoperability performance category if they were found to have committed information blocking. Regarding the recommendation to tie the disincentive to a reduction of 10 points in the performance category, and the recommendation to tie a point reduction to the severity of the information blocking conduct referred by OIG, we note that we did not propose these alternatives for the reasons stated above.
Comments. One commenter recommended CMS consider additional incentives within the Promoting Interoperability performance category to promote the flow of electronic health information and to deter information blocking.
Response. We appreciate this input and may consider it in future rulemaking. In recent years, we have added measures to the Promoting Interoperability performance category such as the Enabling Exchange under the Trusted Exchange Framework and Common Agreement (TEFCA) measure, to encourage the bi-directional exchange of patient information ( 87 FR 70067 ).
Comments. Another commenter requested CMS clarify how cases in which MIPS eligible clinicians transition from reporting traditional MIPS to MIPS Value Pathways (MVPs) during the OIG investigation would be addressed and whether penalties would be imposed given the different participation options within the MVP framework, expressing concern about confusion and implementation challenges.
Response. The MIPS Promoting Interoperability performance category is a foundational component of every MVP. As such, if a finding of information blocking is referred to CMS by OIG, we would apply the disincentive to the MIPS eligible clinician participating in an MVP.
After consideration of the public comments, CMS has finalized our proposal to revise the definition of “meaningful EHR user” for MIPS at 42 CFR 414.1305 to state that a MIPS eligible clinician is not a meaningful EHR user in a performance period if OIG refers a determination that the clinician committed information blocking, as defined at 45 CFR 171.103 , during the calendar year of the performance period. CMS has also finalized minor technical modifications to this definition as proposed ( 88 FR 74961 ). Consistent with our discussion in section III.C.3.b.(1), CMS has finalized this definition to also exclude a qualified audiologist from application of this disincentive. We originally noted this exclusion in the regulation text we proposed in the Disincentive Proposed Rule ( 88 FR 74968 ). Therefore, CMS has finalized the amendment to the regulatory definition of meaningful EHR user for MIPS at 42 CFR 414.1305 generally as proposed, with a modification to address group reporting as discussed in section III.C.3.c.(1) of this rule.
CMS has finalized our proposal that if OIG refers a determination to CMS that the MIPS eligible clinician is found to have committed information blocking, the MIPS eligible clinician will not earn a score in the Promoting Interoperability performance category (a zero score), which is typically a quarter of the total MIPS score. Further, CMS has finalized the proposal that we will apply the disincentive to the MIPS payment year associated with the calendar year in which OIG referred its determination to CMS. To codify this policy, CMS also has finalized its proposal to amend the requirements for earning a score for the MIPS Promoting Interoperability performance category at 42 CFR 414.1375(b) as proposed.
Lastly, CMS has finalized its proposal that, if multiple information blocking violations are identified as part of OIG's determination (including over multiple years) and referred to CMS, each referral of an information blocking determination by OIG would only affect a MIPS eligible clinician's status as a meaningful EHR user in a single performance period during the calendar year when the determination of information blocking was referred to CMS by OIG.
The final policies in this rule will become effective 30 days after the final rule appears in the Federal Register . As noted in section III.B.1. of this final rule, OIG will not begin investigating health care providers until after the effective date of this rule, and will exercise its enforcement discretion not to make any determinations regarding conduct occurring prior to the effective date of this rule for information blocking disincentives. As OIG will not make a determination on conduct occurring prior to the effective date, OIG will not refer any health care providers based on a determination of conduct occurring prior to the effective date of this rule for information blocking disincentives. This means that CMS will not impose the disincentive finalized under the MIPS Promoting Interoperability performance category on information blocking conduct occurring before the effective date of this final rule.
In the Disincentives Proposed Rule, CMS proposed that, if data for the MIPS Promoting Interoperability performance category is submitted as a group or virtual group, then the application of the disincentive would be made at that level ( 88 FR 74962 ). CMS referred readers to our prior rulemaking governing groups and virtual groups ( 81 FR 77073 through 77077 ) and our regulations at 42 CFR 414.1305 (defining MIPS eligible clinicians as including groups as well as separately defining groups and virtual groups) and 414.1315 (governing virtual groups). Additionally, we refer readers to SSA section 1848(q)(1)(D), which provides the Secretary with authority to establish and apply a process to assess the performance of MIPS eligible clinicians in a group practice as a whole group under MIPS, including the group's performance in the Promoting Interoperability performance category.
In the Disincentives Proposed Rule, CMS explained that MIPS eligible clinicians who submit data as a part of a group, virtual group, or individually will be evaluated as an individual or as a group for all performance categories ( 88 FR 74962 ). We clarify in this final rule that if a MIPS eligible clinician reports data for MIPS as a group and an individual, the payment adjustment will be based on the highest final score. [ 35 ] Beginning with the CY 2021 performance period/2023 MIPS payment year, if a TIN/NPI has a virtual group final score associated with it, CMS will use the virtual group final score to determine the MIPS payment adjustment; if a TIN/NPI does not have a virtual group final score associated with it, we will use the highest available final score associated with the TIN/NPI to determine the MIPS payment adjustment ( 85 FR 84917 through 84919 ). CMS noted that it would apply the MIPS payment adjustment factor to the Medicare Part B claims during the Start Printed Page 54700 MIPS payment year for the MIPS eligible clinicians in the group or virtual group. Thus, CMS proposed that, if CMS is calculating a final score and MIPS payment adjustment factor for a group or virtual group and OIG refers a finding of information blocking to CMS, CMS would apply the proposed disincentive to the whole group.
Comments. A few commenters requested clarification on the proposal to apply the disincentive at the group level. Others requested clarification on how OIG would address a group practice that committed information blocking, but that does not participate in MIPS at the group level. Another commenter requested additional information on how CMS would address instances in which a MIPS eligible clinician that is found to have committed information blocking reports both as a group and as an individual, how this policy will be applied to subgroups when a subgroup is identified, and whether the appropriate disincentive will be applied to an entire group, regardless of whether the information blocking practice was limited to a particular subgroup.
Response. In situations where OIG refers a determination of information blocking for multiple NPIs we would apply the disincentive to each NPI. If OIG determines a group consisting of one or more MIPS eligible clinicians has committed information blocking and the MIPS eligible clinicians submit data as a group, the disincentive would be applied at the group level. However, as discussed in more detail below, consistent with PHSA section 3022(a)(6), if OIG determines a single MIPS eligible clinician within a group has committed information blocking (and not the group itself), then we would seek to apply the disincentive to the individual MIPS eligible clinician.
Comments. Several commenters expressed concern that this proposal would discourage group, virtual group, and subgroup reporting, which commenters stated would undermine CMS' goals of reducing the overall reporting burden and increasing participation in value-based payment models. Commenters expressed that the proposal could dissuade health care providers from reporting at the group level, due to concerns about being unfairly penalized for the actions of one bad actor in a group and may impact participation in virtual groups even more because clinicians may practice in different locations and may use different EHR systems.
Response. We disagree with the commenters that finalizing this disincentive policy will discourage group submissions, as we believe the benefits of group reporting outweigh the potential risk of being subject to a disincentive, as MIPS eligible clinicians that comply with the information blocking regulations will not be subject to a disincentive. We have finalized that, if OIG determines the group has committed information blocking, then we will apply the disincentive to the group. However, as discussed in more detail below, consistent with PHSA section 3022(a)(6), if OIG determines a single MIPS eligible clinician within a group has committed information blocking (and not the group itself), then we would seek to apply the disincentive to the individual MIPS eligible clinician.
Comments. Many commenters did not support the proposal to apply the disincentive at the group level, noting that the proposal is overly punitive. Some commenters noted that in large groups hundreds or thousands of MIPS eligible clinicians could be penalized for the action of one within the group. Some commenters noted that a TIN serves many purposes and cannot be easily undone to avoid a disincentive for a group.
Response. We thank commenters for their feedback but decline to modify our proposal in response to these comments. MIPS eligible clinicians do not have to report data as a group; it is a choice that they make. However, as discussed in more detail below, consistent with PHSA section 3022(a)(6), if OIG determines a single MIPS eligible clinician within a group has committed information blocking (and not the group itself), then we would seek to apply the disincentive to the individual MIPS eligible clinician.
Comments. Another commenter requested clarification on how a case would be handled in which a health care provider commits information blocking during a specific MIPS performance period, and then moves to a new practice before the application of the MIPS payment adjustment.
Response. We will apply the disincentive to the MIPS payment year 2 years after CMS receives the information blocking referral from OIG. The application of the disincentive will follow the MIPS eligible clinician. [ 36 ] As discussed in more detail below, consistent with PHSA section 3022(a)(6), if OIG determines a single MIPS eligible clinician within a group has committed information blocking (and not the group itself), then we would seek to apply the disincentive to the individual MIPS eligible clinician.
Comments. Several commenters recommended that CMS apply the disincentive only to the health care provider(s) that were found to have committed information blocking rather than the entire group or virtual group. Some noted that an entire group or individuals not practicing in the same location or have a direct relationship should not be punished for the actions of another individual that may be beyond their control. A few commenters recommended individual physicians found to be information blockers could be excluded from the group data or be required to report and be assessed separately. One commenter contended that punishing the entire group for the behavior of one individual appears to be contrary to the definitions at PHSA 3022(a)(6). One commenter requested that CMS look at the details of the case, determine the extent of and institutional role of the information blocking, and provide appropriate corrective action recommendations and education. One commenter recommended disincentives be applied to individual health care providers unless the subgroup or group has adopted enterprise-wide policies or taken actions as an enterprise that constitute information blocking. Some commenters requested that CMS work to determine a more equitable way to apply a disincentive in these situations, including a later application of the disincentive.
Response. We acknowledge commenters' concerns with the policy we proposed for group reporting. PHSA section 3022(a)(6) relates to limiting what conduct can be determined to constitute information blocking. We will comply with PHSA section 3022(a)(6) in applying the disincentive we have finalized for the MIPS Promoting Interoperability performance category. If OIG determines that a group [ 37 ] has committed information blocking and the group reports at the group level, then we would apply the disincentive to the group. If OIG determines that multiple individual MIPS eligible clinicians within a group have committed information blocking and they report at the individual level, then we would apply the disincentive to each MIPS eligible clinician individually. However, if OIG determines an individual MIPS Start Printed Page 54701 eligible clinician within a group has committed information blocking (and not the group itself), then we would seek to apply the disincentive to the individual MIPS eligible clinician.
To clarify this intent, we are finalizing our proposed amendment to the definition of meaningful EHR user for MIPS at § 414.1305 with modification. Specifically, we are adding language reflecting the requirement at PHSA section 3022(a)(6), providing that the term “information blocking,” with respect to an individual MIPS eligible clinician or group, shall not include an act or practice other than an act or practice committed by such individual MIPS eligible clinician or group. We will seek to address in future rulemaking how we will effectuate this requirement, including how we may disaggregate an individual MIPS eligible clinician's data from a group's data if OIG determines that only the individual MIPS eligible clinician (and not the group) committed information blocking.
Comments. A few commenters specifically expressed concern that the existing MIPS review process would not address the underlying information blocking determination or cause of the zero score for the MIPS Promoting Interoperability performance category because it would not address the information blocking finding itself. One commenter expressed concern that there would be no mechanism for physicians to appeal the appropriateness of the specific disincentives chosen by CMS once it has received an information blocking determination referral from OIG. One commenter requested additional clarification on how the targeted review process within MIPS would apply to information blocking disincentives.
Response. As discussed in section III.B.2. of this final rule, the Cures Act did not provide instruction regarding appeals of disincentives for health care providers established under PHSA section 3022(b)(2)(B). Therefore, any right to appeal administratively a disincentive, if available, would be provided under the authorities used by the Secretary to establish the disincentive through notice and comment rulemaking. We refer readers to the targeted review process we established at 42 CFR 414.1385(a) in accordance with SSA section 1848(q)(13)(A).
After consideration of the public comments, we have finalized our proposed amendment to the definition of meaningful EHR user for MIPS at § 414.1305 with modification. Specifically, we have added language reflecting the requirement at PHSA section 3022(a)(6), providing that the term “information blocking,” with respect to an individual MIPS eligible clinician or group, shall not include an act or practice other than an act or practice committed by such individual MIPS eligible clinician or group. We will seek to address in future rulemaking how we will effectuate this requirement, including how we may disaggregate an individual MIPS eligible clinician's data from a group's data if OIG determines that only the individual MIPS eligible clinician (and not the group) committed information blocking.
In the Disincentives Proposed Rule we noted that CMS has established policies that result in the reweighting of the Promoting Interoperability performance category for certain MIPS eligible clinicians at 42 CFR 414.1380(c)(2) ( 88 FR 74962 ). These include but are not limited to hospital-based clinicians ( 81 FR 77238 through 77420 , 82 FR 53684 , and 82 FR 53686 through 53687 ) and Ambulatory Surgical Center-based clinicians ( 82 FR 53684 ). CMS did not propose changes to its existing reweighting policies for MIPS eligible clinicians in the Disincentives Proposed Rule.
Starting with the CY 2022 performance period/2024 MIPS payment year performance period CMS automatically reweights small practices for the Promoting Interoperability performance category ( 86 FR 65485 through 65487 ; 42 CFR 414.1380(c)(2)(i)(C) ( 9 )). CMS did not propose changes to our existing policy for MIPS eligible clinicians in small practices in the Disincentives Proposed Rule.
CMS noted in the Disincentives Proposed Rule that if these MIPS eligible clinicians choose to submit data for the Promoting Interoperability performance category, their reweighting is canceled, and they could be subject to a disincentive if OIG refers a determination of information blocking to CMS ( 88 FR 74962 ).
Comments. A few commenters supported CMS' decision to not propose any changes to the existing MIPS reweighting policies.
Response. We thank commenters for their support.
Comments. Several commenters requested that CMS clarify how the existing significant hardship exemptions for the MIPS Promoting Interoperability performance category will interact with the proposed MIPS disincentives.
Response. CMS did not propose any changes to the existing reweighting policies for significant hardship or other types of exceptions for the MIPS Promoting Interoperability performance category set forth at 42 CFR 414.1380(c)(2)(i)(C) . These reweighting policies provide bases by which CMS may reweight the 25 percent weight assigned to the MIPS Promoting Interoperability performance category and redistribute that weight to other categories on which the MIPS eligible clinician may be scored in accordance with 42 CFR 414.1380(c)(2)(ii) . If CMS reweights the Promoting Interoperability performance category to zero percent in accordance with these reweighting policies, then the Promoting Interoperability performance category is not assigned any score (zero or otherwise) and is not included in CMS's calculation of the MIPS eligible clinician's final score.
To clarify, if the Promoting Interoperability performance category is reweighted to zero percent for a given performance period/MIPS payment year in accordance with these policies, then CMS does not assess whether the MIPS eligible clinician is a meaningful EHR user and, therefore, does not include any score for the performance category in the MIPS eligible clinician's final score. In this circumstance, this disincentive would not affect the MIPS eligible clinician's final score.
Comments. One commenter requested guidance on how CMS would decide which disincentive to apply to a case in which a hospitalist is found to have engaged in information blocking. One commenter also supported CMS' proposal to not impact the status or MIPS scoring of “non-patient facing” and “hospital-based” MIPS eligible clinicians, or other MIPS eligible clinicians automatically reweighted from the Promoting Interoperability performance category.
Response. A hospitalist likely may be a licensed physician meeting the definition of MIPS eligible clinician set forth at 42 CFR 414.1305 . We refer readers to our discussion in section III.C.3.b.(1) of the Disincentives Proposed rule ( 88 FR 74959 ) and this final rule regarding the alignment of definitions of MIPS eligible clinician and health care provider under the PHSA.
Whether an individual or group is subject to MIPS and its requirements will be determined in accordance with the applicable statute at SSA section 1848(q) and our regulations at 42 CFR part 414, subpart O . We note that, in the Disincentives Proposed Rule, CMS did not propose any changes to the MIPS reweighting policies at 42 CFR 414.1380(c)(2) ( 88 FR 74962 ). Therefore, Start Printed Page 54702 if a hospitalist meets the definition of a hospital-based MIPS eligible clinician at 42 CFR 414.1305 , CMS may continue to reweight the Promoting Interoperability performance category to zero percent for the hospitalist in accordance with 42 CFR 414.1380(c)(2)(i)(C) ( 6 ), subject to any other applicable requirements.
We did not make any proposals in this section. We note that, if a MIPS eligible clinician submits data for the Promoting Interoperability performance category, their reweighting may be cancelled in accordance with 42 CFR 414.1380(c)(2)(i)(C) , and they could be subject to a disincentive if OIG refers a determination of information blocking to CMS.
In the Disincentives Proposed Rule we noted that after OIG has determined that a health care provider has committed information blocking and referred that health care provider to CMS, CMS would notify the MIPS eligible clinician that OIG determined that the eligible clinician committed information blocking as defined under 45 CFR 171.103 , and thus the MIPS eligible clinician was not a meaningful EHR user for the performance period in the calendar year when OIG referred its information blocking determination to CMS ( 88 FR 74962 ). We stated that we would apply the proposed disincentive to the MIPS payment year associated with the calendar year in which the OIG referred its determination to CMS. We noted that this notice would be issued in accordance with the notice requirements for disincentives proposed in 45 CFR 171.1002 (see also section III.B.2. of the Disincentives Proposed Rule and this final rule).
CMS invited public comment on this proposal.
Comments. One commenter expressed concern that applying disincentives within MIPS without providing the physician an opportunity to correct the issue would cause financial harm to practices, reduce the resources practices have available to develop robust information sharing capabilities, and disincentivize quality reporting and improvement efforts.
Response: We did not propose a mechanism by which MIPS eligible clinicians could engage in a corrective action plan or other activity to demonstrate compliance and avoid a disincentive. We remind readers that the definition of information blocking in PHSA section 3022(a) requires that a health care provider “knows” that a practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information.
After consideration of the public comments, CMS has finalized its proposal to notify a MIPS eligible clinician that OIG determined that the MIPS eligible clinician committed information blocking as defined under 45 CFR 171.103 , and, in accordance with the definition of “meaningful EHR user,” that the MIPS eligible clinician was not a meaningful EHR user for the performance period in the calendar year when OIG referred its information blocking determination to CMS.
In the Disincentives Proposed Rule, we explained that Section 3022 of the Patient Protection and Affordable Care Act (PPACA) ( Pub. L. 111-148 , Mar. 23, 2010) added section 1899 to the Social Security Act (SSA) ( 42 U.S.C. 1395jjj ), which established the Medicare Shared Savings Program (Shared Savings Program) ( 88 FR 74963 ). In accordance with the statute, groups of providers of services and suppliers (referred to herein as “ACO participants”) and their associated health care providers (referred to herein as “ACO providers/suppliers”) meeting criteria specified by the Secretary may work together to manage and coordinate care for Medicare fee-for-service beneficiaries through an ACO. ACOs that meet quality performance standards established by the Secretary are eligible to receive payments for shared savings the ACO generates for Medicare and to avoid sharing losses at the maximum level. One condition of participation required by the statute is for the ACO to define certain processes, including a mandate to “define processes to promote evidence-based medicine and patient engagement, report on quality and cost measures, and coordinate care, such as through the use of telehealth, remote patient monitoring, and other such enabling technologies” (Social Security Act section 1899(b)(2)(G)).
In the Disincentives Proposed Rule, we explained that the Shared Savings Program regulations at 42 CFR part 425 set forth, among other things, requirements for ACO eligibility, quality reporting, and other program requirements and beneficiary protections ( 88 FR 74963 ). [ 38 ] The regulations at 42 CFR 425.116 require that an ACO, as a condition of participation in the Shared Savings Program, must effectuate an agreement with its ACO participants and ACO providers/suppliers (as defined at 42 CFR 425.20 ). This agreement must expressly require the ACO participant to agree, and to ensure that each ACO provider/supplier billing through the TIN of the ACO participant agrees, to participate in the Shared Savings Program and to comply with the requirements of the Shared Savings Program and all other applicable Federal laws and regulations including, but not limited to: (1) Federal criminal law; (2) The False Claims Act ( 31 U.S.C. 3729 et seq. ); (3) The anti-kickback statute ( 42 U.S.C. 1320a-7b(b) ); (4) The civil monetary penalties law ( 42 U.S.C. 1320a-7a ); and (5) The physician self-referral law ( 42 U.S.C. 1395nn ).
CMS has interpreted the requirement at section 1899(b)(1)(G) of the SSA that an ACO coordinates care for assigned beneficiaries using enabling technologies to require an ACO (and, by agreement, an ACO participant and ACO provider/supplier) to, among other things, define its methods and processes established to coordinate care across and among health care providers both inside and outside the ACO and have a written plan to “encourage and promote use of enabling technologies for improving care coordination for beneficiaries” ( 42 CFR 425.112(b)(4)(i) and (b)(4)(ii)(C) ). Enabling technologies may include one or more of the following: electronic health records and other health IT tools; telehealth services, including remote patient monitoring; electronic exchange of health information; and other electronic tools to engage beneficiaries in their care. The ACO must ensure that ACO participants and ACO providers/suppliers comply with and implement the defined care coordination process, including the encouragement and promotion of enabling technologies, and the remedial processes and penalties (including the potential for expulsion) applicable to ACO participants and ACO providers/suppliers for failure to comply with and implement the required process (see 42 CFR 425.112(a)(3) ). Sharing health information using enabling technologies across all health care providers engaged in a beneficiary's care (both inside and Start Printed Page 54703 outside the ACO) for purposes of care coordination and quality improvement is an essential aspect of the ACO's activities. Moreover, this type of information sharing among health care providers (both inside and outside the ACO) supports quality measurement and quality reporting activities, which are necessary for the ACO to be eligible to share in savings and are also used in determining the amount of shared losses.
Before the start of an agreement period, before each performance year thereafter, and at such other times as specified by CMS, the ACO must submit to CMS an ACO participant list and an ACO provider/supplier list (see 42 CFR 425.118(a) ; citing 42 CFR 425.302(a)(2) ). The ACO must certify the accuracy, completeness, and truthfulness of the data and information contained in the submitted lists annually. All Medicare-enrolled individuals and entities that have reassigned their right to receive Medicare payment to the TIN of the ACO participant must be included on the ACO provider/supplier list and must agree to participate in the ACO and comply with the requirements of the Shared Savings Program before the ACO submits the ACO participant list and the ACO provider/supplier list.
CMS may deny an ACO, ACO participant, and/or an ACO provider/supplier participation in the Shared Savings Program if the entity or individual has a history of program integrity issues (see 42 CFR 425.305(a)(2) ). CMS screens ACOs, ACO participants, and ACO providers/suppliers during the Shared Savings Program application process and periodically thereafter (for example, during the annual certification of the ACO participant and ACO provider/supplier lists) with regard to their program integrity history (including any history of Medicare program exclusions or other sanctions and affiliations with individuals or entities that have a history of program integrity issues) (see 42 CFR 425.305(a)(1) ). In the Medicare Shared Savings Program Final Rule ( 76 FR 67802 ), CMS stated that the results of the screening would be considered in light of the relevant facts and circumstances. CMS did not draw a bright line regarding when an entity's history of program integrity issues would justify denial of a Shared Savings Program participation agreement. CMS stated instead that we would likely consider the nature of the applicant's program integrity issues (including the program integrity history of affiliated individuals and entities), the available evidence, the entity's diligence in identifying and correcting the problem, and other factors. CMS stated that we intended to ensure that ACOs, ACO participants, and ACO providers/suppliers would not pose a risk of fraud or abuse within the Shared Savings Program while recognizing that some program integrity allegations may not have been fully adjudicated.
CMS may terminate an ACO's Shared Savings Program participation agreement if the ACO, its ACO participants, or its ACO providers/suppliers or other individuals or entities performing functions or services related to ACO activities fail to comply with any of the requirements of the Shared Savings Program under 42 CFR part 425 (§ 425.218(a) and (b)). This includes, but is not limited to, violations of the physician self-referral prohibition, CMP law, Federal anti-kickback statute, antitrust laws, or any other applicable Medicare laws, rules, or regulations that are relevant to ACO operations. Similarly, CMS requires that the agreement the ACO effectuates with its ACO participants must permit the ACO to take remedial action against the ACO participant, and must require the ACO participant, in turn, to take remedial action against its ACO providers/suppliers, including imposition of a corrective action plan, denial of incentive payments, and termination of the ACO participant agreement, to address noncompliance with the requirements of the Shared Savings Program and other program integrity issues, including program integrity issues identified by CMS ( 42 CFR 425.116(a)(7) ). Taken together, these regulations ensure that CMS may take appropriate enforcement actions when CMS' screening process or oversight of an ACO reveals a history of program integrity issues and when an ACO, an ACO participant or an ACO provider/supplier and other individuals or entities performing functions or services related to ACO activities fail to comply with the requirements of the Shared Savings Program, including failure to comply with other Federal laws that are relevant to the ACO's operations, such as the Cures Act's information blocking provision (PHSA section 3022).
In the Disincentives Proposed Rule, CMS proposed to revise the Shared Savings Program regulations to establish disincentives for health care providers, including ACOs, ACO participants, or ACO providers/suppliers, that engage in information blocking ( 88 FR 74964 ). CMS proposed that a health care provider that OIG determines has committed information blocking may not participate in the Shared Savings Program for a period of at least 1 year.
In the Disincentives Proposed Rule, we discussed that information blocking runs contrary to the care coordination goals of the Shared Savings Program ( 88 FR 74964 ). ACO participants and their ACO providers/suppliers participating in an ACO in the Shared Savings Program use enabling technologies (such as electronic health records) to improve care coordination for beneficiaries. The ability of ACO providers/suppliers to exchange information between health care providers (both inside and outside the ACO) is essential for the operations of the ACO, including for effective coordination of care and quality improvement activities and services for assigned beneficiaries.
In the Disincentives Proposed Rule, first, CMS proposed to amend 42 CFR 425.208(b) to include a specific reference to the Cures Act information blocking provision codified in the PHSA ( 88 FR 74964 ). We noted that the provision would be one of many laws with which ACOs (and by agreement, their ACO participants and ACO providers/suppliers) must comply. [ 39 ] We noted that in this case, compliance is required because a Medicare enrolled “health care provider,” to which an information blocking disincentive may apply, includes ACO providers/suppliers (See 42 CFR 400.202 and 425.20 and 45 CFR 171.102 ). We explained that the effect of adding a specific reference to the information blocking provision would be to require that, as a condition of participation in the Shared Savings Program, an ACO must specifically agree (and must require its ACO participants, ACO providers/suppliers, and other individuals or entities performing functions or services related to the ACO's activities to agree) to not commit information blocking as defined in PHSA section 3022(a).
Second, CMS proposed to revise 42 CFR 425.305(a)(1) to specify that the program integrity history on which ACOs, ACO participants, and ACO providers/suppliers are reviewed during the Shared Savings Program application process and periodically thereafter includes, but is not limited to, a history of Medicare program exclusions or other sanctions, noncompliance with the requirements of the Shared Savings Program, or violations of laws specified at 42 CFR 425.208(b) ( 88 FR 74964 ). We Start Printed Page 54704 explained that this revision would provide the basis for CMS to deny participation in the Shared Savings Program to a health care provider that is an ACO, an ACO participant, or an ACO provider/supplier when the health care provider has engaged in information blocking, as determined by OIG.
Third, CMS proposed to make a conforming modification to the provision related to the grounds for CMS to terminate an ACO at 42 CFR 425.218(b)(3) based on “[v]iolations of the physician self-referral prohibition, civil monetary penalties (CMP) law, Federal anti-kickback statute, antitrust laws, or any other applicable Medicare laws, rules, or regulations that are relevant to ACO operations.” CMS proposed to replace this language with “[v]iolations of any applicable laws, rules, or regulations that are relevant to ACO operations, including, but not limited to, the laws specified at § 425.208(b)” ( 88 FR 74964 ).
Pursuant to CMS' authority under 42 CFR 425.206(a)(1) to deny an ACO's participation in the Shared Savings Program, CMS' authority under 42 CFR 425.118(b)(1)(iii) to deny the addition of a health care provider to an ACO's participation list, and CMS' authority under 42 CFR 425.305(a) to screen for program integrity issues, CMS proposed to screen ACOs, ACO participants, and ACO providers/suppliers for an OIG determination of information blocking and deny the addition of such a health care provider to an ACO's participation list for the period of at least 1 year ( 88 FR 74964 ). In the case of an ACO that is a health care provider, CMS proposed to deny the ACO's application to participate in the Shared Savings Program for the period of at least 1 year. We noted that if the ACO were to re-apply to participate in the Shared Savings Program in a subsequent year, then CMS would review whether OIG had made any subsequent determinations of information blocking with respect to the ACO as a health care provider as well as any evidence that indicated whether the issue had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence, as part of the ACO's application process. CMS therefore proposed in the Disincentives Proposed Rule that, in cases where the result of the program integrity screening identifies that an ACO (acting as a health care provider), ACO participant, or ACO provider/supplier, has committed information blocking, as determined by OIG, CMS would take the following actions, as applicable:
- Pursuant to 42 CFR 425.118(b)(1)(iii) , CMS would deny the request of the ACO to add an ACO participant to its ACO participant list on the basis of the results of the program integrity screening under 42 CFR 425.305(a) .
- Pursuant to 42 CFR 425.116(a)(7) and (b)(7) , CMS would notify an ACO currently participating in the Shared Savings Program if one of its ACO participants or ACO providers/suppliers is determined by OIG to have committed information blocking so that the ACO can take remedial action—removing the ACO participant from the ACO participant list or the ACO provider/supplier from the ACO provider/supplier list—as required by the ACO participant agreement.
- Pursuant to 42 CFR 425.305(a)(2) , CMS would deny an ACO's Shared Savings Program application if the results of a program integrity screening under 42 CFR 425.305(a)(1) reveal a history of program integrity issues or other sanctions and affiliations with individuals or entities that have a history of program integrity issues.
- Pursuant to 42 CFR 425.218(a) and (b)(3) , CMS would terminate an ACO participation agreement in the case of a failure to comply with requirements of the Shared Savings Program, including violations of any applicable laws, rules, or regulations that are relevant to ACO operations, including, but not limited to, the laws specified at 42 CFR 425.208(b) ( 88 FR 74964 and 74965 ).
In the Disincentives Proposed Rule, CMS noted that each of these actions would deter information blocking consistent with the discussion of an appropriate disincentive in section III.A.3. of the Disincentives Proposed Rule ( 88 FR 74965 ). We noted that restricting the ability for these entities to participate in the Shared Savings Program for at least 1 year would result in these health care providers potentially not receiving revenue that they might otherwise have earned if they had participated in the Shared Savings Program.
In the Disincentives Proposed Rule, CMS stated that the period of time of the disincentive would be at least 1 performance year ( 88 FR 74965 ). We explained that we would determine if it would be appropriate for the period to exceed 1 year if OIG has made any subsequent determinations of information blocking (for example, CMS would be unlikely to impose a disincentive greater than 1 year if the information blocking occurred in the past and there was evidence that the information blocking had stopped) and whether safeguards have been put in place to prevent the information blocking that was the subject of OIG's determination. We noted that prior to imposing any disincentive arising from an OIG determination of information blocking, CMS would provide a notice in accordance with the notice requirements proposed in 45 CFR 171.1002 ( 88 FR 74953 ) that would specify the disincentive would be imposed for at least 1 performance year.
In the Disincentives Proposed Rule, CMS proposed to apply the disincentive no sooner than the first performance year after we receive a referral of an information blocking determination from OIG and in which the health care provider is to participate in the Shared Savings Program ( 88 FR 74965 ). We explained in the Disincentives Proposed Rule that CMS performs a program integrity screening of ACOs, ACO participants, and ACO providers/suppliers as part of the annual application/change request process for new and existing ACOs, which typically occurs between May and October during the performance year. In the case of the new addition of an ACO participant (TIN) to an ACO's participant list, CMS stated that we would prevent the TIN from joining the ACO as an ACO participant if the program integrity screening reveals that the TIN has engaged in information blocking, as determined by OIG. In the case of an existing ACO participant, CMS stated that we would notify the ACO that an ACO participant or an ACO provider/supplier had committed information blocking, as determined by OIG, so the ACO can remove the ACO participant or ACO provider/supplier from its ACO participant list or ACO provider/supplier list, as applicable. If the TIN were to remain on the ACO participant list or ACO provider/supplier list when the ACO certifies its ACO participant list for the next performance year, we stated that then CMS would issue a compliance action to the ACO. We noted that continued noncompliance (for example, failure to remove the TIN) would result in termination of the ACO's participant agreement with CMS, as the ACO would have failed to enforce the terms of its ACO participant agreement.
In the Disincentives Proposed Rule, CMS stated that applying the disincentive prospectively is the most appropriate timing for the disincentive ( 88 FR 74965 ). We noted that it would be impractical and inequitable for CMS to apply the disincentive retrospectively or in the same year in which CMS received a referral from OIG. Applying the disincentive to a historical performance year or a performance year Start Printed Page 54705 contemporaneous to the OIG's determination would unfairly affect other ACO participants that did not commit the information blocking and likely were not aware of the information blocking. CMS recognized, however, that the prospective application of the disincentive means that it may be applied to a health care provider substantially after the information blocking occurred, during the provider's first attempt to participate in the Shared Savings Program, and after the provider was previously subject to a disincentive in another program, such as MIPS. As discussed in the Disincentives Proposed Rule ( 88 FR 74966 ) and below, CMS contemplated an approach under which a health care provider could participate in the Shared Savings Program if a significant amount of time (for example, 3 to 5 years) had passed between the occurrence of the information blocking and OIG's determination, and the provider had given assurances in the form and manner specified by CMS that the issue had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence.
In the Disincentives Proposed Rule, CMS explained that after the completion of the last performance year in which the disincentive was applied, an ACO may submit a change request to add the TIN or include the NPI on its ACO participant list or ACO provider/supplier list, as applicable, for a subsequent performance year, and CMS would approve the addition, assuming that all other Shared Savings Program requirements for adding a TIN or NPI are met, so long as (1) OIG has not made any additional determinations of information blocking, and (2) the ACO provides assurances (in the form and manner required by CMS) that the information blocking is no longer ongoing and that the ACO has put safeguards in place to prevent the information blocking that was the subject of the referral ( 88 FR 74965 ). If, however, OIG made and referred an additional information blocking determination (that is either related or unrelated to the previous OIG referral) in a subsequent year or the ACO cannot provide assurance that the information blocking has ceased, we discussed that CMS would continue to deny participation.
In addition, in the Disincentives Proposed Rule, we stated that CMS would notify ACOs about an ACO participant or ACO provider/supplier that had committed information blocking, as determined by OIG, so that the ACO could take remedial action—removing the ACO participant from the ACO participant list or the ACO provider/supplier from the ACO provider/supplier list—as required by the ACO participant agreement ( 88 FR 74965 ). We noted that ACOs are well-positioned to take remedial action against ACO participants and ACO providers/suppliers that have been found by OIG to have committed information blocking as a result of their ACO participant agreements, which provide for the ACO to take remedial action against the ACO participant, and require the ACO participant to take remedial action against its ACO providers/suppliers, including imposition of a corrective action plan, denial of incentive payments, and termination of the ACO participant agreement, to address noncompliance with the requirements of the Shared Savings Program and other program integrity issues.
By way of example, consider if in January 2025, OIG determined that an ACO participant has committed information blocking as recently as 2024 and referred this determination to CMS. In the Disincentives Proposed Rule, CMS explained that under the proposal, the ACO participant would be able to remain on the ACO's certified participant list for the duration of the 2025 performance year ( 88 FR 74965 ). However, we explained that CMS would notify the ACO that an ACO participant had been determined to have committed information blocking by OIG and that CMS expected the ACO to take remedial action by removing the ACO participant from its ACO participant list for a specified period of time. To determine if removal was warranted for a period in addition to performance year 2026, CMS stated that it would consider whether there was any evidence to suggest that that information blocking was still occurring (for example, whether OIG had made a subsequent determination of information blocking) and whether safeguards had been put in place to prevent the information blocking that was the subject of the referral. In the Disincentives Proposed Rule, we noted that upon a review of these criteria, CMS may require the affected ACO to remove the ACO participant prior to recertification of the ACO participant list for additional performance years. If the ACO participant were to remain when the ACO certifies its ACO participant list for performance year 2026, we explained that CMS would inform the ACO that it was obligated to take remedial action against the ACO participant by removing it from the ACO participant list for performance year 2026; if it failed to do so, CMS would remove the ACO participant from the ACO's participant list and take compliance action against the ACO up to terminating the ACO pursuant to 42 CFR 425.218(b)(1) and (3) . In the case of a disincentive that was applied only for performance year 2026, we explained that if the ACO were to submit a change request to add the ACO participant for performance year 2027 or a subsequent year, then CMS would review whether OIG had made any subsequent determinations of information blocking with respect to the ACO participant as well as any evidence that indicated whether the issue had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence, prior to approving the ACO participant to participate in the ACO for performance year 2027 or the subsequent year.
In the Disincentives Proposed Rule, we explained that if an ACO applicant or a renewal ACO applicant that is itself a health care provider (for example, a large multi-specialty practice that forms a single participant ACO using its existing legal entity and governing body under 42 CFR 425.104 ) is the subject of an OIG information blocking determination, CMS would deny the ACO's application for participation in the Shared Savings Program for the upcoming performance year for which it was applying to participate ( 88 FR 74966 ). CMS noted that should OIG make a determination of information blocking with respect to an ACO that is already participating in the Shared Savings Program and refer the determination to us for the application of a disincentive, CMS may terminate the ACO's participation agreement for the upcoming performance year. We stated that CMS would assess a subsequent application from an ACO to which the disincentive had been applied under the same criteria described for assessing the return of an ACO participant or ACO provider/supplier. We noted that the ACO may participate in the Shared Savings Program after the duration of the disincentive so long as OIG had not made a subsequent determination of information blocking applicable to the health care provider and whether there was evidence that the issue had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence, prior to approving the ACO's application to participate in the Shared Savings Program in a subsequent performance year.
In the Disincentives Proposed Rule, CMS also considered an alternative policy in which CMS would not apply a disincentive in certain circumstances despite an OIG information blocking Start Printed Page 54706 determination. CMS explained that under this alternative policy, the Shared Savings Program would consider OIG's referral of an information blocking determination in light of the relevant facts and circumstances before denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), or denying an ACO's application to participate in the Shared Savings Program ( 88 FR 74966 ). We explained that the relevant facts and circumstances could include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, the time since the OIG's determination of information blocking, and other factors. We noted that this alternative policy would offer some flexibility in certain circumstances, where prohibiting an ACO, ACO participant, or ACO provider/supplier from participating in the Shared Savings Program would distort participation incentives and therefore be less appropriate. We noted that we were particularly concerned about situations in which many years have passed since an ACO participant or ACO provider/supplier was found to be an information blocker and such an issue had long been remediated. We noted that in such a case, the ACO participant or ACO provider/supplier might be incentivized to apply to the Shared Savings Program for a year in which it did not actually intend to participate merely to avoid being barred from doing so at a future date when it did intend to participate, wasting the resources of the ACO and CMS. We explained that, under such an alternative policy, a health care provider could participate in the Shared Savings Program if a significant amount of time had passed between the occurrence of the information blocking and the OIG's determination, and the provider had given assurances in the form and manner specified by CMS that the issue had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence.
In the Disincentives Proposed Rule, we noted that an ACO may be able to appeal the application of an information blocking disincentive in the Shared Savings Program ( 88 FR 74966 ). An ACO may appeal an initial determination that is not prohibited from administrative or judicial review under 42 CFR 425.800 by requesting a reconsideration review by a CMS reconsideration official ( 42 CFR 425.802(a) ). To the extent it is not barred by 42 CFR 425.800 , an ACO may appeal the removal or denial of a health care provider from an ACO participant list as a result of the referral by OIG of an ACO participant that OIG had determined to be an information blocker. Subject to the same limitation, an ACO applicant or ACO may appeal the denial of the ACO applicant's application or termination of the ACO's participation agreement as a result of the referral by OIG of the ACO applicant or ACO that the OIG had determined to be an information blocker. We explained that the underlying information blocking determination made by OIG, however, would not be subject to the Shared Savings Program's reconsideration process. We noted that the OIG determination is not an initial determination made by CMS, but a determination made by another agency. The Shared Savings Program reconsideration process may not negate, diminish, or otherwise alter the applicability of determinations made by other government agencies (see 42 CFR 425.808(b) ).
In the Disincentives Proposed Rule, we reminded all health care providers and ACOs that it is possible that a health care provider or any entity, such as an ACO, may meet the definition of a HIN/HIE, which is a functional definition, or the definition of a health IT developer of certified health IT, codified in 45 CFR 171.102 ( 88 FR 74966 ). We noted that if it is found by OIG that such health care provider or entity meets either definition and, while under the same set of facts and circumstances, is also found by OIG to have committed information blocking, then the health care provider or entity would be subject to a different intent standard and civil money penalties administered by OIG (see generally 88 FR 42820 ; see 88 FR 42828 and 42829 ).
CMS invited public comment on these proposals and on whether additional actions should be taken.
Comments. Several commenters supported CMS' proposed disincentive for the Shared Savings Program. These commenters explained that the proposed disincentive is consistent with the intent of the Cures Act and that it will help promote widespread electronic exchange of health information across the healthcare spectrum. Commenters also explained that the proposal is consistent with an ACO's goal to utilize technologies like EHRs to facilitate care coordination, quality improvement activities, and patient-centered care. One commenter supported the proposed disincentive for the Shared Savings Program because it would impact a wider set of health care providers and thus have a greater deterrent effect among health care providers compared to the proposed disincentives for the Medicare Promoting Interoperability Program and MIPS. Another commenter specifically supported the proposal to apply the disincentive for at least 1 year and explained that the proposed approach is appropriate and consistent with the other disincentives proposed in this rulemaking.
Response. We agree that sharing health information using enabling technologies across all health care providers engaged in a beneficiary's care (both inside and outside the ACO) for purposes of care coordination and quality improvement is an essential activity for health care providers participating in an ACO in the Shared Savings Program. This type of information sharing among health care providers (both inside and outside the ACO) supports care coordination, quality measurement, and quality reporting activities, which are necessary in order for the ACO to participate in the Shared Savings Program. We appreciate commenters' support for the proposal to revise the Shared Savings Program regulations to establish disincentives for health care providers, including ACOs, ACO participants, or ACO providers/suppliers, that engage in information blocking. We agree that the proposal meets the objectives of the Cures Act by establishing appropriate disincentives for health care providers, as defined in 45 CFR 171.102 , that have been determined by OIG to have committed information blocking.
Comments. Many commenters opposed the proposal to deny ACOs, ACO participants, and ACO providers/suppliers from participating in the Shared Savings Program if they are determined by OIG to have committed information blocking. Commenters stated that the proposal would reduce the number of health care providers and ACOs participating in the Shared Savings Program, which would effectively impede progress towards delivering care based on outcomes, rather than volume, while also disrupting improvements in patient care and diminishing resources that ACOs use to improve patient care. Other commenters stated that prohibiting participation in the Shared Savings Program would disrupt patient care and worsen healthcare quality and Start Printed Page 54707 outcomes, explaining that CMS' proposal would deny Medicare patients access to enhanced services that ACOs offer, such as care coordination and case management services. These commenters further explained that if a health care provider is excluded from the Shared Savings Program, it would be impossible to deliver many of those services because providers would no longer receive claims data for their patients from the Shared Savings Program. Several commenters expressed concern that if a health care provider was removed from an ACO, patients assigned to an ACO would no longer have access to that provider or the patient would be forced to find an alternative provider, which could cause treatment delays and disrupt care continuity. Additionally, many commenters explained that the proposal would undermine CMS' goal of having all Medicare beneficiaries in an accountable care relationship by 2030 and would prevent CMS from effectively addressing healthcare costs and quality. Several commenters expressed concern that the proposed disincentive would disproportionately affect health care provider participation in ACOs serving patients in rural areas, dual-eligible beneficiaries, and patients with disabilities. These commenters also raised concerns about the impact on Medicare beneficiaries in rural areas, stating that specialist health care providers participating in ACOs are often the only specialists available to serve these communities.
Response. While we appreciate the commenters' concerns about the potential negative consequences resulting from application of the disincentive, such as reduced participation in value-based care and a reduction of care coordination services, the purpose of the proposal is to implement the Cures Act by creating a disincentive that deters health care providers from committing information blocking. We disagree with commenters' concerns as we do not expect that the proposal would reduce the number of health care providers and ACOs participating in the Shared Savings Program by a significant amount. Removal from, or denial of approval to participate in, the Shared Savings Program would be limited to those health care providers that have committed information blocking, as determined by OIG. Removal is an appropriate disincentive because it protects beneficiaries and denies health care providers the opportunity to benefit financially and reputationally from participation in the Shared Savings Program.
We disagree with commenters' concerns that application of the disincentive could disrupt patient care and compromise beneficiary outcomes. Beneficiary care would already be negatively affected by information blocking; this disincentive thus is intended to prevent negative outcomes from occurring. Information blocking runs counter to the goals of value-based care, such as care coordination and quality improvement, and health care providers that engage in information blocking may harm beneficiaries by denying them the benefits of value-based care. Furthermore, beneficiaries receiving care from ACO providers/suppliers that regularly engage in information blocking might not receive the full benefits of value-based care because the information blocking may prevent the sharing of information critical to care coordination and quality improvement among the beneficiary's health care providers. With respect to commenters' concerns about how to reconcile the disincentive with CMS' goal of having 100 percent of people with Original Medicare in a care relationship with accountability for quality and total cost of care by 2030, [ 40 ] the proposal aims to deter health care providers from information blocking and hold accountable those health care providers that engage in such practices. In doing so, the proposal supports CMS' broader goal of incentivizing health care providers to coordinate care effectively across care settings so that they can improve patient outcomes and lower costs.
Regarding commenters' concerns that the removal of a health care provider from an ACO due to information blocking would result in ACO beneficiaries no longer having access to their provider, we clarify that this is not the case. The denial of approval to participate in or removal of a health care provider from the Shared Savings Program through the application of this disincentive does not exclude the provider from Medicare. A Medicare beneficiary aligned to an ACO may see the Medicare enrolled health care provider of his or her choice, regardless of whether the provider is a participant or provider/supplier in an ACO. Similarly, we clarify that Medicare beneficiaries in rural areas, dual-eligible beneficiaries, and patients with disabilities, could continue to see a Medicare enrolled health care provider of their choice, irrespective of whether that health care provider is an ACO participant or ACO provider/supplier.
Based on the comments we received, however, we recognize that denial of approval to participate in or removal from the Shared Savings Program is not warranted in every instance. For this reason and for the additional reasons discussed below, we have finalized the proposal with modifications to incorporate the alternative discussed in the Disincentives Proposed Rule. This will enable us to consider an OIG information blocking determination in light of the relevant facts and circumstances of the information blocking determination and subsequent remediation before applying the disincentive. This approach is consistent with the Cures Act's command to implement “appropriate disincentives” and balances CMS' efforts to improve the quality and efficiency of items and services provided to beneficiaries through value-based care.
Comments. Many commenters supported CMS' alternative policy for the Shared Savings Program in which CMS would consider an OIG information blocking determination in light of the relevant facts and circumstances before denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), or denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken. Commenters explained that this alternative policy would provide CMS with flexibility to consider an information blocking determination in light of the relevant facts and circumstances, such as whether the health care provider subject to the information blocking determination had taken corrective action and established safeguards to prevent future instances of information blocking or if significant time had passed since the information blocking occurred. One commenter recommended that CMS always consider information blocking determinations in light of the relevant facts and circumstances, including during the initial screening process when CMS reviews ACOs' program integrity history for OIG determinations of information blocking. Another commenter supported the alternative policy, noting that education and remediation would be more appropriate than applying the disincentive. One Start Printed Page 54708 commenter agreed with CMS that the disincentive as proposed may distort participation incentives and that the alternative proposal may help ameliorate these concerns. Another commenter stated that the alternative policy would help CMS balance the need to prevent information blocking while ensuring the financial stability of ACOs and providers participating in the Shared Savings Program. A few commenters recommended that CMS also consider the size of the practice, number of eligible clinicians in the practice, and relationship between the ACO and the entity found to have committed information blocking when applying the disincentive.
Response. We agree with commenters that the alternative policy will allow us to consider an OIG information blocking determination in light of the relevant facts and circumstances before applying a disincentive, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), or denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken. For an ACO that is already participating in the Shared Savings Program, the alternative policy will also allow us to consider an OIG information blocking determination in light of the relevant facts and circumstances prior to terminating the ACO's participation agreement with CMS for the upcoming performance year. The relevant facts and circumstances include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether the provider was previously subject to a disincentive in another program, and other factors. In the case of an ACO applicant, a renewal ACO applicant, or an ACO participant TIN that would be a new addition to an ACO's participant list, we would request information from the ACO that indicated whether the information blocking had been corrected and appropriate safeguards had been put in place to prevent its reoccurrence. For ACOs, ACO participants, or ACO providers/suppliers that are already participating in the Shared Savings Program, we would issue a compliance action, which would include a request for the same information.
Additionally, we agree with commenters that if the risk of reoccurrence of information blocking has been mitigated, it would be beneficial to take that into consideration before imposing a disincentive that could interrupt the care coordination benefits of beneficiaries receiving care from ACO participants and ACO providers/suppliers. For example, removal of a large ACO participant TIN that had corrected the information blocking that occurred 10 years prior to OIG's determination and had imposed safeguards to prevent its reoccurrence, could result in the multi-TIN ACO falling below the 5,000 assigned beneficiary threshold required by 42 CFR 425.110(a)(1) . Having the discretion to consider the facts and circumstances of the information blocking provider's remediation efforts and past disincentives prior to imposing a disincentive will allow the Shared Savings Program to best determine if removal from, or denial of approval to participate in the Shared Savings Program, is warranted while minimizing unintended consequences for ACOs, ACO participants, and ACO providers/suppliers that had no involvement in the information blocking activity that was the subject of OIG's determination. For these reasons, we have finalized the proposal with modifications to incorporate the alternative policy.
Comments. Many commenters urged CMS to consider implementing less severe disincentives that would encourage compliance with the information blocking regulations without discouraging participation in value-based care models. These commenters recommended that CMS partner with ACOs to identify and remediate cases of information blocking instead of implementing disincentives that affect participation in the Shared Savings Program. The commenters explained that ACOs already have expertise in data sharing and reporting instances of information blocking, thus ACOs are well-positioned to assist HHS in advancing their interoperability goals. A few commenters stated that the proposed disincentive creates arbitrary penalties that neither address the underlying causes of information blocking nor allow health care providers to rectify the behaviors that led to information blocking. Several commenters explained that the proposed disincentive is excessive and disproportionate to the offense and that it may cause more harm than the underlying instance of information blocking.
Response. While we appreciate the commenters' concerns about the perceived severity and appropriateness of the proposed disincentives, information blocking can result in serious and adverse effects on beneficiary care and outcomes. For this reason, the denial of approval to participate or removal of health care providers that have been determined by OIG to have committed information blocking is both appropriate and proportional to the underlying information blocking activity. We disagree that the proposed disincentive creates arbitrary penalties that fail to address the underlying causes of information blocking and do not permit health care providers to rectify the behaviors that led to information blocking. To the contrary, the proposal would impose a clear disincentive—denial of approval to participate in or removal from the Shared Savings Program for at least 1 year—on the specific health care provider that committed information blocking, as determined by OIG.
Further, the disincentive would not prohibit a health care provider that had committed information blocking, as determined by OIG, from correcting the information blocking activity and participating in the Shared Savings Program in the future. The intent of the proposal is to implement PHSA section 3022(b)(2)(B) by creating a disincentive that discourages health care providers from committing information blocking. It is not clear that merely requiring that a healthcare provider take corrective action would adequately discourage repeated information blocking when one considers that substantial time that may elapse between the information blocking and an OIG determination. With respect to the suggestions that CMS partner with ACOs to identify and remediate cases of information blocking, we encourage ACOs to report any instances of information blocking to ONC or OIG. Given that ACOs are engaged in care coordination and quality improvement activities, they may encounter instances of information blocking as they seek to achieve the goals of accountable care in the Shared Savings Program.
We agree with commenters that depending upon the circumstances of the case, CMS may need more flexibility in applying a disincentive under the Shared Savings Program than was provided for under the proposal. We have therefore finalized the proposal with modifications to incorporate the alternative policy discussed in the Disincentives Proposed Rule ( 88 FR 74966 ). This will allow us to consider an OIG information blocking determination in light of the relevant facts and circumstances before applying a disincentive, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/ Start Printed Page 54709 supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken, or terminating an ACO's participation agreement with CMS. We reiterate that the relevant facts and circumstances include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether the provider was previously subject to a disincentive in another program, and other factors. As discussed above, this approach achieves the balance of disincentivizing information blocking in the Shared Savings Program while ensuring that CMS can consider whether a health care provider who has committed information blocking, received disincentives elsewhere for it, and corrected the conduct should be barred from participating in the Shared Savings Program prior to imposing a disincentive.
Comments. Commenters expressed concern that the proposal would inadvertently discourage or deter participation in value-based care models, such as the Shared Savings Program, because ACOs and ACO participants face significant disruption and financial instability if they are removed from the Shared Savings Program. Many commenters were concerned that the proposed policy would have negative financial and operational consequences for ACOs. One commenter explained that if an ACO is suspended from the Shared Savings Program or if a large ACO participant or health care provider is removed from an ACO, the resulting financial impact could be the loss of millions of dollars in potential shared savings revenue, which could result in the ACO collapsing completely.
Other commenters expressed concern that the proposed disincentive could upend ACO operations and greatly compound the financial instability of the ACO and participating physician participants. One commenter expressed concern that the proposed disincentive would be especially detrimental for ACOs operating in rural areas, where Medicare Advantage enrollment exceeds traditional Medicare enrollment. The commenter stated that removing even one participant TIN could force the entire ACO to collapse, severely disrupting patient care and punishing not only the TIN that committed information blocking, but also all ACO participants. A few commenters explained that the potential financial impacts of the proposed disincentive are not aligned with the severity of the underlying information blocking offense. Commenters suggested that the financial disincentives imposed on ACOs should more closely correspond to the severity of the information blocking violation.
A few commenters stated that suspending ACOs from the Shared Savings Program would also cause the government to lose millions of dollars in shared savings. Several commenters also expressed concern that suspension of ACOs from the Shared Savings Program may also make ACOs ineligible for Advanced APM bonus payments. Commenters emphasized that ACOs depend on these bonus payments to cover investment and care coordination costs. Another commenter questioned how the proposal would impact physicians who participate in an ACO but do not meet the Advanced APM threshold for exemption from the MIPS Program. Specifically, the commenter inquired if these physicians who have been found by OIG to have committed information blocking would be removed as an ACO participant and subject to disincentives under the MIPS program. A few commenters expressed concerns that the proposed disincentive would hinder overall data exchange and information sharing that is essential to ACO operations and structure. Another commenter expressed concern that the disincentive would cause adverse financial impacts to the healthcare system and contribute to hospital closures. Finally, one commenter stated that the disincentive may hinder an ACO's ability to meet network adequacy requirements if health care providers who have committed information blocking are removed from the ACO.
Response. We appreciate the commenters' concerns regarding the potential financial and operational impacts on ACOs of disincentives for information blocking. The proposed disincentive will serve as a deterrent to information blocking by health care providers participating in the Shared Savings Program. Commenters' concerns about the negative financial consequences for health care providers and ACOs of the disincentive, as well as how disruptive it may be, support this conclusion.
A strong disincentive is likely to be most appropriate in deterring information blocking given the nature of the activity and its effect on essential aspects of Shared Savings Program. Information blocking is not an inadvertent practice. A health care provider has only committed information blocking if the provider engaged in a practice that the provider “[knew] is unreasonable and likely to interfere with access, exchange, or use of electronic health information” ( 45 CFR 171.103 ). As discussed above, information blocking runs contrary to the overarching goals of the Shared Savings Program, as the ability of ACO participants and ACO providers/suppliers to exchange information between health care providers (both inside and outside the ACO) is essential for the operations of the ACO, including for effective coordination of care, quality improvement activities, and related services for assigned beneficiaries. If health care providers participating in the Shared Savings Program are determined by OIG to have committed information blocking, it is reasonable to remove or prevent any such health care providers from participating in the Shared Savings Program for at least one performance year, given that the health care providers intentionally acted in a manner that may have impaired activities central to the Shared Savings Program: care coordination and improvement in the quality and efficiency of beneficiary care.
We disagree with the commenters' statement that suspending ACOs from the Shared Savings Program would also cause the government to lose millions of dollars in shared savings. The participation of information blockers in the Shared Savings Program undermines the integrity of the program and may harm an ACO's efforts to coordinate and improve the quality and efficiency of beneficiary care. Moreover, if ACOs that have committed information blocking (as determined by OIG) are removed from the Shared Savings Program for at least one performance year, their removal may actually prevent losses by shifting government resources to ACOs that are focused on care coordination and quality improvement activities. With respect to the impact the proposed disincentive will have on cost savings in the Medicare program, as discussed in the Regulatory Impact Statement of the Disincentives Proposed Rule ( 88 FR 74967 ), the expected benefits of this rule would be to deter information blocking that interferes with effective health information exchange and negatively impacts many important aspects of healthcare. We refer readers to the impact analysis of the benefits of prohibiting and deterring information blocking in the ONC Cures Act Final Rule ( 85 FR 25936 ).
Regarding whether clinicians who have been found by OIG to have committed information blocking would Start Printed Page 54710 be removed as an ACO participant and subject to disincentives under the MIPS program, we confirm that such clinicians could be removed as ACO participants and, if they are MIPS eligible clinicians, they could also be subject to the information blocking disincentive under MIPS. While we acknowledge the commenters' concerns that removing or denying an ACO participant from an ACO could result in downstream effects that have implications for eligibility of Advanced APM incentive payments and scoring under MIPS, we reiterate that the approach is to deter information blocking by health care providers participating in the Shared Savings Program through the imposition of appropriate disincentives consistent with the requirements of the Cures Act.
It is important to clarify that there is no network adequacy requirement in the Shared Savings Program. Unlike other healthcare programs, such as managed care plans, the Shared Savings Program does not limit beneficiaries to receiving care from only the providers and suppliers that participate in the ACO. Thus, there is no need in the Shared Savings Program to impose network adequacy requirements on participating ACOs. Concerns about the effect of the disincentive as it relates to network adequacy are thus unwarranted.
Comments. Many commenters did not support the proposed disincentive on the basis that it would be unfair and inappropriate to penalize the entire ACO for the actions of one individual ACO participant or ACO provider/supplier determined by OIG to have committed information blocking. Some commenters stated that if one ACO participant or ACO provider/supplier is determined to have committed information blocking, then the entire ACO entity would be prohibited from participating in the Shared Savings Program. Commenters expressed concern that excluding an entire ACO would harm patients who rely on those ACOs for their healthcare. The commenters explained that by denying participation to all health care providers in an ACO due to the actions of a few, patients' access and continuity of care would ultimately suffer. One commenter stated that ACO participants who did not engage in information blocking themselves would likely be unaware of and unable to control the actions of other participants who did commit information blocking and that it would be unfair to penalize the broader group for the actions of a few individuals. Another commenter expressed concern about an ACO being banned from the Shared Savings Program if a single health care provider within the ACO is found by OIG to have committed information blocking, especially if the information blocking activity is inconsistent with documented ACO policies and practices.
Response. The concerns expressed by the commenters indicate that there might be a misunderstanding about the proposed disincentive. Our intention is not to penalize the entire ACO entity for the actions of a single ACO participant or ACO provider/supplier that is the subject of an OIG information blocking determination. Instead, the proposal would impose a disincentive on the specific health care provider that committed information blocking, as determined by OIG. In the Disincentives Proposed Rule ( 88 FR 74965 ), we explained that CMS would notify ACOs about an ACO participant or ACO provider/supplier that had committed information blocking, as determined by OIG, so that the ACO could take remedial action—removing the ACO participant from the ACO participant list or the ACO provider/supplier from the ACO provider/supplier list—as required by the ACO participant agreement ( 88 FR 74965 ). ACOs are expected to take remedial action against ACO participants and ACO providers/suppliers that have been found by OIG to have committed information blocking through their ACO participant agreements, which must permit the ACO to take remedial action against the ACO participant, and require the ACO participant to take remedial action against its ACO providers/suppliers, including imposition of a corrective action plan, denial of incentive payments, and termination of the ACO participant agreement, to address noncompliance with the requirements of the Shared Savings Program and other program integrity issues. Should the ACO fail to take the appropriate remedial action against the ACO participant or ACO provider/supplier, CMS may take action against the ACO consistent with its authority at 42 CFR 425.216 and 425.218 .
While it is true that consequences may extend to ACO participants or ACO providers/suppliers if the ACO itself is found by OIG to have committed information blocking, our focus is on imposing disincentives for information blocking on the specific health care provider that has committed information blocking, not on imposing disincentives on entire groups of health care providers or ACO participants that had no involvement in the activity that resulted in an information blocking determination by OIG. We also understand the concerns raised about fairness and patient access, and we agree with commenters that there could be a negative impact to an ACO if an ACO participant with a large number of assigned beneficiaries is found by OIG to have committed information blocking, requiring removal of the ACO participant from the ACO participant list as a result of the proposed disincentive. However, it is important that ACOs make their own assessment of potential ACO participants—and the potential ACO participant's commitment to information sharing for the purposes of care coordination, quality measurement, and quality reporting activities—prior to contracting with them. We reiterate that the goal of the proposal is to ensure that appropriate disincentives are imposed on health care providers that have committed information blocking, as determined by OIG, while minimizing unintended consequences for ACOs and Medicare beneficiaries. We have finalized the proposal with modifications so that we will consider an OIG information blocking determination in light of the relevant facts and circumstances before applying a disincentive.
Comments. Several commenters expressed concerns with CMS' proposal to remove ACO participants and ACO providers/suppliers at the TIN level rather than at the individual or NPI level. Commenters stated that implementing disincentives at the TIN level would negatively affect not only health care providers who engaged in information blocking, but also those who did not. One commenter expressed concern that this approach could undermine existing contractual agreements between CMS and ACOs while another commenter stated that applying the disincentive at the TIN-level would negatively impact patient attribution calculations and the beneficiaries receiving services from that TIN. A few commenters requested that CMS clarify how the proposed disincentive and the removal of ACO providers/suppliers would impact patient attribution and who would subsequently assume responsibility for those patients' care. Other commenters requested clarification on how ACO suspension would impact health care providers and suppliers in relation to Shared Savings Program rules allowing gradual progression from one-sided to two-sided risk arrangements over certain time periods.
Response. While we appreciate the concerns raised by commenters regarding the application of disincentives at the ACO participant Start Printed Page 54711 TIN level, it is important to clarify that the approach is designed to hold accountable the health care provider OIG determined to be responsible for information blocking, whether that is at the ACO participant TIN or NPI level. While we understand that not every individual within an ACO participant TIN may be directly involved in information blocking activities, holding the ACO participant TIN accountable (if the ACO participant TIN is the entity found by OIG to have committed information blocking) is required under PHSA section 3022(b)(2)(B), which specifies that health care providers (individuals or entities) that have been determined by OIG to have committed information blocking shall be subject to appropriate disincentives. Please refer to the discussion of the definition of health care provider at 45 CFR 171.102 in section II.B.1. of this rule. Should OIG determine that information blocking has occurred at the NPI level (in other words, that an ACO provider/supplier has committed information blocking), we would notify the ACO so that it could take remedial action—removing the ACO provider/supplier from the ACO's provider/supplier list—as required by the ACO participant agreement. We would not impose a disincentive at the ACO participant TIN level or the ACO level if only an ACO provider/suppler was determined by OIG to have committed information blocking.
With respect to how the removal of an ACO participant or ACO providers/suppliers could affect an ACO's assigned beneficiary population, it is important to note that CMS assigns beneficiaries to an ACO as a whole; beneficiaries are not assigned to a particular ACO participant TIN or ACO provider/supplier. We acknowledge that removal or denial of an ACO participant or ACO provider/suppler as a result of an OIG information blocking determination could impact the number of beneficiaries assigned to an ACO, and we expect the risk of this occurring is a valuable deterrent against information blocking that may lead to the implementation of ACO operating procedures that proactively prevent information blocking. As discussed above, however, this would not affect beneficiary access to care. Medicare beneficiaries may continue to see the health care provider of his or her choice, regardless of whether the provider is a participant or provide/supplier in an ACO, or the beneficiary is assigned to a particular ACO.
The termination of an ACO from the Shared Savings Program for at least one performance year as a result of an information blocking determination would interrupt the ACO's progression along the BASIC track's glide path from a one-sided to two-sided risk arrangement, and the ACO would need to meet eligibility determinations regarding what level of participation they would be eligible for when reentering their participation in the Shared Savings Program. We do not foresee, however, similar challenges to progress to two-sided risk for ACO participants or ACO providers/suppliers that are prevented from joining or that are removed from an ACO as a result of an information blocking determination.
After the completion of the last performance year in which the disincentive was applied, an ACO may submit a change request to add the TIN or include the NPI on its ACO participant list or ACO provider/supplier list, as applicable, for a subsequent performance year, and CMS would approve the addition, assuming that all other Shared Savings Program requirements for adding a TIN or NPI are met, so long as (1) OIG has not made any additional determinations of information blocking, and (2) the ACO provides assurances (in the form and manner required by CMS) that the information blocking is no longer ongoing and that the ACO has put safeguards in place to prevent the information blocking that was the subject of the referral.
Comments. One commenter expressed concern about the impacts of the proposed disincentive on skilled nursing facilities (SNFs) specifically. The commenter explained that because SNFs have been excluded from Federal health IT incentive programs, SNFs may not have the requisite technology to be able to share information as required under the information blocking regulations. As a result, the commenter recommended that OIG and CMS consider each ACO health care provider's unique situation and not apply a one-size-fits-all standard approach to all providers participating in an ACO. The commenter further recommended that CMS provide certain health care providers with exemptions from the proposed disincentive for the Shared Savings Program. Specifically, the commenter requested that CMS exclude SNFs from the proposed disincentive if the SNF is the only health care provider in a rural or underserved location and all other ACO participation requirements are met. The commenter stated that this exception would ensure that Medicare beneficiaries are not denied access to nearby SNFs and post-acute care. The commenter also requested that CMS exclude SNFs or any ACO providers/suppliers if their ACO participant agreements are structured so that they do not receive the ACO's shared savings from the proposed disincentive. The commenter noted that ACOs are not required to share incentive payments and earned shared savings with ACO health care providers in their network, such as SNFs. Therefore, applying the disincentive without this exemption would further deter SNF participation in ACOs.
Response. We appreciate the commenter's concerns regarding the potential impact of the proposed disincentive on SNFs participating in the Shared Savings Program. We recognize that these facilities were not eligible for participation in the Medicare and Medicaid EHR Incentive Programs. However, it is important to clarify that SNFs are explicitly included in the definition of health care provider defined in 45 CFR 171.102 (which codifies the definition of health care provider in section 3000(3) of the PHSA) for which the Cures Act instructs the Secretary to establish appropriate disincentives for information blocking. While it is true that the initial implementation of appropriate disincentives in this rule, through the Shared Savings Program, MIPS, and the Medicare Promoting Interoperability Program, may not reach all types of health care providers defined at 45 CFR 171.102 , to exempt a single type of health care provider participating in one of these programs from the disincentive would be particularly inequitable and thwart the purpose of the rule. For these reasons, we are unwilling and unable to grant any exemptions for SNFs that are ACO participants or SNF affiliates from the proposed disincentive, as requested by the commenter. We nonetheless recognize the vital role SNFs play in providing post-acute care, particularly in rural or underserved areas, and we recognize that it is important to clarify that Medicare beneficiaries may continue to utilize the SNF of his or her choice, regardless of whether the SNF, or the health care providers rendering serves at the SNF, is an ACO participant or ACO provider/supplier in an ACO.
More broadly, we agree with the commenter that it is important to consider the unique circumstances of health care providers when implementing the proposed disincentive under the Shared Savings Program, and we agree that a one-size-fits-all approach may not be suitable for all health care providers, especially those facing technological limitations. For this reason, finalizing the proposal with modifications to incorporate the Start Printed Page 54712 alternative policy will allow us to consider the unique circumstances of the health care provider when applying this disincentive, and we will consider an OIG information blocking determination in light of the relevant facts and circumstances before applying a disincentive, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken, or terminating an ACO's participation agreement with CMS.
Comments. Several commenters stated that the proposed disincentive is excessive, redundant, and constitutes a double penalty because health care providers found by OIG to have committed information blocking will be subject to disincentives under MIPS and may also be subject to removal from the Shared Savings Program for at least 1 year. One commenter expressed concern that cumulative disincentives could be more pronounced for hospitals based on removal from the Shared Savings Program in the violation year and receiving a market basket decrease the following year under MIPS.
Response. We understand commenters' concerns about the potential for cumulative disincentives for health care providers found by OIG to have committed information blocking. We have finalized the proposed policy with modifications to incorporate the alternative policy we outlined in the Disincentives Proposed Rule ( 88 FR 74966 ), under which we will consider OIG's referral of an information blocking determination in light of the relevant facts and circumstances, including the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether a health care provider was previously subject to a disincentive in another program, before denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program, or terminating an ACO's participation agreement with CMS. This approach furthers the Shared Savings Program's goal of imposing appropriate disincentives for information blocking consistent with the Cures Act, while ensuring relevant facts and circumstances are used to inform decisions made under the Shared Savings Program.
Comments. A few commenters recommended that CMS adopt specific revisions to the proposal. One commenter expressed concern that requiring ACOs to remove ACO participants or ACO health care providers could impose undue administrative burdens on ACOs. The commenter recommended that CMS assume the responsibility of removing entities that have committed information blocking from the ACO and notify the affected ACO when it has taken such actions. One commenter suggested that, prior to imposing any disincentives on ACOs, we provide accommodations for hardship, have a well-defined investigative process, and establish a graduated disincentive structure that accounts for the impact ACOs have on the communities they serve. Another commenter recommended that CMS establish more than one disincentive option for ACOs, ACO participants, and ACO providers/suppliers to provide flexibility in determining the disincentive appropriate for each case.
Response. While we understand that removing ACO participants or ACO providers/suppliers that have committed information blocking, as determined by OIG, could result in additional work for the ACO, CMS expects ACOs to be equipped to take remedial action against their ACO participants under their agreements with the ACO participants. We also expect ACO participants, in turn, to take remedial action against its ACO providers/suppliers, including imposition of a corrective action plan, denial of incentive payments, and termination of the ACO participant agreement, to address noncompliance with the requirements of the Shared Savings Program and other program integrity issues, including program integrity issues identified by CMS ( 42 CFR 425.116(a)(7) ). For these reasons, the remedial action CMS expects ACOs and ACO participants to take in the case of an OIG determination of information blocking is consistent with their existing obligations under the Shared Savings Program and should not represent an undue burden.
Regarding the suggestion that CMS provide hardship accommodations prior to imposing any disincentives on ACOs and that CMS have a well-defined investigative process and establish a graduated disincentive structure that accounts for the impact ACOs have on the communities they serve, we have finalized the proposed policy with modifications to incorporate the alternative policy so that we will consider OIG's referral of an information blocking determination in light of the relevant facts and circumstances. This approach will require that we carefully consider the unique circumstances of an ACO prior to imposing any disincentive, and it obviates the need for a hardship accommodation or a graduated disincentive structure. While we appreciate the suggestion to establish multiple disincentive options for ACOs, ACO participants, and ACO providers/suppliers, we decline to do so. As mentioned above, the alternative policy we are adopting provides CMS with the discretion to consider the relevant facts and circumstances before applying a disincentive, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken, or terminating an ACO's participation agreement with CMS. This approach provides adequate flexibility in the application of appropriate disincentives under the Shared Savings program.
Comments. Several commenters opposed to the proposal urged us to consider alternative disincentives. Many commenters recommended that CMS allow ACOs, ACO participants, and ACO providers/suppliers to take remedial or corrective action rather than removal from the Shared Savings Program. Commenters explained that remedial actions could include a probation period, a reduction or withhold of shared savings or incentives, corrective action plans, or mandatory education for those who have engaged in information blocking. Commenters further explained that allowing ACOs, ACO participants, and ACO providers/suppliers to take corrective action would allow CMS to impose disincentives on health care providers determined by OIG to have committed information blocking while still allowing those providers to participate in the Shared Savings Program. Several commenters also recommended that CMS and ONC provide education and technical assistance to ACOs, ACO participants, and ACO providers/suppliers on the proposed disincentive and its potential Start Printed Page 54713 impacts. They also suggested that CMS consider a tiered or scaled approach that accounts for the circumstances and frequency of misconduct when determining the appropriate disincentive to apply. A few commenters recommended that CMS delay implementing disincentives specific to ACOs or the Shared Savings Program and instead introduce disincentives in a separate rule once the risks to patient outcomes are better understood. Another commenter recommended that CMS consult closely with ACOs to ensure that CMS understands the potential impacts of any proposed disincentives. One commenter suggested that instead of limiting ACO participation in the Shared Savings Program, CMS should expand safe harbor protections to facilitate EHR information sharing between hospitals, health systems, and ACOs. The commenter explained that this would more effectively promote interoperability compared to the proposed disincentive. Another commenter recommended that ACOs should only be excluded from the Shared Savings Program if the determination of information blocking is related to activity that is integral to the function or operations of the ACO. In addition, a few commenters recommended that CMS consider disincentives that reduce the Advanced Alternative Payment Model (APM) incentive payment or conversion factor for health care providers. For example, these commenters recommended that health care providers in an Advanced APM found by OIG to have committed information blocking receive only 75 percent of their eligible Advanced APM bonus payment. The commenters explained that this alternative would better align with the disincentive proposed for MIPS eligible clinicians and would not deny access to care for beneficiaries.
Response. We appreciate commenters' suggestions for alternative disincentives but, for the reasons previously noted and for the additional reasons discussed below, we have finalized the proposal with modifications to incorporate the alternative policy discussed in the Disincentives Proposed Rule. In accordance with PHSA section 3022(b)(2)(B), we are required to impose disincentives for health care providers that are found by OIG to have committed information blocking. While we understand the benefits of an approach that would impose remedial or corrective actions rather than denial of approval to participate in or removal from the Shared Savings Program, those approaches may not have any deterrent effect, which is a fundamental aspect of any disincentive. In addition, the relevance of remedial and corrective actions may be limited in light of the time that may elapse between the underlying information blocking conduct and OIG's investigation. The disincentive we are adopting strikes a careful balance between deterring information blocking through meaningful consequences and ensuring that health care providers who have committed information blocking and corrected their actions are not permanently barred from participating in the Shared Savings Program.
We appreciate the recommendation to delay implementation of the proposed disincentive until patient outcomes are better understood. We are concerned, however, that delaying implementation of the disincentive could adversely affect patient care, as information blocking could impede effective care coordination and quality improvement activities within ACOs. Moreover, the proposed disincentive will serve as a deterrent to information blocking by health care providers participating in the Shared Savings Program. For these reasons, we decline to delay the implementation of disincentives for information blocking. In addition, the information blocking regulations in the ONC Cures Act Final Rule were released on May 1, 2020, and it is not necessary to further delay the establishment of disincentives for health care providers that have been found by OIG to have committed information blocking. While expanding safe harbor protections for EHR information sharing may facilitate data sharing and interoperability, we did not propose any such safe harbor expansion in the Disincentives Proposed Rule; therefore, this suggestion is beyond the scope of the disincentive proposed by the Shared Savings Program. Regarding the suggestion to exclude ACOs from the Shared Savings Program only if the determination of information blocking is related to integral ACO activities, we recognize the importance of considering the context of information blocking incidents, which is why we have finalized the proposed policy with modifications to incorporate the alternative policy, under which we will consider whether to impose a disincentive under the Shared Savings Program in light of the relevant facts and circumstances. Our use of a consistent standard in the Shared Savings Program for all instances of information blocking will ensure fairness in the application of disincentives for ACOs, ACO participants, and ACO providers/suppliers.
While we appreciate the recommendation to reduce Advanced APM incentive payments for health care providers found to have committed information blocking, we have not identified authority that would permit us to alter APM incentive payments issued pursuant to section 1833(z)(1) of the Social Security Act and 42 CFR 414.1450 . Finalizing the proposed disincentive with modifications to incorporate the alternative policy is an effective way to impose disincentives for information blocking and to promote interoperability among ACOs, ACO participants, and ACO providers/suppliers.
Comments. A few commenters requested clarification on which disincentives will apply in specific situations such as: whether a disincentive would apply to an ACO if a hospitalist is found to be information blocking and the hospital participates in an ACO; if a hospitalist is found to be information blocking would the health care provider and the hospital receive disincentives; and, if a physician, who is a MIPS eligible clinician and a participant in a Shared Savings Program ACO, is an information blocker could the physician potentially be penalized under MIPS and also removed from the ACO for a year.
Response. As discussed above, the proposal imposes a disincentive on the specific health care provider that committed information blocking, as determined by OIG. Whether the hospitalist or the hospital has committed information blocking will be determined by OIG through its investigation. If a hospitalist is determined by OIG to have committed information blocking and CMS is applying the disincentive, CMS would notify the ACO so that the ACO and ACO participant could take remedial action—removing the hospitalist from either the ACO participant list or the ACO provider/supplier list, as applicable, pursuant to the ACO participant agreement.
We understand commenters' concerns about the potential for cumulative disincentives for health care providers found by OIG to have committed information blocking, such as a MIPS eligible clinician participating in an ACO. As discussed above, we have finalized the proposed policy with modifications to incorporate the alternative policy we outlined in the Disincentives Proposed Rule, under which we will consider OIG's referral of an information blocking determination Start Printed Page 54714 in light of the relevant facts and circumstances, including the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether a health care provider was previously subject to a disincentive in another program, before applying a disincentive, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program, or terminating an ACO's participation agreement with CMS ( 88 FR 74966 ). This approach furthers the Shared Savings Program's goal of imposing disincentives for information blocking consistent with the Cures Act, while ensuring relevant facts and circumstances are used to inform decisions made under the Shared Savings Program.
Comments. One commenter expressed concern with the timing of the disincentive. The commenter explained that because OIG investigations of information blocking can take years to complete, ACO participants that have committed information blocking may no longer be participating in the ACO or the Shared Savings Program by the time CMS receives the referral. The commenter recommended that CMS clarify that if OIG refers to CMS a finding that a former ACO participant committed information blocking, the disincentive should not apply to the ACO or its remaining ACO participants.
Response. We appreciate the commenter's concern and request for additional information about the timing of a disincentive for information blocking. We want to clarify that if a former ACO participant is determined by OIG to have committed information blocking, we would not impose a disincentive on the ACO or the remaining ACO participants. As we explained in the Disincentives Proposed Rule, applying the disincentive prospectively is the most appropriate timing for the disincentive, as it would be impractical and inequitable for CMS to apply the disincentive retrospectively or in the same year in which CMS received a referral from OIG ( 88 FR 74965 ). Prospective application of the disincentive will also enable ACOs to remove any ACO participant TINs or ACO providers/suppliers during the annual application and change request cycle that have committed information blocking, as determined by OIG. Applying the disincentive to a historical performance year or a performance year contemporaneous to OIG's determination would unfairly affect other ACO participants that did not commit the information blocking and likely were not aware of the information blocking ( 88 FR 74965 ).
Comments. A few commenters expressed concern that ACO participants would only be able to appeal the application of the disincentive but not the actual information blocking determination. One commenter expressed concern that an appeal process may not be available under existing rules for Shared Savings Program ACO participants. Another commenter noted that a finding of information blocking could have future program integrity implications. A few commenters specifically requested that HHS clarify the rights of both ACOs and clinicians within an ACO to appeal an information blocking finding and provide extenuating information, such as why they contend an exception applied.
Response. As discussed in the Disincentives Proposed Rule ( 88 FR 74966 ), an ACO may appeal an initial determination that is not prohibited from administrative or judicial review under 42 CFR 425.800 by requesting a reconsideration review by a CMS reconsideration official ( 42 CFR 425.802(a) ). Individual ACO participants do not have the right to request an appeal under the Shared Savings Program regulations. To the extent it is not barred by 42 CFR 425.800 , an ACO may appeal (on behalf of an ACO participant) the removal or denial of a health care provider from an ACO participant list as a result of the referral by OIG of an ACO participant that OIG had determined to be an information blocker. Subject to the same limitation, an ACO applicant or ACO may appeal the denial of the ACO applicant's application or termination of the ACO's participation agreement as a result of the referral by OIG of the ACO applicant or ACO that the OIG had determined to be an information blocker. The underlying information blocking determination made by OIG, however, is not subject to the Shared Savings Program's reconsideration process. The OIG determination is not an initial determination made by CMS, but a determination made by another agency and the Shared Savings Program reconsideration process may not negate, diminish, or otherwise alter the applicability of determinations made by other government agencies (see 42 CFR 425.808(b) ). In the Disincentives Proposed Rule, we noted that we considered OIG to be a separate and distinct agency from CMS for the purposes of this provision ( 88 FR 74966 ). The Shared Savings Program's reconsideration process would thus not be the appropriate forum to seek reconsideration of OIG's determination.
After consideration of the public comments, CMS has finalized the alternative policy that will consider an OIG information blocking determination in light of the relevant facts and circumstances before applying a disincentive, such as denying the addition of an ACO participant to an ACO participant list (or an ACO provider/supplier to the ACO provider/supplier list), informing an ACO that remedial action should be taken against the ACO participant (or ACO provider/supplier), denying an ACO's application to participate in the Shared Savings Program if the remedial action is not taken, or terminating an ACO's participation agreement with CMS. The relevant facts and circumstances include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether the provider was previously subject to a disincentive in another program, and other factors.
CMS notes that the final policies in this rule will become effective 30 days after the official publication date. However, we note that section III.B.1. of this final rule states that OIG will not begin investigating health care providers until after the effective date of this rule, and that OIG will exercise its enforcement discretion not to make any determinations regarding conduct occurring prior to the effective date of this rule for information blocking disincentives. As OIG will not make a determination on conduct occurring prior to the effective date, OIG will not refer any health care providers based on a determination of conduct occurring prior to the effective date of this rule for information blocking disincentives. This means that CMS will not impose the disincentive in the Shared Savings Program for information blocking committed prior to the effective date of this final rule. We further clarify that any disincentives under the Shared Savings Program for information blocking determinations referred by OIG would be imposed after January 1, 2025.
As discussed in section III.C.1. of the Disincentives Proposed Rule, we recognize that the disincentives we proposed would only apply to a subset of health care providers as defined in 45 Start Printed Page 54715 CFR 171.102 ( 88 FR 74954 and 74955 ). However, we believe it is important for HHS to establish appropriate disincentives that would apply to all health care providers, as such providers are defined in 45 CFR 171.102 . This would ensure that any health care provider, as defined in 45 CFR 171.102 , that has engaged in information blocking would be subject to appropriate disincentives by an appropriate agency, consistent with the disincentives provision at PHSA section 3022(b)(2)(B).
We requested information from the public on additional appropriate disincentives that we should consider in future rulemaking, particularly disincentives that would apply to health care providers, as defined in 45 CFR 171.102 , that are not implicated by the disincentives proposed in the Disincentives Proposed Rule ( 88 FR 74966 and 74967 ). We encouraged commenters to identify specific health care providers (for example, laboratories, pharmacies, post-acute care providers, etc.) and associated potential disincentives using authorities under applicable Federal law. We also requested information about the health care providers that HHS should prioritize when establishing additional disincentives.
We received 32 submissions on this RFI. We thank commenters for their comments. We have shared all the comments received with the appropriate agencies and offices for consideration in subsequent rulemaking to establish additional disincentives for specific health care providers.
This document does not impose any new information collection requirements, that is, reporting, recordkeeping or third-party disclosure requirements. Consequently, there is no need for review by the Office of Management and Budget under the authority of the Paperwork Reduction Act of 1995 ( 44 U.S.C. 3501 et seq. ).
We have examined the impacts of this final rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), Executive Order 14094 entitled “Modernizing Regulatory Review” (April 6, 2023), the Regulatory Flexibility Act (RFA) (Pub. L. 96-354, September 19, 1980), section 1102(b) of the Social Security Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4 ), Executive Order 13132 on Federalism (August 4, 1999), and the Congressional Review Act ( 5 U.S.C. 804(2) ).
Executive Order 12866 , as amended by Executive Order 14094 published on April 6, 2023, directs agencies to assess all costs and benefits of available regulatory alternatives and, if regulations are necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, and public health and safety effects; distributive impacts; and equity). A regulatory impact analysis must be prepared for major rules with significant effects (for example, $200 million or more in any given year). This is not a major rule as defined at 5 U.S.C. 804(2) ; it is not significant under section 3(f)(1) of Executive Order 12866 because it does not reach that economic threshold, nor does it meet the other criteria outlined in the Executive order.
This final rule would implement provisions of the Cures Act through changes to 45 CFR part 171 and 42 CFR parts 414 , 425 , and 495 . For the reasons set forth below, we believe that the likely aggregate economic effect of these regulations would be significantly less than $200 million.
The expected benefits of this final rule would be to deter information blocking that interferes with effective health information exchange and negatively impacts many important aspects of healthcare. We refer readers to the impact analysis of the benefits of deterring information blocking in the ONC Cures Act Final Rule, which encompasses all anticipated benefits without differentiation among actors ( 85 FR 25936 ).
We anticipate that OIG would incur some costs associated with investigation as authorized by the Cures Act. The Consolidated Appropriations Act, 2022, provides OIG the authority to use its existing funding to conduct information blocking activities ( Pub. L. 117-103 , March 15, 2022). OIG has not received additional appropriations or increased funding levels specific to information blocking.
Additionally, investigated parties may incur some costs in response to an OIG investigation or in response to the application of a disincentive by an agency with the authority to impose a disincentive. Absent information about the frequency of prohibited practices, including the number of OIG determinations of information blocking in a given year that could be referred to an appropriate agency, we are unable to determine the potential costs of this regulation.
The monetary value of the disincentives finalized in this rule, if imposed on a health care provider by an appropriate agency, would be considered transfers. We are unable to reliably estimate the aggregate value of potential disincentive amounts because the value of the disincentive may vary based on other provisions specific to the authority under which the disincentive has been established, as discussed in section III.C.1. of this final rule. For instance, the value of a disincentive imposed on an eligible hospital under the disincentive finalized in section III.C.2. of this final rule would depend on the amount of IPPS payment received by the eligible hospital.
We invited public comment on potential impacts of the rulemaking. The following is a summary of the comments we received and our responses.
Comments. A few commenters expressed disagreement with ONC's assertion that the proposed rule will have economically insignificant effects. These commenters expressed that the Disincentives Proposed Rule underestimated the potential financial impact to entities operating under the authorities in section III.C. of the proposed rule. One commenter stated that health care providers with a larger share of Medicare patients could face financial costs approximately ten times greater than the estimated median impact. Additionally, this commenter expressed that the potential loss of savings to the Medicare Trust Fund as a result of barring participation in the Shared Savings Program would likely result in the rule having an annual economic effect exceeding $200 million, citing the significant amount of aggregate savings to the Medicare Trust Fund and average savings per ACO. One commenter recommended delaying the rule until HHS conducts an assessment of the rule's impact on clinicians and patient access, expressing concern that the proposed financial disincentives might negatively impact access to care.
Response. We acknowledge commenters' concerns about the impact that applying disincentives may have on individual health care providers. In the Disincentives Proposed Rule, we provided illustrative estimates of the monetary value of the proposed disincentive for eligible hospitals under the Medicare Promoting Interoperability Program ( 88 FR 74956 and 74957 ) and for eligible clinicians under the MIPS Promoting Interoperability performance category ( 88 FR 74960 ). While we presented median values, as well as 95 Start Printed Page 54716 percent ranges of estimates, in both cases, we acknowledge that there may be outlier examples that result in monetary values that are significantly higher than the figures presented in the analysis. However, we disagree that these figures, or other information commenters may provide about potential impacts on individual health care providers, directly impact our analysis of whether this is a significant regulatory action. As noted above, we are unable to reliably estimate either the frequency of prohibited practices, including the number of OIG determinations of information blocking in a given year that could be referred to an appropriate agency as a subset of all prohibited practices that could be determined to be information blocking, or the aggregate value of potential disincentive amounts, because the value of the disincentive may vary based on other provisions specific to the authority under which the disincentive has been established. Regarding the potential loss of savings to the Medicare Trust Fund associated with the disincentive finalized under the Shared Savings Program, we disagree that this would indicate that the rule would have an annual economic effect exceeding $200 million. The figures cited by the commenter of aggregate savings of the Shared Savings Program and average savings per ACO do not provide information about the amount of savings that would be lost due to the imposition of disincentives under the Shared Savings Program, as disincentives would only be imposed on an ACO that is a health care provider, an ACO participant, or an ACO provider/supplier that has been determined by OIG to have committed information blocking, referred to CMS as the appropriate agency to be subject to disincentives. As CMS has finalized in section III.C.4., CMS will also determine whether to impose a disincentive under the Shared Savings Program based on relevant facts and circumstances. As stated above, we are unable to reliably estimate the frequency of prohibited practices or the aggregate value of potential disincentive amounts, and commenters provided no additional information or data for their assertion that the costs will be higher.
The RFA and the Small Business Regulatory Enforcement and Fairness Act of 1996, which amended the RFA, require agencies to analyze options for regulatory relief of small businesses. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and Government agencies.
The Department considers a rule to have a significant impact on a substantial number of small entities if it has an impact of more than 3 percent of revenue for more than 5 percent of affected small entities. This final rule would not have a significant impact on the operations of a substantial number of small entities, as these changes would not impose any new requirement on any party. We have concluded that this final rule likely would not have a significant impact on a substantial number of small entities and that a regulatory flexibility analysis is not required for this rulemaking. Additionally, the Secretary certifies that this final rule would not have a significant impact on a substantial number of small entities.
In addition, section 1102(b) the SSA ( 42 U.S.C. 1302 ) requires us to prepare a regulatory impact analysis if a rule under Titles XVIII or XIX or section B of Title XI of the SSA may have a significant impact the operations of a substantial number of small rural hospitals. We have concluded that this final rule would not have a significant impact on the operations of a substantial number of small rural hospitals because these changes would not impose any requirement on any party. Therefore, a regulatory impact analysis under section 1102(b) of the SSA is not required for this rulemaking. Therefore, the Secretary has certified that this final rule will not have a significant impact on the operations of a substantial number of small rural hospitals.
Section 202 of the Unfunded Mandates Reform Act of 1995, Public Law 104-4 , requires that agencies assess anticipated costs and benefits before issuing any rule that may result in expenditures in any 1 year by State, local, or Tribal governments, in the aggregate, or by the private sector, of $100 million, adjusted annually for inflation. There are no significant costs associated with these finalized proposals that would impose mandates on State, local, or Tribal governments or the private sector resulting in an expenditure of $183 million in 2024 (after adjustment for inflation) or more in any given year. A full analysis under the Unfunded Mandates Reform Act is not necessary.
Executive Order 13132 , Federalism, establishes certain requirements that an agency must meet when it promulgates a rule that imposes substantial direct requirements or costs on State and local governments, preempts State law, or otherwise has federalism implications. In reviewing this rule under the threshold criteria of Executive Order 13132 , we have determined that this final rule would not significantly affect the rights, roles, and responsibilities of State or local governments. Nothing in this final rule imposes substantial direct requirements or costs on State and local governments, preempts State law, or otherwise has federalism implications. We are not aware of any State laws or regulations that are contradicted or impeded by any of the provisions in this final rule.
- Administrative practice and procedure
- Health facilities
- Health professions
- Reporting and recordkeeping requirements
- Health maintenance organizations (HMO)
- Health records
- Computer technology
- Electronic health record
- Electronic information system
- Electronic transactions
- Health care provider
- Health information exchange
- Health information technology
- Health information network
- Health insurance
- Public health
For the reasons set forth in the preamble, HHS amends 42 CFR chapter IV and 45 CFR part 171 as follows:
1. The authority citation for part 414 continues to read as follows:
Authority: 42 U.S.C. 1302 , 1395hh , and 1395rr(b)(l) .
2. Amend § 414.1305 by revising the definition of “Meaningful EHR user for MIPS” to read as follows:
Meaningful EHR user for MIPS means a MIPS eligible clinician that possesses CEHRT, uses the functionality of CEHRT, reports on applicable objectives and measures specified for the Promoting Interoperability performance category for a performance period in the form and manner specified by CMS, does not knowingly and willfully take action (such as to disable functionality) to limit or restrict the compatibility or interoperability of CEHRT, and engages in activities related to supporting providers with the performance of CEHRT. In addition, a MIPS eligible clinician (other than a qualified audiologist) is not a meaningful EHR user for a performance period if the HHS Inspector General refers a determination that the MIPS eligible clinician committed information blocking as defined at 45 CFR 171.103 during the calendar year of the performance period. The term “information blocking,” with respect to an individual MIPS eligible clinician or group, shall not include an act or practice other than an act or practice committed by such individual MIPS eligible clinician or group.
3. Amend § 414.1375 by revising paragraph (b) introductory text to read as follows:
(b) Reporting for the Promoting Interoperability performance category. To earn a performance category score for the Promoting Interoperability performance category for inclusion in the final score, a MIPS eligible clinician must be a meaningful EHR user for MIPS and:
4. The authority citation for part 425 continues to read as follows:
Authority: 42 U.S.C. 1302 , 1306 , 1395hh , and 1395jjj .
5. Amend § 425.208 by adding paragraph (b)(6) to read as follows:
(6) The information blocking provision of the 21st Century Cures Act ( 42 U.S.C. 300jj-52 ).
6. Amend § 425.218 by revising paragraph (b)(3) to read as follows:
(3) Violations of any applicable laws, rules, or regulations that are relevant to ACO operations, including, but not limited to, the laws specified at § 425.208(b).
7. Amend § 425.305 by revising paragraph (a)(1) to read as follows:
(1) ACOs, ACO participants, and ACO providers/suppliers are reviewed during the Shared Savings Program application process and periodically thereafter with regard to their program integrity history, including any history of Medicare program exclusions or other sanctions and affiliations with individuals or entities that have a history of program integrity issues. Program integrity history issues include, but are not limited to, a history of Medicare program exclusions or other sanctions, noncompliance with the requirements of the Shared Savings Program, or violations of laws specified at § 425.208(b).
8. The authority citation for part 495 continues to read as follows:
Authority: 42 U.S.C. 1302 and 1395hh .
9. Amend § 495.4 in the definition of “Meaningful EHR user” by revising paragraph (1) introductory text and adding paragraph (4) to read as follows:
Meaningful EHR user * * *
(1) Subject to paragraphs (3) and (4) of this definition, an eligible professional, eligible hospital or CAH that, for an EHR reporting period for a payment year or payment adjustment year—
(4) An eligible professional, eligible hospital or CAH is not a meaningful EHR user in a payment adjustment year if the HHS Inspector General refers a determination that the eligible hospital or CAH committed information blocking as defined at 45 CFR 171.103 during the calendar year of the EHR reporting period.
10. The authority citation for part 171 continues to read as follows:
Authority: 42 U.S.C. 300jj-52 ; 5 U.S.C. 552 .
11. Amend § 171.102 by adding, in alphabetical order, the definition of “Appropriate agency” and “Disincentive” to read as follows:
Appropriate agency means a government agency that has established disincentives for health care providers that the Office of Inspector General (OIG) determines have committed information blocking.
Disincentive means a condition specified in § 171.1001(a) that is imposed by an appropriate agency on a health care provider that OIG determines has committed information blocking for the purpose of deterring information blocking practices.
12. Add and reserve subparts E through I.
13. Add subpart J to read as follows:
This subpart sets forth disincentives that an appropriate agency may impose on a health care provider that OIG determines has committed information blocking, and certain procedures related to those disincentives.
(a) Centers for Medicare & Medicaid Services may apply the following disincentives:
(1) An eligible hospital or critical access hospital (CAH) as defined in 42 CFR 495.4 is not a meaningful electronic health record (EHR) user as also defined in 42 CFR 495.4 .
(2) A Merit-based Incentive Payment System (MIPS) eligible clinician as defined in 42 CFR 414.1305 , who is also a health care provider as defined in § 171.102, is not a meaningful EHR user for MIPS as defined in 42 CFR 414.1305 . Start Printed Page 54718
(3) Accountable care organizations (ACOs) who are health care providers as defined in § 171.102, ACO participants, and ACO providers/suppliers will be removed from, or denied approval to participate, in the Medicare Shared Savings Program as defined in 42 CFR part 425 for at least 1 year.
(b) [Reserved]
Following referral of a determination of information blocking by OIG, an appropriate agency that imposes a disincentive or disincentives specified in § 171.1001 shall send a notice to the health care provider subject to the disincentive or disincentives, via usual methods of communication for the program or payment system under which the disincentive is applied, that includes:
(a) A description of the practice or practices that formed the basis for the determination of information blocking referred by OIG;
(b) The basis for the application of the disincentive or disincentives being imposed;
(c) The effect of each disincentive; and
(d) Any other information necessary for a health care provider to understand how each disincentive will be implemented.
14. Add subpart K to read as follows:
Authority: 42 U.S.C. 300jj-11(c)(4) .
This subpart sets forth the information that will be posted on the Office of the National Coordinator for Health Information Technology's (ONC) public website about actors that have been determined by the HHS Office of Inspector General to have committed information blocking.
(a) Health care providers. (1) ONC will post on its public website the following information about health care providers that have been subject to a disincentive in § 171.1001(a) for information blocking:
(i) Health care provider name;
(ii) Business address;
(iii) The practice, as the term is defined in § 171.102 and referenced in § 171.103, found to have been information blocking, including when the practice occurred;
(iv) Disincentive(s) applied; and
(v) Where to find any additional information about the determination of information blocking that is publicly available via HHS or, where applicable, another part of the U.S. Government.
(2) The information specified in paragraph (a)(1) of this section will not be posted prior to a disincentive being imposed or the completion of any administrative appeals process pursued by the health care provider, and will not include information about a disincentive that has not been applied.
(3) Posting of the information specified in paragraph (a)(1) of this section will be conducted in accordance with existing rights to review information that may be associated with a disincentive specified in § 171.1001.
(b) Health IT developers of certified health IT and health information networks or health information exchanges. (1) ONC will post on its public website the following information, to the extent applicable, about health information networks/health information exchanges and health IT developers of certified health IT (actors) that have been determined by the HHS Office of Inspector General to have committed information blocking:
(i) Type of actor;
(ii) Actor's legal name, including any alternative or additional trade name(s) under which the actor operates;
(iii) The practice, as the term is defined in § 171.102 and referenced in § 171.103, found to have been information blocking or alleged to be information blocking in the situation specified in paragraph (b)(2)(i) of this section, and including when the practice occurred; and
(iv) Where to find any additional information about the determination (or resolution of information blocking as specified in paragraph (b)(2)(i) of this section) of information blocking that is publicly available via HHS or, where applicable, another part of the U.S. Government.
(2) The information specified in paragraph (b)(1) of this section will not be posted until one of the following occurs:
(i) OIG enters into a resolution of civil money penalty (CMP) liability; or
(ii) A CMP imposed under subpart N of 42 CFR part 1003 has become final consistent with the procedures in subpart O of 42 CFR part 1003 .
Xavier Becerra,
Secretary, Department of Health and Human Services.
1. Except if noted in reference a particular statutory authority or CFR section, we use in this rule “health care provider,” “provider,” and “provider type” as inclusive of individuals and entities that may be characterized for purposes of Medicare enrollment or particular reimbursement policies as providers or suppliers—or both across different contexts such as specific services furnished in particular settings.
2. As January 12, 2017, was the thirtieth day after December 13, 2016, conduct occurring on or after January 13, 2017, that otherwise meets the PHSA section 3022(a) definition of “information blocking,” would be included in that definition.
3. We use the term “civil money penalty” here, rather than “civil monetary penalty” as used in PHSA section 3022(b)(2)(A) for consistency with OIG's usage in the OIG CMP Final Rule ( 88 FR 42820 ).
4. As defined in 42 U.S.C. 300 -jj, the term “health care provider” includes a hospital, skilled nursing facility, nursing facility, home health entity or other long term care facility, health care clinic, community mental health center (as defined in section 300x-2(b)(1) of this title), renal dialysis facility, blood center, ambulatory surgical center described in section 1395l(i) of this title, emergency medical services provider, Federally qualified health center, group practice, a pharmacist, a pharmacy, a laboratory, a physician (as defined in section 1395x(r) of the title), a practitioner (as described in section 1395u(b)(18)(C) of the title), a provider operated by, or under contract with, the Indian Health Service or by an Indian tribe (as defined in the Indian Self-Determination and Education Assistance Act [ 25 U.S.C. 5301 et seq. ]), tribal organization, or urban Indian organization (as defined in section 1603 of title 5), a rural health clinic, a covered entity under section 256b of this title, an ambulatory surgical center described in section 1395l(i) of this title, a therapist (as defined in section 1395w-4(k)(3)(B)(iii) of the title), and any other category of health care facility, entity, practitioner, or clinician determined appropriate by the Secretary. See also this guidance document: https://www.healthit.gov/sites/default/files/page2/2020-08/Health_Care_Provider_Definitions_v3.pdf .
5. In the ONC Cures Act Final Rule, ONC defined the term “health IT developer of certified health IT” in 45 CFR 171.102 , instead of using the term that appears in PHSA 3022(a)(1): “health IT developer.” ONC explained that, because title XXX of the PHSA does not define “health information technology developer,” ONC interpreted section 3022(a)(1)(B) in light of the specific authority provided to OIG in section 3022(b)(1)(A) and (b)(2). ONC noted that section 3022(b)(2) discusses developers, networks, and exchanges by referencing any individual or entity described in section 3022(b)(1)(A) or (C). Section 3022(b)(1)(A) states, in relevant part, that OIG may investigate any claim that a health information technology developer of certified health information technology or other entity offering certified health information technology engaged in information blocking ( 85 FR 25795 , emphasis added).
6. In January 2024, ONC finalized a definition of what it means to “offer health IT,” and finalized a corresponding update to the “health IT developer of certified health IT” definition. These policies are part of a final rule titled Health Data, Technology, and Interoperability: Certification Program Updates, Algorithm Transparency, and Information Sharing ( 89 FR 1354 through 1358 ) (HTI-1 Final Rule).
7. For more information about the USCDI, see: https://www.healthit.gov/isa/united-states-core-data-interoperability-uscdi .
8. For more information, see: https://inquiry.healthit.gov/support/plugins/servlet/desk/portal/6 .
9. For more information, see: https://www.healthit.gov/faqs .
10. For more information, see: https://www.healthit.gov/topic/interoperability .
11. For more information on exceptions to information blocking, see ONC's website: https://www.healthit.gov/topic/information-blocking .
12. Subsequent to receiving this comment, the HIPAA Privacy Rule To Support Reproductive Health Care Privacy final rule ( 89 FR 32976 ) appeared in the Federal Register on April 26, 2024.
13. The Health Data, Technology, and Interoperability: Certification Program Updates, Algorithm Transparency, and Information Sharing final rule ( 89 FR 1192 ) appeared in the Federal Register on January 9, 2024.
14. For more information, see: https://www.healthit.gov/topic/information-blocking .
15. For more information, see: https://www.healthit.gov/topic/information-blocking .
16. 45 CFR parts 160 and 164, subparts A, C, D, and E.
17. “Practice,” as defined in 45 CFR 171.102 , means an act or omission by an actor (health care provider, health IT developer of certified health IT, health information network or health information exchange).
18. For more information, see: “Information Blocking Claims: By the Numbers,” https://www.healthit.gov/data/quickstats/information-blocking-claims-numbers .
19. Ibid.
20. For more information, see: https://www.healthit.gov/ .
21. For more information, see: https://www.healthit.gov/data/quickstats/information-blocking-claims-numbers .
22. For more information, see: https://www.healthit.gov/topic/information-blocking .
23. See: https://www.healthit.gov/buzz-blog/information-blocking/supporting-information-privacy-for-patients-now-and-always-four-reminders-of-how-hhs-information-blocking-regulations-recognize-privacy-rules .
24. 597 U.S. 697 (2022).
25. West Virginia v. EPA, 597 U.S. 697, 721 (2022).
26. See Exxon Shipping Co. v. Baker, 554 U.S. 471, 493 (2008), (noting one “aim” of “punishment” is “deterrence”); Hudson v. United States, 522 U.S. 93, 102 (1997), (“[A]ll civil penalties have some deterrent effect.”).
27. Section 1814(l)(3) of the SSA applies to critical access hospitals the standard for determining a meaningful EHR user in section 1886(n)(3).
28. For MIPS, SSA section 1848(o)(4) defines CEHRT as a qualified electronic health record (as defined in PHSA section 3000(13)) that is certified by ONC pursuant to PHSA section 3001(c)(5) as meeting standards adopted under PHSA section 3004 that are applicable to the type of record involved, as determined by the Secretary. CMS has codified the definition of CEHRT, including additional criteria it must be certified as meeting, that MIPS eligible clinicians must use at 42 CFR 414.1305 .
29. In the Disincentives Proposed Rule, we referred to the Quality Payment Program as a payment incentive program ( 88 FR 74958 ). Within the Quality Payment Program, MIPS is more appropriately described as a value-based payment system, and we have revised this statement for clarity and precision.
30. In the Disincentives Proposed Rule, we only noted that these scoring weights apply to the CY 2024 performance period/2026 MIPS payment year ( 88 FR 74958 ). However, as set forth in SSA section 1848(q)(5)(E), these scoring weights applied beginning 6 years after MIPS began applying to Medicare Part B payments (CY 2017 performance period/2019 MIPS payment year) and continue to apply for each subsequent year thereafter. Accordingly, we amended this description in this final rule for clarity and accuracy to note that these scoring weights continue to apply, provided CMS does not assign a different scoring weight pursuant to applicable exceptions.
31. In the Disincentives Proposed Rule ( 88 FR 74958 ), this word was inadvertently omitted from the quote of the statutory provision.
32. As provided in 42 CFR 414.1320(h) , for purposes of the 2024 MIPS payment year and each subsequent MIPS payment year, the performance period for the MIPS Promoting Interoperability performance category is a minimum of a continuous 90-day period within the calendar year that occurs 2 years prior to the applicable MIPS payment year, up to and including the full calendar year. In 42 CFR 414.1305 , CMS has defined the “MIPS payment year” as the calendar year in which the MIPS payment adjustment factor is applied to Medicare Part B payments. In the CY 2024 Physician Fee Schedule proposed rule, CMS proposed that, beginning with the 2026 MIPS payment year, the performance period for the MIPS Promoting Interoperability performance category would be a minimum of a continuous 180-day period within the calendar year that occurs 2 years prior to the applicable MIPS payment year, up to and including the full calendar year ( 88 FR 52578 through 52579 ). Since the Disincentives Proposed Rule appeared in the Federal Register , CMS finalized this proposal for amending the performance period for the MIPS Promoting Interoperability performance category, to a minimum of a continuous 180-day period, in the CY 2024 Physician Fee Schedule final rule and codified this amendment as proposed at 42 CFR 414.1320(i) ( 88 FR 79351 through 79353 ).
33. Although this statement was not part of this explanation in the Disincentives Proposed Rule ( 88 FR 74962 ), we have added it for clarity. We believe this statement is logically inferred from the original proposal.
34. We refer readers to Table 60 in the CY 2024 Physician Fee Schedule final rule ( 88 FR 79379 ) for an illustration of the potential range of MIPS payment adjustment factors that may be calculated and applied based on comparison of a MIPS eligible clinician's final score to the applicable performance threshold. For instance, a final score of 0 to 18.75 points for the CY 2024 performance period/2026 MIPS payment year may result in negative 9 percent MIPS payment adjustment factor; a final score of 18.76 to 74.99 may result in a MIPS payment adjustment factor between negative 9 percent and zero percent.
35. Although CMS did not include this clarification in the Disincentives Proposed Rule, this statement is consistent with existing MIPS policies governing individual and group reporting. See the CY 2017 Quality Payment Program final rule ( 81 FR 77330 through 77332 ).
36. For more information, see: the CY 2017 Quality Payment Program Final Rule ( 81 FR 77330 through 77332 ).
37. We define this term in our regulation at 42 CFR 414.1305 as a single TIN of two or more eligible clinicians (including at least one MIPS eligible clinician), as identified by their individual NPI, who have reassigned their billing rights to the TIN.
38. Shared Savings Program regulations generally specify standards for an ACO, which is bound by its participation agreement to the standards. CMS generally specifies standards applicable to an ACO participant and ACO provider/supplier that is participating in the ACO through its regulation of the ACO.
39. CMS notes that the list of laws included at 42 CFR 425.208(b) with which an ACO must comply is not an exclusive list. ACOs, ACO participants, and ACO providers/suppliers must continue to comply with all applicable Federal laws.
40. A description of the Innovation Center's strategy to support primary care can be found here: https://www.cms.gov/blog/cms-innovation-centers-strategy-support-high-quality-primary-care .
[ FR Doc. 2024-13793 Filed 6-26-24; 4:15 pm]
BILLING CODE 4150-45-P
- Executive Orders
Reader Aids
Information.
- About This Site
- Accessibility
- No Fear Act
- Continuity Information

COMMENTS
The methods used to study the problem; The initial aim of a title is to capture the reader's attention and to highlight the research problem under investigation. Create a Working Title Typically, the final title you submit to your professor is created after the research is complete so that the title accurately captures what has been done. The ...
Introduction. This article deals with drafting a suitable "title" and an appropriate "abstract" for an original research paper. Because the "title" and the "abstract" are the "initial impressions" or the "face" of a research article, they need to be drafted correctly, accurately, carefully, meticulously, and consume time and energy.[1,2,3,4,5,6,7,8,9,10] Often, these ...
You will use these on the next activities to create your research problem. Among the titles of the previous lesson you have created, which of those you think should be addressed. ... Propose Research Title: Background of the Problem: Reasons: See answer Advertisement Advertisement 224001140432 224001140432 ... Get the Brainly App
In addressing this concern, the proposed research paper will delve into the following components: Background to the research, where it will outline the trajectory of the pandemic and its overall effects on workplaces; the Significance of the Problem, highlighting the gravity of mental health issues on individuals and organizations; the ...
The research problem, therefore, is the main organizing principle guiding the analysis of your research. The problem under investigation establishes an occasion for writing and a focus that governs what you want to say. It represents the core subject matter of scholarly communication and the means by which scholars arrive at other topics of ...
A research problem is a specific issue or gap in existing knowledge that you aim to address in your research. You may choose to look for practical problems aimed at contributing to change, or theoretical problems aimed at expanding knowledge. Some research will do both of these things, but usually the research problem focuses on one or the other.
A good research question is essential to guide your research paper, dissertation, or thesis. All research questions should be: Focused on a single problem or issue. Researchable using primary and/or secondary sources. Feasible to answer within the timeframe and practical constraints. Specific enough to answer thoroughly.
Providing background information in the introduction of a research paper serves as a bridge that links the reader to the research problem.Precisely how long and in-depth this bridge should be is largely dependent upon how much information you think the reader will need to know in order to fully understand the problem being discussed and to appreciate why the issues you are investigating are ...
Key Takeaways. Developing a research proposal involves the following preliminary steps: identifying potential ideas, choosing ideas to explore further, choosing and narrowing a topic, formulating a research question, and developing a working thesis. A good topic for a research paper interests the writer and fulfills the requirements of the ...
1) A brief discussion on what is known about the topic under investigation; 2) An articulation of the research gap or problem that needs to be addressed; 3) What the researcher would like to do or aim to achieve in the study (research goal); 4) The thesis statement, that is, the main argument or claim of the paper; and.
It puts the proposal in context. 3. The introduction typically begins with a statement of the research problem in precise and clear terms. 1. The importance of the statement of the research problem 5: The statement of the problem is the essential basis for the construction of a research proposal (research objectives, hypotheses, methodology ...
In this example, the problem does not reveal the relevance of why you are investigating the fact there is no hospital in the community [e.g., perhaps there's a hospital in the community ten miles away]; it does not elucidate the significance of why one should study the fact there is no hospital in the community [e.g., that hospital in the ...
Answer: The rationale of your research problem is the reason for conducting the study. The rationale should answer the need for conducting the said research. It is a very important part of your publication as it justifies the significance and novelty of your research problem. To write your rationale, you should first write a background on all ...
The introduction leads the reader from a general subject area to a particular topic of inquiry. It establishes the scope, context, and significance of the research being conducted by summarizing current understanding and background information about the topic, stating the purpose of the work in the form of the research problem supported by a hypothesis or a set of questions, explaining briefly ...
Specifically, the Board proposed changing the word "that" to "any" immediately before the reference to the registered public accounting firm that commits the primary violation. After due consideration, the Board has decided not to adopt any changes to Rule 3502 to implement this aspect of the Proposal, for two primary reasons.
The title should be concise and descriptive, capturing the essence of the research problem and conveying the scope of the study. It should be informative and intriguing, capturing the reader's attention and interest. A good research problem and title will set the stage for the research project and help guide the study's methodology and analysis.
Scenario 3: Time Off from Work Betsay, a young, single mother, had been working long hours at the restaurant. She hadn't had a day off in over a month …
The relevant facts and circumstances include the nature of the health care provider's information blocking, the health care provider's diligence in identifying and correcting the problem, the time since the information blocking occurred, whether the provider was previously subject to a disincentive in another program, and other factors.
The research problem I propose to investigate is the impact of social media on mental health among teenagers. I chose this topic because of the growing concern about the influence of social media on young people's well-being. The pervasive use of social media platforms among teenagers has raised concerns about its impact on mental health.
To create a research problem and title, you must identify a topic of interest, narrow it down to a specific question or statement that addresses a gap in knowledge or a problem in the field, and use relevant literature to frame the research problem.. Why are research problem and titles important?. Research problem and titles are important because they provide a clear direction for a research ...