

30 Examples: How to Conclude a Presentation (Effective Closing Techniques)
By Status.net Editorial Team on March 4, 2024 — 9 minutes to read
Ending a presentation on a high note is a skill that can set you apart from the rest. It’s the final chance to leave an impact on your audience, ensuring they walk away with the key messages embedded in their minds. This moment is about driving your points home and making sure they resonate. Crafting a memorable closing isn’t just about summarizing key points, though that’s part of it, but also about providing value that sticks with your listeners long after they’ve left the room.
Crafting Your Core Message
To leave a lasting impression, your presentation’s conclusion should clearly reflect your core message. This is your chance to reinforce the takeaways and leave the audience thinking about your presentation long after it ends.
Identifying Key Points
Start by recognizing what you want your audience to remember. Think about the main ideas that shaped your talk. Make a list like this:
- The problem your presentation addresses.
- The evidence that supports your argument.
- The solution you propose or the action you want the audience to take.
These key points become the pillars of your core message.
Contextualizing the Presentation
Provide context by briefly relating back to the content of the whole presentation. For example:
- Reference a statistic you shared in the opening, and how it ties into the conclusion.
- Mention a case study that underlines the importance of your message.
Connecting these elements gives your message cohesion and makes your conclusion resonate with the framework of your presentation.
30 Example Phrases: How to Conclude a Presentation
- 1. “In summary, let’s revisit the key takeaways from today’s presentation.”
- 2. “Thank you for your attention. Let’s move forward together.”
- 3. “That brings us to the end. I’m open to any questions you may have.”
- 4. “I’ll leave you with this final thought to ponder as we conclude.”
- 5. “Let’s recap the main points before we wrap up.”
- 6. “I appreciate your engagement. Now, let’s turn these ideas into action.”
- 7. “We’ve covered a lot today. To conclude, remember these crucial points.”
- 8. “As we reach the end, I’d like to emphasize our call to action.”
- 9. “Before we close, let’s quickly review what we’ve learned.”
- 10. “Thank you for joining me on this journey. I look forward to our next steps.”
- 11. “In closing, I’d like to thank everyone for their participation.”
- 12. “Let’s conclude with a reminder of the impact we can make together.”
- 13. “To wrap up our session, here’s a brief summary of our discussion.”
- 14. “I’m grateful for the opportunity to present to you. Any final thoughts?”
- 15. “And that’s a wrap. I welcome any final questions or comments.”
- 16. “As we conclude, let’s remember the objectives we’ve set today.”
- 17. “Thank you for your time. Let’s apply these insights to achieve success.”
- 18. “In conclusion, your feedback is valuable, and I’m here to listen.”
- 19. “Before we part, let’s take a moment to reflect on our key messages.”
- 20. “I’ll end with an invitation for all of us to take the next step.”
- 21. “As we close, let’s commit to the goals we’ve outlined today.”
- 22. “Thank you for your attention. Let’s keep the conversation going.”
- 23. “In conclusion, let’s make a difference, starting now.”
- 24. “I’ll leave you with these final words to consider as we end our time together.”
- 25. “Before we conclude, remember that change starts with our actions today.”
- 26. “Thank you for the lively discussion. Let’s continue to build on these ideas.”
- 27. “As we wrap up, I encourage you to reach out with any further questions.”
- 28. “In closing, I’d like to express my gratitude for your valuable input.”
- 29. “Let’s conclude on a high note and take these learnings forward.”
- 30. “Thank you for your time today. Let’s end with a commitment to progress.”
Summarizing the Main Points
When you reach the end of your presentation, summarizing the main points helps your audience retain the important information you’ve shared. Crafting a memorable summary enables your listeners to walk away with a clear understanding of your message.
Effective Methods of Summarization
To effectively summarize your presentation, you need to distill complex information into concise, digestible pieces. Start by revisiting the overarching theme of your talk and then narrow down to the core messages. Use plain language and imagery to make the enduring ideas stick. Here are some examples of how to do this:
- Use analogies that relate to common experiences to recap complex concepts.
- Incorporate visuals or gestures that reinforce your main arguments.
The Rule of Three
The Rule of Three is a classic writing and communication principle. It means presenting ideas in a trio, which is a pattern that’s easy for people to understand and remember. For instance, you might say, “Our plan will save time, cut costs, and improve quality.” This structure has a pleasing rhythm and makes the content more memorable. Some examples include:
- “This software is fast, user-friendly, and secure.”
- Pointing out a product’s “durability, affordability, and eco-friendliness.”
Reiterating the Main Points
Finally, you want to circle back to the key takeaways of your presentation. Rephrase your main points without introducing new information. This reinforcement supports your audience’s memory and understanding of the material. You might summarize key takeaways like this:
- Mention the problem you addressed, the solution you propose, and the benefits of this solution.
- Highlighting the outcomes of adopting your strategy: higher efficiency, greater satisfaction, and increased revenue.
Creating a Strong Conclusion
The final moments of your presentation are your chance to leave your audience with a powerful lasting impression. A strong conclusion is more than just summarizing—it’s your opportunity to invoke thought, inspire action, and make your message memorable.
Incorporating a Call to Action
A call to action is your parting request to your audience. You want to inspire them to take a specific action or think differently as a result of what they’ve heard. To do this effectively:
- Be clear about what you’re asking.
- Explain why their action is needed.
- Make it as simple as possible for them to take the next steps.
Example Phrases:
- “Start making a difference today by…”
- “Join us in this effort by…”
- “Take the leap and commit to…”
Leaving a Lasting Impression
End your presentation with something memorable. This can be a powerful quote, an inspirational statement, or a compelling story that underscores your main points. The goal here is to resonate with your audience on an emotional level so that your message sticks with them long after they leave.
- “In the words of [Influential Person], ‘…'”
- “Imagine a world where…”
- “This is more than just [Topic]; it’s about…”
Enhancing Audience Engagement
To hold your audience’s attention and ensure they leave with a lasting impression of your presentation, fostering interaction is key.
Q&A Sessions
It’s important to integrate a Q&A session because it allows for direct communication between you and your audience. This interactive segment helps clarify any uncertainties and encourages active participation. Plan for this by designating a time slot towards the end of your presentation and invite questions that promote discussion.
- “I’d love to hear your thoughts; what questions do you have?”
- “Let’s dive into any questions you might have. Who would like to start?”
- “Feel free to ask any questions, whether they’re clarifications or deeper inquiries about the topic.”
Encouraging Audience Participation
Getting your audience involved can transform a good presentation into a great one. Use open-ended questions that provoke thought and allow audience members to reflect on how your content relates to them. Additionally, inviting volunteers to participate in a demonstration or share their experiences keeps everyone engaged and adds a personal touch to your talk.
- “Could someone give me an example of how you’ve encountered this in your work?”
- “I’d appreciate a volunteer to help demonstrate this concept. Who’s interested?”
- “How do you see this information impacting your daily tasks? Let’s discuss!”
Delivering a Persuasive Ending
At the end of your presentation, you have the power to leave a lasting impact on your audience. A persuasive ending can drive home your key message and encourage action.
Sales and Persuasion Tactics
When you’re concluding a presentation with the goal of selling a product or idea, employ carefully chosen sales and persuasion tactics. One method is to summarize the key benefits of your offering, reminding your audience why it’s important to act. For example, if you’ve just presented a new software tool, recap how it will save time and increase productivity. Another tactic is the ‘call to action’, which should be clear and direct, such as “Start your free trial today to experience the benefits first-hand!” Furthermore, using a touch of urgency, like “Offer expires soon!”, can nudge your audience to act promptly.
Final Impressions and Professionalism
Your closing statement is a chance to solidify your professional image and leave a positive impression. It’s important to display confidence and poise. Consider thanking your audience for their time and offering to answer any questions. Make sure to end on a high note by summarizing your message in a concise and memorable way. If your topic was on renewable energy, you might conclude by saying, “Let’s take a leap towards a greener future by adopting these solutions today.” This reinforces your main points and encourages your listeners to think or act differently when they leave.
Frequently Asked Questions
What are some creative strategies for ending a presentation memorably.
To end your presentation in a memorable way, consider incorporating a call to action that engages your audience to take the next step. Another strategy is to finish with a thought-provoking question or a surprising fact that resonates with your listeners.
Can you suggest some powerful quotes suitable for concluding a presentation?
Yes, using a quote can be very effective. For example, Maya Angelou’s “People will forget what you said, people will forget what you did, but people will never forget how you made them feel,” can reinforce the emotional impact of your presentation.
What is an effective way to write a conclusion that summarizes a presentation?
An effective conclusion should recap the main points succinctly, highlighting what you want your audience to remember. A good way to conclude is by restating your thesis and then briefly summarizing the supporting points you made.
As a student, how can I leave a strong impression with my presentation’s closing remarks?
To leave a strong impression, consider sharing a personal anecdote related to your topic that demonstrates passion and conviction. This helps humanize your content and makes the message more relatable to your audience.
How can I appropriately thank my audience at the close of my presentation?
A simple and sincere expression of gratitude is always appropriate. You might say, “Thank you for your attention and engagement today,” to convey appreciation while also acknowledging their participation.
What are some examples of a compelling closing sentence in a presentation?
A compelling closing sentence could be something like, “Together, let’s take the leap towards a greener future,” if you’re presenting on sustainability. This sentence is impactful, calls for united action, and leaves your audience with a clear message.
- How to Build Rapport: Effective Techniques
- Active Listening (Techniques, Examples, Tips)
- Effective Nonverbal Communication in the Workplace (Examples)
- What is Problem Solving? (Steps, Techniques, Examples)
- 2 Examples of an Effective and Warm Letter of Welcome
- 8 Examples of Effective Interview Confirmation Emails
How to End a Presentation (+ Useful Phrases)
Table of Contents
Most people are aware of the power of first impressions.
However, our appearance and the first words we utter are only one part of the impact we have on others.
Arguably, the final words we exchange during an interaction can have an even more lasting effect . And that applies to public speaking, too.
Obviously, the way you introduce yourself and the topic you’ll be discussing is important.
However, the end of a presentation should also be recognized as a crucial part of the experience .
With that in mind, this article will walk you through some:
- Things you should consider before drafting your conclusion,
- Tips for ending a presentation memorably,
- Mistakes you should avoid, and
- Phrases you can use to wrap up your speech.
But, before we discuss how to end a presentation, let’s establish why having an impactful conclusion is so essential.
Why is it important to have an impactful ending for your presentation?
In our article about starting a presentation , we explained how the steps of the motivated sequence framework correspond to the structure of the average presentation or speech.
As we have established, the introduction of a presentation mirrors the first step of that model. That means that one of its main goals is to get the listeners’ attention .
The central part of the speech, or the body , corresponds to the second, third, and fourth steps of the motivated sequence framework. In other words, it has to:
- Introduce the audience’s need (or identify a problem the listeners are having),
- Offer a way to satisfy (or resolve) that need, and
- Help the listeners visualize the successful implementation of the speaker’s solution.
Having checked off these points, we arrive at the conclusion , i.e., the subject of this article.
That stage of a presentation corresponds to the final step of the motivated sequence model — which consists of the call to action .
So, the conclusion of a presentation allows the speaker to drive their point home and nudge the audience toward performing a specific action.
However, that’s not the only purpose of a conclusion.
According to the authors of Business Communication: Process & Product , the final section of a presentation should achieve 3 goals . It should:
- Summarize the main themes of the presentation,
- Leave the audience with a specific and noteworthy takeaway (i.e. propose a specific course of action), and
- Include a statement that allows the speaker to leave the podium (or pass the mic) gracefully.
Above all, the ending of a presentation should be memorable , akin to the punchline of a joke.
Having said that, let’s talk about some factors you should consider as you’re writing the conclusion of your speech.
Things to consider before crafting the conclusion of your presentation
If you’re trying to figure out how to end a presentation, knowing the goals of a conclusion should help.
However, those objectives are only one part of the puzzle. To get the others, you should also consider:
- Your audience’s demographic breakdown,
- The general purpose of your presentation ,
- The specific purpose of your presentation , and
- Your thesis statement .
With that in mind, let’s see how each of these factors can help you develop an impactful conclusion for your presentation.
Factor #1: The demographic breakdown of the audience
As we have noted in our article about starting presentations, understanding the demographic breakdown of one’s audience is a crucial part of drafting a speech .
After all, the audience affects all of the choices we make — from the way we present ourselves to the vocabulary and the supporting materials we use during our presentations.
In our quest to learn more about the effect an audience can have on a presentation, we spoke to Persuasion Strategist Juliet Huck .
Having spent a significant portion of her professional career preparing people to take the witness stand, Huck knows a thing or two about adjusting one’s messaging to fit the preferences of one’s audience. She says:

“[The] ending [of] every presentation should be different and always based on the background of your audience. This should not be a blanket statement. It also depends on if you are educating your audience or persuading them to make a decision in your favor. You must do the homework on your audience prior to giving a presentation and end by leading them to your desired conclusion by giving them a conclusion they can relate to.”
But, if you’re not entirely sure how to take your audience into account when drafting your conclusion, consider the following questions:
- How will your audience connect to the topic you’re discussing?
- How can you relate the information you’re sharing to the listeners’ needs?
- What would make your audience think back on your presentation in positive terms?
- What would be the most effective way to get your point across to this specific audience?
Knowing whether your audience is friendly, neutral, uninterested, or hostile will also help you adjust your approach.
If nothing else, it’ll tell you whether you should stick to the facts or feel free to deliver a more casual or rousing speech.
Examples of different audience breakdowns
In our article about starting a presentation, we demonstrated our tips through 3 fictional speakers. So, let’s use the same presenters to illustrate this point.
- Nick Mulder is talking about the dangers of phishing. He introduced himself as the head of the security department. So, we can assume that he’s speaking to an audience of fellow employees, perhaps even through video conferencing software. Therefore, he was addressing an internal problem the company was having in front of a fairly receptive audience.
- Joan Miller is talking about how artificial intelligence is changing the future of the marketing industry. In her introduction, she mentioned having over four decades of experience in marketing. Consequently, we can infer that she’s speaking to an audience of marketing specialists who were previously unaware of her credentials.
- Milo Green is talking about employee retention. In his introduction, he indicated that the audience may know him as the founder of Green & Co. So, he’s probably famous enough to be recognized by at least a portion of his audience. Between that and the subject of his presentation, we can assume that he’s talking to the upper management of other companies.
From our examples, we can see how the identity of the speaker and their level of familiarity with the listeners might affect the way they prepare their presentations .
Factor #2: The general purpose of your presentation
Understanding the general purpose of a speech brings you one step closer to knowing how to end a presentation.
According to the authors of Communicating at Work , most presentations can be sorted into one of 3 categories based on that factor. In that regard, your presentation could be:
- Informative , aiming to expand the listeners’ knowledge and/or help them acquire a specific skill,
- Persuasive , with the goal of changing the listeners’ opinions or encouraging them to behave a certain way, or
- Entertaining , which is good for getting the audience to relax and look forward to upcoming speakers or events.
The general purpose of your presentation will naturally affect your conclusion because it will change what you choose to emphasize.
💡 Pumble Pro Tip
The basic goal of your presentation could correspond with the type of presentation you’re giving. To learn more about presentation types and styles, check out this article:
- Presentation types and styles explained
Examples of defining the general purpose of a presentation
Let’s see how our imaginary presenters would define the general purpose of their presentations.
- The general purpose of our phishing expert’s presentation is informative . The speaker’s primary goal is to teach his coworkers how to recognize and defend themselves against phishing attempts.
- Our marketing expert’s presentation is persuasive . She wants to change her listeners’ minds and make them more open to using AI in their marketing campaigns.
- The last speaker’s presentation about employee retention is also persuasive . After all, the speaker is attempting to show his listeners how they can increase the employee retention rate at their own companies. However, depending on the circumstances surrounding the speech, it could also take on some entertaining qualities.
Factor #3: The specific purpose of your presentation
The specific purpose of a presentation is essentially the outcome you’re looking to achieve with your speech. Defining this goal will require you to know the answers to the following questions :
- Who do you want to influence?
- What do you want them to think or do?
- How, when, and where do you want them to do it?
Ideally, the specific goal you come up with should be realistic and highly specific .
To that end, the authors of Communicating at Work recommend setting measurable goals . So, for example, instead of thinking: “ I want to get approval for my project. ”,
“I want my manager to let me set aside one day per week to work on this project. I also want them to let me ask one or two other people to help me with it.”
Having this kind of goal in mind will help you figure out how to wrap up your presentation.
Examples of defining the specific purpose of a presentation
So, how would our 3 speakers specify the desired outcomes of their presentations in measurable terms? Let’s see:
“I want the people in my company to understand the dangers of phishing attacks. They should learn the exact steps they need to take when they see a suspicious email in their inbox.”
“I want these marketing experts to be more knowledgeable about the way artificial intelligence works right now and understand how they can incorporate that software into their professional practice.”
“I want managers and HR professionals to know how they can make their companies a better place to work so they can keep their employee retention rate high.”
Free team communication software
Try Pumble, a secure, reliable, and easy-to-use communication tool.
FREE FOREVER • UNLIMITED COMMUNICATION

Factor #4: Your thesis statement
Ultimately, defining the general and specific goals of your presentation is a great way to keep yourself on track when crafting your speech.
However, the audience doesn’t need to know those goals.
Instead, they can hear your thesis statement — a summary of your overall message .
You can treat this statement as the throughline of your presentation. It will appear at least once in the introduction, followed by a few repetitions throughout the body of the presentation.
Finally, you’ll also want to include that same idea in your conclusion at least once.
In addition to keeping you, as the speaker, grounded, that repetition also keeps your audience from wondering what your presentation is about .
Examples of defining the thesis statement of a presentation
So, what would a thesis statement look like in practice? Let’s hear it from our fictional presenters:
“Identifying and reporting phishing emails will save the company’s information and money in the long term.”
“Right now, artificial intelligence isn’t as advanced as people think it is. However, we can still use it for marketing purposes as long as we make sure the process doesn’t begin and end with AI.”
“Improving your employee retention rate makes employees more engaged with their work and saves the company time and money that would otherwise go to training new personnel.”
How to end a presentation with a bang: 10 tips + examples
Now that we know why having an impactful conclusion is so crucial, it’s time to find the right way to achieve your goals.
To that end, we have highlighted 10 tips that might help you wrap up your presentation .
- Reiterate the key points and your core message.
- Mirror your opening statement.
- Elicit a response.
- Engage the audience.
- Call to action.
- Hand out materials.
- Acknowledge your contributors.
- Provide contact information.
- Thank the audience.
- Ask for feedback.
Of course, many of these methods we’ll discuss can be combined. However, your choices may be limited depending on the factors we have previously mentioned.
Tip #1: Reiterate the key points and your core message
Making sure the audience remembers your main points is one of the most important objectives your conclusion should accomplish.
With that in mind, you should dedicate some time at the end of your speech to reinforcing what you were trying to say throughout your presentation.
Take it from Mark Beal , Assistant Professor of Professional Practice, Communication, at Rutgers University:

“Every presentation should deliver and consistently reinforce three key message points. Most audience members will not recall more than three messages. Some may only recall one or two. With that [in mind], an engaging and effective presentation should conclude with the three messages the presenter wants the audience to take away.”
In essence, you’ll want to summarize your presentation by reiterating up to 3 key points and then repeating your thesis statement.
You could even translate this tip to your presentation slides. As Juliet Huck says:
“Your last slide should always draw your audience to your desired conclusion. [It] should be your billboard message , as we remember 70% of what we see and 20% of what we hear.”
We can see what that might look like through the example of our imaginary presentation on the dangers of phishing, below.

Tip #2: Mirror your opening statement
According to the authors of Communicating at Work , splitting a narrative between the introduction and the conclusion of your presentation is a good way to keep your audience’s attention.
Assistant Professor of Rhetorical Communication at the State University of New York, Dr. Lee M. Pierce , agrees:

“Psychological closure is looping back to the beginning to give the audience a sense of a closed circle. Don’t add new information in the conclusion, just tie the presentation up with a bow. [For example,] I always customize my closings based on the opening of the speech. During a TEDx Talk on Beyoncé’s ‘Formation,’ I began by walking out to the introduction to the song, and then I ended by walking off to the end of the song.”
The above quote demonstrates that this tip can be useful no matter which method you used to start your presentation .
You can use it to put a new spin on a statistic you shared in the introduction, give a story you told a different ending, or finish the punchline of a joke you started with.
Overall, coming back to the theme you introduced at the beginning of your speech should make your presentation seem more complete and intentional .
Phrases you can use to reflect the introduction of your presentation in the conclusion
With all that being said, let’s see how our imaginary speakers would mirror the opening lines of their presentations in their conclusion.
Having started with a phishing statistic, our first speaker might say:
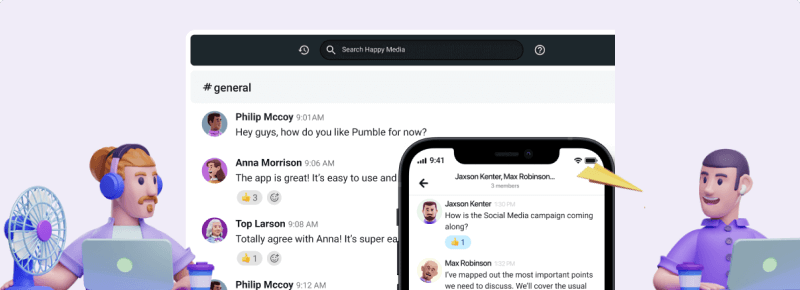
“Going back to the number we started with, remember that the Anti-Phishing Working Group has recorded 1,270,883 individual phishing attacks in the third quarter of 2022 — and that number is always on the rise. Luckily, you now have all the information you need to avoid becoming a part of that statistic.”
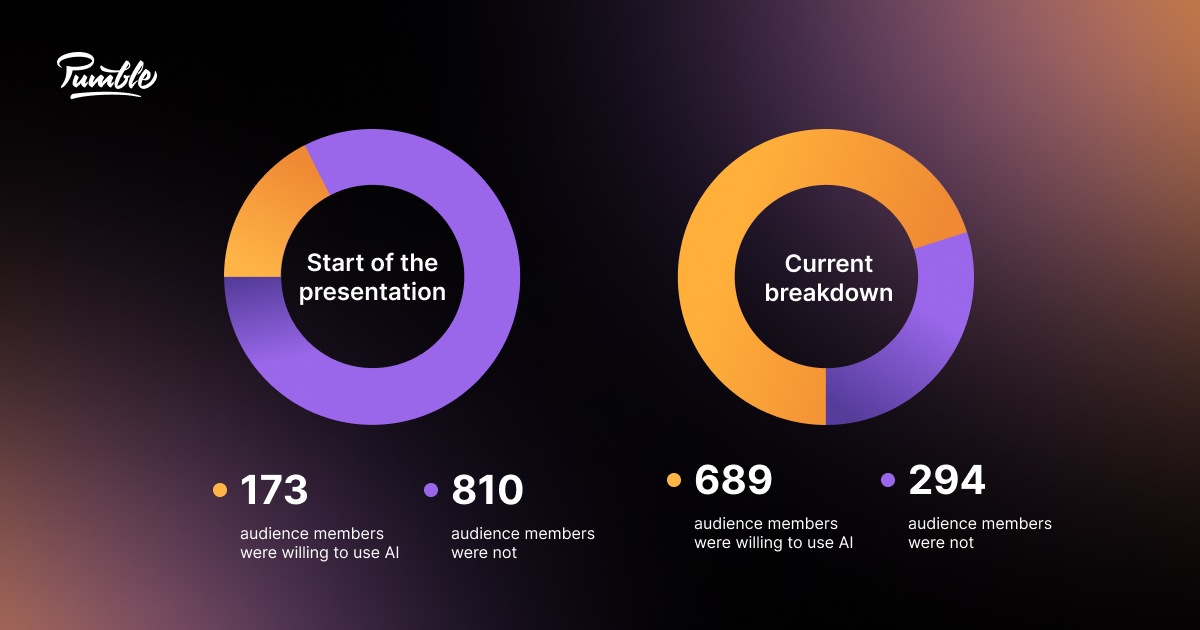
Our second speaker would have announced her plans to survey her listeners at the beginning of her presentation. In her conclusion, she might say:
“At the beginning of my presentation, I asked you to answer a quick survey on whether you’d be willing to work with AI. If you look back at your phones, you’ll see a different link in the #general channel on Pumble . Let’s see if this talk has managed to sway some opinions!”
Lastly, our final speaker might refer back to a humorous statement he made about chaining one’s employees to their desks to ensure that employee retention rates stay high.
“Once you start making your company a better place to work, your employees will happily perform their daily tasks — without being glued to their desks.”
Tip #3: Elicit a response
Making an audience experience strong emotions is always a good thing, but especially as the presentation comes to a close.
Putting the listeners in a contemplative mood or, even better, a cheerful one, means that they’ll be more likely to remember you and the points you made after your presentation ends.
On top of that, concluding your presentation in this manner would allow you to step off the stage gracefully, which is one of the main goals your conclusion should accomplish.
Now, depending on the type of presentation you’re delivering and, indeed, your style of presenting, you could elicit a response by:
- Ending with a short but powerful statement ,
- Asking a thought-provoking rhetorical question ,
- Relying on an impactful statistic or a quote , or even
- Inserting a funny picture or a meme on your final presentation slide.
Any one of these methods could help you solidify yourself and your message in the minds of the audience.
Phrases you can use to elicit a response from the audience
So, how would our 3 presenters try to get a response from their audiences? Well, they might use the following statements.
“Ultimately, the best defense against phishing attacks is human intelligence. You, alone, can ensure that your information remains secure by implementing the checklist I’ve shared today.”
“So, let me ask you again. Would you be willing to incorporate AI into your marketing campaign?”
“Hey, if the conditions you’re offering to your employees are good enough — there’s no need to keep them glued to their desks.”

Tip #4: Engage the audience
As we’ll discuss later on, having a Q&A session at the end of your presentation doesn’t always pan out the way you want it to.
Even so, getting your audience — or at least a few select listeners — to verbally respond to you can go a long way toward making you seem like a more engaging speaker.
Still, you can’t implement this tip without a strategy. You want to lead your audience to a certain type of response .
Professional speaker, career change consultant, and host of the Career Relaunch® podcast, Joseph Liu , had this to say:

“I often invite attendees to share what action they’re going to take amongst the potential ones I’ve covered throughout the presentation or to at least commit to taking some sort of action.”
Speaker, author, and editorial producer at CNN, Nadia Bilchik , agrees:

“If time allows, I always ask participants to share their biggest takeaway.”
The quote above also highlights the importance of being aware of the time as you are concluding a presentation — which is another thing we’ll talk about later.
For now, we’ll just boil this tip down to the following statement: if possible, try to make people verbalize or at least think about the knowledge they’re taking away from your speech .
Phrases you can use to engage the audience
Going back to our imaginary speakers, let’s see how this tip might work in practice.
“As we approach my conclusion, I’d like for us to reflect on everything we’ve learned here today. So, let me turn the spotlight on you all. Does anyone remember how to recognize a phishing email without opening it?”
“Now, I’m sure everyone here has some idea of how they might incorporate AI into their next marketing campaign. Is anyone willing to share their strategy?”
“Alright! Pop quiz time — don’t worry, I won’t grade you. Can you all shout out the main 3 ways to increase employee retention? Number 1?”
Tip #5: Call to action
Once you have finished reiterating your core message and making sure you have your audience’s attention, you need to be able to direct the listeners to the next step.
As Michelle Gladieux , author of Communicate with Courage and President of Gladieux Consulting, an employee coaching provider, would put it:

“What can the audience DO with the information you’ve shared? Suggest a positive, fruitful next step or, even better, suggest several, and let your presentation participants choose among options that have panned out well for others.”
In her workshops, Gladieux says:
“We ask participants to document at least one goal for behavior change that is specific, measurable, and time-based, and take a bonus step of inviting them to name one person they’ll tell about their goal for added accountability.”
According to the authors of Communicating at Work , there are 2 ways to deliver a call to action at the end of your presentation. Namely, you can either phrase it as:
- An appeal or a question (e.g. “If any of this sounds interesting, you can learn more by signing up for our newsletter through the link on the screen behind me.” ), or
- A challenge or a demand (e.g. “Now, you can keep doing what you’re doing and getting lackluster results. Or, you can sign up for our newsletter to receive tips that will help you upgrade your strategy.” ).
As always, your choice will depend on the factors we have listed at the top of this article.
Phrases you can use to call the audience to action
Let’s see what our fictional speakers’ calls to action might look like.
“Remember, even if you happen to open a phishing email, you’ll be able to deal with it easily by forwarding it to this email address. That’s the main thing you need to remember from this talk.”
“I bet many of you could come up with even more creative ways to incorporate AI into your marketing campaigns. So, how about this: if you fill out the form I’m about to send you, I’ll check in with you in about three months. Those of you who succeed in using AI in a meaningful way will get a chance to share your insights on this very stage next year!”
“I have a challenge for those of you who are ready to meet me at my level. I want you to sign a pledge, promising to boost your employee retention rate by 10% in the next year. We had a similar experiment at one of my talks a couple of years back, and even I was surprised by the results.”
If you decide to accompany this part of your speech with a call to action slide, keep Juliet Huck’s advice in mind:
“A call to action slide is not always persuasive. Persuasion is not a call to action — it is a directed action. To ‘call’ means someone can say no, but to ‘persuade’ [is to] direct your audience to your desired conclusion based on a number of steps.”
In effect, that means that your call to action should be the final step of your persuasion strategy.
You should start building to that desired outcome well before you get to the end of your presentation.
Tip #6: Hand out materials
The ending of a presentation is the perfect time to give the audience a keepsake of your speech .
But, keep in mind that a memento doesn’t have to be a physical item. As Michelle Gladieux would say:
“I like to direct my audiences to free downloadable resources on our website for those who want to continue their personal and professional growth as leaders and communicators.”
So, sharing resources through email or a business messaging app would work just as well.
Of course, you don’t have to hold off until the conclusion of your presentation to give your audience something to remember you by. Gladieux also shared a method she used in her workshops:
“[Most of our] participants have our high-quality original workbooks in hand during the presentation and available later as a tangible resource. Folks add notes, take short assessments, and work on case studies when we teach using workbooks. If we use presentation slides, we keep the content as engaging visually as possible and short on words.”
If your budget allows you to do something similar, that might be a good way to make the audience remember you.
Phrases you can use before handing out materials
In the scenarios we have conjured up, the speakers might introduce their additional materials like so.
“If you’re interested in learning more about phishing and how you can defend yourself from future attacks, you’ll find more information by following the link on the screen.”
“Now, at this point, I see that my associates have already started delivering some additional materials and miscellaneous goodies to you. I hope you’ll use them to workshop further ideas for using AI in your marketing strategies.”
“I’ll go ahead and forward these presentation slides as well as some additional resources for improving employee retention to you all.”
If you’re looking for a convenient way to deliver additional resources to the attendees of your speech, Pumble is a great option. This article offers some practical tips for using business messaging software for educational purposes — including online conferences:
- Using Pumble for teaching and learning
Tip #7: Acknowledge contributors
If you’re delivering a business presentation as a representative of a team or a department, you can also use the final moments of your speech to acknowledge everyone who worked on the presentation with you.
On the one hand, you could simply thank your team in general terms and leave it at that.
Alternatively, you could highlight the individual contributions of specific team members if you want to make sure their effort doesn’t go unnoticed.
Phrases you can use to acknowledge your contributors
Here’s how our fictitious presenters might acknowledge the people who helped them create their presentations:
“Before I sign off, I’d like to take a moment to thank Jill and Vanessa from the security team, who helped me compile the data and create the slides you just saw.”
“Finally, I’d like to acknowledge that this presentation wouldn’t be half as informative without the experts who helped me understand the technical side of AI.”
“Now, let’s all give it up for my wonderful team, who helped me organize this lecture.”
Improve communication and collaboration for increased team efficiency with Pumble.
Tip #8: Provide contact information
Business presentations often double as networking opportunities , both for presenters and for audience members.
With that in mind, you might want to put your contact information on one of your closing slides.
For one, doing so would show the audience how they can get in touch with you after your presentation ends. After all, they may have additional questions or even interesting business opportunities for you.
On top of that, putting your contact information on the last slide is also a good way to remind the audience of your name and credentials .
For that reason, our second imaginary speaker might have “Joan Miller — Chief Marketing Officer at Happy Media” on her final slide.
Phrases you can use to provide contact information
So, how would our presenters encourage their audience to keep in touch? Well, they might say:
“I’m always happy to answer any of your security or phishing-related questions on Pumble. You’ll find me by clicking the plus sign next to the direct messages section and searching my name, Nick Mulder.”
“If you all have any follow-up questions for me or one of the AI experts I’ve spoken to, you’ll find all of our contact information on this slide.”
“If you want to stay up to date on Green & Co’s latest news, follow us on LinkedIn.”
Tip #9: Thank the audience
Many presenters find a way to incorporate a “ thank you ” slide at the end of their presentations.
If you want to express your appreciation to your audience members , you could do the same thing.
However, as we’ll soon discuss, many of the experts we’ve spoken to would advise against having pointless visuals at the end of your presentation.
After all, you want to leave the audience with something memorable to take away from your speech.
Still, if you want to thank the audience, you could always make that final slide serve multiple functions .
For example, a “thank you” slide can also contain the speaker’s contact information, as well as additional resources.

This example “thank you” slide above features a QR code (you can create one using a QR code generator ) leading to more resources — it prompts the audience to find the speaker on various social media platforms.
Tip #10: Ask for feedback
Lastly, some speakers might benefit from knowing what the audience thinks about their delivery and other aspects of their presentation.
That’s why some of the experts we’ve spoken to suggest that conducting a brief survey of the audience could be a good activity to end a presentation with.
Rutgers University professor, Mark Beal, says that:
“Offering audience members the opportunity to take a concise survey at the conclusion of a presentation will result in valuable insights that will inform how to consistently evolve and improve a presentation. […] We use the last few minutes of seminars to allow participants to answer a few questions about what was most useful in our content and delivery, and what, in that individual’s opinion, could improve.”
Michelle Gladieux is also an advocate for audience surveys, saying:
“I’ve delivered thousands of training workshops and keynotes and never miss an opportunity to ask for feedback formally (in writing), informally (in conversation), or both. As you might guess, I advise every presenter reading this to do the same.”
You could encourage this type of feedback by:
- Asking attendees to share their thoughts on your presentation after you step off the stage,
- Setting up a notebook near the door and asking people to jot down their thoughts as they exit,
- Having a suggestion box for hand-written feedback notes, or
- Creating an anonymous survey online and linking to it on your presentation slides.
Most presenters nowadays tend to rely on technology to compile audience feedback, but the method you use will depend on the circumstances surrounding your presentation.
If you’ve never had to ask for feedback before, you might find this article interesting:
- How to ask your manager for feedback
The worst ways to end a presentation
Having gone through the best practices for concluding a presentation memorably, we also wanted to know what are some of the mistakes speakers should avoid as they reach the end of their speech.
The experts we have spoken to have identified 5 of the worst ways to end a presentation :
- Overloading your final slide.
- Settling for a lackluster closer.
- Ending with a Q&A session.
- Not having time for any questions at all.
- Going over your time.
So, let’s see what makes these mistakes so bad.
Mistake #1: Overloading your final slide
Overloading your presentation slides isn’t a mistake you can make only at the end of your presentation.
Professional speakers know that slides are only there to accompany your speech — they shouldn’t be the main event.
As Nadia Bilchik says:

“Slides are only there to support your message. Towards the end of the presentation, I may even stop the slideshow entirely and just have a black screen. At the very end of the presentation, my suggestion is to have a slide up with the next steps or a call to action.”
Dr. Lee M. Pierce also tends to use blank slides:

“I always end and begin with blank slides. As a speaker, you’re trying to build connection and rapport between you and the audience, not between the audience and your slide deck.”
Therefore, putting too much information onto a single slide can make the speaker seem unprepared, in addition to overwhelming the audience.
When in doubt, remember Kawasaki’s 10/20/30 rule :
- No more than 10 slides per presentation,
- Keep your presentations under 20 minutes, and
- The text on your slides should never be smaller than 30-point font.
Mistake #2: Settling for a lackluster closer
If your goal is to become a proficient speaker, you’ll have to stop using uninspired closers like:
- “Well, I guess that’s it.”
- “That’s pretty much all I had to say.”
- “That’s about it from me. Can we get some applause?”
The audience will respond if you say something deserving of a response.
Instead of using these bland lines, remember Juliet Huck’s advice:
“Never end your presentation without closing the loop of your beginning theme and being specific when asking for your desire conclusion.”
As we have established, it’s best to conclude your speech by bringing back your thesis statement and key points.
Finishing with weak visuals is similarly offensive — and here we’re not just talking about presentation slides.
Remember, body language is an important component of our communication .
Fidgeting as your presentation comes to a close or slumping your posture as soon as you’re finished speaking won’t do.
As Michelle Gladieux would say:
“Never end a presentation seeming happy to be done, even if you are! Be certain you’re happy to be the presenter before you begin, or find someone else to do it.”
In other words, try not to show signs of anxiety during your presentation .
Maintain a confident demeanor for as long as you remain on stage or as long as you’re on camera, in the case of virtual meetings .
Mistake #3: Ending with a Q&A session
One of the experts we have spoken to, Nadia Bilchik, was particularly adamant about not ending presentations with Q&A sessions.
“Never ever end a presentation on a question-and-answer session. I have seen numerous presenters end by asking ‘Any questions?’ Too often there are no questions, and the presenter is left looking deflated and muttering ‘Thank you.’ [If there are] no questions, you can always say ‘A question I’m often asked is…’ or ‘Something I would like to reiterate is…’ Never end your presentation without your audience being clear about what they are expected to do with the information you have just shared.”
Adding that you can:
“Ask for questions, comments, and concerns, and only then end with a quick wrap-up. The goal is to end with your audience being clear on their next steps.”
Even if the listeners do have questions, there’s a good reason not to have a Q&A session at the very end of your presentation.
Namely, there’s always a chance that someone will ask a question that completely derails the conversation.
If you have the Q&A portion right before your conclusion, you’ll have time to reiterate your core message and proceed with a memorable closing statement .
For reference, you can ask for questions by saying:
“Before I close out this lecture, do you guys have any questions for me?”
Then, if there are no questions, you can still proceed to your conclusion without losing face.
A Q&A session is one of the best ways to make your presentations more interactive — but it’s not the only way to go about it. To learn more, check out this article:
- 18 Ways to make presentations more interactive and engaging
Mistake #4: Not having time for any questions at all
Ending with a Q&A session could be a problem — but, perhaps, not as big of a problem as not taking questions at all.
As Mark Beal would say:
“Not giving the audience the opportunity to participate in the presentation via a question and answer session is another ineffective way to end a presentation. Audiences want to have a voice in a presentation. They will be more engaged with the presentation content and recall it more effectively if given the opportunity to participate in the presentation and interact with the presenter.”
Dr. Lee M. Pierce adds:
“It’s always good to leave at least 15 minutes for questions. Leaving 5 minutes is annoying and pointless. Also, be prepared that the audience may not have questions or not feel comfortable just jumping in, so have some of your own questions ready to offer them. You can say something like, ‘Just to put it out there, if I were going to ask me a question, I’d ask…’ ”
Now, both Nadia Bilchik and Lee M. Pierce have mentioned phrases you can use if no one comes forth with a question.
You’ll notice that the sentences they have come up with will require you to consider the questions you may be asked ahead of time .
In addition to helping you create a better presentation, doing this will also allow you to answer any questions effortlessly.
Mistake #5: Going over your time
Last but not least, many of the professional speakers we have interviewed have stressed the importance of ending one’s presentation on time.
Michelle Gladieux said it best:
“The best way to end a presentation is ON TIME. Respect others’ time commitments by not running over. You can always hang around for a while to speak with people who have more to say or more to ask.”
Dr. Lee M. Pierce agrees:
“The worst thing you can do is run over time. If you were given 45 minutes for a presentation plus 15 minutes for Q & A, you should end at 45 minutes — better if you end at 35 or 40.”
Then again, according to Guy Kawasaki’s 10/20/30 rule, even going over the 20-minute mark could risk boring and alienating one’s audience.
Useful phrases for ending a presentation
In the course of our research, we’ve found many practical phrases one might use to wrap up a presentation.
We even had experts send in their suggestions. For example, Nadia Bilchik says:
“I always end with a very quick summary of the content, a definitive call to action, and a reiteration of the benefits to the audience. This is a superb model, and I have shared it with thousands of individuals who have found it immensely valuable. Use this as your framework: What I have looked at today… What I am asking you to do… The benefits are…”
Other phrases you might use at the end of your presentation include:
“To recap, we’ve discussed…”
“Throughout this presentation, we talked about…”
“In other words,…”
“To wrap up/conclude,…”
“In short, I’d like to highlight…”
“To put it simply,…”
“In conclusion…”
“In summary, the goal of my presentation…”
“If there’s one thing you take away from my presentation…”
“In bringing my presentation to a close, I wanted to…”
If you’d like to incorporate a call to action, you might say:
“I’m counting on you to…”
“After this presentation, I’d like to ask you to…”
“Please take a minute to…”
“Next time you (see a suspicious email), remember to (forward it to this email address).”
To end with a quote, you could say:
“Let me leave you with this quote…”
“That reminds me of the old saying…”
Lastly, more useful phrases include:
“Feel free to reach out if you have any questions.”
“For more information, head to the link on the screen.”
“Thank you for your time/attention.”
“I hope you found this presentation informative/useful/insightful.”
Remember: the last words you say should make it abundantly clear that your presentation has ended.
What should your final slide look like?
If you don’t want to leave your final slide blank as some of the experts we have talked to would recommend, there are other ways to fill that space.
Joseph Liu told us:
“I tend to make it very clear the presentation is coming to an end by having a slide that says, ‘Closing Thoughts’ or something to that effect. I recommend ending with a recap of your content, reconnecting with the initial hook you used at the start, and finally, some sort of call to action.”
Mark Beal has a similar formula for his closing slides, saying:
“The final slides of my presentation include: A slide featuring three key messages/takeaways, A question and answer slide to engage the audience at the conclusion in the same manner a presenter wants to engage an audience at the start of a presentation, and A final slide including the presenter’s contact information and a website address where they can learn more information. This slide can include a QR code that the audience can screenshot and access the presenter’s website or another digital destination.”
Between these two suggestions and the many examples we have included throughout our guide, you ought to have a clear picture of what your final slide might look like.
End your presentations with a bang on Pumble
Knowing how to end a presentation effectively is a skill like any other — you’re bound to get better through practice and repetition.
To get the most out of your presentations, make sure to give them on Pumble.
Pumble — a team communication and collaboration app — allows you to have the most interactive, efficient presentations thanks to:
- The video conferencing feature that allows you to share your knowledge with a large group of people,
- The screen sharing feature that allows you share your presentation,
- The in-call message feature, to ensure your audience can participate (and send questions for the FAQ partition of the presentation, for example), and
- The blur background feature, that ensures your audience’s attention is always on you and you alone.
Secure, real-time communication for professionals.

Olga Milicevic is a communication researcher and author dedicated to making your professional life a bit easier. She believes that everyone should have the tools necessary to respond to their coworkers’ requests and communicate their own professional needs clearly and kindly.
What's on your to-do?
START COLLABORATING
with Pumble

Related posts
Using im apps can cause information leaks & confusion — here’s why.
Discover why using IM apps for business communication is counterproductive and learn about a better alternative….
Drive Innovation with Better Collaboration in 3 Steps
Learn effective strategies for boosting innovation through streamlined collaboration. …
Harness the Power of HR Hilarity: 60+ Funny HR Memes for 2024
Like any other job, HR has its ups and downs, with plenty of humor to be found on either end of the spectrum. These funny HR memes should prove our po…
60+ Absurdly Funny Meeting Memes to Share With Your Work Buddies
The next time you’re stuck in a meeting that has no end, feel free to peruse our compilation of funny meeting memes. …
10+ Tips for Conducting an Effective Job Interview
Learn how to conduct an effective job interview and improve a candidate’s experience with expert tips. …
60+ Hilarious Quips to Use As Your Joke of the Day for Work
Looking for the perfect joke of the day for work? Here are 60+ hilarious jokes for work. …
Need better team communication??
Pumble is an all-in-one team collaboration app. Send messages and files, and start video conferencing with one click, and reduce emails. Free forever.
Free team chat app
Improve collaboration and cut down on emails by moving your team communication to Pumble.
Unlimited users • Unlimited chat history • Free forever

SpeakUp resources
How to end a presentation in english: methods and examples.
- By Matthew Jones
Naturally, the way you end a presentation will depend on the setting and subject matter. Are you pitching an idea to your boss? Are you participating in a group presentation at school? Or are you presenting a business idea to potential investors? No matter the context, you’ll want to have a stellar ending that satisfies your audience and reinforces your goals.
So, do you want to learn how to end a presentation with style? Wondering how to end an informative speech? Or do you want to know how to conclude a Powerpoint presentation with impact? We’re here to help you learn how to end a presentation and make a great impression!
How to End a Presentation: 3 Effective Methods
Every presentation needs a great beginning, middle, and end. In this guide, we will focus on crafting the perfect conclusion. However, if you’d like to make sure that your presentation sounds good from start to finish, you should also check out our guide on starting a presentation in English .
Though there are many ways to end a presentation, the most effective strategies focus on making a lasting impression on your audience and reinforcing your goals. So, let’s take a look at three effective ways to end a presentation:
1. Summarize the Key Takeaways
Most presenters either make an argument (i.e. they want to convince their audience to adopt their view) or present new or interesting information (i.e. they want to educate their audience). In either case, the presentation will likely consist of important facts and figures. The conclusion gives you the opportunity to reiterate the most important information to your audience.
This doesn’t mean that you should simply restate everything from your presentation a second time. Instead, you should identify the most important parts of your presentation and briefly summarize them.
This is similar to what you might find in the last paragraph of an academic essay. For example, if you’re presenting a business proposal to potential investors, you might conclude with a summary of your business and the reasons why your audience should invest in your idea.
2. End with a CTA (Call-To-Action)
Ending with a Call-To-Action is one of the best ways to increase audience engagement (participation) with your presentation. A CTA is simply a request or invitation to perform a specific action. This technique is frequently used in sales or marketing presentations, though it can be used in many different situations.
For example, let’s say that you’re giving an informational presentation about the importance of hygiene in the workplace. Since your goal is to educate your audience, you may think that there’s no place for a CTA.
On the contrary, informational presentations are perfect for CTA’s. Rather than simply ending your presentation, you can direct your audience to seek out more information on the subject from authorities. In this case, you might encourage listeners to learn more from an authoritative medical organization, like the World Health Organization (WHO).
3. Use a Relevant Quote
It may sound cliche, but using quotes in your closing speech is both memorable and effective. However, not just any quote will do. You should always make sure that your quote is relevant to the topic. If you’re making an argument, you might want to include a quote that either directly or indirectly reinforces your main point.
Let’s say that you’re conducting a presentation about your company’s mission statement. You might present the information with a Powerpoint presentation, in which case your last slide could include an inspirational quote. The quote can either refer to the mission statement or somehow reinforce the ideas covered in the presentation.
Formatting Your Conclusion
While these 3 strategies should give you some inspiration, they won’t help you format your conclusion. You might know that you want to end your presentation with a Call-To-Action, but how should you “start” your conclusion? How long should you make your conclusion? Finally, what are some good phrases to use for ending a presentation?<br>
Examples of a Good Conclusion
In conclusion, I believe that we can increase our annual revenue this year. We can do this with a combination of increased efficiency in our production process and a more dynamic approach to lead generation. If we implement these changes, I estimate that annual revenue will increase by as much as 15%.
The example above shows a good conclusion for a business presentation. However, some people believe that the term in conclusion is overused. Here’s how to end a presentation using transition words similar to in conclusion .
Transition words help your audience know that your presentation is ending. Try starting your conclusion with one of these phrases:
- To summarize
However, transition words aren’t always necessary. Here are a few good ways to end a presentation using a different approach.
- Summarize Key Takeaways : There are two things that I’d like you to remember from today’s presentation. First, we are a company that consults startups for a fraction of the cost of other consultation services. And second, we have a perfect record of successfully growing startups in a wide variety of industries. If anything was unclear, I’d be happy to open the floor to questions.
- Make a Call-To-Action : I am very passionate about climate change. The future of the planet rests on our shoulders and we are quickly running out of time to take action. That said, I do believe that we can effect real change for future generations. I challenge you to take up the fight for our children and our children’s children.
- Use a Relevant Quote: I’d like to end my presentation with one of my favorite quotes: “Ask not what your country can do for you — ask what you can do for your country.”
As you can see, your conclusion does not need to be very long. In fact, a conclusion should be short and to the point. This way, you can effectively end your presentation without rambling or adding extraneous (irrelevant) information.
How to End a Presentation in English with Common Phrases
Finally, there are a few generic phrases that people frequently use to wrap up presentations. While we encourage you to think about how to end a presentation using a unique final statement, there’s nothing wrong with using these common closing phrases:
- Thank you for your time.
- I appreciate the opportunity to speak with you today.
- I’ll now answer any questions you have about (topic).
- If you need any further information, feel free to contact me at (contact information).
We hope this guide helps you better understand how to end a presentation ! If you’d like to find out more about how to end a presentation in English effectively, visit Magoosh Speaking today!
Matthew Jones

Free practice (Facebook group)
Phone: +1 (510) 560-7571
Terms of Use
Privacy Policy
Company Home

- Video Marketing
- Case Studies
- Create a video

How to End a Presentation? [Top 8 Strategies with Examples]
Guru - May 9, 2023 - Leave your thoughts. 9 min read
animaker deck , presentation , presentation ideas , Presentation Software , presentation tips
How you end a presentation is just as crucial as its opening. It can make or break the impression that you leave on your audience.
A strong conclusion can reinforce your key message and ensure that your audience remembers it even after the presentation is over.
A well-concluded presentation can leave your audience impressed, energized, and motivated to take action.
So now, are you wondering what’s the best way to conclude your presentation? Don’t worry! You have come to the right place!
To help you make a powerful ending to your presentation, we have compiled a list of 8 different strategies in this blog post.
Each of these strategies is designed to help you create a memorable and impactful conclusion to your presentation.
By choosing the most appropriate one for your presentation, you can ensure that your audience remembers your key message and feels motivated to take action.
Let’s jump right in,
1. Emphasize the core message 2. Mirror your opening statement 3. Pose an open-ended question 4. End with a Call to action 5. Thank the audience 6. End with a powerful quote 7. Acknowledge your contributors 8. Ask for feedback
1. Emphasize the core message:
One of the most important aspects of any presentation is ensuring your audience understands your core message.
Reiterating your main points and summarizing your message at the end of your presentation can reinforce this and leave a lasting impression.
It helps to ensure that your audience understands the purpose of your presentation and has a clear takeaway from the information you have provided.
In this video, the speaker restates her topic to conclude her speech firmly and gives a pause, resulting in tremendous applause from the audience.
Similarly, by restating your core message, you can also create a sense of cohesion and give your presentation a firm closure.
This can be particularly important if you want to motivate your audience to take action or influence their behavior in some way.
However, it's important not to repeat EVERYTHING you have said. Instead, focus on the most crucial elements and highlight them in a concise and clear manner.
2. Mirror your opening statement:
A great way to end your presentation is by mirroring your opening statement in your conclusion.
Highlighting your presentation's key message at the end and emphasizing the central idea you aimed to communicate will help your audience to retain it in their memory.
During the conclusion of the presentation, the speaker effectively utilized the technique of mirroring the opening example she had presented - ordering a pizza on the phone by herself.
The speaker demonstrated the remarkable transformation she had undergone in terms of personal growth and confidence, which strongly reinforced her message to the audience.
By mirroring her opening example, she created a sense of familiarity and connection with her audience while simultaneously driving home the key message of her presentation.
This technique allowed the audience to understand better and relate to the speaker's personal journey and the message she was conveying.
Similarly, you can also use this strategy to conclude your presentation. This can be particularly effective if you are trying to reinforce a specific theme or idea throughout your presentation.
3. Pose an open-ended question:
One of the best ways to conclude your presentation is to elicit a response from your audience using an open-ended question that can effectively engage them and make your presentation more memorable.
Look at how the speaker concludes her speech with an open-ended question in this video.
Similarly, you can also raise open-ended questions to help your audience look from a different perspective and encourage them to investigate more thoroughly on the information presented.
Most importantly, ensuring that your question is relevant to your presentation and doesn't detract from your overall message is essential when eliciting a response.
So make sure that you kindle your audiences’ thoughts and ideas with the open-ended question at the end. This helps create a good long-lasting impression of your presentation.
4. End with a Call to action:
One of the best ways to end your presentation is by concluding with a call to action slide.
Incorporating a call to action into your presentation can be a powerful way to encourage your audience to take the next step.
Whether it's signing up for a program, making a purchase, or supporting a cause, a clear call to action is essential to achieving your desired outcome.
Similarly, according to your type of presentation, you can include a relevant call to action.
For example, this might involve providing specific instructions or offering an incentive for taking action, such as a discount or free trial.
It's essential that you understand their pain points and make your call to action compelling. Ensure that your core message and the needs of your audience are aligned so that they are motivated enough to act.
5. Thank the audience:
At the end of your presentation, it's essential to recognize that your audience has taken time out of their busy schedules to attend and listen to your message.
Thanking your audience for their time and attention can create a positive impression and make them feel appreciated.
It's essential to make your gratitude genuine and sincere rather than a superficial gesture. For example, consider expressing your gratitude with a personal anecdote or acknowledging specific individuals in the audience.
This simple act of gratitude can also create a sense of personal connection and signal to your audience that the presentation has reached its conclusion, paving the way for future interactions with them.
6. End with a powerful quote:
One effective strategy to end your presentation on a high note is by leaving the audience with a powerful quote.
However, it's crucial to choose a quote that is not only impactful but also unique and relevant to your topic.
Using a commonly known quote may come across as unoriginal and irrelevant, losing the attention and interest of your audience in most cases.
In this presentation, Steve Jobs concludes his speech with an inspiring and powerful message, “Stay Hungry! Stay Foolish”. Thereby emphasizing that you should never stop learning, pursue more goals, and never stop being satisfied.
Similarly, in your conclusion, consider using a relevant quote to make an impact.
7. Acknowledge your contributors:
Another best way to conclude your presentation is by showing gratitude to your contributors.
For example, if you deliver a business presentation on behalf of a team or a department, it's essential to recognize the collective effort that went into creating the presentation.
The concluding moments of your speech are the perfect opportunity to acknowledge your team members' hard work and dedication.
You can express gratitude to your team as a whole, thanking them for their contribution to the presentation.
However, if you want to ensure that the individual efforts of team members are recognized, highlighting specific contributions may be a better approach.
Some examples include:
"Join me in giving a round of applause to my incredible team, who played a significant role in arranging this pitch deck."
"Finally, I would like to mention that my tech team experts provided me with insight into the technical nuances, and without their contribution, this presentation would not have been as informative as it is now."
"As I conclude, I want to express my gratitude to Mark and Serene from the Marketing team, whose assistance in gathering the data and designing the slides was invaluable."
By acknowledging individual team members, you are demonstrating your appreciation for their work and giving them the recognition they deserve.
This will not only make them feel valued but also motivate them to continue contributing to the success of future presentations.
So be sure to end your presentation with the required acknowledgment for all the contributions.
8. Ask for feedback:
You can conclude your presentation seamlessly by thanking the audience and asking for feedback from them.
Encouraging feedback from your audience can greatly benefit your future presentations. It allows you to understand how your message was received and how you can improve for the next time.
So, how can you gather feedback effectively?
Firstly, ask attendees to share their thoughts on your presentation after you finish speaking. This can be done by initiating a Q&A session or by approaching individuals directly.
Another option is to set up a QR code near the exit and ask people to scan and jot down their thoughts on the online form as they leave. This allows attendees to provide their feedback in a confidential and hassle-free manner.
Also, consider having a suggestion box for handwritten feedback notes or creating an anonymous online survey that links to your presentation slides. This method is beneficial if you want to gather feedback from a large audience or if you prefer to have quantitative data.
By actively seeking feedback, you show your audience that you value their input and are committed to improving your presentation skills.
However, this strategy does not apply to all the general presentations. So use this way of concluding your presentation where it makes more sense to you and the audience.
In summary, an impactful conclusion is vital to wrap up your presentation successfully.
Each of these strategies serves a unique purpose, and by combining them, you can create a conclusion that is both engaging and impactful.
By incorporating the 8 critical strategies mentioned in this guide, you can leave a lasting impression on your audience, ensuring that your message stays with them even after the presentation has ended.
Now that you have learned the pro strategies of how to end a presentation, take a look at this guide on “How to start a presentation” as well and nail your presentation from start to end!
If you are still uncertain about how to make a presentation from the ground up, we suggest checking out Animaker Deck - the world's first avatar-driven presentation software.
With over 40 distinct and creatively designed templates at your disposal, we are confident you will find it worth trying!
We hope this article was helpful. Do let us know your thoughts on which strategy worked best for you, and also suggest your own ways of ending a presentation.
Related Posts

How to Embed a Video in Powerpoint? [Supercharge your PPTs Now]

Top 10 Presentation Software for 2023

25 Best Presentation Templates for the Perfect Pitch [For 2023]
Subscribe to get awesome DIY tips that could break the boundaries of other DIY tools.
Create Engaging Animated Videos On Your Own
Our drag-and-drop builder makes it easy for anyone to create pro-level animated videos using pre-made templates and ready-to-go assets with zero technical skills.
ATLANTA, MAY 23-24 PUBLIC SPEAKING CLASS IS ALMOST FULL! RESERVE YOUR SPOT NOW

- Public Speaking Classes
- Corporate Presentation Training
- Online Public Speaking Course
- Northeast Region
- Midwest Region
- Southeast Region
- Central Region
- Western Region
- Presentation Skills
- 101 Public Speaking Tips
- Fear of Public Speaking
How to End Your Presentation with a Bang

So you’ve spent days (maybe weeks) putting together a killer presentation. Now, you stand up with confidence, present every bullet point with poise, and then you get all the way to the end… and the presentation just fizzles.
It’s like a marathon runner who trains for months (maybe years), then just a half mile before the finish line, starts to cramps and can’t finish the race.
The last thing that you tell your audience will most likely be what they remember. So, you want to end your presentation with a bang!
In this post, we will cover three things that you should absolutely avoid when you close your presentation. In addition, we will also cover 6 killer ways to end on a positive note.
By the way, for more details about how to organize a good speech, see the following. 7 Foolproof Ways to Start a Presentation . | How to Design a Presentation Quickly .
Eliminate these “Show Stoppers” from Your Presentation Conclusion

Avoid Ending Your Presentation with a Question & Answer Period.
One of the things that drives me up the wall is ending a fantastic presentation with a Q & A session that has a high propensity to just flop.
It reminds me of some sage advice from my jr high school football coach. He was an old-school running game type of coach. He’d say,
“In football, when you pass the ball, only three things can happen and two of them are bad.”
I kind of feel the same way about Question & Answer periods. There are only three ways that Q & A sessions can end, and two of them are bad .
Yes, If your audience asks you great questions, you can end your presentation on a high note. However, if your audience asks you odd questions or uninteresting questions, you can end on a low note. Even worse than getting crappy questions, though is getting no questions. Now, the ending will just seem odd.
When I present, I encourage people to ask questions DURING my presentation . That way, I can use a more dynamic way to end my presentation with a bang.
Don’t End by Thanking the Audience for Their Time.
When you stand up to speak, you should have the attitude that your audience is there to hear from you because you have important information that they need. When you thank your audience for their time, you are conceding that their time is more important than your time.
Also Avoid an Abrupt Ending with No Conclusion.
This happened to me early in my career. The first time that I really bombed a speech, I made two really big mistakes. The first was that I sped through the information so quickly that I finished in less than half of the allotted time. Then, I just ran out of things to say, so I sat down. The people in the audience were confused. I had more time and the ending was so abrupt, that they weren’t sure if I was finished.
So, spend time preparing your conclusion. Practice it a few times, and you will end on a high note.
Bonus Tip: Warn Your Audience Ahead of Time that Your Speech is Coming to a Close.
Our brains are wired to look for structure in things. That’s why people get frustrated with cliffhangers in movies. Only in movies, there’s a sequel. In speeches and presentations, the end is the end.
Give a hint that you are nearing a close a couple of slides or paragraphs before you actually do. Saying something like, “So let’s review what we’ve discussed so far”, “As I wrap up this presentation” or “In conclusion”.
Signaling the close prepares your audience for the ending. Ironically, it also makes the ending more memorable.
Secrets to a Powerful Presentation Ending – 6 Ways to End Your Presentation with a Bang
Not that we have covered what NOT to do, let’s focus on a few, turnkey ways to end your presentation with a bang.
(1) End Your Presentation with a Brief Summary You Key Points.

This technique works really well because it allows you to repeat your key points a few times. This repetition helps your audience remember the content better.
An Example of Using a Summary to End Your Presentation with a Bang!
A couple of months ago, I had a class member that used this technique really well. She worked for a local TV station that was trying to attract new viewers. Here is the presentation outline that she created:
We Can Increase the Number of Young Viewers by Focusing More on Our Social Media Platforms Teens get most news from social media. Increase coverage w/ teens increases interest in station. Making social media selective will make us stand out against competition.
[Introduction] “My topic today is about how we can increase the number of young viewers by focusing more on social media. The things that we are going to cover are, how teens get most of their news from social media, that if we increase our coverage with teens there will also be a corresponding increase in interest in our TV station, and how making our social media selective will allow us to stand out from the competition.”
After the introduction, the speaker would then cover the “meat” of the presentation by going through each point with specific examples and evidence about how each of those points is true.
At the conclusion, the speaker could just recap by saying, “So in conclusion, since teens get most of their news via social media, if we increase our coverage with teens, we will also increase interest in our station, and if we make our social media selective we will stand out from the crowd, I believe that we can increase the number of young viewers by focusing more on social media.”
The summary technique is a very easy way to conclude your speech, and it will also increase the retention of your audience.
For additional examples, see How to Write a Speech in Just a few Steps .
(2) End with an Example, Story, or Anecdote.

I spoke for another 45 minutes, and then I finished the presentation by describing the success story of one of my class members. He had implemented the very content that I had just delivered to that breakout session group. However, he was delivering a very data-intense presentation for the Center for Disease Control. (So his content was even more boring than the type of content the audience had to deliver.) The story showed the group how a speaker can take even boring, data-filled material and deliver it well.
Those contrasting stories — the one at the start of my presentation, and the one at the end, work really well together. They bookend the entire presentation.
An Easy Way to Find a Funny Anecdote to End Your Presentation.
Sometimes a good anecdote or funny story can be a good way to end on a positive as well. A good place to get funny anecdotes is from Reader’s Digest . (RD has a great book published that has just funny work-related stories. You can purchase it here: Laughter the Best Medicine @ Work: America’s Funniest Jokes, Quotes, and Cartoons )
This is kind of an embarrassing incident, but it shows that if you get a little creative, any type of story can be a great ending.
I was training an instructor years ago, and I had her just pick a random funny anecdote from Reader’s Digest. I told her that, no matter what the story was about, I’d find some way to insert the funny story into our class. Here is the story that she picked…
A woman went to her boss saying that she was going to go home early because she was feeling sick. The boss, having just gotten over a cold said that he hoped it wasn’t something that he had given to her. A coworker overhearing the conversation said, ‘I hope not. She has morning sickness.'”
(Obviously, this instructor-in-training also had a sense of humor, as well.) I thought about it a while, and I just ended the session with, “So, in summary, one of the most important parts of the presentation design process is knowing your audience. In fact, that reminds me of a story…” I then just added the anecdote word-for-word, and I got a big laugh.
I created a whole series of posts on storytelling starting with Storytelling in Public Speaking .
(3) Finish Your Speech by Telling the End of an Earlier Story.

Then, I finished the presentation by telling how, just a year later, after a little outside training, I had to stand in front of over 400 people to give an acceptance speech for an award. This time, I was calm, and I used my humor to win over the audience, and I killed it. By continuing the story and providing a positive result at the end, it makes for a pretty nice presentation ending.
So start with a story where you had a challenge and end with a success story about how you overcame that challenge.
(4) End Your Presentation with an Open-Ended Question.

That’s why people are drawn to thought-provoking questions. So a great way to end your speech is with a well-designed, thought-provoking question.
When I teach a class, I use this technique before almost every break. For instance, if I teach an hour-long session, it will be easy for the audience to forget a lot of the content if it isn’t reinforced right away. So, by asking a thought-provoking question about the content, it stimulates the content in the minds of the audience.
When you ask questions, though, avoid easy questions where the answer is an obvious “yes” or “no.” Instead, ask open-ended questions. The easiest way to do this is to ask for the audience members’ opinions.
For instance, if my title is “Starting with a 3-Point Outline Will Help You Save Time When You Design Presentations,” I could end the speech with a question like, “Based on what we’ve talked about today, how can you see starting with a three-point outline helping you save time?”
Any answers that the audience provides will help me prove my point. The more the better.
(5) Give the Audience a Call-to-Action at the End of Your Speech.

Just as an FYI, here, though, if you ask them to do a single thing, they are more likely to do it. If you ask them to do a second thing, they are more likely to do neither. Sp, to prevent that and to inspire your audience, challenge them to do one specific thing from your speech.
If your presentation is about why your company should invest in advertising, make your call to action very specific. “So, my suggestion is that we increase our advertising budget by 10% and use that budget for additional re-targeting ads.”
The thing to keep in mind here is that the more calls to action that you have, the less likely they will do anything. So, make your call to action just a single item. And make the item easy to implement.
(6) The Echo Close Is an Inspirational Way to End Your Speech with a Bang.

A wise man once said, “The mind is not a vessel to be filled, but a fire to be kindled.” So, when you present, kindle the fire of knowledge. Kindle the fire of enthusiasm. Kindle the fire of humor. Kindle the fire of empathy. And you will kindle the fire of learning from your audience.
Another example might be.
So, in conclusion, brevity in public speaking is pretty important. In fact, George Orwell once said, “If it is possible to cut a word out of your speech, always cut it out.” So, when you create a presentation, cut the fluff. Cut the repetitive bullets. Cut the platitudes. And when you do, you will cut the confusion from your audience.
It is an easy technique if you prepare the ending and practice it a few times.
So that concludes the six ways that you can end your presentation with a bang. However… There is…
“One More Thing”
Steve Jobs was famous for concluding his keynotes with “One more thing…” then following it up with a surprising fact, feature, or innovation.
Why is this effective? Because it leaves people talking.

Regardless of how you choose to end your presentation, spend a little time on the ending. Make it flawless, and you will leave your audience wanting more! If you do, you will end your presentation with a bang!
Choose the Best Presentation Ending for Your Presentation Purpose
With all of the great choices, how do we know which presentation ending to use? Luckily, we have created a free handout to help you pick the best presentation ending. Although many of the tips above will work in many different types of speeches, the handout will help you identify which ending will accomplish specific purposes for your specific presentation.
For instance, if your goal is to help your audience retain the content, then summarizing your key points is a great choice. If your purpose is to inspire the audience, you might try the Call to Action or Echo technique instead. Just complete the form below for instant access!
Download the Free “How to End Your Presentation” Handout!

Podcasts , presentation skills
View More Posts By Category: Free Public Speaking Tips | leadership tips | Online Courses | Past Fearless Presentations ® Classes | Podcasts | presentation skills | Uncategorized
Contact Brian
Brian Krogh
How to Write a Great Conclusion for Your Next Presentation

Imagine you are at the end of a long flight.
Your pilot signals her initial descent, lowers the landing gear, and nears the runway. You squirm in your seat, and think about how you cannot wait to get off the plane. As you longingly peer out the window you see the white stripes on the runway streaking past you. Surprisingly, just before the wheels touch down, the pilot revs the engines and quickly ascends into the clouds.
You immediately wonder what is wrong. The pilot, however, comes over the speakers and says, "hi folks, don't worry, nothing is wrong, I just felt like flying a little more today."
Now imagine the pilot does this four or five more times. How frustrated are you?
Presentations and flights have something in common - the best ones conclude well. As an audience member it can be frustrating when a speaker struggles to land the plane at the end of an otherwise effective presentation.
Have you ever sat in a meeting and heard the presenter say "in conclusion" only to keep rambling for fifteen more minutes? How about the speaker who says "let me finish with this" six times before they stop talking?
Phrases like these indicate a speaker has not properly planned their landing and the audience, who is ready to leave, quickly grows frustrated.
Perhaps even more ineffective, is a conclusion that is little more than a slide that says, "Thank You." That is like cutting power to the engines right before the wheels touch down and letting the wheels slam violently onto the runway.
It leaves the audience wondering, "what was that all about?"
A conclusion is your lasting impression on your audience. If your presentation is to be memorable, your conclusion must be designed and executed well.
An effective conclusion will do three things.
- Offer a clear ACTION STEP
- Sum up the presentation with one BIG IDEA
- CEMENT the idea with a compelling story or data point.
It's as simple as ABC: Action step, Big Idea, Cement with a story.
How you end will determine if your audience leaves feeling inspired or indignant.
As a presenter it is tempting to focus on your introduction and the body of your presentation and overlook the conclusion. If you are to inspire others to action you need to write out your conclusion every time you speak and you need to leave time to deliver a conclusion without going over your allotted time limit. It's that important.
When you speak, land the plane smoothly and you will be amazed at the results.
Not sure how to talk to your team about presenting your company's most important information?
I would love to meet you and provide you with some value whether or not we work together long term. Let’s put something on the calendar.
Book a Free Call
Say it Better
© 2023, Brian Krogh
- Student Login:

How to Close Your Presentation in English Powerfully [+ FREE Presentation Checklist]
May 9, 2018 | Business Professional English , Free Resource , Public Speaking & Presentations

This lesson has been updated from its original posting in 2016.
You’re giving your presentation in English. You have just two minutes left. And it’s time for the conclusion …
Did you know most people only remember the first and last things you tell them? It’s true.
If you are giving a presentation in English, then you definitely want people to remember what you say at the end. And this means your closing must be powerful!
You’ve worked hard on your presentation. You searched for information online. You couldn’t sleep at night. You felt nervous about making mistakes. You spent hours preparing. You reviewed the grammar and vocabulary. You worried about someone asking a question. You practiced and practiced and practiced.
And now it’s the last two minutes. This is the last opportunity for your audience to hear your key points. It is the last chance you have to help your audience remember your comments.
A closing in a presentation should be short and clear. It should summarize your key points. And, most importantly, it should be powerful.
In today’s lesson, you’re going to learn about 3 ways to make your closing more powerful. Plus you’ll learn useful key expressions you can use in your presentation.
3 steps to a powerful closing in your presentation.
Lesson by Annemarie
3 Strategies to Close Your Presentation Powerfully
Use these 3 strategies in your conclusion to:
- recapture your audience’s attention
- get your audience to focus and remember your key points
- help your audience connect with you and your topic
- end your presentation powerfully
One: Include a Call to Action (CTA)
Is there something you want your audience to do or think after your presentation. Do you want them to take action? Tell your audience exactly what you want them to do with a Call to Action.
Here’s my example:
“ After you finish today’s lesson, please take 2 minutes to leave a comment about your experience with presentations. You can share your thoughts or ask questions in the comments section at the bottom of this lesson – it’s the perfect place to join a discussion on this topic.”
A couple useful expressions to help you introduce your CTA is:
- To close, I’d like to ask you to do this one thing…
- And finally, before you leave the conference today, please take two minutes to…
Two: End with a Powerful/Inspirational Quote
Is there one thing you really want your audience to remember? Or is there a specific feeling you want your audience to have after your presentation?
Using a powerful quote can help you do that. You could introduce a great quote or interesting statistic with:
- I’d like to finish with this powerful/interesting/wonderful/inspiring/ quote from …
- And finally, let’s finish up today’s discussion with this surprising/useful/shocking/hopeful statistic …
Here are some example quotes that might help people be prepared to take action or to think differently. But remember! Always match the quote or statistic to your topic:
“In the end, we will remember not the words of our enemies, but the silence of our friends.” – Martin Luther King, Jr. “Sometimes we stare so long at a door that is closing that we see too late the one that is open.” – Alexander Graham Bell
Three: Add a Surprising Fact or Statistic
Is there something you’d love for your audience to think about after your presentation? Is there a statistic or fact that will help someone remember your key points?
A surprising fact can also help re-engage your audience, it will snap their attention back to you.
For example:
Did you know that the human brain’s capacity is limitless – that’s great new right? BUT … did you also know that a person is likely to remember only 25% of a presentation after 24 hours?
Uh oh. That is why it’s SO important to have a powerful ending! Remember: the key is to find a statistic or fact that connects directly to your topic.
Useful Language to Close Your Presentation
Summarize Your Key Points & Close Your Presentation
- That brings us to the end of the presentation. I’d like to summarize by saying …
- That concludes my presentation. However, I’d like to quickly summarize the main points or takeaways.
- And on that final note, that concludes my presentation.
- To quickly recap, I’d like you to remember these key points …
- To summarize …
- In conclusion …
- I’d like to bring this presentation to a close with …
- I’d like to close this talk with …
- So, this concludes the focus of discussion today. To end, I’d like to highlight …
- This concludes [name/title of the section] so let’s move on to the final comments.
Thank Your Audience
- I sincerely appreciate your attention today/this evening/this morning.
- And that brings us to the end. I’d like to thank you for your time and attention today.
- Thank you so much for your interest and attention.
- At this time, I’d like to have my colleague speak so I’ll finish up by saying thank you for your attention.
- I can see that our time is just about up so to finish I’d like to say thank you.
- I sincerely appreciate that I’ve had this opportunity to present to you.
- If there is one thing I would like you to remember from today’s presentation it’s …
Take Questions
- If anyone has any questions, I’d be happy to open up the discussion.
- If anyone has any questions, please feel free to ask now and I’ll do my best to answer.
- Would anyone like to ask any questions?
- I would now be interested to hear from you with your thoughts or questions.
- Now let’s move on to some Q&A. (Q&A = Questions and Answers)
Provide Next Steps or Contact Information
- If you would like more information, here is a list of useful resources/websites.
- If anyone who like more information or has questions, please feel free to contact me at: [include contact info]
- Here is a list for further reading on this topic. (Include the list of books or websites.)
Get the complete Presentations in English Series:
Part 1: How to Prepare for Your Presentation in English
Part 2: How to Start with a Great Introduction in Your Presentation
Part 3: How to Organize Your Presentation in English
Part 4: How to End Your Presentation Powerfully
After you’ve watched the video and reviewed the lesson, I’d love to hear from you!
Tell me about the best presentation you ever heard. Who gave the presentation? And why do you remember it? Share what you remember in the comments section below.
And for the bonus question!! Have you given a presentation in English? What tips or advice would you like to share with others? You can add your advice in the comments section.
Thank you so much for joining me!
~ Annemarie
Get the Confidence to Say What You Want in English
Follow my 3-step solution to speak English with clarity, fluency, and freedom so you can say what you want with confidence.
You'll also get my Confident English lessons delivered by email every Wednesday and occasional information about available courses. You can unsubscribe any time.
More Like This

5 Smart Questions to Ask in an English Job Interview
It’s the last question in your job interview in English and you hear: Do you have any questions for me? What should you say? Is it okay to ask a question in a job interview? Find out exactly what you should do plus 5 smart questions to ask.

How to Disagree in English Politely
Want to say “I disagree” without creating tension in the conversation? Master the art of disagreement in this lesson on, “How to Disagree in English Politely.”

#310: The Right Grammar for English Introductions
Get your English introductions just right with this step-by-step video on Grammar for English Introductions when you’re meeting someone new.

#309: How to Go Off Topic in English | English Conversation Skills
Learn how to gracefully go off topic in English without losing your audience. Whether you’re in a meeting or chatting with friends, in this lesson we dive deep into the art of smoothly navigating tangents while enhancing your English conversation skills.
![in an effective presentation conclusion #308: How to Use ‘Though’ in English [+ FREE Worksheet]](https://www.speakconfidentenglish.com/wp-content/uploads/2024/04/How-to-Use-Though-in-English-400x250.png)
#308: How to Use ‘Though’ in English [+ FREE Worksheet]
Learn and practice how to correctly use though, although, even though, and as thought in your English conversations.

#307: How to Use English Abbreviations in Emails, Texts, and Conversations
Follow this comprehensive guide to learn how to use English abbreviations for emails, texts, and conversations.
© Copyright 2014-2024 Speak Confident English | Privacy Policy | Terms & Disclaimer | Online Class Policies
I’m glad to hear it was helpful!
This was very helpful
Thanks, Ma’am/Sir. This helped me a lot…
Same here ma’am
This is so helpful. Thank you so much
This helped a lot. Thank you so much <3
I accidentally found your page while working on my English video presentation. It’s really helpful. Thanks soooo much 🙂
I’m very glad to know it was helpful!
Hi! I found your page very insightful. Thank you very much!
I’m glad to hear it!
great video series. thank you so much. you mentioned that you had a downloadable checklist in the final video. where could I find this thanks?
Hi Ellie, I’m glad the series was helpful.
When you visit the lesson, there should be an image that pops up with an opportunity to get the download. If you don’t see it, please let me know so I can fix it.
Helped a lot! Thank you very much <33
thank you so much
I love your method
Hello, I have a 5 minute oral presentation of a fictional book, w/the main focus on the leadership traits of the characters. I enjoyed the book, and suspect others might, so to that end, is it OK to NOT share the ending? Thank you
Thanks for your help 🙂
Great website. I found a typo in on the presentation closings page “Useful Langauge to Close Your Presentation”.
Good eyes! Thanks so much for the note. We’ve fixed the typo.
Dear Annemarie, thank you so much for sharing.
Dear Annemarie, thank you so much for sharing. I learned so much from your 4 videos and I will work on improving my presentation skills. Love your spirit of excellence. For me as a presenter, its important i am passionate about the topic i share and audience will be able to apply some of the learnings in their life. Thank you Annemarie. I love your voice too. Stay blessed.
I watch continuously watched ur 4 videos and U r a great teacher.Thanks for making such purposeful videos.
I am so happy , I have more form you thank you very much
You are absolutely wonderful and your website is extremely useful and also quit impressive i habe my english A-levels in December i copied this text i sinisterly appreciate that i have had this opportunity to present to you and i also add something * it was a honor for me so thank you ☺️
Thanks, Jasmin! I’m so glad to know my lessons are helpful to you.
hey Annemarie could you help me in ending my presentation on mental health. it is a school presentation for MUN
If you’d like editing help, please see our options for 1:1 classes .
You are my favorite speaker. ☺
Hi Anna, that’s so kind of you. Thank you. 🙂
It’s so useful to us…… I’m so happy by this
I’m glad it was helpful to you, Kalpana.
I was holistically stuck about how to give my first ever presentation, but this gave me an impetus and confidence. Thanks a lot for this exquisite info
Awesome. I’m glad this helped you to move forward.
Thank YOU for tour tips. They are really inspiring. I Will try to put them into practise.
Hi Nancy, Wonderful! I’m glad they’re helpful to you!
It’s so useful to us…… I’m so happy by this
do you have Presentation course
Hi Hammad, I don’t at this time but it’s definitely something I’m thinking about.
Pin It on Pinterest

Presentation Conclusions: Signal to the End
Strong start, strong finish.

3 reasons to include a signal to the end in your presentation conclusion
- Wake up the audience. Many times the audience loses focus and is daydreaming towards the end of the presentation. This shows them that things are ending soon, and it is time to pay attention again.
- Reset your own focus. Sometimes it is easy to go so in-depth on topics we know well that we lose focus on what our audience wants to hear. The signal to the end not only wakes up the audience, but the speaker as well and allows them to deliver a strong presentation conclusion.
- Clarify your structure. Presentations need clear structure so that the audience can focus on key points and follow along. When you use effective transitional phrases such as a signal to the end, it creates clarity in your structure and helps the audience stay with you.
3 phrases to use as a signal to the end in your presentation conclusion
- “ This brings me to the end of my presentation. To summarize my main points,…”
- “ Well, that is all I have for today. Let me now summarize what I talked about…. ”
- “ I have now come to the end of my presentation. In summary, I spoke about…”
3 results of using a signal to the end in your presentation conclusion
- Get your points across a final time. At the end of a good presentation, you will have mentioned your main points in your introduction, your body, and finally in your conclusion summary. A good signal to the end focuses the audience’s attention one last time, so that you can mention your main points again as well as your recommendation. People tend to remember what they hear at the end of a presentation more than at the beginning or middle.
- Set yourself up to finish strong. By clearly defining you are starting your conclusion, it will help you focus and go through the correct steps in your conclusion. This will leave your audience with a favorable impression of your speech.
- Be a better public speaker. So many people give poorly structured presentations, and especially end their presentations on a low note. Having a clear structure will help you to look more professional and get the results you want out of your presentation.
Having a strong presentation conclusion will leave your audience with a focused, positive view of your message and a good signal to the end is key to starting it well. Let us know of any ideas you have in the comment areas below. Want more on presenting with impact? Click here .
FOR MORE INFORMATION
- Presenting with impact seminar
- Interactive presentations
- Storytelling in presentations
You might also like

This is a really important point Matt – and again it is often forgotten.
To give even more emphasis to your ending, raise / lower your voice or make a change to your voice to wake up the audience – but don’t shout!
Something like:
OK! (with a bit more volume) SO! (with a rise and then a fall in your voice) Right! (with a clap of your hands – if appropriate – and a rise in your voice)
Now your audience is awake, you can finish with a bang using one of the sentences above from Matt.
Comments are closed.
Our solutions.
- Virtual Delivery
- Blended Learning
- In-house Business English
- Case Studies
Target Training GmbH
- Kopernikusstrasse 13
- 63071 Offenbach am Main
- Tel: +49 69 8484 79 0

Privacy Policy

- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
What It Takes to Give a Great Presentation
- Carmine Gallo

Five tips to set yourself apart.
Never underestimate the power of great communication. It can help you land the job of your dreams, attract investors to back your idea, or elevate your stature within your organization. But while there are plenty of good speakers in the world, you can set yourself apart out by being the person who can deliver something great over and over. Here are a few tips for business professionals who want to move from being good speakers to great ones: be concise (the fewer words, the better); never use bullet points (photos and images paired together are more memorable); don’t underestimate the power of your voice (raise and lower it for emphasis); give your audience something extra (unexpected moments will grab their attention); rehearse (the best speakers are the best because they practice — a lot).
I was sitting across the table from a Silicon Valley CEO who had pioneered a technology that touches many of our lives — the flash memory that stores data on smartphones, digital cameras, and computers. He was a frequent guest on CNBC and had been delivering business presentations for at least 20 years before we met. And yet, the CEO wanted to sharpen his public speaking skills.
- Carmine Gallo is a Harvard University instructor, keynote speaker, and author of 10 books translated into 40 languages. Gallo is the author of The Bezos Blueprint: Communication Secrets of the World’s Greatest Salesman (St. Martin’s Press).
Partner Center
We use essential cookies to make Venngage work. By clicking “Accept All Cookies”, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Manage Cookies
Cookies and similar technologies collect certain information about how you’re using our website. Some of them are essential, and without them you wouldn’t be able to use Venngage. But others are optional, and you get to choose whether we use them or not.
Strictly Necessary Cookies
These cookies are always on, as they’re essential for making Venngage work, and making it safe. Without these cookies, services you’ve asked for can’t be provided.
Show cookie providers
- Google Login
Functionality Cookies
These cookies help us provide enhanced functionality and personalisation, and remember your settings. They may be set by us or by third party providers.
Performance Cookies
These cookies help us analyze how many people are using Venngage, where they come from and how they're using it. If you opt out of these cookies, we can’t get feedback to make Venngage better for you and all our users.
- Google Analytics
Targeting Cookies
These cookies are set by our advertising partners to track your activity and show you relevant Venngage ads on other sites as you browse the internet.
- Google Tag Manager
- Infographics
- Daily Infographics
- Popular Templates
- Accessibility
- Graphic Design
- Graphs and Charts
- Data Visualization
- Human Resources
- Beginner Guides
Blog Beginner Guides How To Make a Good Presentation [A Complete Guide]
How To Make a Good Presentation [A Complete Guide]
Written by: Krystle Wong Jul 20, 2023

A top-notch presentation possesses the power to drive action. From winning stakeholders over and conveying a powerful message to securing funding — your secret weapon lies within the realm of creating an effective presentation .
Being an excellent presenter isn’t confined to the boardroom. Whether you’re delivering a presentation at work, pursuing an academic career, involved in a non-profit organization or even a student, nailing the presentation game is a game-changer.
In this article, I’ll cover the top qualities of compelling presentations and walk you through a step-by-step guide on how to give a good presentation. Here’s a little tip to kick things off: for a headstart, check out Venngage’s collection of free presentation templates . They are fully customizable, and the best part is you don’t need professional design skills to make them shine!
These valuable presentation tips cater to individuals from diverse professional backgrounds, encompassing business professionals, sales and marketing teams, educators, trainers, students, researchers, non-profit organizations, public speakers and presenters.
No matter your field or role, these tips for presenting will equip you with the skills to deliver effective presentations that leave a lasting impression on any audience.
Click to jump ahead:
What are the 10 qualities of a good presentation?
Step-by-step guide on how to prepare an effective presentation, 9 effective techniques to deliver a memorable presentation, faqs on making a good presentation, how to create a presentation with venngage in 5 steps.
When it comes to giving an engaging presentation that leaves a lasting impression, it’s not just about the content — it’s also about how you deliver it. Wondering what makes a good presentation? Well, the best presentations I’ve seen consistently exhibit these 10 qualities:
1. Clear structure
No one likes to get lost in a maze of information. Organize your thoughts into a logical flow, complete with an introduction, main points and a solid conclusion. A structured presentation helps your audience follow along effortlessly, leaving them with a sense of satisfaction at the end.
Regardless of your presentation style , a quality presentation starts with a clear roadmap. Browse through Venngage’s template library and select a presentation template that aligns with your content and presentation goals. Here’s a good presentation example template with a logical layout that includes sections for the introduction, main points, supporting information and a conclusion:

2. Engaging opening
Hook your audience right from the start with an attention-grabbing statement, a fascinating question or maybe even a captivating anecdote. Set the stage for a killer presentation!
The opening moments of your presentation hold immense power – check out these 15 ways to start a presentation to set the stage and captivate your audience.
3. Relevant content
Make sure your content aligns with their interests and needs. Your audience is there for a reason, and that’s to get valuable insights. Avoid fluff and get straight to the point, your audience will be genuinely excited.
4. Effective visual aids
Picture this: a slide with walls of text and tiny charts, yawn! Visual aids should be just that—aiding your presentation. Opt for clear and visually appealing slides, engaging images and informative charts that add value and help reinforce your message.
With Venngage, visualizing data takes no effort at all. You can import data from CSV or Google Sheets seamlessly and create stunning charts, graphs and icon stories effortlessly to showcase your data in a captivating and impactful way.

5. Clear and concise communication
Keep your language simple, and avoid jargon or complicated terms. Communicate your ideas clearly, so your audience can easily grasp and retain the information being conveyed. This can prevent confusion and enhance the overall effectiveness of the message.
6. Engaging delivery
Spice up your presentation with a sprinkle of enthusiasm! Maintain eye contact, use expressive gestures and vary your tone of voice to keep your audience glued to the edge of their seats. A touch of charisma goes a long way!
7. Interaction and audience engagement
Turn your presentation into an interactive experience — encourage questions, foster discussions and maybe even throw in a fun activity. Engaged audiences are more likely to remember and embrace your message.
Transform your slides into an interactive presentation with Venngage’s dynamic features like pop-ups, clickable icons and animated elements. Engage your audience with interactive content that lets them explore and interact with your presentation for a truly immersive experience.

8. Effective storytelling
Who doesn’t love a good story? Weaving relevant anecdotes, case studies or even a personal story into your presentation can captivate your audience and create a lasting impact. Stories build connections and make your message memorable.
A great presentation background is also essential as it sets the tone, creates visual interest and reinforces your message. Enhance the overall aesthetics of your presentation with these 15 presentation background examples and captivate your audience’s attention.
9. Well-timed pacing
Pace your presentation thoughtfully with well-designed presentation slides, neither rushing through nor dragging it out. Respect your audience’s time and ensure you cover all the essential points without losing their interest.
10. Strong conclusion
Last impressions linger! Summarize your main points and leave your audience with a clear takeaway. End your presentation with a bang , a call to action or an inspiring thought that resonates long after the conclusion.
In-person presentations aside, acing a virtual presentation is of paramount importance in today’s digital world. Check out this guide to learn how you can adapt your in-person presentations into virtual presentations .
Preparing an effective presentation starts with laying a strong foundation that goes beyond just creating slides and notes. One of the quickest and best ways to make a presentation would be with the help of a good presentation software .
Otherwise, let me walk you to how to prepare for a presentation step by step and unlock the secrets of crafting a professional presentation that sets you apart.
1. Understand the audience and their needs
Before you dive into preparing your masterpiece, take a moment to get to know your target audience. Tailor your presentation to meet their needs and expectations , and you’ll have them hooked from the start!
2. Conduct thorough research on the topic
Time to hit the books (or the internet)! Don’t skimp on the research with your presentation materials — dive deep into the subject matter and gather valuable insights . The more you know, the more confident you’ll feel in delivering your presentation.
3. Organize the content with a clear structure
No one wants to stumble through a chaotic mess of information. Outline your presentation with a clear and logical flow. Start with a captivating introduction, follow up with main points that build on each other and wrap it up with a powerful conclusion that leaves a lasting impression.
Delivering an effective business presentation hinges on captivating your audience, and Venngage’s professionally designed business presentation templates are tailor-made for this purpose. With thoughtfully structured layouts, these templates enhance your message’s clarity and coherence, ensuring a memorable and engaging experience for your audience members.
Don’t want to build your presentation layout from scratch? pick from these 5 foolproof presentation layout ideas that won’t go wrong.

4. Develop visually appealing and supportive visual aids
Spice up your presentation with eye-catching visuals! Create slides that complement your message, not overshadow it. Remember, a picture is worth a thousand words, but that doesn’t mean you need to overload your slides with text.
Well-chosen designs create a cohesive and professional look, capturing your audience’s attention and enhancing the overall effectiveness of your message. Here’s a list of carefully curated PowerPoint presentation templates and great background graphics that will significantly influence the visual appeal and engagement of your presentation.
5. Practice, practice and practice
Practice makes perfect — rehearse your presentation and arrive early to your presentation to help overcome stage fright. Familiarity with your material will boost your presentation skills and help you handle curveballs with ease.
6. Seek feedback and make necessary adjustments
Don’t be afraid to ask for help and seek feedback from friends and colleagues. Constructive criticism can help you identify blind spots and fine-tune your presentation to perfection.
With Venngage’s real-time collaboration feature , receiving feedback and editing your presentation is a seamless process. Group members can access and work on the presentation simultaneously and edit content side by side in real-time. Changes will be reflected immediately to the entire team, promoting seamless teamwork.

7. Prepare for potential technical or logistical issues
Prepare for the unexpected by checking your equipment, internet connection and any other potential hiccups. If you’re worried that you’ll miss out on any important points, you could always have note cards prepared. Remember to remain focused and rehearse potential answers to anticipated questions.
8. Fine-tune and polish your presentation
As the big day approaches, give your presentation one last shine. Review your talking points, practice how to present a presentation and make any final tweaks. Deep breaths — you’re on the brink of delivering a successful presentation!
In competitive environments, persuasive presentations set individuals and organizations apart. To brush up on your presentation skills, read these guides on how to make a persuasive presentation and tips to presenting effectively .

Whether you’re an experienced presenter or a novice, the right techniques will let your presentation skills soar to new heights!
From public speaking hacks to interactive elements and storytelling prowess, these 9 effective presentation techniques will empower you to leave a lasting impression on your audience and make your presentations unforgettable.
1. Confidence and positive body language
Positive body language instantly captivates your audience, making them believe in your message as much as you do. Strengthen your stage presence and own that stage like it’s your second home! Stand tall, shoulders back and exude confidence.
2. Eye contact with the audience
Break down that invisible barrier and connect with your audience through their eyes. Maintaining eye contact when giving a presentation builds trust and shows that you’re present and engaged with them.
3. Effective use of hand gestures and movement
A little movement goes a long way! Emphasize key points with purposeful gestures and don’t be afraid to walk around the stage. Your energy will be contagious!
4. Utilize storytelling techniques
Weave the magic of storytelling into your presentation. Share relatable anecdotes, inspiring success stories or even personal experiences that tug at the heartstrings of your audience. Adjust your pitch, pace and volume to match the emotions and intensity of the story. Varying your speaking voice adds depth and enhances your stage presence.

5. Incorporate multimedia elements
Spice up your presentation with a dash of visual pizzazz! Use slides, images and video clips to add depth and clarity to your message. Just remember, less is more—don’t overwhelm them with information overload.
Turn your presentations into an interactive party! Involve your audience with questions, polls or group activities. When they actively participate, they become invested in your presentation’s success. Bring your design to life with animated elements. Venngage allows you to apply animations to icons, images and text to create dynamic and engaging visual content.
6. Utilize humor strategically
Laughter is the best medicine—and a fantastic presentation enhancer! A well-placed joke or lighthearted moment can break the ice and create a warm atmosphere , making your audience more receptive to your message.
7. Practice active listening and respond to feedback
Be attentive to your audience’s reactions and feedback. If they have questions or concerns, address them with genuine interest and respect. Your responsiveness builds rapport and shows that you genuinely care about their experience.

8. Apply the 10-20-30 rule
Apply the 10-20-30 presentation rule and keep it short, sweet and impactful! Stick to ten slides, deliver your presentation within 20 minutes and use a 30-point font to ensure clarity and focus. Less is more, and your audience will thank you for it!
9. Implement the 5-5-5 rule
Simplicity is key. Limit each slide to five bullet points, with only five words per bullet point and allow each slide to remain visible for about five seconds. This rule keeps your presentation concise and prevents information overload.
Simple presentations are more engaging because they are easier to follow. Summarize your presentations and keep them simple with Venngage’s gallery of simple presentation templates and ensure that your message is delivered effectively across your audience.

1. How to start a presentation?
To kick off your presentation effectively, begin with an attention-grabbing statement or a powerful quote. Introduce yourself, establish credibility and clearly state the purpose and relevance of your presentation.
2. How to end a presentation?
For a strong conclusion, summarize your talking points and key takeaways. End with a compelling call to action or a thought-provoking question and remember to thank your audience and invite any final questions or interactions.
3. How to make a presentation interactive?
To make your presentation interactive, encourage questions and discussion throughout your talk. Utilize multimedia elements like videos or images and consider including polls, quizzes or group activities to actively involve your audience.
In need of inspiration for your next presentation? I’ve got your back! Pick from these 120+ presentation ideas, topics and examples to get started.
Creating a stunning presentation with Venngage is a breeze with our user-friendly drag-and-drop editor and professionally designed templates for all your communication needs.
Here’s how to make a presentation in just 5 simple steps with the help of Venngage:
Step 1: Sign up for Venngage for free using your email, Gmail or Facebook account or simply log in to access your account.
Step 2: Pick a design from our selection of free presentation templates (they’re all created by our expert in-house designers).
Step 3: Make the template your own by customizing it to fit your content and branding. With Venngage’s intuitive drag-and-drop editor, you can easily modify text, change colors and adjust the layout to create a unique and eye-catching design.
Step 4: Elevate your presentation by incorporating captivating visuals. You can upload your images or choose from Venngage’s vast library of high-quality photos, icons and illustrations.
Step 5: Upgrade to a premium or business account to export your presentation in PDF and print it for in-person presentations or share it digitally for free!
By following these five simple steps, you’ll have a professionally designed and visually engaging presentation ready in no time. With Venngage’s user-friendly platform, your presentation is sure to make a lasting impression. So, let your creativity flow and get ready to shine in your next presentation!
Discover popular designs

Infographic maker

Brochure maker

White paper online

Newsletter creator

Flyer maker
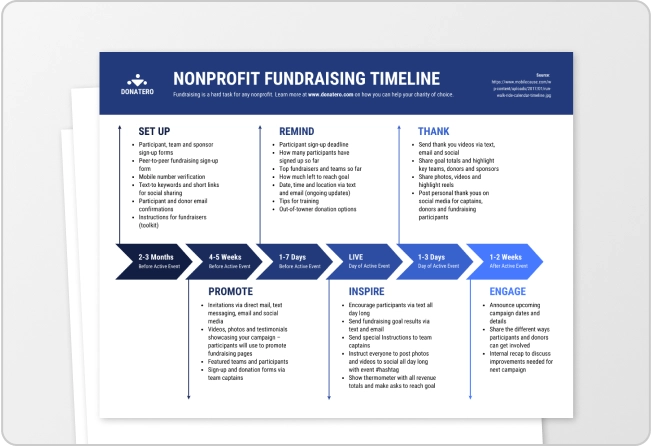
Timeline maker

Letterhead maker
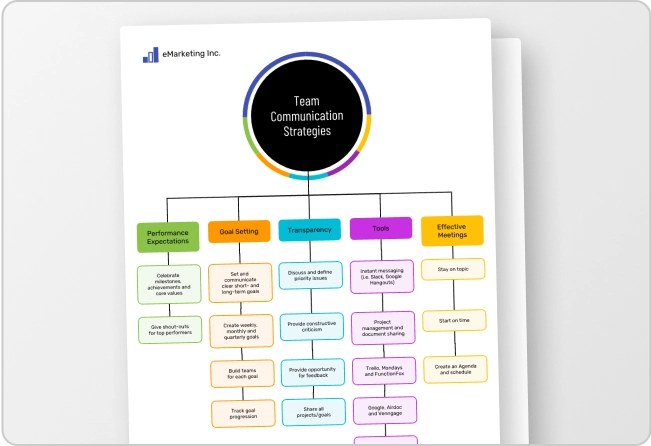
Mind map maker

Ebook maker

Conclusions
What this handout is about.
This handout will explain the functions of conclusions, offer strategies for writing effective ones, help you evaluate conclusions you’ve drafted, and suggest approaches to avoid.
About conclusions
Introductions and conclusions can be difficult to write, but they’re worth investing time in. They can have a significant influence on a reader’s experience of your paper.
Just as your introduction acts as a bridge that transports your readers from their own lives into the “place” of your analysis, your conclusion can provide a bridge to help your readers make the transition back to their daily lives. Such a conclusion will help them see why all your analysis and information should matter to them after they put the paper down.
Your conclusion is your chance to have the last word on the subject. The conclusion allows you to have the final say on the issues you have raised in your paper, to synthesize your thoughts, to demonstrate the importance of your ideas, and to propel your reader to a new view of the subject. It is also your opportunity to make a good final impression and to end on a positive note.
Your conclusion can go beyond the confines of the assignment. The conclusion pushes beyond the boundaries of the prompt and allows you to consider broader issues, make new connections, and elaborate on the significance of your findings.
Your conclusion should make your readers glad they read your paper. Your conclusion gives your reader something to take away that will help them see things differently or appreciate your topic in personally relevant ways. It can suggest broader implications that will not only interest your reader, but also enrich your reader’s life in some way. It is your gift to the reader.
Strategies for writing an effective conclusion
One or more of the following strategies may help you write an effective conclusion:
- Play the “So What” Game. If you’re stuck and feel like your conclusion isn’t saying anything new or interesting, ask a friend to read it with you. Whenever you make a statement from your conclusion, ask the friend to say, “So what?” or “Why should anybody care?” Then ponder that question and answer it. Here’s how it might go: You: Basically, I’m just saying that education was important to Douglass. Friend: So what? You: Well, it was important because it was a key to him feeling like a free and equal citizen. Friend: Why should anybody care? You: That’s important because plantation owners tried to keep slaves from being educated so that they could maintain control. When Douglass obtained an education, he undermined that control personally. You can also use this strategy on your own, asking yourself “So What?” as you develop your ideas or your draft.
- Return to the theme or themes in the introduction. This strategy brings the reader full circle. For example, if you begin by describing a scenario, you can end with the same scenario as proof that your essay is helpful in creating a new understanding. You may also refer to the introductory paragraph by using key words or parallel concepts and images that you also used in the introduction.
- Synthesize, don’t summarize. Include a brief summary of the paper’s main points, but don’t simply repeat things that were in your paper. Instead, show your reader how the points you made and the support and examples you used fit together. Pull it all together.
- Include a provocative insight or quotation from the research or reading you did for your paper.
- Propose a course of action, a solution to an issue, or questions for further study. This can redirect your reader’s thought process and help them to apply your info and ideas to their own life or to see the broader implications.
- Point to broader implications. For example, if your paper examines the Greensboro sit-ins or another event in the Civil Rights Movement, you could point out its impact on the Civil Rights Movement as a whole. A paper about the style of writer Virginia Woolf could point to her influence on other writers or on later feminists.
Strategies to avoid
- Beginning with an unnecessary, overused phrase such as “in conclusion,” “in summary,” or “in closing.” Although these phrases can work in speeches, they come across as wooden and trite in writing.
- Stating the thesis for the very first time in the conclusion.
- Introducing a new idea or subtopic in your conclusion.
- Ending with a rephrased thesis statement without any substantive changes.
- Making sentimental, emotional appeals that are out of character with the rest of an analytical paper.
- Including evidence (quotations, statistics, etc.) that should be in the body of the paper.
Four kinds of ineffective conclusions
- The “That’s My Story and I’m Sticking to It” Conclusion. This conclusion just restates the thesis and is usually painfully short. It does not push the ideas forward. People write this kind of conclusion when they can’t think of anything else to say. Example: In conclusion, Frederick Douglass was, as we have seen, a pioneer in American education, proving that education was a major force for social change with regard to slavery.
- The “Sherlock Holmes” Conclusion. Sometimes writers will state the thesis for the very first time in the conclusion. You might be tempted to use this strategy if you don’t want to give everything away too early in your paper. You may think it would be more dramatic to keep the reader in the dark until the end and then “wow” them with your main idea, as in a Sherlock Holmes mystery. The reader, however, does not expect a mystery, but an analytical discussion of your topic in an academic style, with the main argument (thesis) stated up front. Example: (After a paper that lists numerous incidents from the book but never says what these incidents reveal about Douglass and his views on education): So, as the evidence above demonstrates, Douglass saw education as a way to undermine the slaveholders’ power and also an important step toward freedom.
- The “America the Beautiful”/”I Am Woman”/”We Shall Overcome” Conclusion. This kind of conclusion usually draws on emotion to make its appeal, but while this emotion and even sentimentality may be very heartfelt, it is usually out of character with the rest of an analytical paper. A more sophisticated commentary, rather than emotional praise, would be a more fitting tribute to the topic. Example: Because of the efforts of fine Americans like Frederick Douglass, countless others have seen the shining beacon of light that is education. His example was a torch that lit the way for others. Frederick Douglass was truly an American hero.
- The “Grab Bag” Conclusion. This kind of conclusion includes extra information that the writer found or thought of but couldn’t integrate into the main paper. You may find it hard to leave out details that you discovered after hours of research and thought, but adding random facts and bits of evidence at the end of an otherwise-well-organized essay can just create confusion. Example: In addition to being an educational pioneer, Frederick Douglass provides an interesting case study for masculinity in the American South. He also offers historians an interesting glimpse into slave resistance when he confronts Covey, the overseer. His relationships with female relatives reveal the importance of family in the slave community.
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
Douglass, Frederick. 1995. Narrative of the Life of Frederick Douglass, an American Slave, Written by Himself. New York: Dover.
Hamilton College. n.d. “Conclusions.” Writing Center. Accessed June 14, 2019. https://www.hamilton.edu//academics/centers/writing/writing-resources/conclusions .
Holewa, Randa. 2004. “Strategies for Writing a Conclusion.” LEO: Literacy Education Online. Last updated February 19, 2004. https://leo.stcloudstate.edu/acadwrite/conclude.html.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
Overnight Sensation
Fast Results through Powerful Communication
Public Speaking: How to Write a Powerful Speech Conclusion

Conventional wisdom in the realm of public speaking is that your opening statement and closing statement must be perfect. While I agree that it’s important to develop these two parts of your speech, I don’t think they are as important as others make them out to be — which is the other extreme.
The goals of an effective speech conclusion:
There are generally two goals for an effective conclusion. They are:
- To summarize the main points of your speech.
- To motivate the audience to take action.
Please note that “leaving the audience with a positive impression of your wit” isn’t there, nor is “win an award for best written speech conclusion.” All too often, people put too much pressure on themselves to find a way to close their speech. “Thank you” is the worst way to end a speech profess many well-intentioned but ill-informed public speaking experts (I’ve heard this dozens of times at Toastmasters clubs during speech evaluations). I don’t see the harm — it’s fine to thank the audience at the end of your talk, as long as it’s sincere. So let’s look at your real goals.
Summarizing your main points is pretty straightforward. Most speeches have three to five main points, so including each of these in your summary is important. I’ve found it helpful to state a memorable fact or two about each point to help the audience retain the things I’ve said about each point — and this critical for longer talks (30 minutes or longer). I typically do my question and answer session right before my conclusion, so I may make some impromptu references to some interesting or related points that came up during Q&A. I also provide a mini-outline of the main points of my talk as a handout for longer talks (I also use it as a marketing tool).
Your Closing Statement:
Now on to the great controversy: your closing statement. Your goal for the closing statement is to get the audience to take action. Depending on your speech and your goals for giving the speech, it can fall into one or more of the following actions:
- Think a certain way about the topic you presented.
- Purchase a product or service.
- Volunteer or sign up to do something.
- Promote your topic.
- Get others excited or interested in your topic.
Again, many people make this step overly difficult. Simply asking the audience in a sincere way to take the action is as effective as a well crafted conclusion. In fact, trying too hard to provide a memorable conclusion can sometimes turn off your audience. For example, if you’re selling a product and you conclude your pitch with:
So as you can see, our product has helped many people in your situation succeed. I hope you’ll give us the chance to help you succeed.
it’s quite different than:
Imagine your competition as tortoises, slowly trudging along the path to success with their blinders on, completely unaware that the resources they need for success are right at their finger tips. Then you, the hare, whiz by them at the speed light and all they feel a gentle breeze — this is the advantage to buying our product.
You can see that both are equally as effective even though the latter one is more descriptive and doesn’t really buy you anything. In fact, some people may find it corny.
So in summary, your conclusion should simply sum up the main points of your talk and encourage the audience to take action. My Dale Carnegie instructor and friend, Bob Arnold, once said that your closing statement can be as simple as something that reads like a newspaper headline. So don’t fret, just close.
And again, it’s perfectly acceptable in my book to thank the audience at the end of your speech.
James Feudo owns the Boston Web Design Agency JVF Solutions and loves blogging about personal development and communication in his spare time.
Share this:
- ← The Speech Introduction
- Public Speaking Benefits: How Public Speaking Skills can Help Your Career →
2 thoughts on “ Public Speaking: How to Write a Powerful Speech Conclusion ”
Nice post, James, simplifying the sometimes overwrought task of writing a conclusion.
I completely agree with you about thanking the audience at the end, by the way. I’ve written about thanking the audience a couple of times (you might say I’m a little obsessed with the issue):
http://coachlisab.blogspot.com/2007/03/do-you-thank-your-audience.html http://coachlisab.blogspot.com/2007/08/when-not-to-say-thank-you.html http://coachlisab.blogspot.com/2008/02/two-minutes-of-thank-yous.html
Pingback: Public Speaking Success: How to Give a Great Speech | Overnight Sensation - Public Speaking, Communication and Personal Development
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Crafting an Effective Presentation Structure: From Introduction to Conclusion

Crafting an Effective Presentation Structure, from introduction to conclusion, is daunting and brings about a great deal of work. A great presentation leaves the audience feeling either inspired or informed on a specific topic. It generally is not because the speaker was knowledgeable or motivating.
Instead, they are aware of how to structure presentations logically and simply, allowing the audience to keep up with them and take away the key aspects of the presentation. A good structure helps the speaker deliver a presentation calmly, stay on topic and avoid any awkward silence between the presentations. This is precisely why individuals should look to enhance and develop effective presentation skills .
Factors that Determine the Presentation Structure
Before choosing and designing a presentation structure, the speaker should address a variety of factors, including:
- Who is the audience, and how knowledgeable are they already?
- Time duration of the presentation.
- The kind of setting in which the presentation is being delivered.
- Aim of the Presentation.
- What are the key points the audience should take away from the presentation?
Typical Presentation Structure
In general, the contents of a presentation include an introduction, body, and conclusion. At times, it may include visual aids. This is the usual flow for crafting an effective presentation structure: from introduction to conclusion, which covers all the necessary sections and allows the audience to follow along easily.
When designing a presentation,
- Create a solid, organized structure for the entire presentation.
- Keep the slides simple and clear to follow.
- Remember to be concise and do not confuse the audience.
- Make sure to add style and visual elements consistently in the presentation.
The Introduction – Greeting the audience & introducing the speaker
Be sure to create a positive environment by welcoming the audience with a friendly greeting. This allows the audience to arrive, settle down, and prepare for the presentation to start. The introduction is essential to establish a relationship between the speaker and the audience and gather their attention .
The introduction should help the audience comprehend and understand the subject and objective of the presentation as well as gain their attention and confidence . It should narrow down from a broad topic to the specifics of the talk.
- Stating the general topic.
- Narrowing to the area of interest/ subject of the presentation.
- Stating the problems/ challenges in the area being discussed.
- Stating the objective and purpose of the presentation.
- Providing a statement of the outcome of the presentation.
- Showing a preview of the content of the presentation.
This is a great time also to explain the length of the talk, indicate if the speaker wants an audience interaction, and inform the audience if they need to jot down the important points from the presentation. With effective presentation skills, people can keep the audience engaged and interested throughout the entire tenure of the presentation.
The Body
The body of the presentation should meet the objective and the information indicated in the introduction. This is an integral part of crafting an Effective Presentation Structure: From the Introduction to the Conclusion should make up about 75% of the total duration of the presentation.
- The topics should be segmented considering the nature of the presentation and then working through them individually for the audience to understand fully.
- The main points should be concise with relevant supportive evidence, statistics, and examples.
- Critical points should be indicated with reasons. Each important idea could be presented several times in different ways to help the audience fully absorb the meaning.
- State clear links between the ideas and internal summaries and always signal when moving on to the next point.
- Allow the audience to make relevant notes. Always remember to summarize the talk’s body and remind the audience of the topic.
After the main part of the presentation, the audience should understand the information and arguments clearly.
The Conclusion
The conclusion is frequently underdeveloped, and a poorly executed closing can completely undermine a successful presentation. However, the best section is to reflect more power onto the messages and create a lasting impression in the audience’s minds. The conclusion determines whether the speaker has achieved the presentation goal.
While crafting an Effective Presentation Structure: From Introduction to Conclusion, keep in mind that the conclusion should make up about 1/3 of the entire presentation and should contain the following elements:
- Summarize the key points: Keeping in mind the goal of the presentation, remember to summarise the main points and their implications. This is a good way to ensure the audience walks away with the precise information the speaker intended to convey.
- Repeating the core message: Repeating the core theme or message of the presentation can create a powerful conclusion. This will signal the end of the talk and will provide an overview of the argument, findings, and overall purpose of the talk.
- Offering a thought-provoking takeaway: Use a powerful and effective quote or saying that relates to the presentation’s theme and resonates with the audience.
- Visuals: Visuals can leave a lasting impression on the audience while the closing remarks are emphasized.
Final Steps – Thanking the audience and inviting questions
Conclude the talk by acknowledging and thanking the people present in the audience. Show them appreciation for their interest and the time that they have invested in the presentation. After this, the audience may be invited to ask any questions. It is best to focus on initially delivering the presentation to set the tone and topics for discussion in the Q&A.
After finishing the entire presentation, the speaker should have built a relationship with their audience such that:
- The audience follows through on the presentation.
- It acts in the direction of the presentation’s goal.
- The audience remembers the presentation.
Collaborate with the expert Orator Academy team to gain more knowledge about this concept and enhance presentation skills. Check the official website of Orator Academy to develop your public speaking skills .

Vineeta Khanna
Vineeta Khanna is one of the most well known and successful public speaking coaches In New York and New Jersey. As the founder of Orator Academy, she has helped hundreds of young students and working professionals to become confident speakers.
Vineeta has worked with hundreds of students of all ages: elementary school students, college students, interns, job seekers, Wall Street professionals, home makers, IT professionals, teachers and more.
Leave a comment Cancel reply
You must be logged in to post a comment.
You May Also Like

How to Stop Saying “Um” and Other Fillers When Speaking

The importance of interpersonal skills training

Inquiry Form
" * " indicates required fields
View E-Book
To View E-Book Please Fill the Form.
Presentation Structures: Everything You Need to Organize Your Talk
Hrideep barot.
- Presentation , Public Speaking , Speech Writing

A presentation structure includes an introduction, context, main body, conclusion, and scope for questions. Depending on the type of presentation you’re doing, this format can change. The article discusses various considerations for each section of a presentation structure.
For presentations to be understood and create a good impression, they can’t be haphazard. It has to have some sort of pre-planned presentation structure that is both logical and simple enough. Depending on the type of presentation you’re doing, there are likely some basic frameworks available that people tend to follow. Before we delve into the format, let’s consider key points to consider when planning a presentation.
How do you structure and plan a presentation?
We plan a presentation by considering the type of presentation, who our audience is, ideating the purpose, and formulating subtopics through research.
Consider the type of presentation
This leads to understanding the ideal flow to convey your content best. For instance, for persuasive presentations, you could use creative ways to convey what is best about a product, such as starting with a story about how it has helped many people achieve something.
On the other hand, for a progress presentation at your workplace, you might have conventions about what is expected, which must be followed precisely.
A few other types of presentations include:
- Informative presentations
- Instructive presentations
- Motivational presentations
- Analytical presentations
You might also want to consider if you want audience interaction and put that into the structure accordingly. While some allow questions mid-presentation for smaller audiences, it is typically left towards the end.
Consider your audience’s knowledge level and interests
This will determine if you can assume a particular knowledge base and not include it in your presentation structure or if you have to start off with basics and build up on that.
For instance, if you’re teaching 1st-year students about something, you might start with basics. But for graduates, a similar format would be unnecessary as they might have already learned about it.
Similarly, if your purpose is to deliver something entertaining, knowing about the interests and values of your audience helps a ton.
The most simple way is demographics. It’s typically quite easy to find out the expected age group, gender, etc of the audience. This information can help you have a basic idea of the sort of experiences they go through, which helps formulate an understanding.
Consider the purpose of your presentation
While this may seem obvious, many of us lose track of the main purpose and spend too much time on remotely related content. This diverts attention from the topic and might even cause boredom.
For example, if you’re advocating for some social action, it would be beneficial to stay on the topic itself, like the pros, cons, what can be done practically, etc. Instead, if the presenters spend more time criticizing others, the presentation will fall short of its purpose.
Few other examples of different purposes your presentation could have:
- Entertainment
- Providing information
- Telling your story
- Proposing ideas
- Discussing future plans for the company
Research your topic and start noting down the subtopics
Skip this if you already know exactly what needs to be a part of your presentation, and plan to include just that. While looking up your topic, you’ll discover the various sub-topics within that field. After you start noting them down, you can organize later what comes under which to build a structure.
Here is a guide on short presentations that you might be interested in.
So with these three considerations and subtopics in mind, we’re good to go over to decide our final structure.

What is the best presentation form?
The best presentation format is one that includes the introduction, context, main body, conclusion, and questions.
Here, we will discuss a template or structure for a typical presentation.
Introduction
- Greet the audience and introduce yourself, e.g., what you do and why you’re here
- The purpose of your presentation
- The flow or outline gives a sense of what they can expect
- Depending on the topic and audience, you might have to provide more or less context about your topic
- This could include a brief history, terminologies, the current market status, the current status of the field, etc.
- Includes the full depth of the primary purpose of the presentation
- All major chunks of data, including examples, evidence like research studies, etc, are included here
- Care needs to be taken at times to ensure that your introduction and context are not taking up so much time that the main body isn’t receiving enough attention. Ever wonder if a presentation can be too short? Check out this article .
- Bring emphasis to the main takeaways
- Thank your audience if they have been a good one
- Take questions and encourage healthy discussion
- End with sharing ways they can address their questions later
To make sure that the structure works out, it is important that you practice your presentation. This will also tell you if you’re falling within the time constraints. Here is a guide on how you can go about practicing your presentation.
5 Ways to Structure Your Presentation
The five ways include ordered, problem-solution, comparative, storytelling, and demonstrating structures.
1. Ordered Structure
The presentation follows a logical sequence starting with an introduction, main points, and then conclusions. This is what this article has focused on, as it’s the most straightforward method and tends to be very clear for the audience. However, for presentations that do not follow a clear progression, this may not be useful.
2. Problem-Solution Structure
This is useful when persuading the audience. You explain the problem (+ its importance and impact) and then provide a solution that motivates the audience to take it. This could be in the form of a product, a particular method of communication, some technical thing, etc. There should be a decent amount of time spent on the benefits of the solution as well as the exact “How?” to implement it to make the audience convinced. It helps to address any questions or barriers you expect them to have during the speech itself.
3. Comparative Method
This is useful when you want to highlight the benefits of something over alternatives . It is ideal to first fully address the alternatives by talking about their benefits and limitations. Then you lastly talk about the solution that you possess that effectively addresses the other limitations or is in some way a better choice than others, based on your arguments.
Alternatively, if you do not want to highlight the benefits of something particular and just form a comparison that demonstrates the pros and cons of different subjects in an unbiased manner, this technique is still used. For instance, how the main benefit of a product is practically useful for the consumer in comparison to the main benefit of another product can be discussed.
4. Storytelling Structure
This is useful when your goal is just to tell a story. This could be to explain the context or history of a company. It could also serve to talk about yourself and how you got there. A story will typically have an introduction, a complicating factor that introduces some challenges, and then an ending that highlights the importance of some action or belief.
You may also go in a timewise order when explaining a story. This might take away from the thrill but is useful nonetheless when it is required for the audience to properly understand what is being conveyed. Storytelling can be done in various ways, so feel free to find your own structure.
5. Demonstration Structure
This is useful when demonstrating products or services . The benefits of the product/service are highlighted and it is demonstrated showing those capabilities. The goal should be on persuading the audience that it is useful to them for their needs.
How to structure a scientific presentation?
Structuring a scientific presentation typically includes an introduction, methods, results, and discussion.
This typically follows the below format, but depending on the university/conference guidelines, you’ll have to adjust accordingly. The rest of the sub-topics revolves around these sections.
- Introduction/Background
- Literature review (if applicable)
- Acknowledgments (often optional)
After this, time is given to take questions.
How do you structure a presentation script?
The presentation never includes the full extent of the information. It’s just a concise version of what you’re speaking that adds as a visual aid at times while also highlighting major points.
The script is where the major content lies. The structure remains the same, but the content is greater in depth .
Sample Presentation Script
To make it easier for you to understand how you can structure your presentation script, here is a sample script for a presentation on the topic: Importance of Public Speaking.
This follows the same flow introduced earlier- introduction, context, main body, conclusion, and questions.
Title: Importance of Public Speaking
Slide 1: Why is Public Speaking Important?
Greetings, ladies, and gentlemen. Today, I will be exploring the importance of public speaking. My name is John, and I’m thrilled to discuss with you how improving our public speaking abilities may make a significant difference in our quality of life in the personal, social, and professional domains.
Slide 2: Introduction
Public speaking involves persuading an audience with a well-organized message. It is an essential part of our daily lives. We use it when we make conversation in social groups as well as when we address enormous crowds at social gatherings. It is a highly multifaceted and effective tool.
I will start off by giving some information about the context, moving on to its benefits, which is the main crux of our presentation, and then we will spend some time concluding.
Slide 3: Context
Effective communication is essential in our globally interconnected society. Speaking in front of an audience enables us to express our views and thoughts clearly and firmly. It facilitates the development of solid bonds and influences others, and acts as a catalyst for constructive change. Public speaking may open doors of opportunity and propel achievement for anyone, whether they are a student, professional, or member of the community.
Slide 4: Personal Development
Public speaking increases self-esteem and confidence, which are quite rudimentary to our self-efficacy. Effective communication skills help us to be more assertive and feel more in control of our lives. Research suggests that having an internal locus of control (i.e., feeling in control) leads to better outcomes in our personal lives as well as greater mental health. As we organize our ideas and arguments through public speaking, it improves critical thinking and organizational abilities. Furthermore, as we interact with others during talks and Q&A sessions, public speaking also enhances our listening abilities.
Slide 5: Professional Advancement
The ability to speak in front of an audience effectively is highly essential in most workplaces.
You ask Why? Well, it is because we are better able to communicate our qualifications and worth to potential employers, which enhances our performance in job interviews. Secondly, our influence within organizations grows when we can make a strong case for our points in meetings and conferences.
Next, for leadership positions, where success depends on inspiring and motivating others, public speaking is critical. And in general, you’ll need public speaking in any meeting or any talk you would typically deliver in front of a bunch of people.
Slide 6: Conclusion
Public speaking is a sought-after, multifaceted, and handy skill across many settings. It gives us the ability to inspire others, tell our stories, and make a lasting impression. Strong public speaking abilities help us communicate clearly and lead with influence in many facets of our lives.
Slide 7: Questions
I appreciate everyone here for being a great audience and cooperating wonderfully throughout the presentation. Now I will be taking any questions you all have. Feel free to discuss this now or reach out to me after the session is over.
Slide 8: Thank you
I want to thank you all for being here today.
I hope that the presentation did well to emphasize the importance of public speaking and perhaps motivated at least some of you to work on improving your abilities. We will end here.
[End of presentation]
Here are some tips for delivering an effective presentation.
We considered a few key points for presentation structure and the typical format that can be followed. We also covered five ways you can structure your presentation and the format for a scientific presentation. Lastly, we covered a sample script for presentations.
Public speaking coaching is a great way to increase your skills and get better at presentations as well.
Enroll in our transformative 1:1 Coaching Program
Schedule a call with our expert communication coach to know if this program would be the right fit for you

Lost Voice? Here’s How to Recover Sore Throat and Speak Again

7 Keys to Emcee Like a Pro: Unlock Your Hosting Potential

8 Ways to Rise Above the Noise to Communicate Better

- [email protected]
- +91 98203 57888
Get our latest tips and tricks in your inbox always
Copyright © 2023 Frantically Speaking All rights reserved
Kindly drop your contact details so that we can arrange call back
Select Country Afghanistan Albania Algeria AmericanSamoa Andorra Angola Anguilla Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bosnia and Herzegovina Botswana Brazil British Indian Ocean Territory Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Colombia Comoros Congo Cook Islands Costa Rica Croatia Cuba Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Faroe Islands Fiji Finland France French Guiana French Polynesia Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guinea Guinea-Bissau Guyana Haiti Honduras Hungary Iceland India Indonesia Iraq Ireland Israel Italy Jamaica Japan Jordan Kazakhstan Kenya Kiribati Kuwait Kyrgyzstan Latvia Lebanon Lesotho Liberia Liechtenstein Lithuania Luxembourg Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Monaco Mongolia Montenegro Montserrat Morocco Myanmar Namibia Nauru Nepal Netherlands Netherlands Antilles New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Panama Papua New Guinea Paraguay Peru Philippines Poland Portugal Puerto Rico Qatar Romania Rwanda Samoa San Marino Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Slovakia Slovenia Solomon Islands South Africa South Georgia and the South Sandwich Islands Spain Sri Lanka Sudan Suriname Swaziland Sweden Switzerland Tajikistan Thailand Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom United States Uruguay Uzbekistan Vanuatu Wallis and Futuna Yemen Zambia Zimbabwe land Islands Antarctica Bolivia, Plurinational State of Brunei Darussalam Cocos (Keeling) Islands Congo, The Democratic Republic of the Cote d'Ivoire Falkland Islands (Malvinas) Guernsey Holy See (Vatican City State) Hong Kong Iran, Islamic Republic of Isle of Man Jersey Korea, Democratic People's Republic of Korea, Republic of Lao People's Democratic Republic Libyan Arab Jamahiriya Macao Macedonia, The Former Yugoslav Republic of Micronesia, Federated States of Moldova, Republic of Mozambique Palestinian Territory, Occupied Pitcairn Réunion Russia Saint Barthélemy Saint Helena, Ascension and Tristan Da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin Saint Pierre and Miquelon Saint Vincent and the Grenadines Sao Tome and Principe Somalia Svalbard and Jan Mayen Syrian Arab Republic Taiwan, Province of China Tanzania, United Republic of Timor-Leste Venezuela, Bolivarian Republic of Viet Nam Virgin Islands, British Virgin Islands, U.S.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Can Fam Physician
- v.69(7); 2023 Jul
- PMC10348791
Simple, safe, and effective treatment for pyogenic granuloma
Edward s. weiss.
Assistant Professor in the Department of Family Medicine at Queen’s University in Kingston, Ont.
Dominic Wood
Master’s student in the Biology Department at Queen’s University.
Pyogenic granuloma (PG) is a relatively common cutaneous condition often seen by family physicians. Patients frequently seek treatment for PG owing to the friable nature of the lesion and resultant nuisance bleeding from minor trauma. It is generally easy to diagnose, as the clinical appearance is fairly typical: a fleshy, ulcerated, reddish papule that grows quickly over the course of weeks to months. 1 Pyogenic granuloma has numerous treatment options, including surgical excision, curettage and electrocautery, and vascular laser, although risk of recurrence is not insubstantial. 1 The differential diagnosis for a lesion resembling PG includes rare cases of amelanotic melanoma, and for this reason it is sometimes recommended that ablative treatments (eg, cryotherapy) be avoided—unless the lesion can be demonstrated to be benign by histologic examination.
Busy clinicians may wish to have a simple method for correctly diagnosing and treating benign PG. Thankfully, there is a quick and easy way to do so, using the most common of household ingredients: table salt. Long recognized for its utility in treating umbilical granulomas, salt application has also been shown to be safe, effective, and well-tolerated for treatment of PG. 2 It is believed that salt acts as a desiccant, causing shrinkage of the small vessels feeding the PG. An amelanotic melanoma would not be expected to regress substantially in the presence of salt.
The following method is recommended:
- Apply a thin layer of petrolatum or paraffin around the PG to protect the perilesional skin.

Step 1 in creating a makeshift salt reservoir to treat pyogenic granuloma: Prepare a piece of tape with a medial slit .

Step 2 in creating a makeshift salt reservoir to treat pyogenic granuloma: Allow the edges of the slit to overlap slightly, creating a reservoir for table salt .

Step 3 in creating a makeshift salt reservoir to treat pyogenic granuloma: Tape the salt reservoir over the lesion .
- Cover the PG with an occlusive bandage or tape and ask the patient to repeat the salt application and occlusion daily for 14 days, or until complete clearance is achieved.
- If the lesion has not diminished after 2 weeks of treatment or is recurrent after treatment, consider a biopsy to rule out a malignant mimic of PG.
Presentations of PG before and after treatment with salt are shown in Figures 2A and 2B 2B , respectively.

Pyogenic granuloma before treatment

Pyogenic granuloma after treatment with salt
This technique is easy to implement in a clinic setting and is cost-effective. Patients usually report no more than a mild burning sensation when the salt is first applied, and they often get rapid relief from bleeding associated with PG. Salt application can also be used during pregnancy, when other interventions may be contraindicated.
Difficulties with this technique may ensue with PGs located in areas that are difficult to occlude with tape, such as among facial hair or on mucosal surfaces. Manual occlusion of the salt with a finger several times daily may still be effective, provided there is adequate contact time for the desiccant action to take effect.
Family physicians are encouraged to try this novel technique the next time they are faced with a potential PG in a patient desiring treatment.
We encourage readers to share some of their practice experience: the neat little tricks that solve difficult clinical situations. Praxis articles can be submitted online at http://mc.manuscriptcentral.com/cfp or through the CFP website ( https://www.cfp.ca ) under “Authors and Reviewers.”
Competing interests
None declared
- Open access
- Published: 21 May 2024
New-generation advanced PROTACs as potential therapeutic agents in cancer therapy
- Chao Wang 1 ,
- Yujing Zhang 2 ,
- Wujun Chen 1 ,
- Yudong Wu 1 &
- Dongming Xing 1 , 3
Molecular Cancer volume 23 , Article number: 110 ( 2024 ) Cite this article
Metrics details
Proteolysis-targeting chimeras (PROTACs) technology has garnered significant attention over the last 10 years, representing a burgeoning therapeutic approach with the potential to address pathogenic proteins that have historically posed challenges for traditional small-molecule inhibitors. PROTACs exploit the endogenous E3 ubiquitin ligases to facilitate degradation of the proteins of interest (POIs) through the ubiquitin–proteasome system (UPS) in a cyclic catalytic manner. Despite recent endeavors to advance the utilization of PROTACs in clinical settings, the majority of PROTACs fail to progress beyond the preclinical phase of drug development. There are multiple factors impeding the market entry of PROTACs, with the insufficiently precise degradation of favorable POIs standing out as one of the most formidable obstacles. Recently, there has been exploration of new-generation advanced PROTACs, including small-molecule PROTAC prodrugs, biomacromolecule-PROTAC conjugates, and nano-PROTACs, to improve the in vivo efficacy of PROTACs. These improved PROTACs possess the capability to mitigate undesirable physicochemical characteristics inherent in traditional PROTACs, thereby enhancing their targetability and reducing off-target side effects. The new-generation of advanced PROTACs will mark a pivotal turning point in the realm of targeted protein degradation. In this comprehensive review, we have meticulously summarized the state-of-the-art advancements achieved by these cutting-edge PROTACs, elucidated their underlying design principles, deliberated upon the prevailing challenges encountered, and provided an insightful outlook on future prospects within this burgeoning field.
Introduction
Proteolysis-targeting chimeras (PROTACs) have emerged as a revolutionary category of therapeutic modalities since their initial documentation in 2001 (Fig. 1 ) [ 1 , 2 , 3 , 4 ]. These innovative molecules are meticulously designed to harness the power of the ubiquitin proteasome system (UPS) for targeted protein degradation, offering a promising approach to treating various diseases. Over the years, the field of PROTAC-mediated protein degradation has experienced exponential growth and garnered considerable attention from researchers and pharmaceutical companies alike. This surge in interest is primarily due to the remarkable translational potential demonstrated by these compounds (Fig. 2 ) [ 5 , 6 ]. Initially developed as chimeric peptide-based compounds, PROTACs have evolved into cell-permeable small molecules that can efficiently enter cells and selectively degrade disease-causing proteins. The ability of PROTACs to specifically target proteins for degradation holds immense therapeutic promise across multiple areas of medicine. By eliminating disease-associated proteins at their source, these molecules offer a unique advantage over traditional drug therapies that often only inhibit or modulate protein activity. Moreover, PROTACs can potentially address previously "undruggable" targets by exploiting the UPS machinery's natural ability to degrade proteins [ 7 , 8 , 9 , 10 ]. Following the successful clinical trials of the first two small-molecule degraders against cancer in 2019, numerous other small-molecule PROTACs (Table 1 ) are now progressing into clinical settings for treating a variety of diseases [ 2 , 11 , 12 , 13 , 14 , 15 ]. However, despite the promising preclinical research outcomes, a significant proportion of PROTACs encounter challenges in advancing to human clinical trials [ 16 , 17 ].

Timeline of PROTAC discoveries (adapted from [ 3 ])

The development history of PROTACs. A The total number of PROTAC publications and the part of that about cancer each year (web of science core collection). B Percent of PROTACs in different disease fields which are in preclinical phase (web of science core collection)
PROTACs effectively redirect the UPS to specifically recognize and degrade proteins of interest (POIs), which frequently play crucial roles in various disease contexts (Fig. 3 ). This UPS-involved cascade is orchestrated through two essential steps: firstly, the covalent attachment of ubiquitin molecules onto the POIs via tagging; secondly, the subsequent degradation of the polyubiquitinated POIs by the proteasome machinery. The utilization of heterobifunctional molecules facilitates the interaction between E3 ubiquitin ligase and POIs, thereby inducing the successive rounds of ubiquitylation for the substrates. This process ultimately results in the generation of a polyubiquitin chain consisting of four or more ubiquitin units, which is catalyzed by a recruited E2 ubiquitin ligase [ 18 , 19 , 20 , 21 ]. In view of their distinctive mechanism of action (MOA), PROTACs comprising regulatory ligands for E3 ubiquitin ligases and POIs that are connected by a unique linker offer multiple advantages in regulating POI-related cell function at the molecular level and controlling intracellular biological processes. These low-immunogenic chimeras reversibly and rapidly deplete target proteins with minimal impact on the transcriptome and genome, making them more promising for in vivo applications and potential drug-like properties compared to nucleic acid protein modulation techniques like CRISPR-Cas9 and RNA interference. Moreover, PROTACs possess the remarkable capability of being recycled subsequent to ubiquitination and degradation of the POIs, thereby enabling these compounds to catalyze the elimination of even more POIs [ 22 , 23 ]. This recyclable attribute exhibited by PROTACs underscores their superiority over conventional small-molecule inhibitors that lack reusability, thus highlighting their potential for advancing therapeutic interventions. Furthermore, PROTACs induce a loss-of-function mechanism by repeatedly and transiently forming ternary complexes comprising a chimera molecule, E3 ubiquitin ligase, and POI. In addition, the binding affinity required for PROTACs is not as stringent or enduring as that needed for small molecule inhibitors which rely on robust occupancy over an extended period of time. Therefore, it is anticipated that numerous PROTACs will effectively surmount the mutation-induced resistance which significantly impacts their small-molecule inhibitor counterparts [ 9 , 24 ]. Since the induction of proximity between E3 ubiquitin ligases and POIs can be achieved with just two binding ligands, PROTAC-mediated degradation exhibits immense potential in targeting a wide array of proteins, particularly those that were previously deemed 'undruggable'. The activity of PROTACs is primarily dictated by the affinity between chimeras and POIs, as well as their interactions with E3 ligases. These two factors intricately influence the stability of the ternary complex, thereby potentially enhancing selectivity over corresponding inhibitors for protein families harboring a conserved active site [ 25 , 26 ]. Ultimately, the modular design of these PROTACs enables researchers to systematically enhance the physicochemical properties and efficacy of these compounds, thereby facilitating their optimization for potential applications in a more precise and targeted manner.

A PROTAC-mediated degradation of target proteins through the UPS; B The tenets of PROTAC targets
PROTACs possess the potential to revolutionize the realm of drug discovery by offering a remarkably precise and universally applicable strategy for targeting POIs. Nevertheless, substantial challenges persist, and various limitations hinder their clinical applicability (Fig. 4 ) [ 27 , 28 ]. Firstly, the occurrence of serious side effects is primarily attributed to off-target biodistribution of PROTACs resulting from non-selective expression of E3 ubiquitin ligases at both the targeted normal tissues and disease site. For example, while the inhibition of Bromodomain and Extra-Terminal (BET) is relatively well-tolerated, complete elimination of these components may lead to evident deterioration in lethargy, skin health, reduced mobility, and spinal hunching as observed in a study involving mice treated with BET PROTAC known as ARV-771 [ 29 ]. Secondly, the poor aqueous solubility of PROTACs with a large molecular weight (> 800 Da) often leads to low systemic bioavailability [ 30 ]. Thirdly, the high PROTACs' polar surface restricts their permeability, greatly hindering their ability to traverse the cell membrane and physiological barriers [ 31 ]. Additionally, the Hook effect—whereby higher intracellular concentrations of PROTACs leading to a higher formation of unproductive binary complexes rather than ternary complexes, compromises the efficacy of target degradation and poses challenges for the rational design of in vivo dosages when precise control over local disease site availability cannot be achieved [ 32 ]. Collectively, the non-specific biodistribution, suboptimal solubility, low bioavailability, limited permeability, and unpredictable Hook effect pose significant challenges to the clinical translation of PROTACs.
The typical shortcomings of PROTACs
While structural modifications within PROTAC molecules hold promise in overcoming certain limitations, the simultaneous enhancement of all physicochemical properties for effective in vivo applications poses a formidable challenge [ 33 , 34 , 35 ]. Instead of relying solely on excessive chemical optimization, the design of the new-generation advanced PROTACs can potentially address these dilemmas (Fig. 5 ). These new-generation PROTACs exhibit restored functionality for degradation of POIs upon stimulation by either exogenous or endogenous stimuli in specific tissues, while remaining inactive elsewhere. This innovative approach holds promise for enabling highly targeted therapies with reduced side effects [ 34 , 36 , 37 , 38 , 39 , 40 ]. For example, the click-release PROTAC prodrugs, enzyme-responsive PROTAC prodrugs, glutathione (GSH)-responsive PROTAC prodrugs, hypoxia-responsive PROTAC prodrugs, photo-activatable PROTAC prodrugs, radiation-responsive PROTA prodrugs, reactive oxygen species (ROS)-responsive PROTAC prodrugs, etc. Furthermore, the advanced PROTACs also exhibit the capability to selectively target particular cells through ligand optimization, encompassing folate, antibody, and aptamer moieties. In addition, the utilization of nanomedicine delivery system in PROTACs offers several advantages, including enhanced accumulation of PROTACs in diseased tissues and improved pharmacokinetic (PK) profile in vivo. This is exemplified by the application of nano-PROTAC polymers. To provide a comprehensive overview of the rapidly evolving field of advanced PROTACs for cancer therapy, we present an in-depth analysis of recent advancements in PROTAC discovery and the development of new-generation PROTACs (small-molecule PROTAC prodrugs, biomacromolecule-PROTAC conjugates, and nano-PROTACs). This review endeavors to augment our comprehension of this burgeoning field and make a substantial contribution to the advancement of PROTAC-based cancer therapies.

Overview of strategies utilized in the advanced PROTAC design
Development of PROTACs
The pioneering concept of PROTACs was initially proposed by Crews et al . in 2001, unveiling the first-generation of PROTACs as a captivating heterobifunctional- molecule encompassing an exquisite 10-amino acid phosphopeptide and a potent angiogenesis inhibitor ovalicin [ 1 ]. This specific peptide sequence is recognized by the F-box protein β-TRCP E3 ubiquitin ligase, which serves as an E3 ligase subunit within the heterotetrameric Skp1-Cullin-F box complex. As anticipated, the first-generation of PROTACs effectively triggers the degradation of MetAP2 by enlisting β-TRCP E3 ubiquitin ligase. However, the advancement of these first-generation PROTACs was hindered due to their instability and limited cell permeability within biological systems. To overcome these challenges, Crews et al . introduced second-generation PROTACs in 2008, which demonstrated successful intracellular degradation of the Androgen Receptor (AR) within HeLa cells at a concentration of 10 μM [ 41 ]. This PROTAC combines nutlin to recruit the E3 ubiquitin ligase human homolog of Mouse Double Molecule 2 (MDM2) with the selective AR modulator (SARM). Subsequently, novel small-molecule PROTACs incorporating Inhibitor of Apoptosis (IAPs), von Hippel-Lindau (VHL), Cereblon (CRBN), as well as DDB1 and CUL4-related factors (DCAF15, DCAF16) ligands have emerged, gaining significant traction in the field. These advancements offer tremendous potential for the exploration and creation of innovative therapeutic agents [ 42 , 43 , 44 ]. Despite recent attempts to advance the clinical application of second-generation PROTACs, the majority of these compounds fail to progress beyond the preclinical stage in drug development. One of the most formidable challenges lies in achieving precise protein degradation of desired targets, which remains inadequately addressed. In response to the growing demand for expediting the translational process, the new-generation of advanced PROTACs has been investigated, including small-molecule PROTAC prodrugs, biomacromolecule-conjugated PROTACs, and nano-PROTAC polymers, with the aim of enhancing in vivo performance. These improved new-generation PROTACs have the capability to mitigate unfavorable physicochemical properties associated with traditional PROTACs, enhance their targetability, and minimize off-target side effects. The advent of these advanced and precise new-generation PROTACs will mark a significant milestone in targeted protein degradation field, paving the way for a promising future.
Small-molecule PROTAC prodrugs
Click-release protac prodrugs.
Traditional PROTACs often exhibit inadequate water solubility, tissue permeability, and off-target side effects primarily due to their distinctive molecular composition and structural characteristics [ 45 , 46 , 47 , 48 ]. To address these limitations and enhance the practicality of PROTACs as therapeutic agents, a modular reactive prodrug (click-release PROTAC prodrug, Fig. 6 A) strategy could be implemented. This approach involves generating a heterobifunctional molecule within cells by combining two smaller precursors. By doing so, this strategy offers several advantages. Firstly, using smaller precursors allows for better control over the physicochemical properties of the resulting molecule. This means that issues such as water solubility and tissue permeability can be optimized during precursor selection and design. Secondly, employing a modular reactive prodrug strategy enables greater flexibility in targeting specific proteins while minimizing off-target effects. The use of two distinct precursors provides an opportunity to fine-tune selectivity towards desired target proteins while reducing interactions with non-specific cellular components. Moreover, this approach allows for potential modifications or adjustments in response to emerging scientific knowledge or new drug development strategies without completely redesigning the entire compound structure from scratch. Implementing a modular reactive prodrug strategy offers a more practical approach to overcome challenges associated with traditional PROTACs.
A Cartoon showing the structure of the click-release PROTAC prodrugs. B Chemical structures of the click-release PROTAC prodrugs (adapted from [ 49 , 50 ]). C Chemical structures of the click-release PROTAC prodrug (adapted from [ 51 ]). D Chemical structures of the click-release PROTAC prodrugs (adapted from [ 52 ]). E Chemical structures of the click-release PROTAC prodrug (adapted from [ 53 ])
For instance, Lebraud et al . devised a click-release PROTAC prodrug (NGP-1) comprising of a trans -cyclo-octene ligand specific to the Bromodomain-Containing Protein 4 (BRD4) and a Tetrazine (Tz)-tagged thalidomide derivative [ 49 ]. NGP-1 (Fig. 6 B) could be efficiently produced within cells by employing click chemistry, specifically the reaction between a small precursor containing Tz group and another trans -cyclo-octene small precursor. Another prerequisite for the activity of POI degradation in formed NGP-1 is the formation of an anticipated ternary complex, which relies on the lengths and positions of the linker between the two ligands [ 50 ]. A comprehensive screening of tagged groups was conducted to discover a CRBN-JQ1 based NGP-1 with a linker containing 25 separate bonds, encompassing short carbamate and methylene functionalities. In addition to the BRD4 protein, other oncoproteins such as Extracellular Signal-Regulated Protein Kinase 1/2 (ERK1/2) have also been utilized to showcase the modifiable efficacy of the click-release strategy. However, it is worth mentioning that these findings solely pertain to cellular-level demonstrations and do not address the limitations associated with PROTAC's high polar surface area and molecular weight, which hinder its penetration and solubility. Moreover, in the intracellular-generated PROTAC strategy for POI degradation, it is crucial to minimize or eliminate the extracellular combination reaction of precursors as it significantly compromises the efficiency of degradation. To tackle this issue, Lebraud et al . successfully optimized the position and rate of click reaction and designed NGP-2 (Fig. 6 B) by separately administering the two precursors with an optimal order and time interval. This approach effectively mitigated the premature formation of PROTACs, demonstrating a significant advancement in the field of targeted protein degradation. However, further investigation is needed to determine the optimal dosage regimen of the two precursors in order to enhance the intracellular generation efficiency of PROTACs. The varying pharmacokinetic profiles of these precursors should be carefully considered in this process, as they can significantly impact their efficacy and potential therapeutic outcomes. The potential clinical application of this approach remains unexplored and requires additional research. It is essential to conduct preclinical studies to evaluate its safety, efficacy, and potential off-target effects before considering its translation into clinical practice. Additionally, exploring different delivery methods and formulations may also be necessary to optimize its bioavailability and tissue distribution for various disease indications. In conclusion, while Lebraud et al . 's findings are promising, there is still much work to be done before this approach can be considered for clinical use. Continued research efforts will be crucial in unlocking the full therapeutic potential of PROTAC-based therapies for treating a wide range of diseases.
Another instance of click-release PROTAC prodrugs was reported by Huang et al . [ 51 ]. They designed and synthesized a bioorthogonal click-release PROTAC (MZ1-O, NGP-3, Fig. 6 C) by incorporating benzyl carbonate onto the VHL domain. This strategic design allowed for controlled activation of the PROTAC through an iEDDA reaction, leading to efficient degradation of the target protein. The click-release PROTAC remained inactive in the absence of Tz but could be triggered upon exposure to Tz, resulting in intracellular BRD4 protein degradation and subsequent induction of tumor cell apoptosis. Furthermore, their research demonstrated that systemic administration of NGP-3 encapsulated within tumor-targeting poly(lactic-co-glycolic acid) (PLGA) nanoparticles proved to be an effective method for delivering the drug to subcutaneous tumor sites. Once localized at the tumor site, these nanoparticles could be selectively activated by Tz embedded in a dissolvable MN, thereby facilitating precise initiation of the iEDDA bioorthogonal reaction. The development and successful application of this bioorthogonal click-release PROTAC represent a significant advancement in targeted cancer therapy. It offers potential for more precise and efficient treatment strategies with reduced off-target effects. This innovative approach holds promise for improving patient outcomes and advancing our understanding of targeted protein degradation as a therapeutic strategy in oncology.
To further enhance the application of bioorthogonal chemistry in PROTAC prodrug design, Chang et al . have successfully devised two novel click-release PROTAC prodrugs that exhibited selective activation within cancer cells and subsequent release of active PROTAC molecules [ 52 ]. Inactive PROTAC prodrugs TCO-ARV-771 (NGP-4, Fig. 6 D) and TCO-DT2216 (NGP-5, Fig. 6 D) have been strategically designed through the conjugation of a trans -cyclooctenes (TCO) bioorthogonal moiety into the VHL ubiquitin ligase ligand, demonstrating a rational approach in their development. The RGD peptide modified with Tz, known as c(RGDyK)-Tz, was designed to specifically target the integrin αvβ3 biomarker found in cancer cells. This modification served as the activating component for click-release of PROTAC prodrugs, enabling targeted degradation of BRD4 and B-Cell Lymphoma-X L (BCL-X L ) proteins in cancer cells while sparing noncancerous normal cells. The findings from studies evaluating the feasibility of this approach demonstrated that the PROTAC prodrugs exhibited selective activation in an integrin αvβ3-dependent manner, leading to the generation of PROTACs that effectively degraded POIs within cancer cells. The click-release PROTAC strategy holds promise as a universal, non-biological method for inducing targeted cell death in cancer through modulation of the ubiquitin–proteasome pathway. This innovative approach could potentially revolutionize cancer therapy by providing a more precise and effective way to target and eliminate cancerous cells without causing harm to healthy tissues. Further research and clinical trials are needed to fully explore the potential of this strategy, but initial findings are promising and warrant continued investigation into its therapeutic applications.
Recently, Bi et al . also developed a new click-release PROTAC prodrug BT-PROTAC (NGP-6, Fig. 6 E) [ 53 ]. While NGP-6 alone does not exhibit degradation of the BRD4 protein, its activity could be triggered by highly reactive Tz precursors. However, it is worth noting that despite NGP-6 exhibiting 100-fold lower efficiency than MZ1 in degrading BRD4, the antitumor efficacy of NGP-6 remains comparable to that of MZ1. MZ1 is composed of three components: the E3 ubiquitin ligase ligand VHL, the BRD4-targeting warhead JQ1, and a PEG linker that connects these two elements. Previous investigations have demonstrated that the side chain carboxyl of JQ1 was located in the solvent-exposed region and modifications to this segment did not impact the crucial binding interactions with POI. Based on their findings, the authors concluded that the antitumor activity of NGP-6 primarily stemmed from the potent inhibition of BRD4 activity by the JQ1 moiety, rather than through degradation of POI. Therefore, considering the limitation associated with incorporating MZ1 into TCO and its inability to fully mask its antitumor activity, future studies should explore alternative caging groups with substantial steric effects that could potentially impede the binding of the JQ1 moiety to POI. Furthermore, the development of this synthetic methodology opens up new possibilities for the integration of TCO into a wide range of previously reported PROCTACs. This not only provides an alternative approach to designing bioorthogonal click-release PROTACs with reduced toxicity, but also offers potential for enhancing their efficacy and specificity in targeting specific proteins or pathways within cells. By expanding the scope of available tools for targeted protein degradation, this study contributes to the advancement of chemical biology and drug discovery efforts aimed at developing more effective therapeutics for various diseases.
Folate-targeting PROTAC prodrugs
Folate receptor α (FOLR1 or FRα) has emerged as a protein of immense interest in the realm of anticancer drug delivery, captivating attention as a precise therapeutic target [ 54 , 55 ]. This is primarily due to its elevated expression levels in various tumor types compared to normal tissues, making it an attractive candidate for targeted therapy. In recent years, researchers have made remarkable progress in developing prodrugs incorporating small-molecule inhibitors with folate [ 56 ]. These prodrugs are designed to specifically target cancer cells that overexpress the FRα, while minimizing damage to healthy tissues. The use of folate as a targeting moiety allows for selective delivery of potent anticancer agents directly to tumor cells, enhancing their efficacy and reducing systemic toxicity. Clinical evaluation of these novel prodrugs is currently underway, offering hope for improved treatment options for patients with different types of cancers. Moreover, this approach has also been extended to the design of PROTAC prodrugs (Fig. 7 A). By conjugating folates onto PROTAC molecules, they can be directed towards cancer cells expressing FRα while sparing normal tissues from potential harm. This strategy not only improves the safety profile but also enhances the therapeutic window by increasing drug accumulation at tumor sites. It offers a promising avenue for developing more effective targeted therapies against various malignancies while minimizing adverse effects on normal tissues.

A Cartoon showing the structure of the folate-targeting PROTAC prodrugs. B Chemical structures of the folate-targeting PROTAC prodrugs (adapted from [ 57 ]). C Chemical structures of the folate-targeting PROTAC prodrug (adapted from [ 58 ])
Liu et al . designed a series of folate-targeting PROTACs to specifically degrade BRDs, Mitogen-Activated Extracellular Signal-Regulated Kinases (MEKs), and Anaplastic Lymphoma Kinases (ALKs) proteins [ 57 ]. In particular, NGP-7 (Fig. 7 B) was synthesized by attaching folate to the hydroxyl group of a potent BRD4 PROTAC ARV-771 through a triazole linker. This design allows for FRα-mediated internalization and subsequent activation by endogenous hydrolases within the cell. NGP-7 exhibited an effective degradation of BRDs that was dependent on FRα. This suggests that targeting folate receptors could be an effective strategy for selectively degrading specific proteins implicated in diseases such as cancer. Furthermore, Liu et al . also synthesized new folate-targeting PROTAC prodrugs NGP-8 (Fig. 7 B) and NGP-9 (Fig. 7 B), which demonstrated robust degradation specifically targeting MEK and ALK proteins respectively. These findings highlight the versatility of this approach in designing targeted therapies against different protein targets. It is worth noting that the large molecular weight of these folate-targeting PROTAC prodrugs might influence their PK properties and oral bioavailability. The size and complexity of these molecules may affect their absorption, distribution, metabolism, and excretion in the body, which could ultimately influence their efficacy as therapeutic agents. Despite this potential limitation, the results of the study suggest that targeting folate with PROTACs holds promise for broader applicability to other VHL-derived PROTACs. This approach has the potential to expand the scope of targeted protein degradation and provide new opportunities for drug development in various disease areas. Continued research in this area will be crucial for elucidating the optimal strategies for utilizing these compounds in therapeutic interventions. By gaining a deeper understanding of how folate-targeting PROTACs can be effectively utilized, we may uncover novel therapeutic approaches for diseases where selective protein degradation is desired. This could lead to the development of innovative treatment options for conditions such as cancer, neurodegenerative disorders, and autoimmune diseases.
Chen et al . pioneered the development of the first pomalidomide-derived folate-targeting PROTAC prodrug NGP-10 [ 58 ]. This innovative molecule, as depicted in Fig. 7 C, combines a folate moiety with a CRBN-based ALK degrader MS4048 through a disulfide reduction-cleavable linker. Their expertise in designing advanced molecular constructs was evident in this groundbreaking development. The chemical linkage of the folate moiety to the glutarimide motif effectively inhibits the interaction between CRBN and pomalidomide, ensuring precise targeting of the desired proteins. Furthermore, intracellular GSH plays a crucial role in reducing the disulfide bond on NGP-10, leading to spontaneous intramolecular cyclization and subsequent release of MS4048 as an active PROTAC. One particularly noteworthy achievement is that NGP-10 has demonstrated its ability to induce ALK-targeted degradation specifically in FRα + cells. This not only showcases the potential utility of folate-targeting PROTAC prodrugs but also opens up new possibilities for targeted therapy. Overall, Chen et al . 's pioneering work with NGP-10 represents a significant advancement in the field of targeted protein degradation and holds promise for future developments in precision medicine and cancer therapy.
Photo-activatable PROTAC prodrugs
Light is a remarkable external control agent with exceptional spatial and temporal resolution, finding extensive applications in biomedicine, neurobiology, biochemistry, volatile release, fluorescence activation, and polymerization [ 59 ]. Photodynamic therapy (PDT) has garnered significant traction as an influential tool for precise drug delivery [ 60 ]. The emergence of photo-activatable PROTAC prodrugs represents another exciting development in targeted protein degradation strategies [ 61 , 62 ]. These innovative compounds combine small molecule ligands that bind disease-causing proteins with an attached photocleavable linker group. Upon exposure to specific wavelengths of light, these linkers are cleaved off, leading to selective degradation of target proteins by cellular machinery. Photo-caged PROTAC prodrugs refer specifically to those compounds where the ligand's activity is masked until it is uncaged upon illumination with appropriate wavelengths of light. On the other hand, photo-switchable PROTAC prodrugs involve ligands that can switch between active and inactive states depending on whether they are exposed or shielded from certain wavelengths of light. Overall, these advancements highlight how harnessing the power of light enables precise control over biological processes and opens up new avenues for therapeutic interventions targeting diseases at their molecular level [ 63 ].
Photo-caged PROTAC prodrugs
Photo-caged PROTAC prodrugs (Fig. 8 A) generally incorporate photocleavable caging groups into the parent PROTACs to suppress binding affinities against E3 ubiquitin ligases or POIs under light-free conditions. Subsequently, upon light irradiation, the active PROTACs are promptly liberated, thereby facilitating the degradation of POIs.

A Cartoon showing the structure of the photo-caged PROTAC prodrugs. B Chemical structures of the photo-caged PROTAC prodrugs (adapted from [ 64 ]). C Chemical structures of the photo-caged PROTAC prodrugs (adapted from [ 65 ]). D Chemical structures of the photo-caged PROTAC prodrugs (adapted from [ 66 ]). E Chemical structures of the photo-caged PROTAC prodrug (adapted from [ 67 ])
Xue et al . developed a novel approach to target BRD4 using a photo-caged PROTAC prodrug NGP-11 (Fig. 8 B), which involved the incorporation of the 4,5-dimethoxy-2-nitrobenzyl group (DMNB) into the BET binding region of dBET1, a potent BRD4 PROTAC degrader [ 64 ]. This strategic modification couldn’t effectively impede the binding capability of BRD4 in the absence of light irradiation, as anticipated. However, the binding affinity was restored upon exposure to UV light at a wavelength of 365 nm, leading to dose-dependent degradation of BRD4 in Ramos cells. Furthermore, in a zebrafish embryo model, NGP-11 demonstrated effective photoinduced degradation of BRD4, resulting in the anticipated phenotypic alterations. Significantly, this strategy was not limited to BRD4 alone; the authors also successfully incorporated the DMNB motif into the imide nitrogen of MT-802, a Bruton's Tyrosine Kinase (BTK) PROTAC, resulting in NGP-12 (Fig. 8 B). Remarkably, upon light activation, this compound exhibited remarkable efficacy in inducing potent degradation of BTK within Ramos cells.
Naro et al . successfully devised a new photo-caged PROTAC prodrug for the degradation of proteins triggered by light, demonstrating their expertise in this field [ 65 ]. The approach involved incorporating a coumarin derivative (DEACM) cage group onto the hydroxyproline of VHL through a carbonate linker, resulting in NGP-13 (Fig. 8 C) that specifically targeted Estrogen Receptor α (ERα). Unsurprisingly, the DEACM group in NGP-13 prevented the degradation of ERα under light-free conditions. However, efficient and fast photoactivation of protein degradation was observed during photolysis (≤ 405 nm). For CRBN-based PROTACs, Naro et al . incorporated a piperonyloxymethyl (NPOM) moiety onto the glutarimide nitrogen of thalidomide to generate photo-caged BRD4 PROTAC NGP-14 (Fig. 8 C). With exposure to light, NGP-14 effectively removed the NPOM group and exhibited potent antiproliferative efficacy in 22Rv1 cells. Therefore, this strategy holds great promise for the development of VHL- and CRBN-based photo-caged PROTAC prodrugs targeting other different kinds of proteins. With further research and development, these photo-caged PROTAC prodrugs could pave the way for more precise and effective treatments for a variety of conditions, ultimately benefiting patients in need of innovative therapeutic options.
Liu et al . developed a series of novel photo-caged PROTAC prodrugs by introducing a nitroveratryloxycarbonyl group (NVOC) on the glutarimide nitrogen of the pomalidomide molecule [ 66 ]. Following this strategic approach, they successfully synthesized photo-caged NGP-15 (Fig. 8 D) utilizing dBET1 as the exemplary template. As anticipated, the CRBN binding affinity of NGP-15 was significantly diminished as a result of the introduction of bulky NVOC substitution. Under UV irradiation at a wavelength of 365 nm, photolysis of NGP-15 resulted in the liberation of the active parent PROTAC (dBET1), thereby facilitating the degradation of BRDs in a dose-dependent manner. Similarly, a photo-caged NGP-16 (Fig. 8 D) was devised to enable light-induced degradation of ALK fusion proteins. Consequently, this strategy holds promise as a versatile platform for the design and development of pomalidomide-based photo-activatable PROTAC prodrugs capable of photo-controllable protein degradation. This strategy opens up new possibilities for targeted therapy and drug delivery, offering opportunities for precise manipulation of protein levels within cells. Furthermore, the development of such photo-controllable prodrugs could lead to advancements in the treatment of various diseases, including cancer and neurodegenerative disorders. This innovative strategy holds great promise for improving therapeutic outcomes and expanding our understanding of targeted protein degradation mechanisms.
Kounde et al . developed an innovative photo-activatable NGP-17 (Fig. 8 E) by incorporating a DMNB group into the crucial hydroxy motif of the VHL-binding moiety [ 67 ]. This novel approach allowed for precise control over protein degradation, as the caged form of NGP-17 exhibited no activity in the absence of light. However, upon irradiation at 365 nm, it triggered the degradation of BRD4, a protein involved in gene regulation. To visualize this process in real time, the researchers employed fluorescence imaging techniques using Green Fluorescent Protein (GFP)-tagged BRD4 in live HEK293 cells. The light-induced reduction in GFP-tagged BRD4 was observed and recorded over time, providing valuable insights into the dynamics and efficiency of protein degradation mediated by NGP-17. This study showcases the potential applications of light activation in targeted protein degradation strategies. By harnessing specific wavelengths of light to activate or deactivate therapeutic agents like PROTACs, researchers can achieve spatiotemporal control over protein levels within cells.
Photo-switchable PROTAC prodrugs
In addition to the photo-caged PROTACs, there has been a recent exploration of utilizing photo-switchable PROTACs (Fig. 9 A) to attain a reversible manipulation of the protein degradation profile offered by the PROTACs [ 68 ]. The azobenzene moiety is commonly utilized as the photo-switchable group due to its exceptional resistance to fatigue, adjustable photothermal capability, and predictable geometric transformation. Thus, upon exposure to specific wavelengths of light, photo-switchable PROTACs can efficiently undergo transformation between the " trans " and " cis " isoforms, resulting in distinct biological activities attributed to significant alterations in the conformational arrangement and topological distance between the warheads of the POIs and the E3 ubiquitin ligase ligands.

A Cartoon showing the structure of the photo-switchable PROTAC prodrugs (adapted from [ 68 ]). B Chemical structures of the photo-switchable PROTAC prodrugs (adapted from [ 68 ]). C Chemical structures of the photo-switchable PROTAC prodrugs (adapted from [ 69 ]). D Chemical structures of the photo-switchable PROTAC prodrugs (adapted from [ 70 ]). E Chemical structures of the photo-switchable PROTAC prodrugs (adapted from [ 71 ])
Pfaff et al . were the first to pioneer the incorporation of photo-switchable handles into VHL-based PROTACs, thereby enabling photo-controllable activity of PROTACs [ 68 ]. In this study, they synthesized photo-switchable PROTAC trans -NGP-18 (Fig. 9 B) by substituting the linear linker in the ARV-771 degrader with a bistable o -F4-azobenzene linker. trans -NGP-18 efficiently facilitated the degradation of BRD2 in Ramos cells while exhibiting selectivity towards preserving BRD4. In contrast, cis -NGP-18 (Fig. 9 B) exhibited a shorter topological linkage length, leading to the inhibition of BRD2 degradation over the concentration range tested. Efficient conversion of both isomers was successfully accomplished through irradiation at 415 and 530 nm. Furthermore, it was observed that the photostationary state (PSS) of both isomers remained persistent without undergoing thermal back-isomerization, thereby demonstrating the dynamic switching capability of NGP-18 between these two states to induce distinct bioactivities. The precise spatiotemporal regulation of photoinduced degradation of target proteins using photo-switchable PROTACs incorporating an o -F4-azobenzene-containing linker represents a valuable tool for investigating intricate protein signaling pathways that remain poorly understood.
Reynders et al . conducted an investigation into the elaborate design of photo-switchable PROTACs and successfully synthesized trans -NGP-19 (Fig. 9 C) by incorporating an azobenzene moiety into the lenalidomide component of a JQ1-based PROTAC [ 69 ]. In this particular case, it was observed that trans -NGP-19 did not exhibit any activity and did not have any impact on the degradation of BET proteins under dark conditions. However, an interesting phenomenon occurred when trans -NGP-19 was exposed to irradiation at 390 nm. It underwent a conversion into cis -NGP-19 (Fig. 9 C), which had a significant effect on reducing the protein levels of BRD2-4 as well as Cellular-Myelocytomatosis Viral Oncogene (c-MYC). It is worth noting that cis -NGP-19 exhibited its inhibitory effects gradually over time and eventually reverted back to its inactive form. This observation suggests that the isomerization process from cis -NGP-19 to trans -NGP-19 is relatively slow. These findings provide valuable insights into the behavior and potential therapeutic applications of these compounds in regulating protein levels associated with BRD2-4 and c-MYC. Further investigations are imperative to unravel the intricate mechanisms underlying this photo-induced transformation and its implications for targeted therapies in various diseases where BET proteins play a crucial role. Using a similar approach, photo-switchable PROTAC trans -NGP-20 (Fig. 9 C) achieved photocontrol of FKBP12 degradation in RS4;11 cells, suggesting that this strategy holds promise for the design of photo PROTACs to mitigate potential systemic toxicity.
Jin et al . developed photo-switchable PROTAC NGP-21 (Fig. 9 D) as a novel photo-switchable PROTAC prodrug to target Breakpoint Cluster Region-Abelson Leukemia Virus (BCR-ABL) fusion and Abelson Leukemia Virus (ABL) proteins [ 70 ]. This novel compound presents a distinctive benefit by allowing the adjustability of both the orientation and length of its azo -linker under varying wavelengths of light. Upon treatment with trans -NGP-21, remarkable reductions in the levels of BCR-ABL and ABL proteins were observed, while no discernible changes in protein levels were detected for the corresponding cis isomer. Upon exposure to visible light, the inactive cis -NGP-21 underwent a remarkable activation and effectively triggered the degradation of POIs within the BCR-ABL-driven K562 cell line. Conversely, UV irradiation of live cells caused trans -NGP-21 to revert back to its inactive state. By targeting ABL and BCR-ABL fusion proteins, NGP-21 holds promise in treating diseases associated with these aberrant proteins such as Chronic Myeloid Leukemia (CML). The ability to selectively degrade these disease-causing proteins using light-controlled adjustments could potentially lead to more effective therapies with fewer side effects. Jin et al . 's development of NGP-21 represents an exciting advancement in protein targeting technology. Its adjustable azo -containing linker under different wavelengths of light offers researchers a powerful tool for investigating protein function while holding potential therapeutic implications for diseases involving ABL and BCR-ABL fusion proteins.
Zhang et al . developed a novel series of photo-switchable PROTACs by incorporating a photoswitch arylazopyrazole into the linker moiety [ 71 ]. Remarkably, trans -NGP-22 (Fig. 9 E) exhibited remarkable degradation efficacy against BRDs, while its cis isomer failed to demonstrate any discernible effect in this regard. In addition, NGP-23 (Fig. 9 E) represents a significant advancement in the field of targeted protein degradation. This represents a groundbreaking instance of a photo-switchable degradation activity in a multikinase targeting PROTAC. NGP-23 was specifically designed to target a wide range of kinases, and it successfully captured 235 different kinases across diverse families using its recruited BET ligand (CTX-0294885). However, despite its impressive kinase capturing abilities, trans -NGP-23 only exhibited degradation activity towards three specific kinases: Aurora Kinase A (AURORA-A), Focal Adhesion Kinase (FAK), and TANK Binding Kinase 1 (TBK1). This work suggests that new arylazopyrazole photo-switchable PROTACs have the potential to achieve a high abundance of PSS isomer, swift switching capability, and an optimal half-life (T 1/2 ). Further research and development are needed to fully understand the mechanism behind this selectivity and explore the full therapeutic potential of photo-switchable PROTACs. Nonetheless, this pioneering work opens up new possibilities for designing future PROTACs with enhanced specificity towards desired targets while minimizing off-target effects.
Radiation-responsive PROTAC prodrugs
Radiotherapy, also known as radiation therapy, is a widely used and highly effective treatment for cancer [ 72 , 73 , 74 ]. This form of treatment is often considered a first-line option due to its ability to provide precise targeting and deep tissue penetration [ 75 ]. One of the important advantages of radiotherapy is its ability to deliver localized release of X-ray-responsive prodrugs in tumor tissue. By combining radiotherapy with these X-ray-responsive prodrugs, it becomes possible to enhance the antitumor effects and potentially achieve superior outcomes. The synergistic effects between active drugs and X-rays further contribute to the effectiveness of radiotherapy. When combined with certain chemotherapy agents or targeted therapies, the simultaneous administration of these treatments can lead to enhanced tumor cell killing. This combination approach has shown promising results in clinical trials, offering new hope for patients battling various types of cancers.
Yang et al . have made a new innovation in the field of radiotherapy by developing a novel approach called radiation-responsive PROTAC (Fig. 10 A) [ 76 ]. This innovative technique involves incorporating an azide-caged into the VHL ligand of the PROTAC moiety, enabling precise and spatiotemporal degradation of specific proteins. To demonstrate the feasibility of their approach, the researchers designed a derivative of ARV771 serving as an exemplary PROTAC prodrug model. This involved modifying the hydroxyl group of VHL ligand with a (4-azido-tetrafluorophenyl) methanol mask group through a carbonate bond. The purpose of this modification was to block the interaction between radiation-responsive PROTAC and E3 ligase. Upon exposure to X-ray radiation, the mask moiety underwent reduction, resulting in the formation of 4-(hydroxymethyl)-tetrafluoroaniline. Subsequently, NGP-24 (Fig. 10 B) was further eliminated through a decarboxylation and elimination reaction. Consequently, NGP-24 was restored to its original form as ARV771 for efficient degradation of the BET. The cleavage of the caged group induced by X-ray radiation was confirmed through ultra-performance liquid chromatography analysis. Western blot assay revealed that NGP-24 had minimal impact on the expression of the POI in the absence of radiation. However, in contrast, significant degradation of POI occurred under conditions of X-ray radiation. Importantly, it is noteworthy that a synergistic inhibition of tumor growth was observed when combining X-ray radiation with NGP-24 treatment in an MCF-7 breast tumor-bearing mouse model. Overall, their pioneering work on developing radiation-responsive PROTAC holds immense promise for improving cancer treatment outcomes through precise and spatiotemporal protein degradation. Their innovative approach may revolutionize how we combat not only cancer but also other diseases characterized by aberrant protein levels.

A Cartoon showing the structure of the radiation-responsive PROTAC prodrugs (adapted from [ 68 ]). B Chemical structures of the radiation-responsive PROTAC (adapted from [ 76 ]). C Chemical structures of the radiation-responsive PROTAC (adapted from [ 77 ])
In a new study, An et al . have made significant advancements in the field of controllable targeted drug design by developing a novel radiation-responsive PROTAC prodrug NGP-25 (Fig. 10 C) for traceless release [ 77 ]. This innovative approach utilizes carbamate-bearing cages that exhibit excellent chemical and proteolytic stabilities, ensuring the stability of NGP-25 in blood. In comparison to other molecules bearing carbonate or ester bonds, these cages provide enhanced stability. The researchers conducted experiments to confirm the efficacy and safety of their radiation-responsive PROTAC prodrug. They found that precise and efficient activation of the prodrug could be achieved through safe dosages of X-ray irradiation. This activation specifically targeted BRD4 degradation in tumors while having minimal impact on normal organs. Such selective targeting is crucial for minimizing side effects and maximizing therapeutic benefits. One particularly noteworthy aspect of this study is its potential application beyond BRD4 degradation in tumors. The caging strategy employed by An et al . can be extended to other disease-related biomarkers such as cathepsin B, aminopeptidase N, and β-galactosidase. This opens up exciting possibilities for designing activatable PROTACs, antibody–drug conjugates (ADCs), prodrugs, and biomaterials tailored towards personalized treatment approaches. Overall, this research represents an important step forward in the development of targeted therapies with improved stability and selectivity. By harnessing radiation responsiveness and utilizing advanced linker chemistry techniques, An et al . 's work has paved the way for more effective treatments that minimize off-target effects while maximizing therapeutic outcomes across various diseases.
Tumor microenvironment-responsive PROTAC prodrugs
Enzyme-responsive protac prodrugs.
Due to the very different microenvironment in which diseased and normal tissues are located, cancer cells exhibit several endogenous hallmarks that differentiate them from healthy cells [ 78 , 79 ]. One of these hallmarks is the overexpression of a large variety of enzymes [ 80 ]. The overexpression of enzymes in cancer cells opens up new possibilities for targeted therapies. By designing PROTAC prodrugs that are specifically activated by these overexpressed enzymes, researchers can selectively degrade proteins within cancer cells while sparing normal cells. This approach holds great promise in improving the efficacy and safety of cancer treatments. Harnessing the abundance of overexpressed enzymes in cancer cells presents an exciting avenue for developing novel therapeutics based on enzyme-responsive PROTAC prodrugs (Fig. 11 A). By capitalizing on this opportunity, scientists can advance our understanding of cancer biology while potentially revolutionizing treatment options for patients affected by this devastating disease.

A Cartoon showing the structure of the enzyme-responsive PROTAC prodrugs (adapted from [ 68 ]). B Chemical structures of the enzyme-responsive PROTAC (adapted from [ 81 ]). C Chemical structures of the enzyme-responsive PROTAC (adapted from [ 81 ]). D Chemical structures of the enzyme-responsive PROTAC (adapted from [ 82 ]). E Chemical structures of the enzyme-responsive PROTAC (adapted from [ 77 ])
Given that, Liang et al . developed a controllable protein degradation strategy that could selectively target cancer cells [ 81 ]. To achieve this, they combined enzyme-responsive chemistry with a PROTAC approach. The researchers incorporated a trimethyl-locked quinone group into the BRD4-targeted PROTAC, which they named Pro-PROTAC (NGP-26, Fig. 11 B). The unique feature of the trimethyl-locked quinone group is its ability to be reduced and removed by an enzyme called NAD(P)H quinone dehydrogenase 1 (NQO1), which is known to be overexpressed in tumor cells. This design allowed the NGP-26 to remain inactive or "inert" in normal tissues where it binds to E3 ligase VHL. However, once inside cancer cells with elevated levels of NQO1, the masked quinone group was reduced by NQO1, leading to self-immolating cleavage and release of the active HaloPROTAC. By incorporating this enzymatic activation mechanism into their design, Liang et al . were able to achieve cell-selective protein degradation. The presence of NQO1 in cancer cells triggered the removal of the trimethyl-locked quinone group from NGP-26, thereby restoring its ability to degrade targeted proteins through interaction with E3 ligase VHL. This innovative strategy holds great promise for future therapeutic applications as it allows for precise control over protein degradation specifically within cancer cells while sparing normal tissues. Further research will undoubtedly explore how this approach can be optimized and applied in various disease contexts for more effective treatment options.
To further enhance the selectivity of protein degradation mediated by NQO1, Liang et al . developed a novel approach that utilizes an NQO1-regulated cascade reaction to specifically activate small-molecule PROTACs in cancer cells [ 81 ]. The key idea behind this strategy is to differentiate and potentiate the intracellular environment of cancer cells, making them more susceptible to the action of PROTACs. In previous studies, it has been demonstrated that the reduction of β-Lapachone by NQO1 can generate abundant ROS within living cells [ 83 , 84 , 85 ]. This observation inspired the researchers to design a NQO1-responsive PROTAC called NGP-27 (Fig. 11 C). In this design, BRD4 PROTAC was chemically caged using an aryl boronic ester that can be cleaved by ROS. Only when both NQO1 and β-Lapachone are overexpressed simultaneously in cancer cells, the NGP-27 becomes activated and effectively degrades BRD4. Compared to the activation mechanism of another type of PROTAC called NQO1-HaloPROTAC, which solely relies on cellular NQO1 activity for removing trimethyl-locked quinone, the activation of NGP-27 requires not only the presence of NQO1 but also β-Lapachone. This dual requirement significantly improves the cell selectivity of NGP-27 as it ensures that only cancer cells with both elevated levels of NQO1 and β-Lapachone will undergo targeted protein degradation. The development and application of such innovative strategies hold great promise for advancing precision medicine approaches in treating various diseases including cancer.
Apart from NQO1, there is a wide range of other highly expressed enzymes in diseased cells that can be harnessed for enzyme-responsive PROTAC prodrug strategies [ 82 ]. These enzymes offer an expanded repertoire in the field of chemical biology, enabling cell-specific protein degradation and potentially revolutionizing targeted drug discovery. By leveraging these abundant overexpressed enzymes, researchers can design PROTAC prodrugs that selectively target disease-associated proteins within specific cell types. This approach holds great promise for developing more effective therapies with reduced off-target effects. For proof-of-concept, Wei et al . synthesized a new Cyclin-Dependent Kinases (CDK) degrader prodrug NGP-28 (Fig. 11 D) as a model enzyme-responsive PROTAC prodrug [ 82 ]. The CRBN ligand’ amino group of NGP-28 was modified with the mask moiety of methyl pivalate via mild condition to block the interaction between the CDK PROTAC and E3 ligase. At the tumor site in vivo, the mask moiety was partially removed under the catalysis of the enzyme, releasing the active E3 ubiquitin ligase ligand molecule. Subsequently, NGP-28 was restored to active CDK PROTAC for degradation of the POI. Western blot assay demonstrated that NGP-28 barely affected CDK expression in vitro. In contrast, the CDK was markedly degraded in vivo. It was worth noting that NGP-28 synergistically inhibited tumor growth in B16F10 melanoma-bearing mouse model. This is the first development of an orally bioavailable NGP-28 with high bioavailability for oral administration testing in animals. It may also provide a generic solution for oral administration of PROTAC molecules.
An et al . developed a novel and versatile approach for synthesizing a phosphatase-responsive PROTAC prodrug with diverse molecular blocks that possess both robustness and cleavable linkers [ 77 ]. This strategy allows for the precise manipulation of protein degradation by introducing a "turn on" feature. By taking advantage of the pathological cue of elevated phosphatase levels, the researchers were able to achieve site-specific activation and untraceable release of the original PROTAC molecule through de-caging and subsequent self-immolative cleavage. This breakthrough enables selective uptake and controlled degradation of target proteins in vitro. The study indicated that this particular NGP-29 (Fig. 11 E) demonstrated long plasma exposure and high solubility, making it an ideal candidate for targeted therapy. What makes NGP-29 even more remarkable is its specific activation by tumor cells that overexpress phosphatase enzymes. The activation of NGP-29 leads to efficient protein degradation within the tumor cells, ultimately resulting in potent tumor remission. This finding opens up new possibilities for personalized treatment approaches in cancer therapy. Furthermore, as more reactive biomarkers are being discovered through clinical practice, there is a growing need for versatile tools to design activatable PROTACs, smart biomaterials, and prodrugs. In this context, the caging library developed by researchers could serve as a valuable resource.
GSH-responsive PROTAC prodrugs
Tumor microenvironment-responsive strategy is a promising approach that allows for specific control of the PROTAC's on-target degradation activity. This strategy takes advantage of the unique characteristics of tumor cells, such as their increased levels of GSH [ 86 , 87 , 88 , 89 ]. GSH has been reported to be significantly elevated in tumor cells compared to normal tissues [ 90 , 91 , 92 ]. In the context of breast cancer (BC) therapeutics, ERα-targeting PROTACs have emerged as a promising and novel modality. These PROTACs are designed to selectively degrade ERα. However, one concern with ERα PROTACs is their potential off-tissue toxicity, meaning they may induce unwanted degradation in normal tissues.
To address this concern, Zhou et al . developed a GSH-responsive ERα PROTAC (Fig. 12 A) [ 93 ]. They achieved this by conjugating an o -benzenesulfonyl group to the hydroxyl group of PROTAC targeting ERα through a nucleophilic substitution reaction. This design allows for selective activation and degradation of ERα only in tumor cells with high GSH levels. The o -benzenesulfonyl group, serving as a protective moiety, effectively impedes the bioactivity of ERα PROTAC, which can be specifically recognized and eliminated by the abundant presence of GSH in cancer cells. One major advantage of using GSH-responsive PROTACs like NGP-30 (Fig. 12 B) is their ability to selectively target cancer cells while sparing normal cells from toxic effects. This selectivity reduces potential side effects and improves the overall safety profile of the treatment. This study highlights the potential value of utilizing tumor microenvironment-responsive PROTACs for BC treatment. By exploiting specific characteristics or components present within cancer cells, such as high levels of GSH, these innovative therapeutic strategies offer new possibilities for more effective and personalized approaches against BC.

A Cartoon showing the structure of the GSH-responsive PROTAC prodrugs (adapted from [ 68 ]). B Chemical structures of the GSH-responsive PROTAC prodrug (adapted from [ 93 ])
Hypoxia-responsive PROTAC prodrugs
Hypoxia is a distinctive characteristic observed in numerous solid tumors and is associated with unfavorable prognosis and drug resistance [ 94 ]. The expression of Nitroreductase (NTR) is elevated in hypoxic solid tumors compared to healthy tissues under normoxic conditions, thereby conferring NTR-responsive prodrugs with excellent selectivity between normal cells and tumor cells [ 95 ]. Evofosfamide (TH-302 or Evo), a hypoxia-responsive prodrug incorporating a 2-nitroimidazole group, has exhibited favorable safety profiles and demonstrated potent antitumor efficacy in clinical trials [ 96 ]. Consequently, the distinctive feature of solid tumors provides an opportune platform for the development of specific PROTAC prodrugs that can effectively target pathological tissues while sparing normal sites.
Cheng et al . focused on developing hypoxia-responsive PROTACs (Fig. 13 A) that specifically target the Epidermal Growth Factor Receptor (EGFR) [ 97 ]. To achieve this, they introduced hypoxia activated leaving groups (HALGs) into the 4-NH position of a gefitinib-based EGFR PROTAC, resulting in the synthesis of precursors NGP-31 (Fig. 13 B) and NGP-32 (Fig. 13 B). As expected, when compared to the parent compound gefitinib, both precursors showed a significant decrease in binding affinity against EGFR Del19 . This suggests that the introduction of HALGs affected their ability to interact with the receptor. However, it is worth noting that NGP-32 exhibited a remarkable capability to induce degradation of EGFR Del19 in HCC4006 cells specifically under hypoxic conditions, while remaining ineffective under normoxic conditions. To further investigate the stability and release mechanism of these hypoxia-responsive PROTACs, UPLC-MS/MS analysis was conducted. The results confirmed that both NGP-31 and NGP-32 remained stable in the absence of NTR, an enzyme responsible for activating hypoxia-responsive PROTACs. However, after incubation with NTR for 20 min, active PROTAC was released from both precursors. Thus, this research presents a novel strategy for developing tumor-targeting PROTAC prodrugs by incorporating HALGs into EGFR degraders. The findings highlight the potential application of hypoxia activation as a means to selectively degrade cancer-associated proteins such as EGFR Del19 under specific physiological conditions like hypoxia. Further research is warranted to explore and optimize this approach for targeted cancer therapy.

A Cartoon showing the structure of the hypoxia-responsive PROTAC prodrugs (adapted from [ 68 ]). B Chemical structures of the hypoxia-responsive PROTACs (adapted from [ 97 ]). C Chemical structures of the hypoxia-responsive PROTAC (adapted from [ 98 ]). D Chemical structures of the hypoxia-responsive PROTAC (adapted from [ 99 ]). E Chemical structures of the hypoxia-responsive PROTACs (adapted from [ 100 ]). F Chemical structures of the hypoxia-responsive PROTAC (adapted from [ 101 ])
Shi et al . proposed a hypoxia-responsive PROTAC prodrug strategy to introduce HALG technology into other POI ligands, offering a more straightforward approach for the development of novel therapeutics [ 98 ]. In their study, they successfully synthesized NGP-33 (Fig. 13 C) by conjugating a VHL binding moiety and nitroimidazole caging group onto an EGFR PROTAC. The unique design of NGP-33 allowed it to specifically release the active PROTAC under hypoxic conditions, which are commonly found in solid tumors due to inadequate oxygen supply. This targeted activation resulted in dose-dependent degradation of EGFR, a key receptor involved in cancer cell growth and survival. The researchers observed robust antiproliferative activity of NGP-33 in HCC-827 cells, indicating its potential as an effective treatment option for lung cancer. Moreover, NGP-33 demonstrated good plasma stability and PK profile, suggesting its suitability for further preclinical and clinical development. To evaluate its therapeutic efficacy in vivo, the researchers conducted experiments using the HCC-827 xenograft tumor model. Remarkably, administration of NGP-33 at a dosage of 20 mg/kg every two days led to significant tumor growth inhibition (TGI) with an impressive TGI rate of 86%. These findings highlight the promising potential of NGP-33 as a novel hypoxia-activated therapy for lung cancer treatment. The successful application of HALG technology not only expands researchers' understanding of ligand-guided drug delivery systems but also provides valuable insights into developing targeted therapies that can selectively act on specific cellular environments within tumors while minimizing off-target effects on healthy tissues.
Recently, Do et al . made significant progress in the development of a new PROTAC approach [ 99 ]. This innovative method involves the localized formation of an active degrader, known as NGP-34 (Fig. 13 D), through the orthogonal cross-linking of two molecules: BRD4 ligand and CRBN binder. Activation with GSH and NTR under hypoxic conditions enables the precise targeting and degradation of BRD4. The application of NGP-34 has shown promising results in inducing specific degradation of BRD4 protein in various cancer cell lines. This targeted degradation mechanism is particularly effective under hypoxia, making it a valuable tool for studying cancer biology and developing new therapeutic strategies. Furthermore, this novel strategy has demonstrated its efficacy not only in living cells but also in zebrafish models and mice with solid tumors. The ability to achieve hypoxia-dependent activity highlights the potential clinical relevance of hypoxia-responsive PROTACs as they can specifically target tumor cells that thrive under low oxygen conditions. One notable advantage offered by this approach is its potential to overcome some limitations associated with conventional large molecular weight PROTACs. By utilizing hypoxia for click chemistry-based conjugation reactions, hypoxia-responsive PROTACs may offer improved pharmacological properties such as enhanced stability, reduced off-target effects, and increased selectivity towards disease-related targets. These findings present an exciting avenue for the advancement of novel therapeutics targeting cancer and various other ailments.
To enhance target selectivity, Cheng et al . incorporated HALGs into the CRBN ligand site of EGFR Del19 PROTAC degrader and successfully designed and synthesized tumor hypoxia-responsive PROTACs NGP-35 (Fig. 13 E) and NGP-36 (Fig. 13 E) [ 100 ]. Western blot analyses revealed that NGP-35 and NGP-36 effectively and selectively degraded EGFR Del19 specifically in tumor hypoxia conditions. Moreover, these compounds exhibited significantly stronger inhibitory activity on cell viability in tumor hypoxia (half maximal inhibitory concentration (IC 50 ) = 1.1 μM and 0.6 μM, respectively) compared to normoxic conditions (IC 50 = 1.9 μM and 1.1 μM, respectively). Furthermore, a cell migration inhibitory assay demonstrated pronounced effects of both compounds under tumor hypoxia conditions. In addition, it was observed that under tumor hypoxia conditions, NGP-35 and NGP-36 exhibited a higher induction of cellular apoptosis compared to normoxia. The reductive activation assay provided confirmation that the active EGFR PROTAC could be released from either NGP-35 or NGP-36. However, the relatively low quantity of the active drug prompted us to consider further optimization. These findings validate the feasibility of incorporating HALGs into CRBN E3 ligands for developing hypoxia-responsive PROTACs as a means to enhance the selectivity of PROTACs.
Xie et al . made a groundbreaking discovery in BC therapy by developing an ERα-targeted hypoxia-responsive PROTAC NGP-37 (Fig. 13 F) [ 101 ]. The innovative PROTAC NGP-37 was designed to specifically target the tumor microenvironment and enhance its safety profile. The researchers incorporated the nitrobenzene hypoxia-activating group into the ERα ligand of active PROTAC. This modification allowed for the selective activation of PROTAC under hypoxic conditions commonly found in solid tumors. By exploiting this characteristic of the tumor microenvironment, the hypoxia-responsive PROTAC NGP-37 demonstrated excellent responsiveness to low oxygen levels and effectively degraded ERα. One significant advantage of NGP-37 is its ability to mitigate cytotoxicity in normal cells. The bioactivity studies conducted by Xie et al . confirmed that these caged compounds possess remarkable potential for precise functional control of PROTAC drugs in BC treatment. Their ability to respond specifically to hypoxic environments not only enhances therapeutic efficacy but also minimizes off-target toxicity. This research opens up exciting possibilities for future development and optimization of targeted therapies using PROTCAs for BC treatment. Additionally, the findings from this study may pave the way for further exploration into the use of similar strategies in other types of cancer treatment, offering hope for more effective and personalized approaches to combating this disease.
ROS-responsive PROTAC prodrugs
In addition to inherent tumor characteristics such as hypoxia, solid tumors are characterized by an elevated level of ROS. The ROS present in the tumor microenvironment typically encompass superoxide, hydroxyl radical, and hydrogen peroxide (H 2 O 2 ) [ 102 , 103 ]. Tumor cells demonstrate significantly elevated levels of H 2 O 2 up to 100 μmol/L compared to normal cells [ 104 ]. Over the years, extensive efforts have been made to exploit the heightened ROS level in solid tumors for the development of ROS activatable drug delivery systems or prodrugs aimed at enhancing cancer therapy. The development and utilization of ROS activatable prodrugs and targeted drug delivery systems possess immense potential in enhancing the efficacy of cancer therapy. They offer a more precise approach towards eradicating malignant cells while reducing systemic toxicity associated with conventional chemotherapy drugs. Understanding and leveraging the elevated levels of ROS present in solid tumors provide valuable insights into novel strategies for combating cancer effectively. Continued research efforts focused on harnessing this unique characteristic will undoubtedly contribute significantly towards advancing personalized medicine approaches for improved patient outcomes in oncology treatments.
For example, Liu et al . initially introduced a ROS-responsive PROTAC (Fig. 14 A) for the degradation of tumor-specific proteins [ 105 ]. NGP-38 (Fig. 14 B) was synthesized through a process of grafting an arylboronic acid onto the amine or glutarimide of CRBN ligand of PROTAC. This innovative approach allows for targeted protein degradation within cells. To restore the PROTAC molecule, the boronic acid group is cleaved from the NGP-38 using H 2 O 2 . Comparative studies were conducted between the parent PROTAC and the designed NGP-38 to evaluate their efficacy in degrading target proteins. The results showed that while both compounds effectively degraded target proteins, there was a notable difference in their responsiveness to ROS. The parent PROTAC exhibited no response to ROS, whereas the designed NGP-38 demonstrated sensitivity. Further investigations were carried out using 293 T human embryonic kidney cells and T47D tumor cells as experimental models. It was observed that both cell types experienced comparable degradation of target proteins when treated with the parent PROTAC. However, when exposed to the designed NGP-38, specifically targeting BRD3 protein, T47D BC cells displayed dose-dependent and time-dependent degradation patterns. These findings suggest that this newly developed NGP-38 has great potential for tumor-targeted protein degradation. By selectively degrading specific proteins involved in cancer progression, it may offer a promising therapeutic strategy for treating BC and potentially other types of tumors as well. Further research is imperative to delve into its complete potential and enhance its efficacy in clinical applications.

A Cartoon showing the structure of the ROS-responsive PROTAC prodrug (adapted from [ 68 ]). B Chemical structures of the ROS-responsive PROTAC prodrug (adapted from [ 105 ]). C Chemical structures of the ROS-responsive PROTAC prodrugs (adapted from [ 106 ])
Recently, the development of ROS-responsive PROTAC precursors NGP-39 (Fig. 14 C) and NGP-40 (Fig. 14 C) by Yu et al . has opened up a new avenue for targeted protein degradation in cancer cells [ 106 ]. These precursors can be activated by endogenous H 2 O 2 in cancer cells to release active PROTACs (BET PROTAC and ER PROTAC), which effectively degrade targeted proteins while leaving normal cells almost unaffected. The higher BRD4 degradation activity and cytotoxicity of NGP-39 towards cancer cells is mainly due to the higher endogenous concentration of H 2 O 2 in these cells, as characterized by the H 2 O 2 -responsive fluorescence probe. This method has been validated through Western blot assays and cytotoxicity experiments that demonstrate its effectiveness in degrading BRD4 more effectively and being more cytotoxic in H 2 O 2 -rich cancer cells than in H 2 O 2 -deficient normal ones. Moreover, this strategy has also been extended to ER-PROTAC precursor NGP-40, showing its ability to induce ER degradation dependent on the presence of H 2 O 2 . Thus, this new approach offers a promising way for inducing targeted protein degradation specifically within cancerous tissues without affecting healthy ones. This research represents an important step forward towards developing effective therapies for treating cancers with minimal side effects on healthy tissues. The use of endogenous molecules such as hydrogen peroxide offers a unique advantage over traditional methods that rely on exogenously administered drugs or chemicals.
Biomacromolecule-PROTAC conjugates
Antibody-protac conjugates.
ADCs have become a highly promising category of anticancer drugs due to their ability to selectively deliver cytotoxic agents to malignant cells while sparing healthy tissues [ 107 , 108 , 109 ]. The success of trastuzumab deruxtecan (DS-8201), which was recently approved by the FDA for the treatment of various cancer types, has further fueled interest in this field [ 110 , 111 ]. Conventional ADCs are composed of an antibody that targets a specific antigen on the surface of cancer cells, a cytotoxic payload that kills the cell upon internalization, and a chemical linker that connects them. This innovative approach enables precise administration of highly potent cytotoxic medications directly to malignant cells, leading to enhanced effectiveness and diminished toxicity in contrast to conventional chemotherapy. In addition to their potent antitumor activity, ADCs also exhibit desirable PK properties similar to those of monoclonal antibodies. They are stable in circulation and can be engineered for prolonged T 1/2 and improved tissue penetration. Inspired by the success of ADCs, drug scientists have begun exploring new ways to harness the advantages of antibodies for other therapeutic modalities. One such approach is antibody-PROTAC conjugates (Fig. 15 A), which combine the specificity and selectivity of antibodies with the ability to induce protein degradation through targeted recruitment of E3 ubiquitin ligases. These developments highlight the growing importance and potential impact of antibody-based therapeutics in oncology research and beyond.

A Cartoon showing the structure of the antibody-PROTAC conjugates (adapted from [ 68 ]). B Chemical structures of the antibody-PROTAC conjugate (adapted from [ 112 ]). C Chemical structures of the antibody-PROTAC conjugate (adapted from [ 113 ]). D Chemical structures of the antibody-PROTAC conjugate (adapted from [ 114 ]). E Chemical structures of the antibody-PROTAC conjugates (adapted from [ 115 ]). F Chemical structures of the antibody-PROTAC conjugate (adapted from [ 116 ]). G Chemical structures of the antibody-PROTAC conjugates (adapted from [ 117 ]). H Chemical structures of the antibody-PROTAC conjugates (adapted from [ 118 ])
To address the challenges posed by the well-established GNE-987 [ 119 ], which is a very potent BRD degrader but exhibits off-tumor toxicity in vivo, Pillow et al . have undertaken innovative measures [ 112 ]. They successfully designed and synthesized the first BRD antibody-PROTAC conjugate NGP-41, as depicted in Fig. 15 B, with a drug-to-antibody ratio (DAR) of 6. This breakthrough was achieved by conjugating the C-type lectin-like molecule-1 (CLL1)-lead antibody to BRD degrader employing a novel disulfide-bonded linker. Remarkably, the success of the novel linker tailored for NGP-41 is evident in its stability over a period of seven days. This extended stability ensures that the NGP-41 compound remains intact and active, allowing for effective targeting and degradation of pathogenesis-related POIs. Furthermore, in vivo studies conducted on mice after a single intravenous injection revealed a PK profile with a T 1/2 exceeding 14 days. The significance of NGP-41 lies not only in its stability and PK profile but also in its remarkable efficacy against tumors. Multiple xenograft studies have consistently shown tumor regression when treated with NGP-41, highlighting its potential as an effective therapeutic agent. Importantly, this tumor regression was found to be both antigen-dependent and dose-dependent, further emphasizing the specificity and potency of NGP-41. In contrast to the impressive activity exhibited by NGP-41, the parent PROTAC did not demonstrate any significant effects. This stark difference underscores the importance of optimizing PROTAC degrader candidates into potent bifunctional compounds like antibody-PROTAC conjugates. The challenges associated with undesirable physicochemical properties and PK profiles of non-conjugated degraders can be overcome by converting inadequate PROTAC degraders into stable and efficacious molecules like antibody-PROTAC conjugates.
Next, Maneiro et al . designed and synthesized a new antibody-PROTAC conjugate (NGP-42, DAR = 4, Fig. 15 C), which targets BRD4 extremely well by utilising an unbreakable linker [ 113 ]. NGP-42 has an important function in specifically targeting the HER2/neu receptor on tumor cells and selectively degrading BRD4 in those cells, particularly in the BT-474 and SK-BR-3 cell lines. This occurs without affecting the levels of BRD4 in normal cells or in the MCF-7 and MDA-MB-231 cell lines. NGP-42 was designed not only for its specific therapeutic benefits, but also to serve as a labeled antibody-PROTAC conjugate derivative that can be used to observe the transport and internalization processes in living cells through confocal microscopy by utilizing fluorescence. This allowed researchers to track the intracellular fate of NGP-42 and gain insights into its mechanism of action at a cellular level. The development of NGP-42 represents a promising advancement in targeted cancer therapy, offering both specific recognition of tumor cell surface receptors and selective degradation of oncogenic proteins within those cells. The ability to monitor its intracellular trafficking further enhances the understanding of how this novel antibody–drug conjugate functions within the complex microenvironment of tumors.
The Surface Antigen Prostate-6-Transmembrane Epithelial Antigen-1 (STEAP-1) has been identified as a crucial marker for the diagnosis and treatment of prostate cancer. It is often overexpressed in patients with this disease, making it an attractive target for therapeutic intervention. Recently, Dragovich et al . developed an antibody-PROTAC conjugate, NGP-43 (Fig. 15 D), which was designed to degrade STEAP1 protein levels within cells [ 114 ]. This unique strategy allowed NGP-43 to achieve a high DAR (DAR = 6). Interestingly, the study's results suggested that a high DAR may be necessary to achieve effective protein degradation. Specifically, NGP-43 demonstrated better intracellular BRD4 degradation than other antibody-PROTAC conjugates did. However, despite these promising findings, the antiproliferative effect of NGP-43 on prostate cancer PC3 cells with elevated levels of STEAP-1 in vitro was not evident. Overall, while NGP-43 represents an innovative approach towards targeting STEAP1 in prostate cancer therapy, further research is needed to determine its efficacy in vivo and potential clinical applications.
Dragovich et al . conducted structural modifications on MZ1, focusing on hybridisation of amine molecular functional groups with BET-binding molecules, PROTAC linkers, or VHL ligands. These chemical treatments were identified as promising targets for modification [ 115 ]. By introducing amide or carbamate linkages, they successfully linked the linker moieties to the MZ1 derivatives efficiently. One notable antibody-PROTAC conjugate that was constructed is NGP-44 (Fig. 15 E), which uses an amine PROTAC linker with a DAR of 6. This particular antibody-PROTAC conjugate demonstrated enhanced degradation of BRD4 compared to another antibody-PROTAC conjugate (DAR = 2). The results highlight the importance of achieving necessary degrader loading in order to enable effective protein degradation through antibody-PROTAC conjugate-mediated mechanisms. Dragovich et al . designed and developed NGP-45 (DAR = 6, Fig. 15 E), in which MZ1 was replaced by the BET-binding molecule GNE-987, which exerted an effective antiproliferative effect on PC3-S1 cells and significantly increased the degradation activity of BRD4. Moreover, in in vivo experiments, NGP-45 exhibited TGI that was dose- and antigen-dependent. Additionally, when conjugated with the CLL1-monoclonal antibody, the same parent drug resulted in robust antigen-dependent anti-tumor effects in in vivo experiments in mice, as demonstrated by NGP-46 (DAR = 6, Fig. 15 E). The above experimental results showed the utilization of this specific antibody-PROTAC conjugate holds substantial potential for treating diverse forms of tumors.
With the continued development of PROTAC technology, Vartak et al . have made significant progress in developing a novel approach to target and degrade the EGFR. Their study aimed to develop a potent antibody-PROTAC conjugate NGP-47 (Fig. 15 F) for the treatment of non-small cell lung cancer (NSCLC) [ 116 ]. To achieve this, they utilized a combination of lysine conjugation and azide-alkyne cyclization click chemistry techniques to bind together two important molecules—cetuximab, an antibody targeting EGFR, and pomalidomide, a small molecule with proteasome inhibitory activity. This innovative combinatorial approach allowed for the creation of NGP-47, which effectively degraded EGFR. The researchers then evaluated the efficacy of NGP-47 by comparing its IC 50 values with those of cetuximab in both EGFR-resistant H1650 cells and EGFR-sensitive HCC827 cells. The results showed that NGP-47 exhibited significantly lower IC 50 values compared to cetuximab alone, indicating its enhanced potency in inhibiting EGFR-mediated signaling pathways. Furthermore, the team conducted multicellular 3D spheroid assays to assess the impact of NGP-47 on cell proliferation. The results demonstrated that NGP-47 not only significantly inhibited cell growth but also induced apoptosis when compared to both antibody treatment alone and control groups. This study represents an important advancement in PROTAC research by successfully developing a novel combinatorial approach for degrading EGFR using an antibody-PROTAC conjugate. The promising results obtained from cellular assays suggest that further exploration is warranted towards utilizing such strategies as potential therapeutic interventions against NSCLC or other cancers driven by aberrant protein expression or activation.
Dragovich et al . conducted a comprehensive investigation on various antibody-PROTAC conjugate degraders that specifically target the ERα protein [ 117 ]. Among these, they successfully developed NGP-48 (DAR = 2, Fig. 15 G), which is a PROTAC designed to recruit XIAP in order to target ERα. NGP-48 efficiently released active PROTAC intracellularly through protease-mediated cleavage of the Val-Cit-PAB linker. The researchers observed that NGP-48 exhibited remarkable antigen-triggered degradation capability when tested against HER2/MCF7-neo cells in vitro. However, when tested in vivo, it unexpectedly showed insufficient PK properties. This limitation was likely attributed to biotransformation occurring at the XIAP binding site. To overcome this challenge and enhance the PK profile of their protein degraders, Dragovich et al . developed two additional antibody-PROTAC conjugates derived from VHL-based PROTACs. These new compounds aimed to improve stability and optimize drug-like properties for potential therapeutic applications. NGP-49 (DAR = 6, Fig. 15 G) and NGP-50 (DAR = 6, Fig. 15 G) were synthesized by incorporating a disulphide-containing or phosphatase-cleavable linker onto the parent PROTAC, respectively. Both antibody-PROTAC conjugates exhibited ERα degradation in an antigen-dependent manner and demonstrated satisfactory in vivo stability. In conclusion, this study expands the scope of antibody-PROTAC conjugate technology for efficient delivery of ER PROTACs to tumor cells, emphasizing the crucial roles played by both ADC linker technology and parent PROTAC properties in the preparation of antibody-PROTAC conjugates.
Although antibody-PROTAC conjugates have shown promising results in selectively degrading BRD4, EGFR, and ER within cells, there are still several important aspects that need further exploration. One such aspect is the targeting of linking chemistries through antibody conjugation. The choice of linker chemistry plays a crucial role in determining the stability and efficacy of the conjugate. Different linkers may exhibit varying degrees of stability or release rates, which can impact the overall effectiveness of targeted protein degradation. In 2023, Chan et al . conducted a study that further advanced the application of antibody-PROTAC conjugates by demonstrating their ability to selectively degrade the Receptor-Interacting Protein Kinase 2 (RIPK2) in HER2 + cell lines [ 118 ]. They developed a novel antibody-PROTAC conjugate called NGP-51, which consisted of a protease-hydrolysable linker connecting the antibody and the degrader. Through their experiments, Chan et al . observed successful degradation of RIPK2 in HER2 + cell lines when treated with NGP-51 (DAR = 4, Fig. 15 H). Interestingly, they also tested an equivalent anti-IL4 antibody-PROTAC conjugate called NGP-52 (DAR = 3.7, Fig. 15 H) but found no degradation at treatment-relevant concentrations. This highlights the specificity and selectivity of antibody-PROTAC conjugates in targeting particular proteins for degradation. Importantly, neither NGP-51 nor NGP-52 showed any significant effect on RIPK2 levels in HER2- cell lines. This suggests that these bioconjugates have the potential for cell-selective delivery, as they only induce protein degradation in cells expressing high levels of specific characteristic proteins like HER2. The results of this research offer invaluable perspectives into the prospective utilization of PROTAC-based therapies for precise protein degradation. By utilizing antibodies as carriers for PROTACs, researchers can achieve selective delivery to specific cell types based on surface protein expression profiles. This opens up new possibilities for developing personalized treatments tailored to individual patients or diseases characterized by distinct molecular markers.
Aptamer-PROTAC conjugates
Aptamers, as single-stranded oligonucleotide sequences designed to selectively bind with target proteins, have gained significant attention in the field of biomedical research [ 120 ]. These "chemical antibodies" offer several advantages over traditional antibodies, including low immunogenicity and high tissue penetration. Researchers have explored the potential of using aptamer-drug conjugates based on AS1411 (the most promising aptamer) for targeted delivery of anticancer drugs such as doxorubicin [ 121 , 122 ]. By attaching the aptamer to these drugs, researchers aim to enhance their tumor-specific delivery and improve their efficacy against cancer cells. Furthermore, there is growing interest in utilizing aptamers for enhancing the antitumor efficacy and tumor-targeting ability of PROTACs. The conjugation of an aptamer with a PROTAC molecule (Fig. 16 A) may provide a dual targeting approach by specifically recognizing cancer cell surface markers through the aptamer while simultaneously promoting protein degradation through PROTAC action. These advancements highlight the potential of using aptamers not only as therapeutic agents themselves but also as tools for improving targeted drug delivery strategies in cancer therapy. Continued exploration and innovation in this domain offer immense potential for propelling the frontiers of precision medicine methodologies and enhancing patient outcomes within the realm of oncology.

A Cartoon showing the structure of the aptamer-PROTAC conjugates (adapted from [ 68 ]). B Chemical structures of the antibody-PROTAC conjugate (adapted from [ 123 ]). C Chemical structures of the antibody-PROTAC conjugate (adapted from [ 124 ]). D Chemical structures of the antibody-PROTAC conjugate (adapted from [ 125 ]). E Chemical structures of the antibody-PROTAC conjugate (adapted from [ 126 ])
The development of the first aptamer-PROTAC conjugate NGP-53 (Fig. 16 B) by He et al . represents a significant advancement in targeted cancer therapy [ 123 ]. By linking MZ1 and AS1411 through an ester-disulfide linkage, NGP-53 is able to selectively target nucleolin (NCL)-rich tumor cells while avoiding healthy cells. This is achieved through the preferential breaking of the disulfide link by endogenous GSH upon import into tumor cells. In addition to its selectivity, NGP-53 has also demonstrated promising anti-tumor effects without significant adverse effects in preclinical studies using the MCF-7 xenograft model. The effective degradation of BRD4 in NCL + MCF-7 cells further highlights the potential therapeutic benefits of this novel approach. While promising, further research is required to enhance the strategy's specificity for tumor tissue and its therapeutic efficacy. Nevertheless, aptamer-PROTAC conjugation offers a new avenue for developing targeted cancer therapies that may overcome some of the limitations associated with traditional approaches. With continued advancements in this field, we may see even greater success in treating various types of cancers with minimal side effects on healthy tissues.
By employing the aptamer AS1411 as a specific binding agent for the nucleosome protein NCL and linking it to the VHL E3 ligand, Zhang et al . have made progress in the development of aptamer-PROTAC conjugates [ 124 ]. In their study, they successfully designed and synthesized a novel aptamer-PROTAC conjugate called NGP-54 (Fig. 16 C). One of the key advantages of NGP-54 is its excellent serum stability and water solubility, which are crucial for its potential therapeutic applications. They found that NGP-54 specifically attaches to and internalizes into BC cells but not normal cells. This selective targeting is attributed to the varying expression levels of NCL on the surface of these cells. By exploiting this differential expression pattern, NGP-54 holds great promise as a specific therapeutic agent for BC treatment. Furthermore, they demonstrated that NGP-54 effectively induces the degradation of NCL both in vitro and in vivo. This degradation process was shown to be mediated by the formation of a VHL-NGP-54-NCL ternary complex within BC cells. The disruption of NCL function through targeted degradation has been proven to inhibit BC cell proliferation and migration. This groundbreaking work highlights the potential use of aptamers in designing specific PROTACs with high selectivity towards target proteins like NCL. Aptamers offer several advantages over traditional small-molecule compounds, including their ability to bind targets with high specificity and affinity while being easily modified for different applications.
Chen et al . also reported a unique approach, utilizing aptamers as targeting warheads, to induce degradation of non-druggable proteins [ 125 ]. To demonstrate the proof of concept, the researchers developed several aptamer-PROTAC conjugates specifically tailored for the degradation of a carcinogen known as NCL. Among these compounds, NGP-55 (Fig. 16 D) showed remarkable efficacy in degrading the NCL protein through a ubiquitin proteasome-dependent mechanism. Furthermore, it was noted that NGP-55 demonstrated effective inhibition of NCL-mediated proliferation in breast cancer cells. Building upon this success, the scientists went on to develop a photo-controllable version of NGP-55 called opto-NGP-55. By introducing a photo easily digestible oligonucleotide into the structure of NGP-55, NGP-55 was able to reduce potential on-target toxicity. Upon UVA irradiation, opto-NGP-55 underwent cleavage and released active NGP-55 molecules. This activation led to efficient degradation of NCL and further validated the versatility and controllability of aptamer-PROTAC conjugates. These exciting results highlight that aptamer-PROTAC conjugates represent an innovative and feasible approach for selectively degrading proteins of interest. The ability to utilize aptamers as targeting warheads provides flexibility in targeting non-druggable proteins that traditional small molecule inhibitors cannot effectively address. With further advancements in this field, PROTAC-based strategies may hold great promise for therapeutic interventions against various diseases where specific protein targets need to be degraded or modulated for desired outcomes.
Lately, Shih et al . also effectively induced the degradation of STAT3 using a novel aptamer-PROTAC conjugate technology [ 126 ]. Their study demonstrated that the decoy aptamer, which was specifically engineered for STAT3, exhibited a high affinity for binding to the E3 conjugate. This interaction resulted in the formation of potent PROTACs, which were able to selectively recruit different E3 ubiquitin ligases. It is worth noting that the efficacy of NGP-56 (Fig. 16 E) in promoting STAT3 degradation is a significant finding in the field of targeted protein degradation. The specific mechanism by which NGP-56 recruits CRBN as its E3 ubiquitin ligase partner sheds light on the potential for developing novel therapeutic strategies for diseases associated with dysregulated STAT3 signaling. Furthermore, the importance of the aptamer sequence in facilitating STAT3 degradation cannot be overstated. By disrupting this sequence and introducing a decoy aptamer targeting STAT3, the effectiveness of NGP-56 was significantly diminished. Moreover, NGP-56-induced STAT3 degradation was inhibited by bortezomib, thalidomide, siRNA, and MLN7243-mediated CRBN deletion. The findings from this study provide valuable insights into the mechanism of STAT3 degradation and its potential as a target for cancer therapy. The identification of CRBN as the responsible E3 ubiquitin ligase sheds light on the specific pathway through which STAT3 is degraded, offering a potential avenue for developing targeted therapies. Furthermore, the observed reduction in NCI-H2087 cell survival after treatment with NGP-56 highlights the potential of this compound as a cytocidal agent for cancer cells that rely heavily on STAT3 signaling. This suggests that targeting STAT3 degradation could be an effective strategy for combating cancers driven by aberrant STAT3 activity. The use of decoy inducer-PROTAC conjugates to induce protein degradation against oncogenic transcription factors such as STAT3 represents an innovative approach to cancer therapy. By specifically targeting these key regulators of tumor cell survival and growth, this strategy holds promise for the development of novel anti-cancer therapies with potentially fewer off-target effects compared to traditional treatments. Overall, these discoveries underscore the therapeutic potential of targeting protein degradation pathways in cancer cells and pave the way for further research into exploiting this approach for developing more effective and selective anti-cancer treatments.
Nano-PROTAC polymers
Nanosized drug delivery systems (NDDS) have emerged as a promising approach for cancer therapy, offering numerous advantages over traditional small molecular drugs. NDDS have been extensively studied and exploited due to their ability to improve the PK profiles of therapeutic agents [ 127 , 128 , 129 , 130 , 131 , 132 ]. Recently, researchers have turned their attention towards investigating the engagement between NDDS and PROTAC prodrugs. By combining NDDS with PROTAC prodrugs, several benefits can be achieved. Firstly, by encapsulating PROTACs within nanocarriers, their blood circulation time can be significantly extended compared to free-form counterparts. This allows for sustained release of active compounds at tumor sites over an extended period. Secondly, utilizing NDDS can enhance tumor distribution by facilitating passive targeting via the enhanced permeability and retention (EPR) effect. Last but importantly, cellular uptake of PROTACs can also be facilitated by employing NDDS strategies. Nanoparticles possess unique characteristics that promote efficient internalization into cells through various mechanisms such as endocytosis or receptor-mediated pathways. Exploring the interaction between NDDS and PROTAC prodrugs holds immense promise in enhancing therapeutic performance against cancerous diseases (Fig. 17 A). Through increasing tumor distribution efficiency, elongating blood circulation time, and facilitating cellular uptake, the combination could potentially revolutionize cancer treatment strategies by maximizing efficacy while minimizing side effects on healthy tissues [ 133 , 134 ].

A Cartoon showing the structure of the nano-PROTAC polymers (adapted from [ 68 ]). B Chemical structures of the structure of the nano-PROTACs (adapted from [ 135 ]). C Chemical structures of the structure of the nano-PROTACs (adapted from [ 136 , 137 ]). D Chemical structures of the structure of the nano-PROTACs (adapted from [ 138 ]). E Chemical structures of the structure of the nano-PROTACs (adapted from [ 139 ]). F Chemical structures of the structure of the nano-PROTACs (adapted from [ 140 ]). G Chemical structures of the structure of the nano-PROTACs (adapted from [ 141 ]). H Chemical structures of the structure of the nano-PROTACs (adapted from [ 142 ]). I Chemical structures of the structure of the nano-PROTACs (adapted from [ 143 ]). J Chemical structures of the structure of the nano-PROTACs (adapted from [ 144 ]). K Chemical structures of the structure of the nano-PROTACs (adapted from [ 145 ]). L Chemical structures of the structure of the nano-PROTACs (adapted from [ 146 ]). M Chemical structures of the structure of the nano-PROTACs (adapted from [ 147 ]). N Chemical structures of the structure of the nano-PROTACs (adapted from [ 148 ])
Gao et al . introduced a groundbreaking concept in their research by developing nano-PROTAC polymer NGP-57 (Fig. 17 B) [ 135 ]. These innovative polymers consist of an amphiphilic structure, with a hydrophilic PEG segment and a hydrophobic segment conjugated with PROTAC molecules. This unique design allows the nano-PROTAC to self-assemble into micellar nanoparticles. The presence of the hydrophilic PEG segment on the surface of the nanoparticles enhances their stability in the bloodstream, preventing rapid clearance by immune cells. As a result, NGP-57 can circulate for extended periods, increasing their chances of reaching tumor tissues. Furthermore, NGP-57 exhibits enhanced accumulation at tumor sites through an EPR effect. Once accumulated at the tumor site, these nano-PROTAC nanoparticles are designed to release active PROTAC molecules for precise degradation of POIs. They chose ARV-771-based PROTAC targeting BRD4 protein as their model PROTAC molecule. By grafting it onto the polymer backbone through a disulfide chain spacer, they ensured controlled release at specific locations. They also incorporated sheddable PEG corona into these nano-PROTAC particles to further enhance their functionality. Activation and dissociation mechanisms were developed using intracellular acidic conditions and extracellular matrix metalloproteinase-2 (MMP-2) respectively. MMP-2 activation triggers nanoparticle dissociation outside cells while GSH-triggered cleavage inside cells releases active PROTAC molecules from the disulfide bond spacer. In vivo experiments demonstrated that NGP-57 effectively degraded BRD4 protein within tumor tissues and exhibited potent inhibition against MDA-MB231 tumor growth models. Their study showcases how nanotechnology can be harnessed to improve targeted therapy strategies, offering promising prospects for precision medicine approaches in cancer treatment.
To further enhance the accumulation of nano-PROTAC nanoparticles specifically in tumor cells, researchers developed a bioorthogonal nano-PROTAC known as N3@nano-PROTAC (NGP-58, Fig. 17 C) [ 136 , 137 ]. This innovative approach involves modifying the azide group on the PEG head of the nanoparticle. The dibenzocyclooctyne (DBCO) groups, which possess high reactivity towards azide-containing molecules, were efficiently delivered to the tumor mass using a sophisticated system of nanoparticles that respond to the extracellular acidity of tumors. The encapsulation of DBCO groups within the hydrophobic core of the pre-targeted nanoparticle allows for their protection in normal conditions. However, when exposure to the acidic tumor microenvironment, these DBCO groups become exposed and ready for further reaction. This unique feature enables the specific trapping of azide group-modified nano-PROTAC nanoparticles in tumor tissue through a click reaction with the pre-delivered DBCO groups. By employing this bioorthogonal strategy, there is a significant increase of fourfold greater tumor localization of PROTAC compared to using free PROTAC alone as a control. Moreover, it is worth noting that the NGP-58 has been further modified by grafting PPa photosensitizer onto its polymer backbone. This addition endows the nanoparticle with PDT properties, which can enhance apoptosis in tumor cells upon activation by light. In vivo antitumor study suggested that combining PDT and BRD4 degradation resulted in highly efficient induction of tumor cell apoptosis and inhibition of tumor growth. The synergistic effect between these two mechanisms provides a promising approach for combating cancer and potentially overcoming drug resistance commonly observed with single-agent therapies. This research showcases a novel approach utilizing targeted nanoparticles loaded with both PROTACs and PDT agents to achieve improved therapeutic outcomes against tumors.
Zhang et al . engineered a semiconducting nano-PROTAC polymer NGP-59 (namely SPNpro, Fig. 17 D) by integrating its properties of activatable protein degradation activity and photo-immunometabolic efficacy [ 138 ]. NGP-59 is composed of two parts, a PEG shell that connects to the peptide-derived PROTAC, and a semiconducting polymer with PDT efficacy that serves as the core via a chelator protein B (CatB) cleavage peptide. CatB was utilized as the stimulus for liberating PROTAC from NGP-59 due to its status as a cancer biomarker that is commonly overexpressed in various tumor cells, melanoma, encompassing prostate cancer, and BC [ 149 ]. The semiconducting polymer component of NGP-59 exhibited PDT efficacy at the tumor site when subjected to laser irradiation, thereby directly inducing immunogenic cell death (ICD) and eliminating tumor cells in these malignant entities. At the same time, the peptide-derived PROTAC was retrieved from NGP-59 at the tumor site via CatB, resulting in the degradation of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO). In a mutually reinforcing manner, PDT-induced immunogenic cell death (ICD) enhanced the immunogenicity of the tumor while simultaneously relieving immunosuppression through IDO degradation. Immunofluorescence assays demonstrated the ability of NGP-59 nanoparticles and peptide-derived PROTAC to degrade IDO via the ubiquitin–proteasome pathway. Significant reduction in IDO levels was observed in both NGP-59 and IPP groups; however, groups lacking either the VHL ligand or target unit exhibited minimal changes in IDO expression. Moreover, co-administration of inhibitors effectively prevented IDO degradation. As a result, the localized immunometabolic intervention mediated by NGP-59, which combines immune stimulation with activatable protein degradation through photo-immune therapy, effectively suppressed tumor growth in a mouse model. In addition to targeting IDO protein, this innovative protein hydrolysis technology can also be utilized to uniquely degrade other immunosuppressive proteins associated with tumors, thereby enhancing cancer therapy by seamlessly linking corresponding moieties endowed with exceptional targeting capabilities.
Given the remarkable agility exhibited by nano-PROTAC polymers, it becomes conceivable to effortlessly manipulate alternative target proteins through the modification of peptide-derived PROTACs' targeted units. Thus, Zhang et al . developed a novel nano-PROTAC (SPN COX , NGP-60, Fig. 17 E) comprising of a peptide-derived PROTAC targeting Cyclooxygenase 1/2 (COX-1/2), a semiconducting polymer core, and a CatB-cleaved peptide as the linker [ 139 ]. The selection of COX-1/2 as the target was based on its metabolite prostaglandin E2 (PGE2) which has been shown to exert beneficial effects on immune suppressor cells including M2-type macrophages (M2 Macs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs). Consequently, by eliminating COX-1/2 and reducing the accumulation of PGE2, this approach holds promise for reprogramming the immunosuppressive tumor microenvironment and downregulating immune suppressor cells. Hence, the reprogramming of the suppressive tumor microenvironment would be achieved through the elimination of COX-1/2 enzymes, thereby mitigating the accumulation of their metabolite (PGE2) and subsequently attenuating immune suppressor cells. In the meantime, the utilization of semiconducting polymer core-mediated PDT not only enables direct elimination of tumor cells but also induces ICD, thereby eliciting an immune response. The author convincingly demonstrates that NGP-60 effectively regresses tumor growth and prevents recurrence through a synergistic approach involving photo-metabolic cancer immunotherapy via PDT and activatable degradation of COX-1/2. This groundbreaking study represents the pioneering development of tumor microenvironment-reprogramming PROTACs, which holds immense potential for advancing cancer immunotherapy applications.
Zhang et al . conducted a new study where they demonstrated the effectiveness of a novel nano-PROTAC NGP-61 (NPRO, Fig. 17 F) for degrading immune checkpoints [ 140 ]. NGP-61 consisted of two components: a photosensitizer called protoporphyrin IX (PpIX) and a PROTAC peptide that targeted Src Homology 2 Domain-Containing Phosphatase 2 (SHP2), which was cleaved by caspase 3. They found that NGP-61 was able to induce ICD through PDT. Additionally, they observed an upregulation of caspase 3 expression in tumor cells, which further promoted the activation of the peptide-derived PROTAC in macrophages and CD8 + T cells. This activation led to the depletion of SHP2, an important protein involved in immunosuppressive checkpoint signaling pathways such as CD47/SIRPa and programmed cell death-1/programmed cell death ligand 1 (PD-1/PD-L1). By blocking these immunosuppressive checkpoint signaling pathways, the degradation of SHP2 reinvigorated antitumor macrophages and T cells. This combination approach of protein degradation-based modulation of checkpoint signals and PDT holds great potential for cancer immunotherapy. They have made significant progress in developing a comprehensive PROTAC platform for cancer immunotherapy. Their findings provide valuable insights into how nanotechnology can be utilized to enhance immune responses against tumors by targeting specific proteins involved in immune checkpoint regulation.
In order to confine the protein degradation activity of PROTACs to cancer lesions, Wang et al . developed a novel approach by preparing nano-PROTAC, known as NAP (NGP-62, Fig. 17 G) [ 141 ]. NGP-62 was created through the self-assembly of an amphiphilic conjugate of PROTAC linked with near-infrared (NIR) photosensitizer (PS) using a self-immolative thioketal linker. The nanoformulation of NGP-62 significantly enhanced the accumulation of PROTAC in tumor tissues. This targeted delivery system ensured that the therapeutic effects were specifically localized to cancer lesions while minimizing off-target effects on healthy cells. Both in vitro and in vivo experiments showcased that only upon NIR photoirradiation could NGP-62 produce singlet oxygen ( 1 O 2 ), which effectively broke the linkage and released active PROTAC molecules. This light-triggered release mechanism allowed for precise control over BRD4 degradation, a key target involved in tumor growth regulation. This work not only opens up new possibilities for photo-regulation of PROTACs in vivo but also provides valuable insights into designing activatable platforms for targeted protein degradation therapies. The ability to precisely control protein levels within cancer cells offers promising prospects for safely and effectively suppressing tumor growth without causing significant harm to normal tissues.
Recently, He et al . reported an innovative approach to achieve controllable target protein degradation using a nano-PROTAC system [ 142 ]. They designed a photocaged-PROTAC called phoBET1 and incorporated it into mesoporous silica nanoparticles based on upconversion nanoparticles (UCNPs) to create UMSNs@phoBET1 (NGP-63, (Fig. 17 H) nanocages. These nanocages can be activated by NIR light with a wavelength of 980 nm. When exposed to NIR light, the NGP-63 nanocages release active PROTAC in a controlled manner. This active PROTAC specifically targets BRD4, which plays a crucial role in cancer cell growth and survival. By inducing BRD4 degradation, the nano-PROTAC effectively triggers apoptosis in MV-4–11 cancer cells. To evaluate the potential of this novel nanoplatform for clinical applications, in vivo experiments were conducted. The results demonstrated that when NIR light was applied to tumor tissues containing NGP-63 nanocages, BRD4 degradation occurred, leading to significant suppression of tumor growth. This breakthrough technology addresses some limitations associated with existing short-wavelength light-controlled PROTACs. Short-wavelength light often suffers from poor tissue penetration and potential damage to surrounding healthy tissues. In contrast, NIR light has deeper tissue penetration capabilities and is considered safer for biomedical applications. The development of this NIR light activatable PROTAC nanoplatform opens up new possibilities for precise regulation of targeted protein degradation within living tissues. It provides researchers with an advanced tool for studying cellular processes involving specific proteins and offers potential therapeutic strategies for diseases such as cancer where abnormal protein expression plays a critical role.
Ferroptosis, a programmed cell death mechanism, plays a key role in the pathogenesis of numerous diseases [ 150 , 151 , 152 ]. The key players in cellular resistance to ferroptosis are glutathione peroxidase 4 (GPX4) and dihydroorotate dehydrogenase (DHODH) [ 153 , 154 ]. Consequently, targeting the inactivation of these proteins presents an exceptional opportunity for efficacious synergistic cancer therapy based on ferroptosis. Yao et al . developed a multifunctional nano-PROTAC called BPNpro (NGP-64, Fig. 17I ), which holds immense potential for targeted treatment of tumors [ 143 ]. NGP-64 is created using a nanoprecipitation method that involves the use of a thermoresponsive liposome. This unique formulation allows for the encapsulation of a GPX4 targeting boron dipyrromethene (Bodipy) probe (BP) inside the liposome, while on its outer surface, a DHODH ROTAC with cathepsin B (CatB)-cleavable peptide modification (DPCP) is attached. One remarkable feature of NGP-64 is its response to near-infrared (NIR) photoirradiation. When exposed to NIR light, NGP-64 undergoes melting and releases BP specifically within tumor cells. Once released, BP acts as an inhibitor by forming a covalent bond with selenocysteine at the active site of GPX4 enzyme activity. This inhibition disrupts the antioxidant defense mechanism employed by cancer cells and renders them vulnerable to oxidative stress-induced cell death. Furthermore, DPCP plays an essential role in achieving enduring degradation of DHODH through activation by CatB that is overexpressed within tumor cells. By selectively degrading DHODH, DPCP contributes to impairing cancer cell metabolism and inhibiting their proliferation. The combined action of GPX4 inhibition through BP and sustained degradation of DHODH via DPCP leads to extensive ferroptosis—an iron-dependent form of regulated cell death characterized by lipid peroxidation and ROS generation. Ferroptosis induction ultimately results in efficient eradication of tumor cells. Both in vivo and in vitro studies have demonstrated promising outcomes for this novel ferroptosis-based therapy approach proposed by Yao et al . . The antitumor effect exhibited by this therapeutic strategy has shown excellent efficacy against various types of cancers tested so far. This breakthrough not only opens up new possibilities for targeted cancer therapies but also highlights the importance and potential applications of nanotechnology in medicine. Further research will undoubtedly focus on optimizing this innovative nano-agent design and exploring its full potential as an effective treatment option for patients battling cancer worldwide.
Therapy-induced DNA damage represents a prevailing approach to impede tumor cell proliferation; however, the therapeutic effectiveness is constrained by the intricate machinery of DNA repair. Carrier-free nano-PROTAC, known as SDNpro (NGP-65, Fig. 17 J), has emerged as a groundbreaking approach to enhance the effectiveness of PDT in cancer treatment. Researchers have developed NGP-65 by combining two key components: chlorine e6 (Ce6), a potent photosensitizer with excellent light absorption properties; and dBET57, a specific PROTAC molecule designed to degrade BRD4 protein involved in DNA repair processes [ 144 ]. Through self-assembly mediated by noncovalent interactions between Ce6 and dBET57, NGP-65 was formed with favorable dispersibility and uniform nanosize distribution without the need for additional drug excipients. Upon exposure to light irradiation at an appropriate wavelength, NGP-65 efficiently produced abundant ROS within tumor cells. These highly reactive molecules induce significant oxidative damage to cellular components including DNA. Simultaneously, the presence of dBET57 leads to degradation of BRD4 protein through targeted proteolysis. This dual mechanism synergistically enhances the efficacy of PDT by intensifying oxidative DNA damage while interrupting crucial DNA repair pathways mediated by BRD4. The unique advantage offered by NGP-65 lies in its ability to suppress tumor growth while minimizing systemic side effects commonly associated with conventional chemotherapy drugs or carrier-based delivery systems. By specifically targeting BRD4 for degradation within cancer cells during PDT treatment, NGP-65 provides a promising strategy for improving clinical outcomes in tumor therapy. In conclusion, the development of carrier-free nano-PROTACs such as NGP-65 represents an innovative approach towards enhancing PDT efficacy against tumors. With further research and refinement, these advancements hold great potential for advancing PROTAC-based therapies into clinical practice for effective tumor treatment without compromising patient safety or well-being.
Hu et al . reported a PROTAC-Cy7, tridentate molecular probe, using a three-in-one molecular design strategy, which incorporated the E3 ligase ligand pomalidomide, a warhead targeting MCL-1, and a heptamethine cyanine linkage connecting the warhead and the ligand within the same scaffold [ 145 ]. To overcome water insolubility, bovine serum albumin (BSA) nanoparticles with exceptional biocompatibility, nonantigenicity, and biosafety were employed to encapsulate PROTAC-Cy7. The resulting PROTACCy7@BSA (NGP-66, Fig. 17 K) exhibited tumor-targeting capabilities and effectively induced degradation of MCL-1 by specifically binding to overexpressed MCL-1 receptors in various tumors. Furthermore, NGP-66 demonstrated successful utilization in noninvasive biomedical vascular imaging within the NIR-II range and enable real-time intraoperative imaging guidance for tumor resection. When exposed to 808 nm laser irradiation, NGP-66 exhibited a synergistic Chemo-Phototherapy effect, resulting in impeccable tumor suppression and a remarkable 100% survival rate throughout the entire two-month monitoring period. This research presents a straightforward and viable approach for the development of multifunctional agents that enable precise imaging-guided therapy targeting tumors through multiple modalities.
The activation of cell cycle progression in cancer cells renders CDK4/6 inhibition-based cell cycle arrest a potent therapeutic strategy for cancer treatment. To optimize therapeutic efficacy, it is imperative to explore combination strategies based on CDK4/6 inhibition. Wang et al . conducted a groundbreaking study that demonstrated the potential of combining CDK4/6 PROTAC with Chlorin e6-based PDT for treating cancer [ 146 ]. When these two therapeutic approaches were combined, leading to enhanced anti-cancer activity. The synergistic impact was facilitated through the accumulation and activation of mitochondria, resulting in augmented generation of ROS and induction of apoptosis. To facilitate the translation of their findings into clinical practice, Wang et al . developed a self-assembled nano-PROTAC system that could co-deliver CDK4/6 PROTAC and Chlorin e6-based PDT agents without the need for additional carriers. This carrier-free nanoparticle system not only improved drug delivery efficiency but also ensured precise targeting of cancer cells. Remarkably, NGP-67 (Fig. 17 L) exhibited even higher levels of apoptosis induction compared to individual treatments alone. This combination therapy had an added benefit—it cooperatively induced ICD and chemotaxis of immune cells. Additionally, NGP-67 remodeled the immunosuppressive tumor microenvironment by enhancing anti-tumor immunity through various mechanisms such as attracting immune cells towards tumors and modulating immunosuppressive factors present in the tumor microenvironment. Overall, this study presents a promising strategy for combining G1 cell cycle blockage with PDT using CDK4/6 PROTACs and Chlorin e6-based agents. The development of carrier-free nanoparticles provides a practical solution for delivering these therapeutics simultaneously while maximizing their efficacy against cancer cells.
PROTACs represent a promising approach for protein degradation via the proteasome pathway, holding significant potential in nanotherapy. However, their efficacy in degrading target proteins remains suboptimal, impeding the achievement of desired outcomes and ultimately leading to therapeutic failure in tumor treatment. Two-dimensional (2D) nanosystem, also known as 2D-nano-PROTAC (NGP-68, Fig. 17 M), has been developed using tantalum telluride (TaTe2) nanosheet loaded with PROTAC (ARV-825) [ 147 ]. The main objective of constructing NGP-68 is to increase the contact probability between the E3 ligase and target protein, thereby enhancing the protein degradation performance. By efficiently degrading BRD4 and C-MYC proteins, NGP-68 can significantly improve the efficacy of photothermal and PDT for tumors. One remarkable feature of NGP-68 is its ability to serve as a photothermal optical coherence tomography (PT-OCT) contrast agent. This means that it can provide high-resolution three-dimensional images of tumor tissue at a micron level. These detailed images are crucial in guiding multimodal tumor therapy approaches. The construction of therapeutic strategies involving NGP-68 has proven to be highly effective in improving the degradation efficiency of PROTACs towards target proteins. This enhanced efficiency ultimately leads to an improved therapeutic effect when utilizing nano preparations for treating tumors. In summary, by harnessing the unique properties of TaTe2 nanosheet and loading them with PROTAC, researchers have successfully developed NGP-68. These systems not only enhance protein degradation performance but also enable precise imaging through PT-OCT technology. With their potential applications in guided multimodal tumor therapy, these advancements hold great promise for improving cancer treatment outcomes.
Liu et al . also introduced a GSH-scavenging nano-PROTAC strategy, which enhances the bioavailability of PROTACs and optimizes their potential to degrade intracellular POIs for tumor therapy [ 148 ]. The nano-PROTACs are formulated by encapsulating PROTACs within disulfide amide GSH-responsive poly polymeric (PDSA) nanoparticles, demonstrating that ARV@PDSA nano-PROTAC (NGP-69, Fig. 17 N), a nanoengineered BRD4 PROTAC known as ARV-771, effectively promotes BRD4 degradation while reducing downstream oncogene c-Myc expression. By leveraging the GSH-scavenging capability to induce cell cycle arrest and amplify c-Myc-related ferroptosis, NGP-69 demonstrates enhanced anti-tumor efficacy when administered at low doses and exhibits exceptional in vivo biocompatibility. These findings unveil the potential of the nano-PROTAC NGP-69 in treating various diseases through targeted dismantling of associated pathogenic proteins.
In recent years, heterobifunctional PROTACs have emerged as a highly promising therapeutic modality. These innovative molecules offer fascinating potentials in the field of drug development. One of the most significant benefits offered by PROTACs lies in their exceptional capacity to selectively target and degrade POIs that were previously deemed impervious to pharmacological intervention. This opens up new possibilities for treating diseases caused by these elusive protein targets. Another exciting aspect of PROTACs is their potential to degrade protein targets catalytically. By harnessing this mechanism, PROTACs can effectively combat drug resistance induced by mutations in the targeted proteins. This provides hope for patients who have developed resistance to traditional drugs and are in need of alternative treatment options. Despite these inspiring advances, there are still challenges when it comes to translating PROTACs into clinical applications. One issue is that always-on PROTACs often exhibit on-target but off-tissue effects. This means that while they effectively degrade the intended protein target, they may also cause systemic toxicity by degrading proteins in normal tissues. Additionally, traditional PROTACs face limitations due to their poor water solubility, high polarity, and limited membrane permeability. These properties hinder their oral administration and limit their potential as convenient treatment options for patients. Therefore, extensive efforts have been dedicated to improving the formulation and delivery methods of PROTACs with the aim of enhancing their bioavailability and reducing side effects.
In addition to optimizing the molecular structure of PROTAC, extensive researches have been conducted on small-molecule PROTAC prodrugs in order to achieve precise control over spatiotemporally tunable protein degradation. The development of intracellular assembled PROTACs (click-release PROTACs) has shown promise in overcoming the limitations caused by the large molecular weight of traditional PROTAC molecules. By utilizing two rationally designed precursor ligands, these click-release PROTACs are able to avoid the disadvantages associated with their size. However, it is important to consider the in vitro reaction efficacy and suitable molecular ratio of these precursors owing to the distinct PK profiles exhibited by each unit. In an effort to enhance the functionality of PROTAC molecules, photo-responsive masks have been integrated into their structure, resulting in photo-activatable PROTAC prodrugs. Unfortunately, one major setback in this approach is that UV light lacks sufficient tissue penetration ability, which hinders the development and application of these photo-activatable prodrugs. To address this issue, researchers have explored alternative strategies for activating PROTAC prodrugs within solid tumors. They have leveraged endogenous hallmarks such as hypoxia and elevated levels of enzymes like GSH and ROS to achieve precise protein degradation. While these approaches show promise, there is still a need for additional responsive moieties in prodrug design. This inevitably leads to an increase in molecular weight for PROTACs, which may pose challenges when it comes to their druggability.
Ligand-modified PROTAC prodrugs have emerged as promising therapeutic agents for targeted protein degradation in tumor cells. These prodrugs, such as folate-targeting PROTAC prodrugs and biomacromolecule-PROTAC conjugates, exhibit high tumor specificity due to their ability to selectively bind to specific receptors or biomolecules expressed on the surface of cancer cells. One key advantage of these ligand-modified PROTAC prodrugs is their ability to deliver the degrader cargoes directly to the diseased tissues. By exploiting the target selection property of the ligands, these conjugates can effectively transport the degraders specifically to cancerous sites while minimizing exposure to healthy tissues. This targeted delivery approach holds great potential for overcoming poor cell permeability issues commonly encountered with traditional small-molecule drugs. Furthermore, ligand modification also allows for responsive release features in these PROTAC prodrugs. This means that once internalized by cancer cells, they can efficiently release their active degrader molecules at the desired site of action. This controlled release mechanism not only enhances efficacy but also minimizes off-tissue toxicities by confining exposure solely within the diseased tissue. However, designing effective ligand-modified PROTAC prodrugs requires careful consideration of several factors. Firstly, selecting an appropriate ligand that exhibits high affinity and selectivity towards its target receptor is crucial for achieving optimal therapeutic outcomes. Additionally, ensuring stability in circulation is essential to prevent premature degradation or loss of activity before reaching the intended target site. Moreover, it is important to design ligands that facilitate efficient release of active degraders upon internalization into cancer cells. The timing and extent of this release should be carefully controlled to maximize therapeutic efficacy while minimizing any potential adverse effects.
The development of advanced PROTACs has been a subject of extensive research, with the nano-PROTAC polymers being investigated as potential candidates. These polymers have shown promising results in their ability to enhance the efficacy and reduce toxicity in disease therapeutics. To achieve this, the PROTACs are reversibly conjugated onto the backbone of an amphiphilic copolymer. This unique design allows for self-assembly into nanoparticles, which can then be utilized as nanomedicine. The advantage of using nanomedicine is its ability to selectively accumulate at the tumor site due to its specific targeting properties. Once accumulated at the tumor site, these nanomedicine systems release their integrated PROTACs inside the tumor cells. This targeted delivery ensures that only cancerous cells are affected by POI degradation, minimizing damage to healthy tissues surrounding the tumor. Thus, the nano-PROTAC polymers have made significant advancements in terms of PK, preferential accumulation in diseased tissues, and ultimately enabling a synergistic enhancement of efficacy while reducing toxicity in disease therapeutics. In addition to collaborating with each other, delivery systems can also benefit from the integration of various responsive mechanisms. This integration allows for a more sophisticated and precise control over the release of PROTACs, leading to enhanced therapeutic outcomes. By orchestrating protein degradation with other therapeutic approaches, nanomedicine systems have the potential to optimize treatment efficacy and mitigate adverse effects. However, despite their promising potential, nano-PROTAC polymers face certain challenges that limit their broad application. One such challenge is ensuring the biocompatibility of the selected materials used in these delivery systems. It is crucial to carefully evaluate and select materials that are safe for use in vivo and do not cause any adverse reactions or toxicity. Another important consideration is quality control reliability. As these delivery systems involve complex formulations and processes, it becomes essential to establish robust quality control measures throughout production. This ensures consistency in drug release profiles and maintains product integrity. Moreover, practicality of volume production is another factor that needs attention when considering widespread application of nano-PROTAC polymers. The scalability and cost-effectiveness of manufacturing processes should be evaluated to ensure efficient large-scale production without compromising product quality.
To further facilitate the clinical application of the new-generation of advanced PROTACs (Table 2 ), it is important to consider various exogenous stimuli that can be used in clinical therapy. For example, ultrasound and radiation are two potential options that could be explored. These methods have been widely used in medical treatments and have proven to be effective in targeting specific areas within the body. In addition to selecting appropriate exogenous stimuli, optimizing the choice of endogenous hallmark of diseased tissue is also crucial for improving disease specificity. This requires researchers to gain a deep understanding of different diseases and their unique characteristics. By doing so, they can identify specific markers or proteins that are present only in diseased tissues and use them as targets for PROTACs. Furthermore, the administration approach of PROTACs plays an important role in their clinical promotion. Patients prefer oral administration over other routes such as injection or infusion due to its convenience and ease-of-use. Therefore, developing smart and precise PROTACs for oral administration should be a top priority for researchers.
This review highlights the significant progress made in the development of new-generation advanced PROTACs for cancer therapy. These innovative prodrugs have been engineered to achieve targeted protein degradation while minimizing side effects, by responding to various endogenous or external stimuli such as light, hypoxia, enzyme, X-ray, ROS, and GSH. One of the key advantages of these stimuli-responsive structures is that they allow for incorporation of multifunctional ligands into the PROTAC prodrug. This enables cell selectivity through targeting specific receptors such as antibodies, folate, or aptamers. By combining this with nanomedicine delivery systems, PROTAC prodrugs can further improve PK profiles and enhance tumor tissue accumulation. The potential therapeutic efficacy of these new-generation PROTACs is particularly exciting when combined with other treatment modalities. The synergistic effect achieved through combination therapies has shown great promise in preclinical studies and could lead to more effective treatments for cancer patients. Given the integration of medicinal chemistry, nanomedicine, and materials science, the development of new-generation advanced PROTACs holds great promise for precise protein degradation within tumor cells. This innovative approach has the potential to revolutionize cancer therapy by enabling targeted treatment strategies. In addition to PROTACs, there are several other emerging technologies that offer alternative methods for manipulating protein levels. For example, AUTACs (autophagy-targeting chimeras) and ATTECs (autophagosome-tethering compounds) are designed to target proteins for degradation through the autophagy pathway. These compounds can be used to selectively remove specific proteins from cells, providing researchers with a powerful tool for studying protein function. Another emerging technology is LYTACs (lysosome-targeting chimeras), which are designed to target proteins for degradation in the lysosome. By directing specific proteins to the lysosome for degradation, LYTACs provide researchers with a new approach for controlling protein levels within cells. It is worth noting that all these protein degradation strategies rely on heterobifunctional chimeras similar to PROTACs. While they hold immense potential in advancing precision medicine, it is crucial to acknowledge that challenges may arise during their translation into clinical applications. Issues such as off-target effects or limited efficacy could hinder their successful implementation. Therefore, it becomes imperative to systematically explore and evaluate the new-generation advanced PROTACs alongside other protein degraders. By doing so, we can not only enhance our understanding of these novel approaches but also pave the way for improved therapies across various disease areas beyond cancer. The continuous advancement in this field will undoubtedly contribute towards expanding our toolbox of targeted therapies and ultimately benefit patients worldwide. Through rigorous research and collaboration between scientists from different disciplines, we can unlock the full potential of these innovative techniques and bring about a new era in personalized medicine.
Availability of data and materials
No datasets were generated or analysed during the current study.
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98(15):8554–9.
Article CAS PubMed PubMed Central Google Scholar
Chirnomas D, Hornberger KR, Crews CW. Protein degraders enter the clinic - a new approach to cancer therapy. Nat Rev Clin Oncol. 2023;20(4):265–78.
Article CAS PubMed Google Scholar
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: The past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200.
Article PubMed PubMed Central Google Scholar
Schneider M, Radoux CJ, Hercules A, Ochoa D, Dunham I, Zalmas LP, Hessler G, Ruf S, Shanmugasundaram V, Hann MM, Thomas PJ, Queisser MA, Benowitz AB, Brown K, Leach AR. The PROTACtable genome. Nat Rev Drug Discov. 2021;20(10):789–97.
Nalawansha DA, Crews CM. PROTACs: an emerging therapeutic modality in precision medicine. Cell Chem Biol. 2020;27(8):998–1014.
Li K, Crews CM. PROTACs: Past, present and future. Chem Soc Rev. 2022;51(12):5214–36.
Li M, Zhi Y, Liu B, Yao Q. Advancing strategies for proteolysis-targeting chimera design. J Med Chem. 2023;66(4):2308–29.
Wang C, Zhang Y, Wu Y, Xing D. Developments of CRBN-based PROTACs as potential therapeutic agents. Eur J Med Chem. 2021;225:113749.
Burslem GM, Crews CM. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181(1):102–14.
An S, Fu L. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 2018;36:553–62.
Wang C, Zhang Y, Wang J, Xing D. VHL-based PROTACs as potential therapeutic agents: Recent progress and perspectives. Eur J Med Chem. 2022;227:113906.
Sun X, Gao H, Yang Y, He M, Wu Y, Song Y, Tong Y, Rao Y. PROTACs: Great opportunities for academia and industry. Signal Transduct Target Ther. 2019;4:64.
He M, Cao C, Ni Z, Liu Y, Song P, Hao S, He Y, Sun X, Rao Y. PROTACs: Great opportunities for academia and industry (an update from 2020 to 2021). Signal Transduct Target Ther. 2022;7(1):181.
Mullard A. Targeted protein degraders crowd into the clinic. Nat Rev Drug Discov. 2021;20(4):247–50.
Garber K. The PROTAC gold rush. Nat Biotechnol. 2022;40(1):12–6.
Wang Y, Jiang X, Feng F, Liu W, Sun H. Degradation of proteins by PROTACs and other strategies. Acta Pharm Sin B. 2020;10(2):207–38.
Kong NR, Jones LH. Clinical translation of targeted protein degraders. Clin Pharmacol Ther. 2023;114(3):558–68.
Bondeson DP, Mares A, Smith IE, Ko E, Campos S, Miah AH, Mulholland KE, Routly N, Buckley DL, Gustafson JL, Zinn N, Grandi P, Shimamura S, Bergamini G, Faelth-Savitski M, Bantscheff M, Cox C, Gordon DA, Willard RR, Flanagan JJ, Casillas LN, Votta BJ, den Besten WI, Famm K, Kruidenier L, Carter PS, Harling JD, Churcher I, Crews CM. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11(8):611–7.
Churcher I. Protac-induced protein degradation in drug discovery: Breaking the rules or just making new ones? J Med Chem. 2018;61(2):444–52.
Gu S, Cui D, Chen X, Xiong X, Zhao Y. PROTACs: An emerging targeting technique for protein degradation in drug discovery. BioEssays. 2018;40(4):e1700247.
Article PubMed Google Scholar
Smith BE, Wang SL, Jaime-Figueroa S, Harbin A, Wang J, Hamman BD, Crews CM. Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat Commun. 2019;10(1):131.
Lai AC, Crews CM. Induced protein degradation: An emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16(2):101–14.
Xiong Y, Zhong Y, Yim H, Yang X, Park KS, Xie L, Poulikakos PI, Han X, Xiong Y, Chen X, Liu J, Jin J. Bridged proteolysis targeting chimera (PROTAC) enables degradation of undruggable targets. J Am Chem Soc. 2022;144(49):22622–32.
Moreau K, Coen M, Zhang AX, Pachl F, Castaldi MP, Dahl G, Boyd H, Scott C, Newham P. Proteolysis-targeting chimeras in drug development: A safety perspective. Br J Pharmacol. 2020;177(8):1709–18.
Wang C, Zhang Y, Xing D, Zhang R. PROTACs technology for targeting non-oncoproteins: Advances and perspectives. Bioorg Chem. 2021;114:105109.
Koroleva OA, Dutikova YV, Trubnikov AV, Zenov FA, Manasova EV, Shtil AA, Kurkin AV. PROTAC: Targeted drug strategy. Principles and limitations. Russ Chem Bull. 2022;71(11):2310–34.
Gao J, Yang L, Lei S, Zhou F, Nie H, Peng B, Xu T, Chen X, Yang X, Sheng C, Rao Y, Pu K, Jin J, Xu Z, Yu H. Stimuli-activatable PROTACs for precise protein degradation and cancer therapy. Sci Bull (Beijing). 2023;68(10):1069–8485.
Chen C, Yang Y, Wang Z, Li H, Dong C, Zhang X. Recent advances in pro-PROTAC development to address on-target off-tumor toxicity. J Med Chem. 2023;66(13):8428–40.
Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AM, Wang J, Chen X, Dong H, Siu K, Winkler JD, Crew AP, Crews CM, Coleman KG. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2016;113(26):7124–9.
Edmondson SD, Yang B, Fallan C. Proteolysis targeting chimeras (PROTACs) in “beyond rule-of-five” chemical space: Recent progress and future challenges. Bioorg Med Chem Lett. 2019;29(13):1555–64.
Matsson P, Kihlberg J. How big is too big for cell permeability? J Med Chem. 2017;60(5):1662–4.
Kiely-Collins H, Winter GE, Bernardes GJL. The role of reversible and irreversible covalent chemistry in targeted protein degradation. Cell Chem Biol. 2021;28(7):952–68.
Pike A, Williamson B, Harlfinger S, Martin S, McGinnity DF. Optimising proteolysis-targeting chimeras (PROTACs) for oral drug delivery: A drug metabolism and pharmacokinetics perspective. Drug Discov Today. 2020;25(10):1793–800.
Chen Y, Tandon I, Heelan W, Wang Y, Tang W, Hu Q. Proteolysis-targeting chimera (PROTAC) delivery system: Advancing protein degraders towards clinical translation. Chem Soc Rev. 2022;51(13):5330–2350.
Zeng S, Huang W, Zheng X, Liyan C, Zhang Z, Wang J, Shen Z. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges. Eur J Med Chem. 2021;210:112981.
Guenette RG, Yang SW, Min J, Pei B, Potts PR. Target and tissue selectivity of PROTAC degraders. Chem Soc Rev. 2022;51(14):5740–2756.
Guedeney N, Cornu M, Schwalen F, Kieffer C, Voisin-Chiret AS. PROTAC technology: A new drug design for chemical biology with many challenges in drug discovery. Drug Discov Today. 2023;28(1):103395.
Liu J, Peng Y, Wei W. Light-controllable PROTACs for temporospatial control of protein degradation. Front Cell Dev Biol. 2021;9:678077.
Dragovich PS. Degrader-antibody conjugates. Chem Soc Rev. 2022;51(10):3886–97.
Salerno A, Seghetti F, Caciolla J, Uliassi E, Testi E, Guardigni M, Roberti M, Milelli A, Bolognesi ML. Enriching proteolysis targeting chimeras with a second modality: When two are better than one. J Med Chem. 2022;65(14):9507–30.
Benowitz AB, Jones KL, Harling JD. The therapeutic potential of PROTACs. Expert Opin Ther Pat. 2021;31(1):1–24.
Paiva SL, Crews CM. Targeted protein degradation: elements of PROTAC design. Curr Opin Chem Biol. 2019;50:111–9.
Li X, Song Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13(1):50.
Zou Y, Ma D, Wang Y. The PROTAC technology in drug development. Cell Biochem Funct. 2019;37(1):21–30.
Alabi SB, Crews CM. Major advances in targeted protein degradation: PROTACs, LYTACs, and MADTACs. J Biol Chem. 2021;296:100647.
Lipinski CA. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–41.
Raina K, Crews CM. Targeted protein knockdown using small molecule degraders. Curr Opin Chem Biol. 2017;39:46–53.
Deshaies RJ. Protein degradation: Prime time for PROTACs. Nat Chem Biol. 2015;11(9):634–5.
Lebraud H, Wright DJ, Johnson CN, Heightman TD. Protein degradation by in-cell self-assembly of proteolysis targeting chimeras. ACS Cent Sci. 2016;2(12):927–34.
Cyrus K, Wehenkel M, Choi EY, Han HJ, Lee H, Swanson H, Kim KB. Impact of linker length on the activity of PROTACs. Mol Biosyst. 2011;7(2):359–64.
Huang J, Yao Z, Li B, Ping Y. Targeted delivery of PROTAC-based prodrug activated by bond-cleavage bioorthogonal chemistry for microneedle-assisted cancer therapy. J Control Release. 2023;361:270–9.
Chang M, Gao F, Pontigon D, Gnawali G, Xu H, Wang W. Bioorthogonal PROTAC prodrugs enabled by on-target activation. J Am Chem Soc. 2023;145(25):14155–63.
Bi T, Liang P, Zhou Y, Wang H, Huang R, Sun Q, Shen H, Yang S, Ren W, Liu Z. Rational design of bioorthogonally activatable PROTAC for tumor-targeted protein degradation. J Med Chem. 2023;66(21):14843–52.
Ruff E, Poh S. Folate targeting peptide conjugates for inflammatory response suppression. Curr Drug Metab. 2023;24(4):283–9.
George S, Srinivasan A, Tulimilli SV, Madhunapantula SV, Palantavida S. Folate targeting self-limiting hyperthermic nanoparticles for controlled photothermal therapy. J Mater Chem B. 2023;11(29):6911–21.
Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol. 2020;17(6):349–59.
Liu J, Chen H, Liu Y, Shen Y, Meng F, Kaniskan H, Jin J, Wei W. Cancer selective target degradation by folate-caged PROTACs. J Am Chem Soc. 2021;143(19):7380–7.
Chen H, Liu J, Kaniskan H, Wei W, Jin J. Folate-guided protein degradation by immunomodulatory imide drug-based molecular glues and proteolysis targeting chimeras. J Med Chem. 2021;64(16):12273–85.
Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–7.
Tao Y, Chan HF, Shi B, Li M, Leong KW. Light: A magical tool for controlled drug delivery. Adv Funct Mater. 2020;30(49):2005029.
Verma S, Manna D. Controlling PROTACs with light. ChemMedChem. 2020;15(14):1258–61.
Hu Z, Crews CM. Recent developments in PROTAC-mediated protein degradation: From bench to clinic. ChemBioChem. 2022;23(2):e202100270.
Wu P, Manna D. Optochemical control of protein degradation. ChemBioChem. 2020;21(16):2250–2.
Xue G, Wang K, Zhou D, Zhong H, Pan Z. Light-induced protein degradation with photocaged PROTACs. J Am Chem Soc. 2019;141(46):18370–4.
Naro Y, Darrah K, Deiters A. Optical control of small molecule-induced protein degradation. J Am Chem Soc. 2020;142(5):2193–7.
Liu J, Chen H, Ma L, He Z, Wang D, Liu Y, Lin Q, Zhang T, Gray N, Kaniskan H, Jin J, Wei W. Light-induced control of protein destruction by opto-PROTAC. Sci Adv. 2020;6(8):eaay5154.
Kounde CS, Shchepinova MM, Saunders CN, Muelbaier M, Rackham MD, Harling JD, Tate EW. A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chem Commun (Camb). 2020;56(41):5532–5.
Pfaff P, Samarasinghe KTG, Crews CM, Carreira EM. Reversible spatiotemporal control of induced protein degradation by bistable photoPROTACs. ACS Cent Sci. 2019;5(10):1682–90.
Reynders M, Matsuura BS, Bérouti M, Simoneschi D, Marzio A, Pagano M, Trauner D. PHOTACs enable optical control of protein degradation. Sci Adv. 2020;6(8):eaay5064.
Jin YH, Lu MC, Wang Y, Shan WX, Wang XY, You QD, Jiang ZY. Azo-PROTAC: Novel light-controlled small-molecule tool for protein knockdown. J Med Chem. 2020;63(9):4644–54.
Zhang Q, Kounde CS, Mondal M, Greenfield JL, Baker JR, Kotelnikov S, Ignatov M, Tinworth CP, Zhang L, Conole D, De Vita E, Kozakov D, McCluskey A, Harling JD, Fuchter MJ, Tate EW. Light-mediated multi-target protein degradation using arylazopyrazole photoswitchable PROTACs (AP-PROTACs). Chem Commun (Camb). 2022;58(78):10933–6.
Vozenin MC, Bourhis J, Durante M. Towards clinical translation of FLASH radiotherapy. Nat Rev Clin Oncol. 2022;19(12):791–803.
Allen C, Her S, Jaffray DA. Radiotherapy for cancer: Present and future. Adv Drug Deliv Rev. 2017;109:1–2.
Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomedicine. 2021;16:1083–102.
Petroni G, Cantley LC, Santambrogio L, Formenti SC, Galluzzi L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat Rev Clin Oncol. 2022;19(2):114–31.
Yang C, Yang Y, Li Y, Ni Q, Li J. Radiotherapy-triggered proteolysis targeting chimera prodrug activation in tumors. J Am Chem Soc. 2023;145(1):385–91.
An K, Deng X, Chi H, Zhang Y, Li Y, Cheng M, Ni Z, Yang Z, Wang C, Chen J, Bai J, Ran C, Wei Y, Li J, Zhang P, Xu F, Tan W. Stimuli-responsive protacs for controlled protein degradation. Angew Chem Int Ed Engl. 2023;62(39):e202306824.
Chang J, Cai W, Liang C, Tang Q, Chen X, Jiang Y, Mao L, Wang M. Enzyme-instructed activation of pro-protein therapeutics in vivo. J Am Chem Soc. 2019;141(45):18136–41.
Fouladi F, Steffen KJ, Mallik S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjug Chem. 2017;28(4):857–68.
Fejerskov B, Jarlstad Olesen MT, Zelikin AN. Substrate mediated enzyme prodrug therapy. Adv Drug Deliv Rev. 2017;118:24–34.
Liang C, Zheng Q, Luo T, Cai W, Mao L, Wang M. Enzyme-catalyzed activation of pro-PROTAC for cell-selective protein degradation. CCS Chemistry. 2022;4(12):3809–19.
Article CAS Google Scholar
Wei M, Zhao R, Cao Y, Wei Y, Li M, Dong Z, Liu Y, Ruan H, Li Y, Cao S, Tang Z, Zhou Y, Song W, Wang Y, Wang J, Yang G, Yang C. First orally bioavailable prodrug of proteolysis targeting chimera (PROTAC) degrades cyclin-dependent kinases 2/4/6 in vivo. Eur J Med Chem. 2021;209:112903.
Parkinson EI, Hergenrother PJ. Deoxynyboquinones as NQO1-activated cancer therapeutics. Acc Chem Res. 2015;48(10):2715–23.
Bey EA, Reinicke KE, Srougi MC, Varnes M, Anderson VE, Pink JJ, Li LS, Patel M, Cao L, Moore Z, Rommel A, Boatman M, Lewis C, Euhus DM, Bornmann WG, Buchsbaum DJ, Spitz DR, Gao J, Boothman DA. Catalase abrogates β-lapachone-induced PARP1 hyperactivation-directed programmed necrosis in NQO1-positive breast cancers. Mol Cancer Ther. 2013;12(10):2110–20.
Kapalatiya H, Madav Y, Tambe VS, Wairkar S. Enzyme-responsive smart nanocarriers for targeted chemotherapy: An overview. Drug Deliv Transl Res. 2022;12(6):1293–305.
Hsu PH, Almutairi A. Recent progress of redox-responsive polymeric nanomaterials for controlled release. J Mater Chem B. 2021;9(9):2179–88.
Yang Y, Sun W. Recent advances in redox-responsive nanoparticles for combined cancer therapy. Nanoscale Adv. 2022;4(17):3504–16.
Chen M, Liu D, Liu F, Wu Y, Peng X, Song F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J Control Release. 2021;332:269–84.
Liu P, Hao L, Liu M, Hu S. Glutathione-responsive and -exhausting metal nanomedicines for robust synergistic cancer therapy. Front Bioeng Biotechnol. 2023;11:1161472.
Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152(1):2–12.
Liu C, Jia S, Tu L, Yang P, Wang Y, Ke S, Shi W, Ye S. GSH-responsive and hypoxia-activated multifunctional nanoparticles for synergetically enhanced tumor therapy. ACS Biomater Sci Eng. 2022;8(5):1942–55.
Ma Z, Gao X, Raza F, Zafar H, Huang G, Yang Y, Shi F, Wang D, He X. Design of GSH-responsive curcumin nanomicelles for oesophageal cancer therapy. Pharmaceutics. 2022;14(9):1802.
Zhou Z, Fan H, Yu D, Shi F, Li Q, Zhang Z, Wang X, Zhang X, Dong C, Sun H, Mi W. Glutathione-responsive PROTAC for targeted degradation of ERα in breast cancer cells. Bioorg Med Chem. 2023;96:117526.
Li Y, Zhao L, Li XF. Targeting hypoxia: Hypoxia-activated prodrugs in cancer therapy. Front Oncol. 2021;11:700407.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410.
Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J Jr, Chiorean EG, Rosen PJ, Ulrich B, Dragovich T, Del Prete SA, Rarick M, Eng C, Kroll S, Ryan DP. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2015;33(13):1475–81.
Cheng W, Li S, Wen X, Han S, Wang S, Wei H, Song Z, Wang Y, Tian X, Zhang X. Development of hypoxia-activated PROTAC exerting a more potent effect in tumor hypoxia than in normoxia. Chem Commun (Camb). 2021;57(95):12852–5.
Shi S, Du Y, Zou Y, Niu J, Cai Z, Wang X, Qiu F, Ding Y, Yang G, Wu Y, Xu Y, Zhu Q. Rational design for nitroreductase (NTR)-responsive proteolysis targeting chimeras (PROTACs) selectively targeting tumor tissues. J Med Chem. 2022;65(6):5057–71.
Do TC, Lau JW, Sun C, Liu S, Kha KT, Lim ST, Oon YY, Kwan YP, Ma JJ, Mu Y, Liu X, Carney TJ, Wang X, Xing B. Hypoxia deactivates epigenetic feedbacks via enzyme-derived clicking proteolysis-targeting chimeras. Sci Adv. 2022;8(50):eabq2216.
Cheng W, Li S, Han S, Miao R, Wang S, Liu C, Wei H, Tian X, Zhang X. Design, synthesis and biological evaluation of the tumor hypoxia-activated PROTACs bearing caged CRBN E3 ligase ligands. Bioorg Med Chem. 2023;82:117237.
Xie B, Xu B, Xin L, Wei Y, Guo X, Dong C. Discovery of estrogen receptor α targeting caged hypoxia-responsive PROTACs with an inherent bicyclic skeleton for breast cancer treatment. Bioorg Chem. 2023;137:106590.
Yu H, Jin F, Liu D, Shu G, Wang X, Qi J, Sun M, Yang P, Jiang S, Ying X, Du Y. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10(5):2342–57.
Tao W, He Z. ROS-responsive drug delivery systems for biomedical applications. Asian J Pharm Sci. 2018;13(2):101–12.
Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3(3):205–14.
Liu H, Ren C, Sun R, Wang H, Zhan Y, Yang X, Jiang B, Chen H. Reactive oxygen species-responsive pre-PROTAC for tumor-specific protein degradation. Chem Commun (Camb). 2022;58(72):10072–5.
Yu D, Fan H, Zhou Z, Zhang Y, Sun J, Wang L, Jia Y, Tian J, Campbell A, Mi W, Sun H. Hydrogen peroxide-inducible PROTACs for targeted protein degradation in cancer cells. ChemBioChem. 2023;24(17):e202300422.
Teicher BA, Morris J. Antibody-drug conjugate targets, drugs, and linkers. Curr Cancer Drug Targets. 2022;22(6):463–529.
Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93.
Jin Y, Schladetsch MA, Huang X, Balunas MJ, Wiemer AJ. Stepping forward in antibody-drug conjugate development. Pharmacol Ther. 2022;229:107917.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494–503.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46.
Pillow TH, Adhikari P, Blake RA, Chen J, Del Rosario G, Deshmukh G, Figueroa I, Gascoigne KE, Kamath AV, Kaufman S, Kleinheinz T, Kozak KR, Latifi B, Leipold DD, Sing-Li C, Li R, Mulvihill MM, O’Donohue A, Rowntree RK, Sadowsky JD, Wai J, Wang X, Wu C, Xu Z, Yao H, Yu SF, Zhang D, Zang R, Zhang H, Zhou H, Zhu X, Dragovich PS. Antibody conjugation of a chimeric bet degrader enables in vivo activity. Chem Med Chem. 2020;15(1):17–25.
Maneiro MA, Forte N, Shchepinova MM, Kounde CS, Chudasama V, Baker JR, Tate EW. Antibody-PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15(6):1306–12.
Dragovich PS, Pillow TH, Blake RA, Sadowsky JD, Adaligil E, Adhikari P, Bhakta S, Blaquiere N, Chen J, Dela Cruz-Chuh J, Gascoigne KE, Hartman SJ, He M, Kaufman S, Kleinheinz T, Kozak KR, Liu L, Liu L, Liu Q, Lu Y, Meng F, Mulvihill MM, O’Donohue A, Rowntree RK, Staben LR, Staben ST, Wai J, Wang J, Wei B, Wilson C, Xin J, Xu Z, Yao H, Zhang D, Zhang H, Zhou H, Zhu X. Antibody-mediated delivery of chimeric BRD4 degraders. Part 1: Exploration of antibody linker, payload loading, and payload molecular properties. J Med Chem. 2021;64(5):2534–75.
Dragovich PS, Pillow TH, Blake RA, Sadowsky JD, Adaligil E, Adhikari P, Chen J, Corr N, Dela Cruz-Chuh J, Del Rosario G, Fullerton A, Hartman SJ, Jiang F, Kaufman S, Kleinheinz T, Kozak KR, Liu L, Lu Y, Mulvihill MM, Murray JM, O’Donohue A, Rowntree RK, Sawyer WS, Staben LR, Wai J, Wang J, Wei B, Wei W, Xu Z, Yao H, Yu SF, Zhang D, Zhang H, Zhang S, Zhao Y, Zhou H, Zhu X. Antibody-mediated delivery of chimeric BRD4 degraders Part 2: Improvement of in vitro antiproliferation activity and in vivo antitumor efficacy. J Med Chem. 2021;64(5):2576–607.
Vartak R, Deore B, Sanhueza CA, Patel K. Cetuximab-based PROteolysis targeting chimera for effectual downregulation of NSCLC with varied EGFR mutations. Int J Biol Macromol. 2023;252:126413.
Dragovich PS, Adhikari P, Blake RA, Blaquiere N, Chen J, Cheng YX, Den Besten W, Han J, Hartman SJ, He J, He M, Rei Ingalla E, Kamath AV, Kleinheinz T, Lai T, Leipold DD, Li CS, Liu Q, Lu J, Lu Y, Meng F, Meng L, Ng C, Peng K, Lewis-Phillips G, Pillow TH, Rowntree RK, Sadowsky JD, Sampath D, Staben L, Staben ST, Wai J, Wan K, Wang X, Wei B, Wertz IE, Xin J, Xu K, Yao H, Zang R, Zhang D, Zhou H, Zhao Y. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα). Bioorg Med Chem Lett. 2020;30(4):126907.
Chan K, Sathyamurthi PS, Queisser MA, Mullin M, Shrives H, Coe DM, Burley GA. Antibody-proteolysis targeting chimera conjugate enables selective degradation of receptor-interacting serine/threonine-protein kinase 2 in HER2+ cell lines. Bioconjug Chem. 2023;34(11):2049–54.
Sang X, Zhang Y, Fang F, Gao L, Tao Y, Li X, Zhang Z, Wang J, Tian Y, Li Z, Yao D, Wu Y, Chu X, Zhang K, Ma L, Lu L, Chen Y, Yu J, Zhuo R, Wu S, Zhang Z, Pan J, Hu S. BRD4 inhibitor GNE-987 exerts anticancer effects by targeting super-enhancer-related gene LYL1 in acute myeloid leukemia. J Immunol Res. 2022;2022:7912484.
Nimjee SM, White RR, Becker RC, Sullenger BA. Aptamers as therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:61–79.
Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86(3):151–64.
Zhu G, Niu G, Chen X. Aptamer-drug conjugates. Bioconjug Chem. 2015;26(11):2186–97.
He S, Gao F, Ma J, Ma H, Dong G, Sheng C. Aptamer-PROTAC conjugates (APCs) for tumor-specific targeting in breast cancer. Angew Chem Int Ed Engl. 2021;60(43):23299–305.
Zhang L, Li L, Wang X, Liu H, Zhang Y, Xie T, Zhang H, Li X, Peng T, Sun X, Dai J, Liu J, Wu W, Ye M, Tan W. Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin. Mol Ther Nucleic Acids. 2022;30:66–79.
Chen M, Zhou P, Kong Y, Li J, Li Y, Zhang Y, Ran J, Zhou J, Chen Y, Xie S. Inducible degradation of oncogenic nucleolin using an aptamer-based PROTAC. J Med Chem. 2023;66(2):1339–48.
Shih PC, Naganuma M, Tsuji G, Demizu Y, Naito M. Development of decoy oligonucleotide-warheaded chimeric molecules targeting STAT3. Bioorg Med Chem. 2023;95:117507.
Yang K, Yang Z, Yu G, Nie Z, Wang R, Chen X. Polyprodrug nanomedicines: An emerging paradigm for cancer therapy. Adv Mater. 2022;34(6):e2107434.
Li Z, Xiao C, Yong T, Li Z, Gan L, Yang X. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem Soc Rev. 2020;49(8):2273–90.
Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64.
Liang C, Xu L, Song G, Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: Animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem Soc Rev. 2016;45(22):6250–69.
Yan WL, Lang TQ, Yuan WH, Yin Q, Li YP. Nanosized drug delivery systems modulate the immunosuppressive microenvironment to improve cancer immunotherapy. Acta Pharmacol Sin. 2022;43(12):3045–54.
Lv L, Shi Y, Wu J, Li G. Nanosized drug delivery systems for breast cancer stem cell targeting. Int J Nanomedicine. 2021;16:1487–508.
Gao J, Wang WQ, Pei Q, Lord MS, Yu HJ. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol Sin. 2020;41(7):986–94.
Moon Y, Jeon SI, Shim MK, Kim K. Cancer-specific delivery of proteolysis-targeting chimeras (PROTACs) and their application to cancer immunotherapy. Pharmaceutics. 2023;15(2):411.
Gao J, Hou B, Zhu Q, Yang L, Jiang X, Zou Z, Li X, Xu T, Zheng M, Chen YH, Xu Z, Xu H, Yu H. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumour-specific protein degradation and precise cancer therapy. Nat Commun. 2022;13(1):4318.
Xie Z, Fan T, An J, Choi W, Duo Y, Ge Y, Zhang B, Nie G, Xie N, Zheng T, Chen Y, Zhang H, Kim JS. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev. 2020;49(22):8065–87.
Wu D, Yang K, Zhang Z, Feng Y, Rao L, Chen X, Yu G. Metal-free bioorthogonal click chemistry in cancer theranostics. Chem Soc Rev. 2022;51(4):1336–76.
Zhang C, Zeng Z, Cui D, He S, Jiang Y, Li J, Huang J, Pu K. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat Commun. 2021;12(1):2934.
Zhang C, He S, Zeng Z, Cheng P, Pu K. Smart Nano-PROTACs reprogram tumor microenvironment for activatable photo-metabolic cancer immunotherapy. Angew Chem Int Ed Engl. 2022;61(8):e202114957.
Zhang C, Xu M, He S, Huang J, Xu C, Pu K. Checkpoint nano-PROTACs for activatable cancer photo-immunotherapy. Adv Mater. 2023;35(6):e2208553.
Wang W, Zhu C, Zhang B, Feng Y, Zhang Y, Li J. Self-assembled nano-PROTAC enables near-infrared photodynamic proteolysis for cancer therapy. J Am Chem Soc. 2023;145(30):16642–9.
He Q, Zhou L, Yu D, Zhu R, Chen Y, Song M, Liu X, Liao Y, Ding T, Fan W, Yu W. Near-infrared-activatable PROTAC nanocages for controllable target protein degradation and on-demand antitumor therapy. J Med Chem. 2023;66(15):10458–72.
Yao L, Yang N, Zhou W, Akhtar MH, Zhou W, Liu C, Song S, Li Y, Han W, Yu C. Exploiting cancer vulnerabilities by blocking of the DHODH and GPX4 pathways: A multifunctional bodipy/PROTAC Nanoplatform for the efficient synergistic ferroptosis therapy. Adv Healthc Mater. 2023;12(26):e2300871.
Zhao LP, Rao XN, Zheng RR, Huang CY, Kong RJ, Cheng H, Li B, Li SY. Carrier-free nano-PROTACs to amplify photodynamic therapy induced DNA damage through BRD4 degradation. Nano Lett. 2023;23(13):6193–201.
Hu Z, Li R, Cui X, Hu C, Chen Z. Tailoring albumin-based theranostic PROTACs nanoparticles for enhanced NIR-II bioimaging and synergistic cancer chemo-phototherapy. Chem Eng J. 2023;469:143883.
Wang T, Zhang Y, Chen K, Huang Y, Liu Y, Xu S, Wang W. CDK4/6 nano-PROTAC enhances mitochondria-dependent photodynamic therapy and anti-tumor immunity. Nano Today. 2023;50:101890.
Liu H, Chen C, Chen H, Mo L, Guo Z, Ye B, Liu Z. 2D-PROTACs with augmented protein degradation for super-resolution photothermal optical coherence tomography guided momentary multimodal therapy. Chem Eng J. 2022;446:137039.
Article Google Scholar
Liu HJ, Chen W, Wu G, Zhou J, Liu C, Tang Z, Huang X, Gao J, Xiao Y, Kong N, Joshi N, Cao Y, Abdi R, Tao W. Glutathione-scavenging nanoparticle-mediated PROTACs delivery for targeted protein degradation and amplified antitumor effects. Adv Sci (Weinh). 2023;10(16):e2207439.
Olson OC, Joyce JA. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15(12):712–29.
Lei G, Mao C, Yan Y, Zhuang L, Gan B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 2021;12(11):836–57.
Xu H, Ye D, Ren M, Zhang H, Bi F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol Med. 2021;27(9):856–67.
Yang F, Xiao Y, Ding JH, Jin X, Ma D, Li DQ, Shi JX, Huang W, Wang YP, Jiang YZ, Shao ZM. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35(1):84-100.e8.
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, Poyurovsky MV, Olszewski K, Gan B. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586–90.
Xu L, Liu Y, Chen X, Zhong H, Wang Y. Ferroptosis in life: To be or not to be. Biomed Pharmacother. 2023;159: 114241.
Download references
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82303590), the Natural Science Foundation of Shandong (ZR2021QH156), the Natural Science Foundation of Qingdao (23-2-1-141-zyyd-jch), the Youth Innovation Team Development Program of Shandong Province (2023KJ227), the China Postdoctoral Science Foundation (2023M741867), and the Qingdao Postdoctoral Application Project (QDBSH20230202076)
This is a review article; and there is no financial support for this work.
Author information
Authors and affiliations.
Cancer Institute, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, 266071, Shandong, China
Chao Wang, Wujun Chen, Yudong Wu & Dongming Xing
The Affiliated Cardiovascular Hospital of Qingdao University, Qingdao University, Qingdao, 266071, Shandong, China
Yujing Zhang
School of Life Sciences, Tsinghua University, Beijing, 100084, China
Dongming Xing
You can also search for this author in PubMed Google Scholar
Contributions
CW and DX presented the conceived idea of the paper. CW and YZ downloaded the material and wrote the paper. WC and YW prepared the figures and proofread the paper. All authors contributed and approved the submitted version.
Corresponding authors
Correspondence to Yujing Zhang , Yudong Wu or Dongming Xing .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, C., Zhang, Y., Chen, W. et al. New-generation advanced PROTACs as potential therapeutic agents in cancer therapy. Mol Cancer 23 , 110 (2024). https://doi.org/10.1186/s12943-024-02024-9
Download citation
Received : 20 February 2024
Accepted : 10 May 2024
Published : 21 May 2024
DOI : https://doi.org/10.1186/s12943-024-02024-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- New-generation PROTACs
- Nanomedicine
- Precise protein degradation
- Cancer therapy
Molecular Cancer
ISSN: 1476-4598
- General enquiries: [email protected]

- Liberty University
- Jerry Falwell Library
- Special Collections
- < Previous Event
- Next Event >
Home > Conferences and Events > Research Week > 2024 > Oral Presentations > 53

Oral Presentations
Sensory Room Design to Prevent Overstimulation in Those with Sensory Processing Disorders
Presenter Information
Mary Schmidt , Liberty University Follow
Oral - Creative and Artistic
Description
Sensory rooms are invaluable resources in assisting people with sensory processing disorders by providing a relaxing escape from the regular stimulation of the world. Designers have an imperative role in creating these rooms specifically for people with these disorders. Well-designed sensory environments have the capability to promote relaxation, reduce stress and anxiety, and improve emotional regulation. This research presentation aims to propose a design solution for a sensory room catered toward college students with sensory processing disorders, especially those that are on the autism spectrum. After touring preexisting sensory spaces and conducting a comprehensive review of literature on sensory environments and sensory processing disorders, the proposed design solution identifies key design considerations for developing an inclusive and effective sensory space. The conclusion reached through this research emphasizes the need for sensory rooms to gently engage all the human senses while being careful to avoid overstimulating the brain. Sensory spaces should be limited to a low number of occupants at one given time to prevent overstimulation and sensory overload. Sensory rooms should also provide a variety of options for engaging the senses in order to appeal to a wide range of sensory preferences. Successful sensory spaces often include low lighting, sound modulation, tactile surfaces, comfortable seating, and a flexible layout that allow users to customize the environment according to their needs. Additionally, this research presentation addresses the possible implementation of a sensory room at Liberty University in the Office of Disability and Accommodation Support within a specific allotted space and budget.
Undergraduate
Since May 20, 2024
To view the content in your browser, please download Adobe Reader or, alternately, you may Download the file to your hard drive.
NOTE: The latest versions of Adobe Reader do not support viewing PDF files within Firefox on Mac OS and if you are using a modern (Intel) Mac, there is no official plugin for viewing PDF files within the browser window.
- Collections
- Faculty Expert Gallery
- Theses and Dissertations
- Conferences and Events
- Open Educational Resources (OER)
- Explore Disciplines
Advanced Search
- Notify me via email or RSS .
Faculty Authors
- Submit Event
- Expert Gallery Login
Student Authors
- Undergraduate Submissions
- Graduate Submissions
- Honors Submissions
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright

Beaty: Finding harmony in the shark-seal debate
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Entertainment

The ecological narrative begins with the gray seal, an apex predator that has made a remarkable comeback after centuries of overhunting and habitat degradation. Their resurgence is a testament to conservation efforts and a reminder of the importance of preserving marine biodiversity. However, this success story has also led to an increase in great white shark sightings, as these apex predators follow their primary food source. This migration pattern has raised concerns among beachgoers and local businesses, fearing the impact on tourism and public safety.
The economic dimension of this issue is equally pressing. Cape Cod’s economy relies heavily on tourism, with beachgoers and water sports enthusiasts flocking to the area each summer. The presence of sharks has led to beach closures and restrictions, potentially jeopardizing the livelihoods of local business owners and employees. Furthermore, the fishing industry, a significant contributor to the local economy, is also affected by the growing seal population, as they compete for fish and shellfish resources.
Beyond ecological and economic considerations, the shark-seal debate raises important social questions. How do we balance human interests with marine conservation? Can we find a middle ground that satisfies both beachgoers and conservationists? The answers lie in innovative solutions that prioritize coexistence and sustainability.
One such solution is the implementation of shark mitigation strategies, such as shark spotters and warning systems, to minimize encounters and attacks. Additionally, public education campaigns can raise awareness about shark behavior and promote responsible wildlife viewing practices. Ecotourism initiatives, focusing on responsible wildlife viewing and marine conservation, can also support local economies while promoting coexistence with marine wildlife.
Another crucial aspect is the need for continued research and monitoring of shark and seal populations. By better understanding their behavior, migration patterns, and ecological roles, we can inform effective management decisions and ensure the long-term conservation of these species.
In conclusion, the Cape Cod conundrum presents a complex web of ecological, economic, and social challenges. By embracing a holistic approach that prioritizes coexistence, sustainability, and innovation, we can find harmony in the shark-seal debate. Through collaborative efforts and a willingness to adapt, we can ensure the preservation of Cape Cod’s marine ecosystem for future generations, while also supporting the local economy and promoting public safety.
Ronald Beaty is a former Barnstable County Commissioner and a lifelong Cape Cod resident.
More in Opinion

Editorial: Somber reminders put prom-goers in the driver’s seat
Saint-louis: the critical need for youth civic engagement.

Ask the Psychic: Signs of a spirit’s presence and how to communicate

SUBSCRIBER ONLY
Editorial: leominster’s flood aid a testament to its dogged efforts.
- Open access
- Published: 14 May 2024
Objective response after immune checkpoint inhibitors in a chemotherapy-refractory pMMR/MSS metastatic rectal cancer patient primed with experimental AlloStim® immunotherapy
- Ariel Hirschfeld 1 ,
- Daniel Gurell 2 &
- Michael Har-Noy ORCID: orcid.org/0000-0002-9342-9337 3
Translational Medicine Communications volume 9 , Article number: 15 ( 2024 ) Cite this article
175 Accesses
Metrics details
Immune Checkpoint Inhibitor (ICI) immunotherapy is most effective in immune effector cell infiltrated ‘hot’ tumor lesions, such as occurs in deficient mismatch repair, microsatellite instability high (dMMR/MSI-H) colorectal cancer (CRC). However, most all metastatic CRC tumors are mismatch repair proficient/microsatellite stable (pMMR/MSS) ‘cold’ lesions, without significant immune cell infiltration, and are unresponsive to ICI. AlloStim®, is an experimental, allogeneic immunomodulatory cell therapy designed to convert ‘cold’ metastatic tumor lesions to ‘hot’ inflamed lesions. After AlloStim® immunotherapy, this cold to hot inflammatory mechanism can make it difficult to distinguish between pseudoprogression and actual progression on restaging CT scans, as inflamed metastatic lesions can appear larger and occult disease can appear as new small lesions.
To explore whether radiological progression after AlloStim® immunotherapy is due to immune-flare or disease progression, we administered a short course of a combination ICI therapy to a pMMR/MSS chemotherapy-refractory metastatic colorectal cancer patient enrolled in the StimVax Phase IIb clinical study that presented with radiological progression after AlloStim® immunotherapy. Our rationale was that an accelerated response to ICI should occur if the lesions were inflamed, while if the enlarged lesions were due to disease progression there would not be a response.
Here we report a rapid, significant reduction in tumor burden in response to ICI administration in an AlloStim® primed pMMR/MSS mCRC patient with retroperitoneal and lung metastases.
This rare objective response to ICIs in a pMMR/MSS mCRC patient supports further evaluation of the combination of AlloStim® with ICI immunotherapy in MSS mCRC and other cold or ICI refractory tumors.
Trial registration
National Library of Medicine (NLM) at the National Institutes of Health (NIH). Registered 22 June 2020, https://clinicaltrials.gov/study/NCT04444622 .
Immune checkpoint inhibitor (ICI)-based regimens have not yet shown meaningful positive outcomes in proficient DNA mismatch repair/microsatellite stable (pMMR/MSS) metastatic colorectal cancers (mCRC). Here we report a rare objective response in a pMMR/MSS heavily pre-treated metastatic colorectal cancer (mCRC) patient subsequent to a short course of an immune checkpoint inhibitor (ICI) combination after first being primed with an experimental immunomodulatory cell therapy drug, AlloStim®, designed to convert immunologically ‘cold’ tumors to ‘hot’ tumors, and a short, low dose course of regorafenib.
The FDA has approved ICI drugs targeting CTLA-4, PD-1, PD-L1 and LAG-3 checkpoint molecules for a variety of solid tumor indications, including melanoma, renal, bladder, lung, gastric, gastroesophageal junction, hepatocellular carcinoma and head and neck cancers. However, ICIs have demonstrated only limited efficacy in mCRC.
An anti-CTLA4 ICI, pembrolizumab, was approved in first line mCRC [ 1 ] and also approved in combination with the anti-PD-1 ICI, nivolumab, for a subset of mCRC patients that have deficient DNA mismatch repair/microsatellite instability-high (dMMR/MSI-H) status [ 2 , 3 , 4 , 5 , 6 , 7 , 8 ]. However, this dMMR/MSI-H subset constitutes only ~ 5% of mCRC patients [ 9 ], while the remaining ~ 95% that present with pMMR/MSS status do not respond to ICI [ 6 , 8 , 10 , 11 , 12 , 13 ].
Most dMMR/MSI-H status tumors are considered immunologically ‘hot’ tumors, while pMMR/MSS status tumors are considered to be ‘cold’ [ 14 ]. ICI have demonstrated greater efficacy in hot tumors, characterized by an inflamed phenotype, including a high level of infiltrating T-cells and NK cells, an interferon-γ (IFN-γ) signature and upregulated PD-L1 expression, while cold tumors have an absence of tumor-infiltrating lymphocytes [ 15 ].
While dMMR/MSI-H mCRC patients are more responsive to ICI therapy, approximately 50% are refractory [ 16 , 17 , 18 ]. Resistance to ICI responsiveness, regardless of MMR/MSI status, is correlated with tumor mutational burden (TMB) [ 19 ]. Since somatic mutations can encode immunogenic neoantigens, high TMB is believed to be more likely to prime for infiltrating tumor-specific effector immune cells. Consistent with this, the dMMR/MSI-H patients that present with lower TMB values have been shown to be the non-responders, whereas patients with the highest TMB values tend to obtain benefit from ICI [ 16 ], particularly with anti-CTLA-4/PD-1 combination ICI immunotherapy [ 20 ].
Present strategies for increasing the effectiveness of ICI immunotherapy in pMMR/MSS mCRC cold tumors include evaluating combinations with other therapeutic methods, such as chemotherapy, targeted therapy, and radiotherapy [ 21 ]. In addition, strategies to convert immunologically cold tumors to hot tumors are thought to be a fruitful line of investigation for increasing ICI efficacy in ICI refractory tumors [ 22 , 23 ].
AlloStim® is an experimental cellular immunotherapy drug designed to convert immunologically cold tumors to hot tumors by mirroring the graft vs. tumor (GVT) mechanism of allogeneic stem cell transplant procedures to create a host vs tumor (HVT) effect without graft vs. host disease (GVHD) toxicity [ 24 ]. AlloStim® is currently being evaluated as a monotherapy in the STIMVAX Phase IIB open label clinical trial in third-line chemotherapy-refractory pMMR/MSS mCRC (NCT04444622). The primary end-point in this study is overall survival (OS) and an exploratory end-point is objective tumor response by RECIST 1.1.
AlloStim® is a living, allogeneic (“off-the-shelf”), non-genetically manipulated, activated Th1-like immune cell therapy derived from CD4 + T-cell precursors isolated from the blood of healthy donors. The STIMVAX protocol provides for three monthly cycles of weekly AlloStim® administration (intradermal and intravenous) designed to increase circulating memory Th1/Th2 ratio [ 25 ], activate circulating memory T cells and NK cells, which in turn causes their extravasation to tumor sites [ 24 ]. The anti-tumor effects are correlated with the establishment of an IFN-γ dominated microenvironment [ 26 ]. The systemic tumor infiltration mechanism serves to convert immunologically cold tumors to hot tumors. The modulation of the tumor microenvironment (TME) can also counter-regulate tumor-mediated immune suppression [ 27 ].
AlloStim®-mediated intratumoral type I cytokine production by infiltrating activated T-cells and NK cells, including IL-12 and IFN-γ, is believed to: upregulate MHC-I on tumor cells making them susceptible to CD8 + T-cell recognition; cause maturation of dendritic cells to DC1 (IL-12 + CD80/86 positive, MHC I and MHC II positive); convert M2 macrophages to M1 [ 28 ]; and, release neoantigens into the TME [ 29 , 30 ]. The release of neoantigens into an inflammatory TME creates the conditions for in-situ vaccination [ 31 ] where immature dendritic cells mature to type I dendritic cells (DC1), process the released chaperoned neoantigens, migrate to the draining lymph nodes, resulting in a patient-specific anti-tumor adaptive immune response [ 32 ].
In previous clinical studies, the inflammatory mechanism of AlloStim® almost always caused post-treatment CT scan images to be read as progressive disease (PD), with systemic increases in size of existing tumor lesions and often the appearance of new small lesions (especially in lungs). However, this PD determination did not always correlate with the clinical status of the patient or with overall survival (OS). As the systemic increase in target lesion size could be due to peritumoral inflammation that occurs when tumor lesions convert from cold to hot and new small lesions could be the result of inflammation of occult disease, it is difficult to distinguish tumor progression from pseudoprogression using CT scan imaging after experimental AlloStim® experimental treatment.
Pseudoprogression after immunotherapy has been observed in patients with various tumor types and is thought to be due to transient immune cell infiltration into the tumor [ 33 ]. The phenomenon of pseudoprogression has led to modification of the RECIST 1.1 evaluation criteria [ 34 ]. The understanding that tumor growth by RECIST does not necessarily translate to disease progression in patients treated with immunotherapy has also led to the development of immune-related response criteria (irRC) to better surveil these patients [ 35 , 36 ].
However, it is still considered challenging to distinguish radiological progression from pseudoprogression and, consequently, to define the best management for these patients. In additional, a new category of “hyper-progression” and dissociated atypical responses have also been described after immunotherapy [ 37 ]. These issues have resulted in some subjects being prematurely removed from immunotherapy clinical trials [ 38 ].
In the CheckMate 142 clinical trial, nivolumab (3 mg/kg) plus low-dose ipilimumab (1 mg/kg) provided durable clinical benefit, and a manageable safety profile in patients with previously treated dMMR/MSI-H metastatic CRC [ 39 ]. Due to the improved safety profile of the lower dose ipilimumab in this combination, we decided to test this regimen in a chemotherapy-refractory mCRC pMMR/MSS patient presenting with radiological progression after 3 cycles of AlloStim® experimental immunotherapy.
We hypothesized that if the radiological progression was due to inflammation within the tumor lesions (hot tumors), a short course of ICI immunotherapy should result in a rapid reduction in tumor burden, due to release of suppression of resident infiltrating effector immune cells. On the other hand, if the enlarged and increased number of lesions were due to true progression and no infiltrating effector immune cells were present, either no response or further progression would be expected to be observed.
Case presentation
A 69 yo Caucasian male presented in March 2011 with blood in the stool. The patient’s medical and social history was significant for type 2 diabetes, hypertension, hypercholesterolemia, atrial fibrillation (s/p ablation) and was a former 2 pack/day smoker. Upon workup, was found to have a rectal mass and subsequently underwent low anterior resection (LAR). 2 of 24 nodes were found to be positive for adenocarcinoma disease with initial staging of pT2N1b. The patient was treated with adjuvant FOLFOX and achieved a complete response (CR). In August 2014, disease recurred and he underwent transanal resection plus radiation therapy (XRT). In April 2016, a right inguinal node was identified as metastatic adenocarcinoma and he received additional XRT. In January 2022, he presented with enlarged retroperitoneal nodes and was treated with 22 cycles of FOLFIRI plus Avastin from January 2022 to November 2022. In January 2023, was treated again with FOLFOX, but developed a reaction to oxaliplatin.
CT scan on April 26, 2023 showed appearance of innumerable bilateral pleural parenchymal lung nodules significantly increased in size and number from prior examination on March 8, 2023 and significant worsening of mediastinal and hilar lymphadenopathy consistent with progressive metastatic disease. No suspicious liver lesions were found and stable non-specific retroperitoneal adenopathy was also noted. Target lesions were identified in the lungs. The retroperitoneal disease was too small (< 15 mm) to be included in the RECIST 1.1 evaluation.
Patient consented on April 21, 2023 to received AlloStim® experimental immunotherapy as part of the STIMVAX Phase IIB clinical trial (NCT04444622). Eligible patients had histologically confirmed pMMR/MSS adenocarcinoma of the colon or rectum; received all available standard systemic therapies (fluoropyrimidines, oxaliplatin, irinotecan, and bevacizumab; cetuximab or panitumumab if RAS wild-type tumors); were aged 18 years or older; had adequate organ function; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and measurable disease. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines after approval by a central institutional review board (IRB) and the ethics board at each institution where applicable.
The dosing and procedure schedule is shown in Table 1 and longitudinal changes in lung target lesions from CT scans are shown in Fig. 1 . Three 28-day cycles of weekly AlloStim® immunotherapy were administered per protocol from May 9, 2023 to September 26, 2023. The CT scan comparison from baseline (April 26, 2023) to completion of the AlloStim® three cycles (October 10, 2023) demonstrated progressive disease (PD) by RECIST 1.1 evaluating target lesions in the lungs and retroperitoneum (sum of diameters of target lesions = + 73%) with increased non-target retroperitoneal adenopathy and innumerable enlarging and new thoracic nodules.
Matched CT scans and biopsies of liver target lesions in two MSS mCRC subjects at baseline and at day 84 after 3 AlloStim® cycles. Subject #1 shows extensive tumor (red circle) on the periphery of areas of fibrosis without immune cell infiltration at baseline. The corresponding CT scan shows the biopsied target tumor in liver After 3 cycles of AlloStim®, the re-staging CT scan on day 84 shows progressive disease. However, the corresponding biopsies show areas of coagulative necrosis and immune cell infiltration. Subject #2 has almost completely solid tumor at baseline. The re-staging CT scan indicates progressive disease, however note the extensive peri-tumoral inflammation. The corresponding biopsy indicates large area of tumor necrosis and tumor admixed with immune cells
From October 11, 2023 to October 23, 2023, a short, reduced dose of regorafenib was administered followed by a short course of nivolumab (240 mg) and low dose ipilimumab (1 mg/kg) administered between October 23, 2023 and December 4, 2023. No treatment was administered from December 12, 2023 and February 8, 2024 during which time a tapering dose of oral prednisone beginning at 60 mg/day was administered for treatment of colitis (adverse effect from the combination ICI immunotherapy), a restaging CT scan was then obtained on February 8, 2024.
The February 8, 2024 scan demonstrated a partial response (PR) by RECIST 1.1 criteria in comparison to the post-AlloStim® scan on October 10, 2023 (change in sum of diameters of target lesions = -48%) with the previously observed innumerable non-target thoracic lesions and retroperitoneal adenopathy uniformly decreased in size and number. Comparison to the April 26, 2023 baseline scan was read as stable disease (SD) with a 10% decrease in sum of diameters of target lesions (see Fig. 2 ).

Longitudinal changes in two lung target lesions (orange arrows). The slices are adjusted to show the view with the measurement in the longest tumor diameter. Red circles indicate presence or absence of non-target lesions in the selected slices. An increase in size of non-target existing lesions and appearance of new non-target lesions seen on post-AlloStim October 10, 2023 compared to April 26, 2023 baseline. Elimination or reduction in size of the non-target lesions seen in the post-ICI February 8, 2024 scan. According to RECIST 1.1, the October 10, 2023 scan compared to April 26, 2023 is scored as PD. The February 8, 2024 scan compared to October 10, 2023 is scored as PR. The February 8, 2024 compared to April 26, 2023 is scored as SD.
Serum levels of IL-12 levels were negligible prior to AlloStim® administration. After AlloStim® dosing, IL-12 became detectable after the first cycle and remained detectible over the 3 cycles of AlloStim® administration. Soluble heat shock protein (HSP)-70 was also negligible at baseline but was elevated throughout the experimental immunotherapy dosing (see Fig. 3 ).

IL-12 and HSP70 serum levels during allostim administration. Whole blood samples were collected longitudinally from subjects in SST Tiger Top tubes. The tubes were spun at 3000 rpm and shipped overnight at 2-80C to the central lab facility where the serum was aseptically transferred to cryotubes and stored at -800C until analysis. For analysis, samples were diluted 1:2 and plated in triplicate on ELISA plates (R&D Systems) and incubated for 2-3h at RT. The plates were read on a Cytation 7 plate reader (Agilent BioTek) at 650nm absorbance. A standard curve was generated using known samples. Quantitative levels were determined by comparing absorbance values to the standard curve. The bar graph shows the mean +/- SE at each sample timepoint
Here we present a rare case of rapid tumor debulking response subsequent to a short course of combination ICI immunotherapy in a heavily pre-treated pMMR/MSS mCRC patient presenting with radiological progression after third-line experimental AlloStim® immunotherapy and a short course of low dose regorafenib. Here we consider the question whether the ICI immunotherapy alone or in combination with previous AlloStim®, or in combination with previous regorafenib alone, or with prior AlloStim® and regorafenib together was most likely responsible for eliciting this rare objective response in a cold tumor indication.
It seems unlikely that the combination ICI immunotherapy could be solely responsible for the observed response. ICI-based regimens both as monotherapy [ 40 , 41 ] or as combination therapies [ 42 ] have not previously shown any meaningful positive outcomes in pMMR/MSS colorectal cancers.
For example, an initial phase II study assessed the efficacy of tremelimumab, a monoclonal antibody against CTLA4, in patients with treatment-refractory mCRC, which resulted in no improvement post-treatment [ 43 ]. Furthermore, two phase I studies of anti-PD-1 [ 44 ] and anti-PD-L1 [ 45 ] in previously-treated mCRC patients produced no responses. ICI regimens also failed as maintenance therapy after first line therapy in the MODUL study [ 46 ].
In general, ICI immunotherapy combining CTLA-4 and PD-L1 inhibitors have also shown very limited clinical benefit in patients with non-selected mCRC. A rare partial response (PR) (1/119) was reported in a randomized phase 2 clinical trial which evaluated the efficacy of combination durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) in patients with advanced refractory mCRC. In this study, 119 patients were assigned to the treatment group and 61 patients were assigned to best supportive care (BSC) alone. Patients in the treatment group received a median of 12 weeks of durvalumab and 12 weeks of tremelimumab [ 47 ], while in the present case only 5 weeks of ICI combination therapy was administered.
The phase II KEYNOTE-016 trial was performed to evaluate the clinical efficacy of single agent pembrolizumab in patients with pMMR/MSS mCRC, dMMR/MSI-H mCRC and or dMMR/MSI-H non-CRC. No responses were noted in 18 patients in the pMMR/MSS mCRC group [ 48 ]. In a clinical study which included 59 pMMR/MSS mCRC patients treated with ICI beyond radiological progression by RECIST 1.1, no patient demonstrated subsequent radiographical tumor shrinkage at a median of 42 days [ 49 ].
It has been reported that a small subset (~ 2%) of patients with pMMR/MSS colorectal cancer with a mutation in POLE and POLD1 enzymes and those without liver metastases have a higher chance of a response to ICI immunotherapy [ 50 ]. While the patient in the present case did not have POLE or POLD1 mutations, no liver metastases were present. Therefore, it is possible this patient was more susceptible to ICI immunotherapy, but seems unlikely that the short course of combination ICI alone was solely responsible for the extensive tumor debulking observed.
ICI strategies in combination with other drugs or procedures are under investigation, including evaluations of ICI in combinations with chemotherapy, radiotherapy, vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) inhibitors, mitogen-activated protein kinase (MEK) inhibitors, and signal transducer and activation of transcription 3 (STAT3) inhibitors [ 51 ]. However, these combination approaches have yet to demonstrate any significant anti-tumor activity in the clinical setting [ 12 , 52 , 53 ].
Could the short course of regorafenib alone or in combination with ICI immunotherapy be responsible for the objective tumor response observed in this case?
Regorafenib is approved for third-line mCRC based on the results of the CORRECT trial which demonstrated only a 1.4 month increase in the median survival compared to a placebo control (6.4 months vs. 5.0 months) [ 54 ]. In the Phase II TEXCAN trial, no objective responses were reported in 35 mCRC patients after 2 months of treatment with regorafenib according to RECIST 1.1, Choi, and modified Choi [ 55 ]. Therefore, it seems unlikely that the prior short course of low dose regorafenib alone could be responsible for the rare objective response reported here.
Regorafenib is a multi-kinase inhibitor that targets several receptor tyrosine kinases involved in angiogenesis and metastases (VEGFR1, VEGFR2, VEGFR3, FGFR1, FGFR2, TIE2, PDGFRs), oncogenesis (KIT, RET, RAF1), and tumor immunity (CSF1R). While regorafenib does not directly convert cold tumors to hot tumors, regorafenib is believed to possibly contribute to shifting the tumor microenvironment toward a more immune-responsive state. This constellation of mechanisms suggests that regorafenib could potentially be a combination partner for ICIs [ 56 ].
There are mixed results on the combination of regorafenib with ICI in clinical trials. Regorafenib in combination with PD‐1 antibody as a third‐line mCRC therapy has been evaluated in several studies. For example, 24 patients with MSS mCRC were included in the REGONIVO study. In this study, regorafenib was administered at 80–160 mg once daily for 21 days on and 7 days off together with nivolumab at 3 mg/kg every 2 weeks. A 33.3% objective response rate was reported with this regimen [ 57 ]. However, this promising activity has not been observed in other studies.
In a single site study, 18 mCRC patients treated with a combination of regorafenib and nivolumab, no objective responses were observed. In this study, 13 patients (69%) had progressive disease, and the median progression-free survival (PFS) was only 2 months. Four out of five patients in this study evaluated with stable disease (SD) occurred in patients without liver metastases, whereas a short disease stabilization was seen in 1 of 14 patients with history of liver metastases [ 58 ].
In another study in MSS mCRC patients, a combination of regorafenib and toripalimab, an anti-PD-1 ICI yielded an objective response rate of 15.2% (5 of 33 patients) with all (3 of 3) with lung-only metastasis responding [ 59 ]. In a retrospective study that involved 14 Chinese medical centers, a partial response rate of 5% (4 of 84 patients) was reported in MSS mCRC patients administered regorafenib combined with ICIs [ 60 ].
In a phase 2 study in patients from the USA with pMMR/MSS mCRC, regorafenib plus nivolumab yielded an objective response rate of 7%, with all responses observed in patients without liver metastases [ 61 ]. In this study, regorafenib was administered at 80 mg/day on a 3 weeks on/1 week off schedule and was increased to 120 mg/day if the 80 mg/day was well tolerated. Nivolumab was administered at 480 mg every 4 weeks.
Based on these data, we cannot rule out the possibility that the regorafenib pre-treatment may have primed for responsiveness to the ICI immunotherapy in this pMMR/MSS mCRC patient that presented without liver metastases.
However, in this case, the doses and frequencies of both regorafenib and of the combination ICI immunotherapy that were actually administered were significantly less that the doses administered in clinical trials where objective responses were observed.
In addition, in the present case, corticosteroids (CS) were administered 6 weeks after start of ICI administration. In a retrospective single institution study, patients were evaluated in two cohorts based on timing of initiation of CS after initiation of ICI immunotherapy (≥ 2 months vs < 2 months). The administration of CS < 2 months after initiation of ICI immunotherapy was found to significantly hinder ICI efficacy [ 62 ].
Since regorafenib does not directly convert cold tumors to hot tumors, which is necessary for priming ICI responsiveness, and the doses and frequencies of both regorafenib and the ICI immunotherapy used in the present case were at sub-optimal therapeutic levels, combined with the early use of CS, we believe, while possible, it is unlikely that the regorafenib priming was responsible for the rare objective tumor response observed here and it is more likely that a combination that converted the cold tumors to hot was responsible.
Therefore, we finally consider whether the experimental AlloStim® priming alone or in combination with regorafenib contributed to the ICI objective response.
We hypothesized that if the restaging CT scan after AlloStim® immunotherapy reported as progressive disease (PD) by RECIST 1.1, was actually pseudoprogression due to ‘hot’ inflammation of the tumor lesions which would make them appear to be larger than the actual tumor burden, that ICI immunotherapy would elicit a rapid tumor debulking response due to resident infiltrating effector immune cell release from suppression.
The present subject was negative for serum IL-12 at baseline. After three cycles of experimental AlloStim® immunotherapy the subject seroconverted to IL-12 positivity, supporting that the host immune system was modulated. We previously reported that IL-12 positivity correlated with long-term survival after AlloStim® immunotherapy [ 63 ].
IL-12 is an effector cytokine that promotes anti-tumor immunity by activating an effector Th1 response, which is required for the activation of cytotoxic T and NK cells [ 64 ]. IL-12 promotes production of IFN-γ which acts to upregulate PD-L1 in the tumor microenvironment (TME), which may make these tumors more susceptible to anti-PD-L1 ICI immunotherapy [ 65 , 66 , 67 ].
The presence of IL-12 can have many beneficial anti-tumor effects, including: increasing production of IFN-γ from NK and T cells [ 68 ]; stimulation of growth and cytotoxicity of activated NK cells and CD8 + and CD4 + T cells [ 69 ], shifting the Th1/Th2 balance in favor of the Th1 phenotype [ 70 ]; induction of antiangiogenic cytokine and chemokine production [ 71 ]; remodeling of the peritumoral extracellular matrix and tumor stroma [ 72 ], reprogramming of myeloid-derived suppressor cells [ 73 ], and increasing expression of MHC class I molecules necessary of cytolytic T-lymphocyte (CTL) recognition of tumor cells [ 74 ].
Soluble heat shock protein (HSP)-70 was also detected in the serum after AlloStim® administration. HSP-70 is a stress-inducible chaperone that is overexpressed within tumor cells, including CRC [ 75 ]. The finding of HSP-70 in serum suggests that tumor cells have been killed in a manner where the cell membrane is disrupted (immunological cell death), releasing the HSP along with danger signals into the tumor microenvironment. Hsp70 extracellular function is believed to be immunogenic and extracellular Hsp70 can serve as an adjuvant to activate the innate immune system [ 76 ] and can eventually lead to tumor-specific adaptive immunity [ 77 ]. Endogenous HSP chaperone all tumor cell antigens, including self- and neo-antigens. Tumors accumulate mutations that can cause tumor-specific neo-antigen expression. Since these neo-antigens are intracellular, they may not have been previously exposed to the immune system, as the tumors sequester these neoantigens. Thus the presence of soluble HSP-70 supports that AlloStim® modified the TME in a manner that caused tumor lysis and release of chaperoned neoantigens. Exposure of tumor neoantigens to the immune system increases responsiveness to ICI [ 78 ].
The mechanism of action of AlloStim® is also consistent with the conclusion that an inflammatory cold to hot conversion occurred which caused the dramatic 73% increase in target lesion size by RECIST 1.1. The immune systems of patients with metastatic cancers are dysregulated resulting in a shift toward Th2 dominance [ 79 , 80 , 81 ]. AlloStim® experimental immunotherapy modulates the dysregulated immune systems of these patients to a Th1 dominance using a strategy of allo-priming [ 82 ]. The STIMVAX protocol incorporated a first series of intradermal injections of AlloStim®. The host rejection of the intentionally mis-matched AlloStim® cells shortly after administration results in increased titers of allo-specific Th1 and CTL cells, modulating the resident Th1/Th2 balance.
These allo-specific cells elicited after intradermal injections are non-specifically activated by cytokine release after intravenous infusion of AlloStim® through a bystander activation mechanism [ 83 ]. Activated T-cells can extravasate and local inflammation attracts these cells into tissues and sites of inflammation, including tumors [ 84 ]. Thus, the intravenous infusion of AlloStim® after allo-priming can convert “cold” tumors into “hot” tumors with extensive infiltration of Th1/CTL memory cells, which could possibly account for the 73% increase in target lesion size.
Inflamed tumor lesions can enlarge and appear as PD by RECIST 1.1. However, the putative anti-tumor mechanism leading to tumor debulking immunity may take several additional months before a radiological response can be detected and patients are often removed from the treatment protocols before a later assessment can be conducted.
We hypothesized that based on the mechanism of action of AlloStim® that radiological progression after 3 cycles likely represents a beneficial immune response that has primed the tumor lesions for an eventual debulking anti-tumor response. In order to support this hypothesis, we administered a short course of combination ICI immunotherapy. Since pMMR/MSS mCRC is known not to be responsive to ICI immunotherapy, we predicted that if a rapid tumor debulking response were observed, this would provide evidence supporting that the tumor lesions had been previously primed with infiltrating effector immune cells.
The available evidence makes it appear likely that AlloStim® played a role in eliciting the objective response observed after regorafenib and combination ICI immunotherapy.
The rare objective response in this case provides support for further investigation of the combination of AlloStim® combined with ICI immunotherapy with/or without regorafenib in ICI resistant patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Twice a day
Basic supportive care
Colorectal cancer
Corticosteroid
Colony stimulating factor receptor-1
Computed tomography
Cytolytic T-lymphocyte
Cytotoxic T-lymphocyte associated protein 4
C-X-C motif chemokine receptor-3
Dendritic cell type 1
Deficient DNA mismatch repair
Deoxyribonucleic acid
Eastern Cooperative Oncology Group
Food and drug administration
Epidermal growth factor receptor-1
Epidermal growth factor receptor-2
Folinic acid, fluorouracil and irinotecan
Folinic acid, fluorouracil and oxaliplatin
Graft vs. host disease
Graft vs. tumor
Immune checkpoint inhibitor
Intradermal
Interleukin
Immune related response criteria
Intravenous
Lymphocyte activation gene
Low anterior resection
- Metastatic colorectal cancer
Microsatellite instability high
- Microsatellite stable
Natural killer cell
Overall survival
Peripheral blood mononuclear cell
Progressive disease
Programmed death receptor-1
Platelet derived growth factor receptor
Programmed death receptor-ligand 1
Progression free survival
Proficient DNA mismatch repair
Polymerase delta-1
Polymerase epsilon
Partial response
Raf-1 proto-oncogene, serine/threonine kinase
Rat sarcoma gene
Response evaluation in solid tumors
Status post
Stable Disease
Signal transducer and activator of transcription 3
T-helper type 1
T-helper type 2
Three times a day
TEK tyrosine kinase, endothelial
Tumor infiltrating lymphocytes
Tumor mutational burden
Tumor microenvironment
United States of America
Vascular endothelial growth factor
Vascular endothelial growth factor receptor
Radiation therapy
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18.
Article CAS PubMed Google Scholar
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9.
Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 Inhibitors in Metastatic dMMR/MSI-H Colorectal Cancer. Front Oncol. 2019;9:396.
Article PubMed PubMed Central Google Scholar
Oh CR, Kim JE, Hong YS, Kim SY, Ahn JB, Baek JY, Lee MA, Kang MJ, Cho SH, Beom SH, Kim TW. Phase II study of durvalumab monotherapy in patients with previously treated microsatellite instability-high/mismatch repair-deficient or POLE-mutated metastatic or unresectable colorectal cancer. Int J Cancer. 2022;150:2038–45.
Mulet-Margalef N, Linares J, Badia-Ramentol J, Jimeno M, Sanz Monte C, Manzano Mozo JL, et al. Challenges and Therapeutic Opportunities in the dMMR/MSI-H Colorectal Cancer Landscape. Cancers (Basel). 2023;15(4):1022. https://doi.org/10.3390/cancers15041022 .
Lumish MA, Cercek A. Immunotherapy for the treatment of colorectal cancer. J Surg Oncol. 2021;123:760–74.
Gorzo A, Galos D, Volovat SR, Lungulescu CV, Burz C, Sur D. Landscape of Immunotherapy Options for Colorectal Cancer: Current Knowledge and Future Perspectives beyond Immune Checkpoint Blockade. Life (Basel). 2022;12(2):229.
Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol. 2018;9:160–9.
Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, Allegra CJ, National Cancer I. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767–72.
Manz SM, Losa M, Fritsch R, Scharl M. Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Therap Adv Gastroenterol. 2021;14:17562848211002018.
Article CAS PubMed PubMed Central Google Scholar
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–75.
Matteucci L, Bittoni A, Gallo G, Ridolfi L, Passardi A. Immunocheckpoint Inhibitors in Microsatellite-Stable or Proficient Mismatch Repair Metastatic Colorectal Cancer: Are We Entering a New Era? Cancers (Basel). 2023;15(21):5189.
Pecci F, Cantini L, Bittoni A, Lenci E, Lupi A, Crocetti S, Giglio E, Giampieri R, Berardi R. Beyond microsatellite instability: evolving strategies integrating immunotherapy for microsatellite stable colorectal cancer. Curr Treat Options Oncol. 2021;22:69.
Bai J, Chen H, Bai X. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J Clin Lab Anal. 2021;35: e23810.
Wang L, Geng H, Liu Y, Liu L, Chen Y, Wu F, Liu Z, Ling S, Wang Y, Zhou L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm. 2020;2023(4): e343.
Google Scholar
Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30:1096–103.
Ooki A, Shinozaki E, Yamaguchi K. Immunotherapy in colorectal cancer: current and future strategies. J Anus Rectum Colon. 2021;5:11–24.
Thomas J, Leal A, Overman MJ. Clinical Development of immunotherapy for deficient mismatch repair colorectal cancer. Clin Colorectal Cancer. 2020;19:73–81.
Article PubMed Google Scholar
Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, Sokol ES, Frampton G, Schrock AB, Anhorn R, Reddy P. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist. 2020;25:e147–59.
Manca P, Corti F, Intini R, Mazzoli G, Miceli R, Germani MM, Bergamo F, Ambrosini M, Cristarella E, Cerantola R, et al. Tumour mutational burden as a biomarker in patients with mismatch repair deficient/microsatellite instability-high metastatic colorectal cancer treated with immune checkpoint inhibitors. Eur J Cancer. 2023;187:15–24.
Li J, Xu X. Immune checkpoint inhibitor-based combination therapy for colorectal cancer: an overview. Int J Gen Med. 2023;16:1527–40.
Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev Cancer. 2020;1874: 188447.
Liu N, Shan F, Ma M. Strategic enhancement of immune checkpoint inhibition in refractory Colorectal Cancer: Trends and future prospective. Int Immunopharmacol. 2021;99: 108017.
Har-Noy M, Slavin S. The anti-tumor effect of allogeneic bone marrow/stem cell transplant without graft vs. host disease toxicity and without a matched donor requirement? Med Hypotheses. 2008;70:1186–92.
Har-Noy M, Zeira M, Weiss L, Fingerut E, Or R, Slavin S. Allogeneic CD3/CD28 cross-linked Th1 memory cells provide potent adjuvant effects for active immunotherapy of leukemia/lymphoma. Leuk Res. 2009;33:525–38.
LaCasse CJ, Janikashvili N, Larmonier CB, Alizadeh D, Hanke N, Kartchner J, Situ E, Centuori S, Har-Noy M, Bonnotte B, et al. Th-1 lymphocytes induce dendritic cell tumor killing activity by an IFN-gamma-dependent mechanism. J Immunol. 2011;187:6310–7.
Janikashvili N, LaCasse CJ, Larmonier C, Trad M, Herrell A, Bustamante S, Bonnotte B, Har-Noy M, Larmonier N, Katsanis E. Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood. 2011;117:1555–64.
Ma K, Jin Q, Wang M, Li X, Zhang Y. Research progress and clinical application of predictive biomarker for immune checkpoint inhibitors. Expert Rev Mol Diagn. 2019;19:517–29.
Zhang Y, Zheng L. Tumor immunotherapy based on tumor-derived heat shock proteins (Review). Oncol Lett. 2013;6:1543–9.
Srivastava PK, Callahan MK, Mauri MM. Treating human cancers with heat shock protein-peptide complexes: the road ahead. Expert Opin Biol Ther. 2009;9:179–86.
Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy. 2016;8:315–30.
Felzmann T, Huttner KG, Breuer SK, Wimmer D, Ressmann G, Wagner D, Paul P, Lehner M, Heitger A, Holter W. Semi-mature IL-12 secreting dendritic cells present exogenous antigen to trigger cytolytic immune responses. Cancer Immunol Immunother. 2005;54:769–80.
Waxman ES, Lee Gerber D. Pseudoprogression and Immunotherapy Phenomena. J Adv Pract Oncol. 2020;11:723–31.
PubMed PubMed Central Google Scholar
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Hales RK, Banchereau J, Ribas A, Tarhini AA, Weber JS, Fox BA, Drake CG. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21:1944–51.
Ippolito D, Maino C, Ragusi M, Porta M, Gandola D, Franzesi CT, Giandola TP, Sironi S. Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy. World J Clin Oncol. 2021;12:323–34.
Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169–78.
Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz HJ, Atasoy A, Shangguan T, Zhao H, El-Rayes B. Safety of Nivolumab plus Low-Dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. 2019;24:1453–61.
Almquist DR, Ahn DH, Bekaii-Saab TS. The Role of immune checkpoint inhibitors in colorectal adenocarcinoma. BioDrugs. 2020;34:349–62.
Wang L, Huang C. Application of immune checkpoint inhibitors in colorectal cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46:894–9.
PubMed Google Scholar
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91.
Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, Saltz LB. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–90.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, Korn WM, Weinberg BA, Hwang JJ, Shields AF, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16:805–12.
Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020;6:831–8.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Parseghian CM, Patnana M, Bhosale P, Hess KR, Shih YT, Kim B, Kopetz S, Overman MJ, Varadhachary GR, Javle M, et al. Evaluating for pseudoprogression in colorectal and pancreatic tumors treated with immunotherapy. J Immunother. 2018;41:284–91.
El Hajj J, Reddy S, Verma N, Huang EH, Kazmi SM. Immune Checkpoint Inhibitors in pMMR/MSS Colorectal Cancer. J Gastrointest Cancer. 2023;54:1017–30.
Lin KX, Istl AC, Quan D, Skaro A, Tang E, Zheng X. PD-1 and PD-L1 inhibitors in cold colorectal cancer: challenges and strategies. Cancer Immunol Immunother. 2023;72:3875–93.
Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf). 2020;8:11–24.
Tintelnot J, Stein A. Immunotherapy in colorectal cancer: Available clinical evidence, challenges and novel approaches. World J Gastroenterol. 2019;25:3920–8.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Lucidarme O, Wagner M, Gillard P, Kim S, Bachet JB, Rousseau B, Mazard T, Louvet C, Chibaudel B, Cohen R, et al. RECIST and CHOI criteria in the evaluation of tumor response in patients with metastatic colorectal cancer treated with regorafenib, a prospective multicenter study. Cancer Imaging. 2019;19:85.
Akin Telli T, Bregni G, Vanhooren M, Saude Conde R, Hendlisz A, Sclafani F. Regorafenib in combination with immune checkpoint inhibitors for mismatch repair proficient (pMMR)/microsatellite stable (MSS) colorectal cancer. Cancer Treat Rev. 2022;110: 102460.
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053–61.
Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and Nivolumab or Pembrolizumab Combination and Circulating Tumor DNA Response Assessment in Refractory Microsatellite Stable Colorectal Cancer. Oncologist. 2020;25:e1188–94.
Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2: 100383.
Yang K, Han L, Wu S, Qu X, Li Q, Zhao C, Zhou J, Jin X, Wang Y, Yan D, et al. Real-world outcomes of regorafenib combined with immune checkpoint inhibitors in patients with advanced or metastatic microsatellite stable colorectal cancer: A multicenter study. Cancer Immunol Immunother. 2022;71:1443–51.
Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Tam R, D’Adamo D, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023;58: 101917.
Maslov DV, Tawagi K, Kc M, Simenson V, Yuan H, Parent C, Bamnolker A, Goel R, Blake Z, Matrana MR, Johnson DH. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer. 2021;9(7):e002261.
Har-Noy M, Lausoontornsiri W, Or R, Katsanis E. Response of Her2+ breast cancer patients to allogeneic cell immunotherapy. J Clin Oncol. 2012;30(suppl): e13013.
Article Google Scholar
Lu X. Impact of IL-12 in Cancer. Curr Cancer Drug Targets. 2017;17:682–97.
Yuan W, Deng D, Jiang H, Tu C, Shang X, He H, Niu R, Dong J. Hyperresponsiveness to interferon gamma exposure as a response mechanism to anti-PD-1 therapy in microsatellite instability colorectal cancer. Cancer Immunol Immunother. 2019;68:257–68.
Kikuchi T, Mimura K, Okayama H, Nakayama Y, Saito K, Yamada L, Endo E, Sakamoto W, Fujita S, Endo H, et al. A subset of patients with MSS/MSI-low-colorectal cancer showed increased CD8(+) TILs together with up-regulated IFN-gamma. Oncol Lett. 2019;18:5977–85.
CAS PubMed PubMed Central Google Scholar
Yuan W, Deng D, Li H, Hu X, Shang X, Hou X, Jiang H, He H. IFNgamma/PD-L1 Signaling Improves the Responsiveness of Anti-PD-1 Therapy in Colorectal Cancer: An in vitro Study. Onco Targets Ther. 2021;14:3051–62.
Otani T, Nakamura S, Toki M, Motoda R, Kurimoto M, Orita K. Identification of IFN-gamma-producing cells in IL-12/IL-18-treated mice. Cell Immunol. 1999;198:111–9.
Zeh HJ 3rd, Hurd S, Storkus WJ, Lotze MT. Interleukin-12 promotes the proliferation and cytolytic maturation of immune effectors: implications for the immunotherapy of cancer. J Immunother Emphasis Tumor Immunol. 1993;14:155–61.
Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–68.
Angiolillo AL, Sgadari C, Tosato G. A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann N Y Acad Sci. 1996;795:158–67.
Kerkar SP, Leonardi AJ, van Panhuys N, Zhang L, Yu Z, Crompton JG, Pan JH, Palmer DC, Morgan RA, Rosenberg SA, Restifo NP. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther. 2013;21:1369–77.
Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–57.
Suzuki S, Umezu Y, Saijo Y, Satoh G, Abe Y, Satoh K, Nukiwa T. Exogenous recombinant human IL-12 augments MHC class I antigen expression on human cancer cells in vitro. Tohoku J Exp Med. 1998;185:223–6.
Kanazawa Y, Isomoto H, Oka M, Yano Y, Soda H, Shikuwa S, Takeshima F, Omagari K, Mizuta Y, Murase K, et al. Expression of heat shock protein (Hsp) 70 and Hsp 40 in colorectal cancer. Med Oncol. 2003;20:157–64.
Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34:4153–61.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–78.
Ma S, Chee J, Fear VS, Forbes CA, Boon L, Dick IM, Robinson BWS, Creaney J. Pre-treatment tumor neo-antigen responses in draining lymph nodes are infrequent but predict checkpoint blockade therapy outcome. Oncoimmunology. 2020;9:1684714.
Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8.
Sato M, Goto S, Kaneko R, Ito M, Sato S, Takeuchi S. Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer Res. 1998;18:3951–5.
CAS PubMed Google Scholar
Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–59.
Har-Noy M, Or R. Allo-priming as a universal anti-viral vaccine: protecting elderly from current COVID-19 and any future unknown viral outbreak. J Transl Med. 2020;18:196.
Paprckova D, Salyova E, Michalik J, Stepanek O. Bystander activation in memory and antigen-inexperienced memory-like CD8 T cells. Curr Opin Immunol. 2023;82: 102299.
Newton P, O’Boyle G, Jenkins Y, Ali S, Kirby JA. T cell extravasation: demonstration of synergy between activation of CXCR3 and the T cell receptor. Mol Immunol. 2009;47:485–92.
Download references
Acknowledgements
The authors wish to thank Axella Clinical Research (CRO) for assistance in coordinating data collection and research blood samples, Dr. Xiaochuan Yang and Eirini Topouzi for analyzing blood samples and assisting with preparation of figures. Dr. Elena Fingerut for managing the manufacturing of the study drug in accordance with good manufacturing practices and Thu Bui for managing blood donor collection pursuant to 21 CFR 1271 and the distribution of the frozen investigational product to the clinical site.
Mirror Biologics, Inc. is the sponsor of ITL-032-MCRC3-STIMVAX Phase IIB clinical trial and has funded the research.
Author information
Authors and affiliations.
Hirschfeld Oncology, 19 Fayette Street, 2Nd Floor, Brooklyn, NY, 11206, USA
Ariel Hirschfeld
University Diagnostic Medical Imaging, PC, 1200 Waters Place, Suite m108, Bronx, NY, 10461, USA
Daniel Gurell
Mirror Biologics, Inc, 2818 Cypress Ridge Blvd, Suite 110, Wesley Chapel, FL, 33544, USA
Michael Har-Noy
You can also search for this author in PubMed Google Scholar
Contributions
MHN wrote the manuscript with input and review from other authors. AH conceived the design of the study and was responsible for implementing the protocol and for patient care. DW reviewed the longitudinal CT scans and determined the RECIST 1.1 conclusions and selected the CT scan images.
Corresponding author
Correspondence to Michael Har-Noy .
Ethics declarations
Ethics approval and consent to participate.
Salus IRB, 2111 W. Braker Lane, Ste 100, Austin, TX 78758, serves as the central ethics committee for this study (ITL-032-MCRC3-STIMVAX). Informed consent was obtained from all individual participants included in the study.
Consent for publication
The patient informed consent form provided consent to publish non-identifiable results.
Competing interests
MHN is the inventor of the investigational AlloStim® product and is the founder of Mirror Biologics, Inc. which sponsored the clinical study. AH and DG declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hirschfeld, A., Gurell, D. & Har-Noy, M. Objective response after immune checkpoint inhibitors in a chemotherapy-refractory pMMR/MSS metastatic rectal cancer patient primed with experimental AlloStim® immunotherapy. transl med commun 9 , 15 (2024). https://doi.org/10.1186/s41231-024-00174-y
Download citation
Received : 25 March 2024
Accepted : 30 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s41231-024-00174-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunotherapy
- Checkpoint inhibitor
Translational Medicine Communications
ISSN: 2396-832X
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Clarifying the cellular mechanisms underlying periodontitis with an improved animal model
Periodontal disease, represented by periodontitis, is the leading cause of tooth loss and affects close to one in five adults worldwide. In most cases, this condition occurs as a result of an inflammatory response to bacterial infection of the tissue around teeth. As the condition worsens, the gums begin to pull away, exposing teeth roots and bone. Notably, the incidence of periodontitis becomes more prevalent with age and with populations worldwide living longer, developing a solid understanding of its underlying causes and progression is important.
In a study recently published in Nature Communications on March 28, 2024, researchers from Tokyo Medical and Dental University (TMDU) found a way to achieve this by improving upon a widely used animal model to study periodontitis.
Studying periodontitis directly in humans is challenging. As a result, scientists often resort to animal models for preclinical research. For instance, the "mouse ligature-induced periodontitis model," since its inception in 2012, has enabled researchers to study the cellular mechanisms underlying this condition. Simply put, with this model, periodontal disease is artificially induced by ligating silk threads onto the molars of mice models, which induces plaque accumulation. While convenient and effective, this model, however, fails to capture the complete picture of periodontitis. "Even though the periodontal tissue is composed of gingiva, periodontal ligament, alveolar bone, and cementum, analyses are usually performed exclusively on gingival samples due to technical and quantitative limitations," remarks lead author Mr. Anhao Liu. "This sampling strategy limits the conclusions that may be drawn from these studies, so methods that allow for the simultaneous analysis of all tissue components are needed."
To address this limitation, the research team developed a modified ligature-induced periodontitis model. Instead of the classic single ligature, they used a triple ligature approach on the upper left molar of male mice. This strategy expanded the range of bone loss without causing severe bone destruction around the second molar, increasing the yield of the different types of periodontal tissue. "We isolated the three main tissue types and evaluated the RNA yield between the two models. The results showed that the triple-ligature model effectively increased the yield, achieving four times the yield of normal peri-root tissue and supporting the high-resolution analysis of different tissue types," explains senior author Dr. Mikihito Hayashi.
After confirming the efficacy of their modified model, the researchers proceeded to investigate the effects of periodontitis on gene expression among the different tissue types over time, focusing on genes related to inflammation and osteoclast differentiation. One of their main findings was that the expression of the Il1rl1 gene was markedly higher in peri-root tissue five days after ligation. This gene encodes the protein ST2 in both receptor and decoy isoform, which binds to a cytokine called IL-33 that is involved in inflammatory and immunoregulation processes.
To gain further insights into the role of this gene, the team induced periodontitis in genetically modified mice that lacked the Il1rl1 or Il33 genes. These mice exhibited accelerated inflammatory bone destruction, highlighting the protective role of the IL-33/ST2 pathway. Further analysis of cells containing the ST2 protein in its receptor form, mST2, revealed that most of them were of macrophage lineage. "Macrophages are typically classified into two main types, pro-inflammatory and anti-inflammatory, based on their activation process. We found that mST2-expressing cells were unique in that they expressed some markers of both types of macrophages simultaneously," comments senior author Dr. Takanori Iwata. "These cells were present in the peri-root tissue before inflammation was triggered, so we named them 'periodontal tissue-resident macrophages.'"
Together, the findings of this study showcase the power of this modified animal model to study the full scope of periodontitis in greater detail, right down to the biomolecular level. "We suggest the possibility that a novel IL-33/ST2 molecular pathway regulating inflammation and bone destruction in periodontal disease, alongside specific macrophages in peri-root tissue, is deeply involved in periodontal disease. This will hopefully lead to the development of new treatment strategies and prevention methods," concludes senior author Dr. Tomoki Nakashima.
- Bone and Spine
- Biotechnology and Bioengineering
- Microbiology
- Periodontal disease
- Adipose tissue
- House mouse
- Biological tissue
- Blood vessel
- Global climate model
Story Source:
Materials provided by Tokyo Medical and Dental University . Note: Content may be edited for style and length.
Journal Reference :
- Anhao Liu, Mikihito Hayashi, Yujin Ohsugi, Sayaka Katagiri, Shizuo Akira, Takanori Iwata, Tomoki Nakashima. The IL-33/ST2 axis is protective against acute inflammation during the course of periodontitis . Nature Communications , 2024; 15 (1) DOI: 10.1038/s41467-024-46746-2
Cite This Page :
Explore More
- Stopping Flu Before It Takes Hold
- Cosmic Rays Illuminate the Past
- Star Suddenly Vanish from the Night Sky
- Dinosaur Feather Evolution
- Warming Climate: Flash Droughts Worldwide
- Record Low Antarctic Sea Ice: Climate Change
- Brain 'Assembloids' Mimic Blood-Brain Barrier
- 'Doomsday' Glacier: Catastrophic Melting
- Blueprints of Self-Assembly
- Meerkat Chit-Chat
Trending Topics
Strange & offbeat.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Optimal timing for the Modified Early Warning Score for prediction of short-term critical illness in the acute care chain: a prospective observational study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-9568-0138 Lars Ingmar Veldhuis 1 , 2 ,
- Merijn Kuit 1 ,
- Liza Karim 1 ,
- Milan L Ridderikhof 3 ,
- Prabath WB Nanayakkara 4 ,
- Jeroen Ludikhuize 5 , 6
- 1 Emergency Department , Amsterdam UMC Locatie AMC , Amsterdam , The Netherlands
- 2 Department of Anaesthesiology , Amsterdam UMC Locatie AMC , Amsterdam , The Netherlands
- 3 Emergency Medicine , Amsterdam UMC - Locatie AMC , Amsterdam , The Netherlands
- 4 Section Acute Medicine, Department of Internal Medicine , Amsterdam Universitair Medische Centra , Amsterdam , The Netherlands
- 5 Department of Internal Medicine , Amsterdam UMC Locatie VUmc , Amsterdam , The Netherlands
- 6 Department of Intensive Care , Haga Hospital , Den Haag , The Netherlands
- Correspondence to Lars Ingmar Veldhuis, Emergency Department, Amsterdam UMC Locatie AMC, Amsterdam, 1105 AZ, The Netherlands; l.i.veldhuis{at}amsterdamumc.nl
Introduction The Modified Early Warning Score (MEWS) is an effective tool to identify patients in the acute care chain who are likely to deteriorate. Although it is increasingly being implemented in the ED, the optimal moment to use the MEWS is unknown. This study aimed to determine at what moment in the acute care chain MEWS has the highest accuracy in predicting critical illness.
Methods Adult patients brought by ambulance to the ED at both locations of the Amsterdam UMC, a level 1 trauma centre, were prospectively included between 11 March and 28 October 2021. MEWS was calculated using vital parameters measured prehospital, at ED presentation, 1 hour and 3 hours thereafter, imputing for missing temperature and/or consciousness, as these values were expected not to deviate. Critical illness was defined as requiring intensive care unit admission, myocardial infarction or death within 72 hours after ED presentation. Accuracy in predicting critical illness was assessed using the area under the receiver operating characteristics curve (AUROC).
Results Of the 790 included patients, critical illness occurred in 90 (11.4%). MEWS based on vital parameters at ED presentation had the highest performance in predicting critical illness with an AUROC of 0.73 (95% CI 0.67 to 0.79) but did not significantly differ compared with other moments. Patients with an increasing MEWS over time are significantly more likely to become critical ill compared with patients with an improving MEWS.
Conclusion The performance of MEWS is moderate in predicting critical illness using vital parameters measured surrounding ED admission. However, an increase of MEWS during ED admission is correlated with the development of critical illness. Therefore, early recognition of deteriorating patients at the ED may be achieved by frequent MEWS calculation. Further studies should investigate the effect of continuous monitoring of these patients at the ED.
- emergency department
- emergency care systems
- care systems
- critical care
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/emermed-2022-212733
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The Modified Early Warning Score (MEWS) is an effective tool to identify deteriorating patients in the acute care chain who might deteriorate.
Although it is increasingly being implemented, the optimal timing for assessing the MEWS is unknown.
WHAT THIS STUDY ADDS
This prospective multicentre study included 790 patients and found that MEWS measured at ED presentation had the highest accuracy in predicting the development of critical illness. However, the performance is moderate and not significantly better compared to MEWS based at other moments in the acute care chain.
However, an increase in MEWS during the ED encounter is highly correlated with the development of critical illness.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
As clinical deterioration and subsequent development of critical illness is highly correlated with an increase of MEWS during the ED stay, we suggest further investigation on the value of continuous monitoring of these patients at the ED
Introduction
Early recognition of the deteriorating patient is of vital importance to reduce the occurrence of serious adverse events (SAEs) including cardiopulmonary arrests, (delayed) intensive care unit (ICU) admissions and death. Prior research indicates that up to 80% of deteriorating patients show physiological abnormalities up to 24 hours before the event. 1–4 Track and trigger systems, including the Early Warning Score (EWS) were developed to recognise the early signs of deterioration. These scoring systems are relatively simple models using the patients’ vital parameters to assess the degree of illness of the patient.
In general, the higher the EWS, the more likely it is that a patient is clinically deteriorating and subsequently becomes critically ill. 5 This use of an EWS has proven to be efficient for detecting deteriorating patients on the wards. 6 When a deteriorating patient is identified, the Medical Emergency Team can be consulted, and more appropriate care can be provided. The implementation of EWS-based systems can lead to a reduction in SAEs and reduced time to ICU admission in deteriorating patients. 7
As the EWS-based system has been shown to be effective in general wards, the model has been increasingly implemented in other aspects of acute care, that is, the prehospital and ED settings. 8–11 Several studies suggest that EWS can be useful in the entire acute care chain. Prior studies showed a MEWS performance in the ED setting of area under the receiver operating characteristics curve (AUROC) 0.65. 12
However, it is unclear what moment in the acute care chain has the highest accuracy in predicting deterioration.
Timely interventions such as administration of antibiotics, and fluid challenges strongly affect vital parameters and overall survival. 13 These interventions may stabilise the patient and prevent further deterioration, which influences the EWS.
The primary aim of this study was to determine at which time point, from the first moment of contact with the EMS to admission to a nursing ward, an EWS is most accurate in detecting a deteriorating patient. Although the National EWS is generally slightly more accurate compared with the Modified Early Warning Score (MEWS), 14 we studied the performance of MEWS, as this is the tool regularly used in the Netherlands.
Study design and population
This was a prospective observational multicentre study, conducted at a university hospital, serving as a level 1 trauma centre with two locations. All adult patients (18 years and older) brought by ambulance to one of these two centres between 11 March and 28 October 2021, were included. Interhospital transfers and patients receiving prehospital cardiopulmonary resuscitation were excluded. Participants gave informed consent before taking part.
Data collection
Data were collected by a researcher present during EMS presentation between 10:00 hours and 18:00 hours on workdays, as during this period most ambulances arrive at the EDs of both centres. As we recorded data up to 3 hours after ED presentation, data were obtained until 21:00 hours. Patient characteristics, including vital parameters measured at four time points were collected on paper forms: prehospital (recorded by the ambulance); at ED admission (±15 min); at 1 hour (±15 min); and at 3 hours (±30 min) after ED arrival. Three-day outcome was obtained from the electronic patient records. All obtained data were processed using a standardised data worksheet. Collected data were anonymously processed using an online data collection system (Castor eClinical Data Management).
Endpoints and definitions
The primary outcome was the performance of MEWS in predicting critical illness for all four time points during which data were collected.
Secondary outcome was the association between the MEWS over time (ie, increase of MEWS 1 hour after ED admission compared with prehospital MEWS) and subsequent development of critical illness.
Critical illness was defined as mortality; ICU admission and/or myocardial infarction (as concluded by a cardiologist) all within 3 days after ED presentation.
Primary and secondary outcome was assessed by investigating the electronic medical records on day 4 after the initial ED admission. MEWS was thereafter calculated using the vital parameters at each time point; see figure 1 for thresholds of the MEWS.
- Download figure
- Open in new tab
- Download powerpoint
Modified Early Warning Score.
Missing data
Previous studies have shown that the temperature and level of consciousness of patients generally remain constant from transportation by EMS to arrival at the ED. 13 Therefore, in any cases where the temperature or level of consciousness of a patient was recorded prehospitally but missing at admission or vice versa, the recorded values for these parameters were used. MEWS was then calculated if a minimum of four out of six vital parameters was available with the one or two missing parameters considered normal. In choosing this method we acted on the assumption that diverging vital parameters would have been registered by the ED nurse. If more than two vital parameters were missing for a certain point in time, the MEWS at that time was not calculated. Patients for whom the MEWS could not be calculated were excluded from analysis for that specific point in time.
Sensitivity analysis
Model performance was tested after excluding patients with SARS-CoV-2 infection, as patients with COVID-19 are known to have relatively stable vital parameters despite being critically ill (as compared with patients without COVID-19). 15
Primary and secondary outcomes
The primary outcome was the performance of MEWS at different periods of time using the outcomes of developing critical illness (as defined above). The secondary outcome was whether an increase in MEWS over time was associated with becoming critically ill.
Statistical analysis
Descriptive and statistical analysis was performed using SPSS V.22.0 (SPSS, Chicago, Illinois, USA). Non-normally distributed continuous variables were described as medians with IQRs and were compared with the Mann-Whitney U test. Categorical variables were described as numbers and percentages and were compared by Pearson’s χ 2 test. The primary outcome was expressed as the AUROC of the MEWS for each time point. Also, for each MEWS between 0 and 5, sensitivity and specificity were calculated.
Using the AUROC derived from MEWS at the different time points, superiority in performance was assessed using the method of Hanley and McNeil. 16 In general, the AUROC is characterised using standard terms, where AUROC 0.6–0.7 is considered a poor testing method, 0.7–0.8 is considered moderate, 0.8–0.9 is good and a test with an AUROC >0.9 is considered an excellent method.
A χ 2 test was used to test whether an increase of MEWS over time had a higher incidence of becoming critically ill compared with a decreased or stable MEWS.
Sample size calculation
For the sample size calculation, the previously reported performance (AUROC 0.65) of MEWS in the ED was used. 12 For the primary outcome (the moment with the highest AUROC of MEWS) based on a 95% CI, 80% power and a 0.1 difference in MEWS, 114 patients were needed to test for statistically significant difference. These calculations were performed in nQuery tool for design of trials, link https://www.statsols.com/nquery .
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Of the 790 patients included in this study, critical illness occurred in 90 patients (11.4%). Prehospital alert calls to the ED were made significantly more often for critically ill patients (88.9% vs 69.4%, p<0.001). Additionally, these patients were assessed more often in either the resuscitation or trauma bay ( table 1 ).
- View inline
Patient characteristics
Of the 90 critically ill patients, 41 patients were directly admitted from the ED to the ICU, 16 patients were initially admitted to the ward then went to the ICU, 15 died and 17 had a myocardial infarction, all within 72 hours after ED presentation. Prior to imputing for missing values, the number of complete MEWS values was limited ( table 2 ). After imputing for missing values, the most complete moment of measurements was at ED arrival (94.8%).
Complete MEWS before and after imputing
Primary outcome
MEWS based on vital parameters measured at ED admission had the highest performance with an AUROC of 0.726 ( table 3 , figure 2 ). MEWS based on vital parameters measured 1 hour and 3 hours after ED admission had lower performance ( table 4 ). The performance of MEWS measured at ED admission was not significantly superior compared with the other time points in predicting critical illness.
AUROCs for the prediction of critical illness within 72 hours
Receiver operating characteristics (ROC) curves for prediction of critical illness within 72 hours.
Sensitivity and specificity for cut-off points of MEWS
Of the 790 patients, 82 had a proven SARS-CoV-2 infection. Excluding patients with a proven SARS-CoV-2 infection did not lead to a significant improvement of MEWS accuracy in predicting critical illness ( table 3 ).
In addition, sensitivity and specificity were calculated for each threshold ( table 4 ). For the MEWS measured at ED admission using a cut-off value of 3, sensitivity was 64.0% (95% CI 60.5% to 67.4%) and specificity was 70.1% (95% CI 66.7% to 73.3%).
Secondary outcome
To estimate the influence of a change over time in MEWS (delta MEWS) on outcome, a χ 2 test was performed. An increase in MEWS between the MEWS measured prehospitally and 1 hour after ED admission had an incidence of 25.7% of critical illness, while stable or decreasing MEWS had an incidence of 7.5%. This difference was significantly different (p<0.05) (see table 5 ).
Changes in MEWS during admission and the development of critical illness
While many studies focus on the performance of EWS in either the prehospital or ED setting, little is known about the best timing to use it in the acute care chain. 8 17 18 Therefore, this prospective multicentre study was performed to attempt to direct clinical practice to the best moment in the acute care chain to measure MEWS to identify subsequent development of critical illness in patients brought to the ED by ambulance. Although MEWS calculated based at presentation had the highest accuracy in predicting the development of critical illness, an AUROC of 0.726 was not significantly superior to MEWS measured prehospitally or 1 hour or 3 hours after ED presentation. Also, excluding patients with proven SARS-CoV-2 infection did not lead to an improvement in model performance. While the performance of MEWS found in this study in predicting critical illness is moderate, this was consistent with other studies. 19
Our secondary outcome was to test the correlation between an increase of MEWS over time and the development of critical illness. Prior studies suggest that the trend of MEWS during the first hours of ED presentation may identify clinically deteriorating patients better compared with a single MEWS calculation. 5 10 20 21 Our results indicate that an increase of MEWS between prehospital and at 1 hour after ED admission was significantly correlated with the development of critical illness, p=0.005. Therefore, we suggest that patients with an increasing MEWS during ED stay should be more intensively monitored and early consultation with the ICU consultant may be justifiable.
Limitations
The study has several limitations which may reduce the generalisability of our data and have most likely influenced our results. First, the study ran during the summer months, so season-specific diseases may have occurred. Furthermore, there was a high percentage of missing data for calculating MEWS. We have excluded patients from analysis if two vital parameters other than temperature or mental status were missing. Also, we only included patients arriving between 10:00 hours and 18:00 hours potentially leading to selection bias. To improve the quality and clinical relevance of the data, future studies should also include cases where MEWS is found to be above the cut-off point, even if there are missing variables. Additionally, it is possible that the data were not missing at random. When a patient has normal vital signs during the first check, their vitals usually do not get monitored as frequently as when a patient initially has abnormal vital signs. Therefore, only including cases with known MEWS at all time points can cause a distorted view of the predictive performance of EWS in the ED, since there is a probability that patients with abnormal vital signs are disproportionately over-represented. It is important to record the full vital parameters set needed to calculate MEWS in clinical practice.
Clinical implication
Implementation of a single standard time point for measurement of MEWS in the prehospital setting or ED is clinically not useful due to its moderate performance. However, patients with an increase of MEWS over time is highly correlated with the development of critical illness. Implementing standard repeated measurements in the acute care chain may result in better prediction of which patients are likely to become critically ill.
In conclusion, MEWS based on vital parameters measured at ED presentation has the highest accuracy in predicting the development of critical illness. However, performance is moderate and not significantly better compared with MEWS measured at other moments in the acute care chain. However, an increase in MEWS during the encounter is highly correlated with the development of critical illness. We, therefore, conclude that it would be valuable to assess MEWS over time, rather than only at a single moment.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants. The Medical Ethics Committee of both locations of Amsterdam UMC waived ethics approval for this study (Waiver: W-19_480 # 19.554). Participants gave informed consent to participate in the study before taking part.
- Goldhill DR ,
- Franklin C ,
- Ludikhuize J ,
- Smorenburg SM ,
- de Rooij SE , et al
- van Galen LS ,
- Dijkstra CC ,
- Ludikhuize J , et al
- Fullerton JN ,
- Silvey NE , et al
- Hobbelink EL ,
- van Tienhoven AJ , et al
- Paterson R ,
- MacLeod DC ,
- Thetford D , et al
- Spencer W ,
- Date P , et al
- Vegting IL ,
- Houben E , et al
- Delgado-Hurtado JJ ,
- Nannan Panday RS ,
- Minderhoud TC ,
- Alam N , et al
- Schinkel M ,
- Bergsma L ,
- Veldhuis LI , et al
- Bein K , et al
- Silcock DJ ,
- Corfield AR ,
- Gowens PA , et al
- Veldhuis L ,
- Ridderikhof ML ,
- Schinkel M , et al
- Hanley JA ,
- Yoshida T ,
- Shinozaki T , et al
- Kivipuro M ,
- Tirkkonen J ,
- Kontula T , et al
- Rutherford P , et al
- Brekke IJ ,
- Puntervoll LH ,
- Pedersen PB , et al
- Kellett J ,
- Woodworth S , et al
Handling editor Kirsty Challen
Contributors LIV and MK: planning, conceptualisation, methodology, data curation and writing original draft. LK: data curation. MLR, PWBN and JL: important intellectual content and guarantor of the article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
30 Example Phrases: How to Conclude a Presentation. 1. "In summary, let's revisit the key takeaways from today's presentation.". 2. "Thank you for your attention. Let's move forward together.". 3. "That brings us to the end. I'm open to any questions you may have.".
Here are some tips for using a story to conclude a presentation: Make sure the story is brief. Choose a story that relates to the main points of the presentation. Stories about a customer experience or successful case study are effective. Make sure the story is relatable and encourages empathy from your audience. 7.
From summarizing key points to engaging the audience in unexpected ways, make a lasting impression with these 10 ways to end a presentation: 1. The summary. Wrap up your entire presentation with a concise and impactful summary, recapping the key points and main takeaways.
But how you end it can make all the difference in your presentation's overall impact. Here are some ways to ensure you end powerfully: Way #1: Include a Strong Call-to-Action (CTA) Way #2: Don't End With a Q&A. Way #3: End With a Memorable Quote. Way #4: Close With a Story. Way #5: Drive Your Main Points Home.
Mistake #5: Going over your time. Last but not least, many of the professional speakers we have interviewed have stressed the importance of ending one's presentation on time. Michelle Gladieux said it best: "The best way to end a presentation is ON TIME. Respect others' time commitments by not running over.
Though there are many ways to end a presentation, the most effective strategies focus on making a lasting impression on your audience and reinforcing your goals. So, let's take a look at three effective ways to end a presentation: 1. Summarize the Key Takeaways. Most presenters either make an argument (i.e. they want to convince their ...
This helps create a good long-lasting impression of your presentation. 4. End with a Call to action: One of the best ways to end your presentation is by concluding with a call to action slide. Incorporating a call to action into your presentation can be a powerful way to encourage your audience to take the next step.
1. A Strong Call to Action. There is always a purpose/goal behind every speech. You engage the audience with your content and establish a connection to achieve that goal. A call to action is a prompt that motivates the target group to take the desired action at the end of your presentation. Some examples of CTA are-.
So, in conclusion, brevity in public speaking is pretty important. In fact, George Orwell once said, "If it is possible to cut a word out of your speech, always cut it out.". So, when you create a presentation, cut the fluff. Cut the repetitive bullets. Cut the platitudes.
An effective conclusion will do three things. Offer a clear ACTION STEP. Sum up the presentation with one BIG IDEA. CEMENT the idea with a compelling story or data point. It's as simple as ABC: Action step, Big Idea, Cement with a story. How you end will determine if your audience leaves feeling inspired or indignant.
3 Strategies to Close Your Presentation Powerfully. Use these 3 strategies in your conclusion to: recapture your audience's attention. get your audience to focus and remember your key points. help your audience connect with you and your topic. end your presentation powerfully. One: Include a Call to Action (CTA)
A good presentation conclusion will have an effective summary, recommendation or call to action, and an opportunity to address any open issues through questions. A part of a presentation conclusion that often gets forgotten is a clear and effective "signal to the end".
A speech's ending should reiterate the key points you want to make, creating a lasting impression in the minds of your audience. How to conclude your presentation Here are some tips for creating an effective conclusion for your next presentation: 1. Restate your thesis Every good presentation should have a goal, thesis statement, or fundamental ...
Here are a few tips for business professionals who want to move from being good speakers to great ones: be concise (the fewer words, the better); never use bullet points (photos and images paired ...
Apply the 10-20-30 rule. Apply the 10-20-30 presentation rule and keep it short, sweet and impactful! Stick to ten slides, deliver your presentation within 20 minutes and use a 30-point font to ensure clarity and focus. Less is more, and your audience will thank you for it! 9. Implement the 5-5-5 rule. Simplicity is key.
The conclusion allows you to have the final say on the issues you have raised in your paper, to synthesize your thoughts, to demonstrate the importance of your ideas, and to propel your reader to a new view of the subject. It is also your opportunity to make a good final impression and to end on a positive note.
Presentation skills are the abilities and qualities necessary for creating and delivering a compelling presentation that effectively communicates information and ideas. They encompass what you say, how you structure it, and the materials you include to support what you say, such as slides, videos, or images. You'll make presentations at various ...
Think a certain way about the topic you presented. Purchase a product or service. Volunteer or sign up to do something. Promote your topic. Get others excited or interested in your topic. Again, many people make this step overly difficult. Simply asking the audience in a sincere way to take the action is as effective as a well crafted conclusion.
Typical Presentation Structure . In general, the contents of a presentation include an introduction, body, and conclusion. At times, it may include visual aids. This is the usual flow for crafting an effective presentation structure: from introduction to conclusion, which covers all the necessary sections and allows the audience to follow along ...
Step 1: Return to your thesis. To begin your conclusion, signal that the essay is coming to an end by returning to your overall argument. Don't just repeat your thesis statement —instead, try to rephrase your argument in a way that shows how it has been developed since the introduction. Example: Returning to the thesis.
Here are some tips for delivering an effective presentation. Conclusion. We considered a few key points for presentation structure and the typical format that can be followed. We also covered five ways you can structure your presentation and the format for a scientific presentation. Lastly, we covered a sample script for presentations.
An effective presentation conclusion should strive to accomplish what three goals? Summarize the main themes, give the audience a memorable take-away, and include a statement that allows for a graceful exit.
Connor opened his presentation to an audience of business owners with this statement: If you want to reduce employee benefit costs by at least 15 percent without hurting employee morale or impacting your work environment, please stand up right now. By asking them to stand, Connor is. capturing listeners' attention and getting them involved.
Step 1 in creating a makeshift salt reservoir to treat pyogenic granuloma: Prepare a piece of tape with a medial slit . Open in a separate window. Figure 1B. Step 2 in creating a makeshift salt reservoir to treat pyogenic granuloma: Allow the edges of the slit to overlap slightly, creating a reservoir for table salt . Open in a separate window.
In conclusion, the development of carrier-free nano-PROTACs such as NGP-65 represents an innovative approach towards enhancing PDT efficacy against tumors. With further research and refinement, these advancements hold great potential for advancing PROTAC-based therapies into clinical practice for effective tumor treatment without compromising ...
This research presentation aims to propose a design solution for a sensory room catered toward college students with sensory processing disorders, especially those that are on the autism spectrum. ... the proposed design solution identifies key design considerations for developing an inclusive and effective sensory space. The conclusion reached ...
By Ronald Beaty. May 22, 2024 at 12:29 a.m. Cape Cod, a picturesque haven renowned for its serene beaches and thriving marine life, is currently grappling with a complex ecological dilemma. The ...
Immune Checkpoint Inhibitor (ICI) immunotherapy is most effective in immune effector cell infiltrated 'hot' tumor lesions, such as occurs in deficient mismatch repair, microsatellite instability high (dMMR/MSI-H) colorectal cancer (CRC). However, most all metastatic CRC tumors are mismatch repair proficient/microsatellite stable (pMMR/MSS) 'cold' lesions, without significant immune ...
Periodontal disease, represented by periodontitis, is the leading cause of tooth loss and affects close to one in five adults worldwide. In most cases, this condition occurs as a result of an ...
Introduction The Modified Early Warning Score (MEWS) is an effective tool to identify patients in the acute care chain who are likely to deteriorate. Although it is increasingly being implemented in the ED, the optimal moment to use the MEWS is unknown. This study aimed to determine at what moment in the acute care chain MEWS has the highest accuracy in predicting critical illness.