Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 18 February 2020

Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies
- Roger D. Klein 1 , 2 &
- Scott J. Hultgren ORCID: orcid.org/0000-0001-8785-564X 1 , 2
Nature Reviews Microbiology volume 18 , pages 211–226 ( 2020 ) Cite this article
16k Accesses
259 Citations
41 Altmetric
Metrics details
- Antimicrobial resistance
- Bacterial adhesion
- Bacterial pathogenesis
Urinary tract infections (UTIs) are common, recurrent infections that can be mild to life-threatening. The continued emergence of antibiotic resistance, together with our increasing understanding of the detrimental effects conferred by broad-spectrum antibiotic use on the health of the beneficial microbiota of the host, has underscored the weaknesses in our current treatment paradigm for UTIs. In this Review, we discuss how recent microbiological, structural, genetic and immunological studies have expanded our understanding of host–pathogen interactions during UTI pathogenesis. These basic scientific findings have the potential to shift the strategy for UTI treatment away from broad-spectrum antibiotics targeting conserved aspects of bacterial replication towards pathogen-specific antibiotic-sparing therapeutics that target core determinants of bacterial virulence at the host–pathogen interface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Urinary tract infections: pathogenesis, host susceptibility and emerging therapeutics
Current and emerging strategies to curb antibiotic-resistant urinary tract infections
Molecular determinants of disease severity in urinary tract infection
Foxman, B., Barlow, R., D'Arcy, H., Gillespie, B. & Sobel, J. D. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10 , 509–515 (2000).
CAS PubMed Google Scholar
Foxman, B. & Brown, P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. North. Am. 17 , 227–241 (2003).
PubMed Google Scholar
Scholes, D. et al. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 182 , 1177–1182 (2000).
Epp, A. et al. Recurrent urinary tract infection. J. Obstet. Gynaecol. Can. 32 , 1082–1090 (2010).
Foxman, B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North. Am. 28 , 1–13 (2014).
Echols, R. M., Tosiello, R. L., Haverstock, D. C. & Tice, A. D. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin. Infect. Dis. 29 , 113–119 (1999).
Hooton, T. M. & Gupta., K. Acute Simple Cystitis in Women (eds Calderwood, S. B. & Bloom, A.) (UpToDate, 2018).
Hooton, T. M. Clinical practice. Uncomplicated urinary tract infection. N. Engl. J. Med. 366 , 1028–1037 (2012).
Katchman, E. A. et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am. J. Med. 118 , 1196–1207 (2005).
Hsu, D. D. & Melzer, M. Strategy to reduce E. coli bacteraemia based on cohort data from a London teaching hospital. Postgrad. Med. J. 94 , 212–215 (2018).
CAS Google Scholar
Seymour, C. W. et al. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 376 , 2235–2244 (2017).
PubMed PubMed Central Google Scholar
Hatfield, K. M. et al. Assessing variability in hospital-level mortality among U.S. Medicare beneficiaries with hospitalizations for severe sepsis and septic shock. Crit. Care Med. 46 , 1753–1760 (2018).
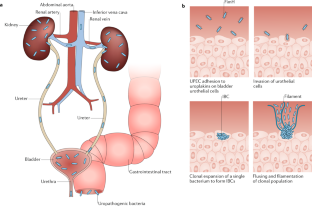
Flores-Mireles, A. L. et al. Fibrinogen release and deposition on urinary catheters placed during urological procedures. J. Urol. 196 , 416–421 (2016). This study illustrates the molecular mechanism by which fibrinogen deposition on urinary catheters facilitates bladder colonization .
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13 , 269–284 (2015).
CAS PubMed PubMed Central Google Scholar
Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7 , 653–660 (2010).
Spees, A. M. et al. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. mBio 4 , e00430-133 (2013).
Google Scholar
Koves, B. et al. Benefits and harms of treatment of asymptomatic bacteriuria: a systematic review and meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur. Urol. 72 , 865–868 (2017).
Yamamoto, S. et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J. Urol. 157 , 1127–1129 (1997).
Mayer, B. T. et al. Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J. Infect. Dis. 212 , 793–802 (2015).
Macklaim, J. M., Clemente, J. C., Knight, R., Gloor, G. B. & Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 26 , 27799 (2015).
Hooton, T. M. et al. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 293 , 949–955 (2005).
Hooton, T. M., Roberts, P. L. & Stapleton, A. E. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA 307 , 583–589 (2012).
Schreiber IV, H. L. et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci. Transl Med. 9 , eaaf12833 (2017).
Subashchandrabose, S. & Mobley, H. L. T. Virulence and fitness determinants of uropathogenic Escherichia coli . Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.UTI-0015-2012 (2015).
Article PubMed Google Scholar
Anderson, G. G. et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301 , 105–107 (2003).
Song, J. et al. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl Acad. Sci. USA 106 , 14966–14971 (2009).
Schwartz, D. J., Chen, S. L., Hultgren, S. J. & Seed, P. C. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect. Immun. 79 , 4250–4259 (2011).
Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A. & Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4 , e329 (2007).
De Nisco, N. J. et al. Direct detection of tissue-resident bacteria and chronic inflammation in the bladder wall of postmenopausal women with recurrent urinary tract infection. J. Mol. Biol. 431 , 4368–4379 (2019).
Robino, L. et al. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog. Dis. 68 , 78–81 (2013).
Robino, L. et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin. Infect. Dis. 59 , e158–e164 (2014).
Duell, B. L. et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J. Immunol. 188 , 781–792 (2012).
Ingersoll, M. A., Kline, K. A., Nielsen, H. V. & Hultgren, S. J. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol. 10 , 2568–2578 (2008).
Sivick, K. E., Schaller, M. A., Smith, S. N. & Mobley, H. L. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J. Immunol. 184 , 2065–2075 (2010).
Schiwon, M. et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156 , 456–468 (2014).
Mulvey, M. A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282 , 1494–1497 (1998).
Schaale, K. et al. Strain- and host species-specific inflammasome activation, IL-1β release, and cell death in macrophages infected with uropathogenic Escherichia coli . Mucosal Immunol. 9 , 124–136 (2016).
Schlager, T. A., LeGallo, R., Innes, D., Hendley, J. O. & Peters, C. A. B cell infiltration and lymphonodular hyperplasia in bladder submucosa of patients with persistent bacteriuria and recurrent urinary tract infections. J. Urol. 186 , 2359–2364 (2011).
Mysorekar, I. U. & Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA 103 , 14170–14175 (2006).
Hannan, T. J. et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1 , 46–57 (2014).
Hannan, T. J., Mysorekar, I. U., Hung, C. S., Isaacson-Schmid, M. L. & Hultgren, S. J. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 6 , e1001042 (2010).
Ferry, S. A., Holm, S. E., Stenlund, H., Lundholm, R. & Monsen, T. J. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand. J. Infect. Dis. 36 , 296–301 (2004).
Yu, L. et al. Mucosal infection rewires TNFɑ signaling dynamics to skew susceptibility to recurrence. eLife 8 , e46677 (2019).
Wurpel, D. J., Beatson, S. A., Totsika, M., Petty, N. K. & Schembri, M. A. Chaperone-usher fimbriae of Escherichia coli. PLoS One 8 , e52835 (2013). This study provides an excellent overview of the phenotypic relationships of CUP pili throughout all E. coli species .
Stapleton, A. E., Stroud, M. R., Hakomori, S. I. & Stamm, W. E. The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor In vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect. Immun. 66 , 3856–3861 (1998).
Dodson, K. W. et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105 , 733–743 (2001).
Hung, C. S. et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 44 , 903–915 (2002).
Backhed, F. et al. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277 , 18198–18205 (2002).
Luterbach, C. L. & Mobley, H. L. T. Cross talk between MarR-like transcription factors coordinates the regulation of motility in uropathogenic Escherichia coli . Infect. Immun. 86 , e00338-18 (2018).
Wurpel, D. J. et al. F9 fimbriae of uropathogenic Escherichia coli are expressed at low temperature and recognise Galbeta1-3GlcNAc-containing glycans. PLoS One 9 , e93177 (2014).
Subashchandrabose, S. et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc. Natl Acad. Sci. USA 111 , 18327–18332 (2014).
Conover, M. S. et al. Inflammation-induced adhesin-receptor interaction provides a fitness advantage to uropathogenic E. coli during chronic infection. Cell Host Microbe 20 , 482–492 (2016). This study elucidates the role of the F9 pilus adhesin FmlH in colonization of chronically infected bladders via interaction with galactose moieties exposed by inflammation .
Spaulding, C. N. et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 546 , 528–532 (2017). This study highlights the efficacy of mannose analogues in the selective and simultaneous extirpation of UPEC from the bladder and gastrointestinal niches without a concomitant disruption of the beneficial microbiota .
Sauer, M. M. et al. Binding of the bacterial adhesin FimH to its natural, multivalent high-mannose type glycan targets. J. Am. Chem. Soc. 141 , 936–944 (2018).
Kalas, V. et al. Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions. Sci. Adv. 3 , e1601944 (2017).
Chen, S. L. et al. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl Acad. Sci. USA 106 , 22439–22444 (2009).
Schwartz, D. J. et al. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc. Natl Acad. Sci. USA 110 , 15530–15537 (2013).
Abraham, S. N. et al. Glycerol-induced unraveling of the tight helical conformation of Escherichia coli type 1 fimbriae. J. Bacteriol. 174 , 5145–5148 (1992).
Aprikian, P. et al. The bacterial fimbrial tip acts as a mechanical force sensor. PLoS Biol. 9 , e1000617 (2011).
Mortezaei, N. et al. Structure and function of enterotoxigenic Escherichia coli fimbriae from differing assembly pathways. Mol. Microbiol. 95 , 116–126 (2015).
Spaulding, C. N. et al. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. eLife 7 , e31662 (2018).
Hospenthal, M. K. et al. The cryoelectron microscopy structure of the Type 1 chaperone-usher pilus rod. Structure 25 , 1829–1838.e4 (2017).
Du, M. et al. Handover mechanism of the growing pilus by the bacterial outer-membrane usher FimD. Nature 562 , 444–447 (2018).
Omattage, N. S. et al. Structural basis for usher activation and intramolecular subunit transfer in P pilus biogenesis in Escherichia coli. Nat. Microbiol. 3 , 1362–1368 (2018).
Pinkner, J. S. et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl Acad. Sci. USA 103 , 17897–17902 (2006).
Miethke, M. & Marahiel, M. A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71 , 413–451 (2007).
Lopez, C. A. & Skaar, E. P. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23 , 737–748 (2018).
Weichhart, T., Haidinger, M., Horl, W. H. & Saemann, M. D. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur. J. Clin. Invest. 38 , 29–38 (2008).
Reigstad, C. S., Hultgren, S. J. & Gordon, J. I. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J. Biol. Chem. 282 , 21259–21267 (2007).
Patras, K. A. et al. Augmentation of urinary lactoferrin enhances host innate immune clearance of uropathogenic Escherichia coli . J. Innate Immun. 11 , 481–495 (2019).
Chaturvedi, K. S., Hung, C. S., Crowley, J. R., Stapleton, A. E. & Henderson, J. P. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 8 , 731–736 (2012).
Robinson, A. E., Heffernan, J. R. & Henderson, J. P. The iron hand of uropathogenic Escherichia coli: the role of transition metal control in virulence. Future Microbiol. 13 , 745–756 (2018).
Henderson, J. P. et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 5 , e1000305 (2009).
Johnson, J. R. et al. Contribution of yersiniabactin to the virulence of an Escherichia coli sequence type 69 (‘‘clonal group A’’) cystitis isolate in murine models of urinary tract infection and sepsis. Microb. Pathog. 120 , 128–131 (2018).
Parker, K. S., Wilson, J. D., Marschall, J., Mucha, P. J. & Henderson, J. P. Network analysis reveals sex- and antibiotic resistance-associated antivirulence targets in clinical uropathogens. ACS Infect. Dis. 1 , 523–532 (2015).
Koh, E. I., Robinson, A. E., Bandara, N., Rogers, B. E. & Henderson, J. P. Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat. Chem. Biol. 13 , 1016–1021 (2017).
Brumbaugh, A. R. et al. Blocking yersiniabactin import attenuates extraintestinal pathogenic Escherichia coli in cystitis and pyelonephritis and represents a novel target to prevent urinary tract infection. Infect. Immun. 83 , 1443–1450 (2015).
Paniagua-Contreras, G. L. et al. Comprehensive expression analysis of pathogenicity genes in uropathogenic Escherichia coli strains. Microb. Pathog. 103 , 1–7 (2017).
Ohlemacher, S. I. et al. Enterobacteria secrete an inhibitor of Pseudomonas virulence during clinical bacteriuria. J. Clin. Invest. 127 , 4018–4030 (2017). This study highlights the importance of metal acquisition in UPEC virulence and identifies a siderophore metabolic by-product that can inhibit iron uptake by competing bacteria .
Welch, R. A. Uropathogenic Escherichia coli-associated exotoxins. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.UTI-0011-2012 (2016).
Marrs, C. F. et al. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J. Med. Microbiol. 51 , 138–142 (2002).
Hannan, T. J. et al. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol. Microbiol. 67 , 116–128 (2008).
Mobley, H. L. et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58 , 1281–1289 (1990).
Nagamatsu, K. et al. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl Acad. Sci. USA 112 , E871–E880 (2015).
Otto, K. & Silhavy, T. J. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl Acad. Sci. USA 99 , 2287–2292 (2002).
Tschauner, K., Hornschemeyer, P., Muller, V. S. & Hunke, S. Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS One 9 , e107383 (2014).
Behr, S., Fried, L. & Jung, K. Identification of a novel nutrient-sensing histidine kinase/response regulator network in Escherichia coli. J. Bacteriol. 196 , 2023–2029 (2014).
Steiner, B. D. et al. Evidence of cross-regulation in two closely related pyruvate-sensing systems in uropathogenic Escherichia coli. J. Membr. Biol. 251 , 65–74 (2018).
Clarke, M. B. & Sperandio, V. Transcriptional autoregulation by quorum sensing Escherichia coli regulators B and C (QseBC) in enterohaemorrhagic E. coli (EHEC). Mol. Microbiol. 58 , 441–455 (2005).
Breland, E. J., Zhang, E. W., Bermudez, T., Martinez, C. R. III & Hadjifrangiskou, M. The histidine residue of QseC is required for canonical signaling between QseB and PmrB in uropathogenic Escherichia coli. J. Bacteriol. https://doi.org/10.1128/JB.00060-17 (2017).
Article PubMed PubMed Central Google Scholar
Kostakioti, M., Hadjifrangiskou, M., Pinkner, J. S. & Hultgren, S. J. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol. Microbiol. 73 , 1020–1031 (2009).
Guckes, K. R. et al. Strong cross-system interactions drive the activation of the QseB response regulator in the absence of its cognate sensor. Proc. Natl Acad. Sci. USA 110 , 16592–16597 (2013).
Guckes, K. R. et al. Signaling by two-component system noncognate partners promotes intrinsic tolerance to polymyxin B in uropathogenic Escherichia coli. Sci. Signal. 10 , eaag1775 (2017).
Shah, C., Baral, R., Bartaula, B. & Shrestha, L. B. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 19 , 204 (2019).
Eberly, A. R. et al. Biofilm formation by uropathogenic Escherichia coli is favored under oxygen conditions that mimic the bladder environment. Int. J. Mol. Sci. 18 , 20777 (2017). This study reveals the mechanisms by which UPEC biofilm formation is triggered within the bladder in response to an oxygen-poor environment .
Beebout, C. J. et al. Respiratory heterogeneity shapes biofilm formation and host colonization in uropathogenic Escherichia coli . mBio 10 , e02400-18 (2019).
Reichhardt, C. & Cegelski, L. Solid-state NMR for bacterial biofilms. Mol. Phys. 112 , 887–894 (2013).
PubMed Central Google Scholar
Chapman, M. R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295 , 851–855 (2002).
Van Gerven, N., Klein, R. D., Hultgren, S. J. & Remaut, H. Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 23 , 693–706 (2015).
Biesecker, S. G., Nicastro, L. K., Wilson, R. P. & Tukel, C. The functional amyloid curli protects Escherichia coli against complement-mediated bactericidal activity. Biomolecules 8 , 5 (2018).
Cegelski, L. et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 5 , 913–919 (2009).
Hollenbeck, E. C. et al. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc. Natl Acad. Sci. USA 115 , 10106–10111 (2018).
Klein, R. D. et al. Structure-function analysis of the curli accessory protein CsgE defines surfaces essential for coordinating amyloid fiber formation. mBio 9 , e01349-18 (2018).
Evans, M. L. et al. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol. Cell 57 , 445–455 (2015).
Schubeis, T. et al. Structural and functional characterization of the curli adaptor protein CsgF. FEBS Lett. 592 , 1020–1029 (2018).
Sleutel, M. et al. Nucleation and growth of a bacterial functional amyloid at single-fiber resolution. Nat. Chem. Biol. 13 , 902–908 (2017).
Nhu, N. T. K. et al. Discovery of new genes involved in curli production by a uropathogenic Escherichia coli strain from the highly virulent O45:K1:H7 lineage. mBio 9 , e01462-18 (2018).
Smith, D. R. et al. The production of curli amyloid fibers is deeply integrated into the biology of Escherichia coli . Biomolecules 7 , 75 (2017).
Majdalani, N., Heck, M., Stout, V. & Gottesman, S. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 187 , 6770–6778 (2005).
Magill, S. S. et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 370 , 1198–1208 (2014).
Rice, L. B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197 , 1079–1081 (2008).
Santajit, S. & Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016 , 2475067 (2016).
Weiner, L. M. et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 37 , 1288–1301 (2016).
Centers for Disease Control and Prevention. 2014 HAI progress report (CDC, 2016).
Daniels, K. R., Lee, G. C. & Frei, C. R. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001–2010. Am. J. Infect. Control. 42 , 17–22 (2014).
Meddings, J., Rogers, M. A., Macy, M. & Saint, S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin. Infect. Dis. 51 , 550–560 (2010).
Delnay, K. M., Stonehill, W. H., Goldman, H., Jukkola, A. F. & Dmochowski, R. R. Bladder histological changes associated with chronic indwelling urinary catheter. J. Urol. 161 , 1106–1108; discussion 1108–1109 (1999).
Peychl, L. & Zalud, R. Changes in the urinary bladder caused by short-term permanent catheter insertion. Cas. Lek. Cesk. 147 , 325–329 (2008).
Guiton, P. S., Hannan, T. J., Ford, B., Caparon, M. G. & Hultgren, S. J. Enterococcus faecalis overcomes foreign body-mediated inflammation to establish urinary tract infections. Infect. Immun. 81 , 329–339 (2013).
Flores-Mireles, A. L., Pinkner, J. S., Caparon, M. G. & Hultgren, S. J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl Med. 6 , 254ra127 (2014).
Puyo, C. A. et al. Mitochondrial DNA induces Foley catheter related bladder inflammation via Toll-like receptor 9 activation. Sci. Rep. 8 , 6377 (2018).
Xu, W. et al. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes 3 , 28 (2017).
La Rosa, S. L., Montealegre, M. C., Singh, K. V. & Murray, B. E. Enterococcus faecalis Ebp pili are important for cell-cell aggregation and intraspecies gene transfer. Microbiology 162 , 798–802 (2016).
Zhanel, G. G. et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 26 , 380–388 (2005).
Al Mohajer, M., Musher, D. M., Minard, C. G. & Darouiche, R. O. Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand. J. Infect. Dis. 45 , 688–695 (2013).
Gilbert, N. M. et al. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob. Adv. Health Med. 2 , 59–69 (2013).
Routh, J. C., Alt, A. L., Ashley, R. A., Kramer, S. A. & Boyce, T. G. Increasing prevalence and associated risk factors for methicillin resistant staphylococcus aureus bacteriuria. J. Urol. 181 , 1694–1698 (2009).
Cheng, A. G. et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23 , 3393–3404 (2009).
Walker, J. N. et al. The Staphylococcus aureus ArlRS two-component system is a novel regulator of agglutination and pathogenesis. PLoS Pathog. 9 , e1003819 (2013).
Walker, J. N. et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl Acad. Sci. USA 114 , E8721–E8730 (2017).
McAdow, M. et al. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 7 , e1002307 (2011).
Warren, J. W., Tenney, J. H., Hoopes, J. M., Muncie, H. L. & Anthony, W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146 , 719–723 (1982).
Armbruster, C. E., Prenovost, K., Mobley, H. L. & Mody, L. How often do clinically diagnosed catheter-associated urinary tract infections in nursing homes meet standardized criteria? J. Am. Geriatr. Soc. 65 , 395–401 (2017). In this study, the authors demonstrate the molecular basis for synergy among members of polymicrobial communities during colonization of urinary catheters in the health-care setting .
Jacobsen, S. M., Stickler, D. J., Mobley, H. L. & Shirtliff, M. E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21 , 26–59 (2008).
Coker, C., Poore, C. A., Li, X. & Mobley, H. L. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2 , 1497–1505 (2000).
Broomfield, R. J., Morgan, S. D., Khan, A. & Stickler, D. J. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J. Med. Microbiol. 58 , 1367–1375 (2009).
Schaffer, J. N., Norsworthy, A. N., Sun, T. T. & Pearson, M. M. Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc. Natl Acad. Sci. USA 113 , 4494–4499 (2016).
Armbruster, C. E. et al. The pathogenic potential of Proteus mirabilis is enhanced by other uropathogens during polymicrobial urinary tract infection. Infect. Immun. 85 , e00808-16 (2017).
Shapiro, D. J., Hicks, L. A., Pavia, A. T. & Hersh, A. L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J. Antimicrob. Chemother. 69 , 234–240 (2014).
Klein, T. et al. FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 53 , 8627–8641 (2010).
Cusumano, C. K. et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl Med. 3 , 109ra115 (2011).
Schonemann, W. et al. Improvement of aglycone pi-stacking yields nanomolar to sub-nanomolar FimH antagonists. ChemMedChem 14 , 749–757 (2019).
Jarvis, C. et al. Antivirulence isoquinolone mannosides: optimization of the biaryl aglycone for FimH lectin binding affinity and efficacy in the treatment of chronic UTI. ChemMedChem 11 , 367–373 (2016).
Mydock-McGrane, L. et al. Antivirulence C-mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J. Med. Chem. 59 , 9390–9408 (2016).
Touaibia, M. et al. Sites for dynamic protein-carbohydrate interactions of O- and C-linked mannosides on the E. coli FimH adhesin. Molecules 22 , 1101 (2017).
Kalas, V. et al. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc. Natl Acad. Sci. USA 115 , E2819–E2828 (2018).
Fimbrion Therapeutics. The opportunity: mannosides as therapeutics. Fimbrion https://www.fimbrion.com/pipeline (2019).
Langermann, S. et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli . J. Infect. Dis. 181 , 774–778 (2000).
Savar, N. S. et al. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J. Biotechnol. 175 , 31–37 (2014).
Sarkissian, C. A., Alteri, C. J. & Mobley, H. L. T. UTI patients have pre-existing antigen-specific antibody titers against UTI vaccine antigens. Vaccine 37 , 4937–4946 (2019).
Mobley, H. L. & Alteri, C. J. Development of a vaccine against Escherichia coli urinary tract infections. Pathogens 5 , 1 (2015).
Flores-Mireles, A. L. et al. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. m Bio 7 , e01653-16 (2016).
Kane, T. L., Carothers, K. E. & Lee, S. W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug. Targets 19 , 111–127 (2018).
Hawkey, C. J. COX-1 and COX-2 inhibitors. Best. Pract. Res. Clin. Gastroenterol. 15 , 801–820 (2001).
Bleidorn, J., Gagyor, I., Kochen, M. M., Wegscheider, K. & Hummers-Pradier, E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? - Results of a randomized controlled pilot trial. BMC Med. 8 , 30 (2010).
Gagyor, I. et al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 351 , h6544 (2015).
Kronenberg, A. et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ 359 , j4784 (2017).
Zinkernagel, A. S., Johnson, R. S. & Nizet, V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 85 , 1339–1346 (2007).
Lin, A. E. et al. Role of hypoxia inducible factor-1alpha (HIF-1alpha) in innate defense against uropathogenic Escherichia coli infection. PLoS Pathog. 11 , e1004818 (2015).
Sunden, F., Hakansson, L., Ljunggren, E. & Wullt, B. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J. Urol. 184 , 179–185 (2010).
Darouiche, R. O. et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 78 , 341–346 (2011).
Koves, B. et al. Rare emergence of symptoms during long-term asymptomatic Escherichia coli 83972 carriage without an altered virulence factor repertoire. J. Urol. 191 , 519–528 (2014).
Stork, C. et al. Characterization of asymptomatic bacteriuria Escherichia coli isolates in search of alternative strains for efficient bacterial interference against uropathogens. Front. Microbiol. 9 , 214 (2018).
Hagan, E. C., Lloyd, A. L., Rasko, D. A., Faerber, G. J. & Mobley, H. L. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6 , e1001187 (2010).
Chen, S. L. et al. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci. Transl Med. 5 , 184ra160 (2013).
Duraj-Thatte, A. M., Praveschotinunt, P., Nash, T. R., Ward, F. R. & Joshi, N. S. Modulating bacterial and gut mucosal interactions with engineered biofilm matrix proteins. Sci. Rep. 8 , 3475 (2018).
Smith, A. L. et al. Treatment and prevention of recurrent lower urinary tract infections in women: a rapid review with practice recommendations. J. Urol. 200 , 1174–1191 (2018).
Dbeibo, L. et al. Evaluation of CpxRA as a therapeutic target for uropathogenic Escherichia coli infections. Infect. Immun. 86 , e00798-17 (2018).
Simmering, J. E., Tang, F., Cavanaugh, J. E., Polgreen, L. A. & Polgreen, P. M. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open. Forum Infect. Dis. 4 , ofw281 (2017).
Wagenlehner, F. M. et al. Diagnosis and management for urosepsis. Int. J. Urol. 20 , 963–970 (2013).
O’Brien, V. P. et al. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nat. Microbiol. 2 , 16196 (2016).
Hannan, T. J. et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 36 , 616–648 (2012).
Sumati, A. H. & Saritha, N. K. Association of urinary tract infection in women with bacterial vaginosis. J. Glob. Infect. Dis. 1 , 151–152 (2009).
Gilbert, N. M., O'Brien, V. P. & Lewis, A. L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 13 , e1006238 (2017).
Stapleton, A. E. et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 52 , 1212–1217 (2011).
Scholes, D. et al. Risk factors associated with acute pyelonephritis in healthy women. Ann. Intern. Med. 142 , 20–27 (2005).
Bautista, C. T. et al. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil. Med. Res. 3 , 4 (2016).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2 , 123–140 (2004).
Touchon, M. et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5 , e1000344 (2009).
Piatti, G., Mannini, A., Balistreri, M. & Schito, A. M. Virulence factors in urinary Escherichia coli strains: phylogenetic background and quinolone and fluoroquinolone resistance. J. Clin. Microbiol. 46 , 480–487 (2008).
Ejrnaes, K. et al. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: phylogenetic groups, virulence factors and biofilm formation. Virulence 2 , 528–537 (2011).
Wang, Y. et al. Drug resistance and virulence of uropathogenic Escherichia coli from Shanghai, China. J. Antibiot. 67 , 799–805 (2014).
Nielsen, K. L. et al. Whole-genome comparison of urinary pathogenic Escherichia coli and faecal isolates of UTI patients and healthy controls. Int. J. Med. Microbiol. 307 , 497–507 (2017).
Mann, R., Mediati, D. G., Duggin, I. G., Harry, E. J. & Bottomley, A. L. Metabolic adaptations of uropathogenic E. coli in the urinary tract. Front. Cell Infect. Microbiol. 7 , 241 (2017).
Lavigne, J. P. et al. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: a French prospective multicentre study. J. Med. Microbiol. 65 , 530–537 (2016).
Chen, Z. et al. The urinary microbiome in patients with refractory urge incontinence and recurrent urinary tract infection. Int. Urogynecol J. 29 , 1775–1782 (2018).
Maddirala, A. et al. Biphenyl Gal and GalNAc FmlH Lectin antagonists of uropathogenic E. coli (UPEC): optimization through iterative rational drug design. J. Med. Chem. 62 , 467–479 (2019). This study provides immunological insight into the long history of epidemiological data suggesting that a history of UTI is the greatest risk factor for the development of subsequent UTI by identifying long-term damage caused by immunomediated urothelial exfoliation .
Download references
Acknowledgements
The authors thank K. W. Dodson and T. J. Hannan for their helpful suggestions and comments on the manuscript. Work in the authors’ laboratory was supported by grants AI099099, AI095542, AI029549 and AI048689 from the US National Institution of Allergy and Infectious Diseases, grants DK051406 and DK108840 from the US National Institute of Diabetes and Digestive and Kidney Diseases and Medical Scientist Training Program Grant T32GM07200 from the US National Institute of General Medical Sciences. The authors apologize to researchers whose work was not included in this Review due to space constraints.
Author information
Authors and affiliations.
Department of Molecular Microbiology, Washington University School of Medicine, St Louis, MO, USA
Roger D. Klein & Scott J. Hultgren
Center for Women’s Infectious Disease Research, Washington University School of Medicine, St Louis, MO, USA
You can also search for this author in PubMed Google Scholar
Contributions
R.D.K and S.J.H. researched data for the article, discussed the content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Correspondence to Scott J. Hultgren .
Ethics declarations
Competing interests.
S.J.H. has an ownership interest in Fimbrion Therapeutics, and may benefit if the company is successful in marketing mannosides. S.J.H. is also the chief scientific officer of QureTech Bio. R.D.K. declares no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
European Association of Urology Guidelines: https://uroweb.org/wp-content/uploads/EAU-Extended-Guidelines-2015-Edn..pdf
Supplementary information
The opening of the urethra through which urine exits in males and females, sometimes referred to as the external urethral orifice.
An isolated infection of the bladder and/or lower urinary tract without signs or symptoms of upper urinary tract or systemic infection in a patient without significant comorbid conditions, such as pregnancy or structural urinary tract abnormalities.
An infection of the upper urinary tract leading to upper urinary tract signs or systemic symptoms, or any urinary tract infection in pregnant women, immunocompromised patients or patients with functional urinary tract abnormalities.
An infection of the renal pelvis, calices and/or cortex.
A glycoprotein released into the bladder lumen in response to inflammation and infection, and which can coat urinary catheters and serve as a nidus for bacterial binding.
Large collections of microbial organisms embedded within a complex extracellular matrix comprising polysaccharides, proteinaceous fibres and extracellular DNA.
Also known as facet cells, umbrella cells are large, polarized superficial cells that line the bladder lumen.
An inbred mouse strain commonly used for the study of a variety of disease processes, including urinary tract infections.
Enlargement of mucosal lymphoid nodules seen via histology.
The presence of bacteria in urine not attributable to contamination. Can be symptomatic or asymptomatic.
Sequestration of nutrients by a host organism to prevent colonization by and proliferation of pathogens.
Low molecular weight compounds secreted by the host systems to bind metal ions and transport them across cellular membranes.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Klein, R.D., Hultgren, S.J. Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat Rev Microbiol 18 , 211–226 (2020). https://doi.org/10.1038/s41579-020-0324-0
Download citation
Accepted : 07 January 2020
Published : 18 February 2020
Issue Date : April 2020
DOI : https://doi.org/10.1038/s41579-020-0324-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Antibacterial properties and urease suppression ability of lactobacillus inhibit the development of infectious urinary stones caused by proteus mirabilis.
- Dominika Szczerbiec
- Katarzyna Bednarska-Szczepaniak
- Agnieszka Torzewska
Scientific Reports (2024)
- Morgan R. Timm
- Seongmi K. Russell
- Scott J. Hultgren
Nature Reviews Microbiology (2024)
Gentamicin loaded niosomes against intracellular uropathogenic Escherichia coli strains
- Jacopo Forte
- Linda Maurizi
- Catia Longhi
Carbapenem-resistant Enterobacteriaceae in the livestock, humans and environmental samples around the globe: a systematic review and meta-analysis
- Barbra Tuhamize
- Joel Bazira
Effect of DMPEI coating against biofilm formation on PVC catheter surface
- Vinícius S. Tarabal
- Yuri K. D. Abud
- Paulo A. Granjeiro
World Journal of Microbiology and Biotechnology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- Search Menu
- Sign in through your institution
- Volume 11, Issue 9, September 2024 (In Progress)
- Volume 11, Issue 8, August 2024
- Advance articles
- Editor's Choice
- Insight Articles
- Novel ID Cases and Teaching Cases
- Supplement Archive
- Cover Archive
- IDSA Journals
- Clinical Infectious Diseases
- The Journal of Infectious Diseases
- Author Guidelines
- Open Access
- Why Publish
- IDSA Journals Calls for Papers
- About Open Forum Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Conclusions, supplementary data.
- < Previous
Definitions of Urinary Tract Infection in Current Research: A Systematic Review
M. P. B. and R. M. H. J. contributed equally to this work.
Potential conflicts of interest. All authors: No reported conflicts.
- Article contents
- Figures & tables
Manu P Bilsen, Rosa M H Jongeneel, Caroline Schneeberger, Tamara N Platteel, Cees van Nieuwkoop, Lona Mody, Jeffrey M Caterino, Suzanne E Geerlings, Bela Köves, Florian Wagenlehner, Simon P Conroy, Leo G Visser, Merel M C Lambregts, Definitions of Urinary Tract Infection in Current Research: A Systematic Review, Open Forum Infectious Diseases , Volume 10, Issue 7, July 2023, ofad332, https://doi.org/10.1093/ofid/ofad332
- Permissions Icon Permissions
Defining urinary tract infection (UTI) is complex, as numerous clinical and diagnostic parameters are involved. In this systematic review, we aimed to gain insight into how UTI is defined across current studies. We included 47 studies, published between January 2019 and May 2022, investigating therapeutic or prophylactic interventions in adult patients with UTI. Signs and symptoms, pyuria, and a positive urine culture were required in 85%, 28%, and 55% of study definitions, respectively. Five studies (11%) required all 3 categories for the diagnosis of UTI. Thresholds for significant bacteriuria varied from 10 3 to 10 5 colony-forming units/mL. None of the 12 studies including acute cystitis and 2 of 12 (17%) defining acute pyelonephritis used identical definitions. Complicated UTI was defined by both host factors and systemic involvement in 9 of 14 (64%) studies. In conclusion, UTI definitions are heterogeneous across recent studies, highlighting the need for a consensus-based, research reference standard for UTI.
Urinary tract infection (UTI) refers to a plethora of clinical phenotypes, including cystitis, pyelonephritis, prostatitis, urosepsis, and catheter-associated UTI (CA-UTI) [ 1 , 2 ]. In both clinical practice and in research, the diagnosis of UTI is based on a multitude of clinical signs and symptoms and diagnostic tests. Signs and symptoms can be further subdivided into (1) lower urinary tract symptoms, such as dysuria, frequency, and urgency; (2) systemic signs and symptoms, such as fever; and (3) nonspecific signs and symptoms, such as nausea and malaise. Commonly used diagnostic tests include urine dipstick for determining the presence of leukocyte esterase and nitrites, microscopy or flow cytometry for quantification of pyuria, and urine and blood cultures.
When defining and diagnosing UTI, numerous combinations of signs, symptoms, and outcomes of diagnostic tests are possible, and this diversity is reflected in various research guidelines. For drug development and approval purposes, the European Medicines Agency (EMA) [ 3 ] and US Food and Drug Administration (FDA) [ 4 , 5 ] have developed guidelines for clinical trials evaluating antimicrobials for the treatment of UTI, summarized in Table 1 . These guidelines provide definitions for uncomplicated UTI, complicated UTI, and acute pyelonephritis. McGeer et al [ 6 ] have developed research guidelines for studies in long-term care facilities (LTCFs). Clinical practice guidelines include the Infectious Diseases Society of America (currently being updated) [ 7 ] and European Association of Urology [ 8 ] guidelines. It is important to distinguish between research guidelines and clinical practice guidelines as the latter are meant for treatment recommendations, and the definitions in these clinical guidelines are generally based on often limited diagnostic information available when assessing a patient in the clinical, near-patient setting.
European Medicines Agency and US Food and Drug Administration Definitions of Uncomplicated and Complicated Urinary Tract Infection
| Category . | uUTI . | cUTI . | ||
|---|---|---|---|---|
| EMA . | FDA . | EMA . | FDA . | |
| Symptoms | A minimum number of symptoms, such as frequency, urgency, and dysuria | ≥2 of dysuria, frequency, urgency, and suprapubic pain (lower abdominal discomfort is also mentioned in another section of the guidance document) Patients should not have signs or symptoms of systemic illness such as fever >38°C, shaking chills, or other manifestations suggestive of cUTI | A minimum number of signs/symptoms compatible with an ongoing process in the urinary tract, such as flank or pelvic pain, CVA tenderness, dysuria, frequency, or urgency | ≥2 of chills or rigors or warmth associated with fever (>38°C), flank or pelvic pain, dysuria, frequency or urgency, CVA tenderness (malaise is also mentioned in another section of the guidance document) |
| Host factors | Female patients | Female patients with normal anatomy of the urinary tract | ≥1 of indwelling catheter, urinary retention, obstruction, neurogenic bladder AP is mentioned separately from cUTI, but it is not further defined | ≥1 of indwelling urinary catheter, neurogenic bladder, obstructive uropathy, azotemia caused by intrinsic renal disease, urinary retention (including retention caused by BPH) AP is a subset of cUTI regardless of underlying abnormalities of the urinary tract |
| Pyuria | >10 leukocytes/μL | “A microscopic evaluation for pyuria or dipstick analysis for leukocytes, nitrites or a catalase test should be performed” | >10 leukocytes/μL | Urine dipstick positive for leukocyte esterase or >10 leukocytes/μL |
| Bacteriuria | >10 CFU/mL of a single relevant pathogen | ≥10 CFU/mL of a single species of bacteria | >10 CFU/mL of a single or no more than 2 relevant pathogens | ≥10 CFU/mL of a single species of bacteria |
| Category . | uUTI . | cUTI . | ||
|---|---|---|---|---|
| EMA . | FDA . | EMA . | FDA . | |
| Symptoms | A minimum number of symptoms, such as frequency, urgency, and dysuria | ≥2 of dysuria, frequency, urgency, and suprapubic pain (lower abdominal discomfort is also mentioned in another section of the guidance document) Patients should not have signs or symptoms of systemic illness such as fever >38°C, shaking chills, or other manifestations suggestive of cUTI | A minimum number of signs/symptoms compatible with an ongoing process in the urinary tract, such as flank or pelvic pain, CVA tenderness, dysuria, frequency, or urgency | ≥2 of chills or rigors or warmth associated with fever (>38°C), flank or pelvic pain, dysuria, frequency or urgency, CVA tenderness (malaise is also mentioned in another section of the guidance document) |
| Host factors | Female patients | Female patients with normal anatomy of the urinary tract | ≥1 of indwelling catheter, urinary retention, obstruction, neurogenic bladder AP is mentioned separately from cUTI, but it is not further defined | ≥1 of indwelling urinary catheter, neurogenic bladder, obstructive uropathy, azotemia caused by intrinsic renal disease, urinary retention (including retention caused by BPH) AP is a subset of cUTI regardless of underlying abnormalities of the urinary tract |
| Pyuria | >10 leukocytes/μL | “A microscopic evaluation for pyuria or dipstick analysis for leukocytes, nitrites or a catalase test should be performed” | >10 leukocytes/μL | Urine dipstick positive for leukocyte esterase or >10 leukocytes/μL |
| Bacteriuria | >10 CFU/mL of a single relevant pathogen | ≥10 CFU/mL of a single species of bacteria | >10 CFU/mL of a single or no more than 2 relevant pathogens | ≥10 CFU/mL of a single species of bacteria |
In the EMA guidelines, bacteriuria definitions were mentioned in the description of the microbiological intention-to-treat population. In the FDA guidelines, they were also mentioned separately, under clinical microbiology considerations.
Abbreviations: AP, acute pyelonephritis; BPH, benign prostatic hyperplasia; CFU, colony-forming units; cUTI, complicated urinary tract infection; CVA, costovertebral angle; EMA, European Medicines Agency; FDA, United States Food and Drug Administration; uUTI, uncomplicated urinary tract infection.
While the aforementioned research guidelines overlap in the sense that they all include a combination of symptoms and evidence of pyuria and/or bacteriuria in the definition of UTI, they also differ. For instance, none of these guidelines include the same set (or minimum number) of symptoms for the diagnosis of UTI. Moreover, the definition of complicated UTI is variable and based on either systemic signs and symptoms or the presence of host factors predisposing the patient to a complicated clinical course (eg, functional or anatomical abnormalities of the urinary tract).
It is probable that this wide range of possible definitions and different research guidelines pose problems for researchers conducting studies with patients with UTI. A uniform research definition increases homogeneity between studies, which is important for the interpretation, synthesis, and comparability of results, and mitigates the risk of misclassification bias. This is especially relevant in an era of rising antimicrobial resistance, in which novel antimicrobials are being investigated in large randomized controlled trials. The aim of this systematic review is to evaluate how UTI is defined in current studies, and to which extent these definitions differ between studies.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines [ 9 ].
Eligibility Criteria
Studies published between January 2019 and May 2022, investigating any therapeutic or prophylactic intervention in adults with (recurrent) UTI, were eligible for inclusion. Given the fact that definitions tend to change over time, this time frame was chosen to reflect the most recent consensus. In addition, updated FDA and EMA guidelines were published in 2019. We excluded studies concerning only prostatitis, CA-UTI, pericatheter or perioperative prophylaxis, or asymptomatic bacteriuria. Studies investigating patients with spinal cord injury or neurogenic bladder were also excluded, because separate UTI definitions are mostly used for patients who are unable to experience (or have altered perception of) lower urinary tract symptoms. Finally, we excluded systematic reviews, meta-analyses, and studies published in non-English-language journals
Search Strategy
Multiple electronic databases (PubMed, Embase, Web of Science, and the Cochrane library) were searched on 16 May 2022. Our search strategy was constructed by a research librarian and was based on a population, intervention, comparison, outcome (PICO)–style approach. We applied language and publication year filters as described above and used an “article” type filter for clinical trials. The complete search strategy is provided in Supplementary Material 1 .
Data Extraction and Analysis
Covidence software was used for screening and data extraction. References were imported and duplicates were removed. Title and abstract screening, full-text screening and data extraction were performed by 2 independent reviewers (M. P. B. and R. M. H. J.). In case of disagreement, a third researcher was consulted (M. M. C. L.) and a final decision was based on consensus.
For each study, the following data were collected: study design, setting, population, intervention, and the type of UTI under investigation. Criteria for the definition of UTI were subdivided into 3 categories: signs and symptoms, urinalysis, and urine culture. For each of these categories, we assessed whether they were required or conditionally required (ie, dependent on the presence of other categories) for the diagnosis of UTI. If categories were not mentioned, or if they were only required for a secondary outcome or definition, they were considered as not required. Definitions were derived from eligibility criteria unless definitions were explicitly stated elsewhere. For signs and symptoms, additional data were collected on minimum number of symptoms and symptom specification (eg, if fever and frequency were further defined). Moreover, we recorded which symptoms were part of the definition of acute cystitis, acute pyelonephritis, and UTI if a clinical phenotype was not mentioned (henceforth described as UTI–phenotype not specified). For the urinalysis category, we extracted which methods were used for determining pyuria, which cutoff values were applied, and whether nitrites were part of the UTI definition. Regarding the urine culture category, we recorded the cutoff value for colony-forming units (CFU)/mL and the maximum number of uropathogens. For all 3 categories, we assessed whether study definitions met FDA and EMA guideline requirements. Concerning complicated UTI, we collected the same components of the definition as described above, but we also assessed whether the definition was based on host factors, systemic involvement, or a combination of both. Finally, we compared definitions between studies, stratified per UTI type. No risk of bias assessment was performed as we studied definitions instead of outcomes. Data are summarized as proportions.
Study Selection and Study Characteristics
The study selection process is summarized in a PRISMA flowchart ( Figure 1 ). We screened 348 reports published between January 2019 and May 2022. Studies that were excluded during title and abstract screening (n = 290) mainly involved patients with CA-UTI or conditions other than UTI (eg, interstitial cystitis), or investigated pericatheter or perioperative prophylaxis. During full-text screening, 7 non-English articles and secondary analyses of articles already included in the study using our search criteria were excluded. A total of 47 randomized controlled trials and cohort studies with a median of 145 participants were included [ 2–56 ].

Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the study selection process. Abbreviation: UTI, urinary tract infection.
Thirty-one studies (66%) investigated antimicrobials for the treatment of UTI, and 15 (32%) evaluated antimicrobial prophylaxis for recurrent UTI. Sixteen studies (34%) only included women, 4 studies (9%) only included men, and 27 studies (57%) included both. Participants were hospitalized in 25 studies (53%) and treated through an outpatient or primary care clinic in 22 studies (47%). None of the included studies were conducted in LTCFs. Twelve studies (26%) included acute cystitis, 16 (34%) included acute pyelonephritis, and 13 (28%) included UTI–phenotype not specified. A table containing details of all included studies is provided in Supplementary Material 2 .
UTI Definition and Heterogeneity
Table 2 shows how UTI was defined across the included studies. In 11 studies (23%) the definition consisted of only signs and symptoms, in 16 studies (34%) the definition consisted of both signs and symptoms and a positive urine culture, and in 5 studies (11%) all 3 components (signs and symptoms, the presence of pyuria, and a positive urine culture) were required for the diagnosis of UTI. None of the studies investigating acute cystitis (n = 12) or UTI–phenotype not specified (n = 13) included the same set of symptoms and diagnostic criteria in their definition. Of the studies defining acute pyelonephritis, 2 (17%) used identical definitions.
Categories of Urinary Tract Infection Definition
| Categories of UTI Definition (n = 47) . | No. (%) . |
|---|---|
| Signs and symptoms | |
| Required | 40 (85) |
| Conditionally required | 1 (2) |
| Not required | 6 (13) |
| Signs and symptoms specified | 34/40 (85) |
| Minimum number of symptoms specified | 24/40 (60) |
| Pyuria | |
| Required | 13 (28) |
| Conditionally required | 4 (9) |
| Not required | 30 (64) |
| Method of establishing pyuria specified | 14/17 (82) |
| Dipstick only | 2 (14) |
| Quantification only | 4 (29) |
| Both methods allowed | 8 (57) |
| Cutoff for pyuria specified | 12/12 (100) |
| >5 leukocytes/HPF | 2 (17) |
| >10 leukocytes/µL or >10 leukocytes/HPF | 10 (83) |
| Urine culture | |
| Required | 26 (55) |
| Conditionally required | 1 (2) |
| Not required | 20 (43) |
| Cutoff for CFU/mL specified | 19/27 (70) |
| >10 CFU/mL | 8 (42) |
| >10 CFU/mL | 4 (21) |
| >10 CFU/mL | 7 (37) |
| Maximum No. of uropathogens specified | 4/27 (15) |
| Urine collection method specified | 12/47 (26) |
| Categories of UTI Definition (n = 47) . | No. (%) . |
|---|---|
| Signs and symptoms | |
| Required | 40 (85) |
| Conditionally required | 1 (2) |
| Not required | 6 (13) |
| Signs and symptoms specified | 34/40 (85) |
| Minimum number of symptoms specified | 24/40 (60) |
| Pyuria | |
| Required | 13 (28) |
| Conditionally required | 4 (9) |
| Not required | 30 (64) |
| Method of establishing pyuria specified | 14/17 (82) |
| Dipstick only | 2 (14) |
| Quantification only | 4 (29) |
| Both methods allowed | 8 (57) |
| Cutoff for pyuria specified | 12/12 (100) |
| >5 leukocytes/HPF | 2 (17) |
| >10 leukocytes/µL or >10 leukocytes/HPF | 10 (83) |
| Urine culture | |
| Required | 26 (55) |
| Conditionally required | 1 (2) |
| Not required | 20 (43) |
| Cutoff for CFU/mL specified | 19/27 (70) |
| >10 CFU/mL | 8 (42) |
| >10 CFU/mL | 4 (21) |
| >10 CFU/mL | 7 (37) |
| Maximum No. of uropathogens specified | 4/27 (15) |
| Urine collection method specified | 12/47 (26) |
If categories were not mentioned, they were considered as not required. Definitions were derived from eligibility criteria unless definitions were explicitly stated elsewhere. Percentages may not add up to 100 due to rounding.
Abbreviations: CFU, colony-forming units; HPF, high-power field; UTI, urinary tract infection.
Signs and Symptoms
Signs and symptoms were required for the diagnosis of UTI in 40 studies (85%). Of these, 34 (85%) specified signs and symptoms in the definition. The different signs and symptoms that were included in the definition of acute cystitis, acute pyelonephritis, and UTI–phenotype not specified are highlighted in Table 3 . FDA guidelines [ 4 ] require a minimum of 2 of the following symptoms for patients with uncomplicated UTI: dysuria, urgency, frequency, and suprapubic pain. Two of 12 studies (17%) met these criteria. Flank pain and/or costovertebral angle tenderness, fever, nausea and/or vomiting, and dysuria were most often included in the definition of acute pyelonephritis. Frequency was not further specified in any study. Perineal and/or prostate pain was part of the definition in 3 of 31 (10%) studies involving men. A specific temperature cutoff for fever was defined in 7 of 17 (65%) studies that included fever in the definition of UTI.
Symptoms and Signs in Different Types of Urinary Tract Infections
| Symptoms and Signs . | Acute Cystitis (n = 12) . | Acute Pyelonephritis (n = 16) . | UTI–Phenotype Not Specified (n = 13) . |
|---|---|---|---|
| Dysuria | 9 (75) | 8 (50) | 9 (69) |
| Urgency | 9 (75) | 6 (38) | 7 (54) |
| Frequency | 9 (75) | 7 (44) | 6 (46) |
| Suprapubic pain | 5 (42) | 0 | 6 (46) |
| Macroscopic hematuria | 4 (33) | 0 | 4 (31) |
| Lower abdominal pain | 2 (17) | 0 | 1 (8) |
| Perineal/prostate pain | 1 (8) | 0 | 2 (15) |
| Pelvic pain | 0 | 2 (13) | 1 (8) |
| Flank pain or CVA tenderness | 1 (8) | 12 (75) | 2 (15) |
| New urinary incontinence | 0 | 0 | 1 (8) |
| Worsening incontinence | 0 | 0 | 1 (8) |
| Fever | 0 | 12 (75) | 2 (15) |
| Chills or rigors | 0 | 7 (44) | 0 |
| Nausea or vomiting | 0 | 8 (50) | 0 |
| Symptoms not specified | 3 (25) | 4 (25) | 2 (15) |
| Symptoms and Signs . | Acute Cystitis (n = 12) . | Acute Pyelonephritis (n = 16) . | UTI–Phenotype Not Specified (n = 13) . |
|---|---|---|---|
| Dysuria | 9 (75) | 8 (50) | 9 (69) |
| Urgency | 9 (75) | 6 (38) | 7 (54) |
| Frequency | 9 (75) | 7 (44) | 6 (46) |
| Suprapubic pain | 5 (42) | 0 | 6 (46) |
| Macroscopic hematuria | 4 (33) | 0 | 4 (31) |
| Lower abdominal pain | 2 (17) | 0 | 1 (8) |
| Perineal/prostate pain | 1 (8) | 0 | 2 (15) |
| Pelvic pain | 0 | 2 (13) | 1 (8) |
| Flank pain or CVA tenderness | 1 (8) | 12 (75) | 2 (15) |
| New urinary incontinence | 0 | 0 | 1 (8) |
| Worsening incontinence | 0 | 0 | 1 (8) |
| Fever | 0 | 12 (75) | 2 (15) |
| Chills or rigors | 0 | 7 (44) | 0 |
| Nausea or vomiting | 0 | 8 (50) | 0 |
| Symptoms not specified | 3 (25) | 4 (25) | 2 (15) |
All symptoms and signs are shown as No. (%). Other symptoms mentioned in studies focusing on acute cystitis or UTI–phenotype not specified were vesical tenesmus (n = 1), malodorous and/or cloudy urine (n = 1), hypogastric pain (n = 1), and nocturia (n = 1). Additional criteria for the definition of acute pyelonephritis not mentioned in the table: elevated serum inflammatory parameters (n = 1), signs of pyelonephritis on ultrasound or computed tomography (n = 1), and hypotension (n = 1).
Abbreviations: CVA, costovertebral angle; UTI, urinary tract infection.
This included all studies investigating acute pyelonephritis, either alone or in conjunction with other types of UTI.
Urinalysis and Urine Culture
The presence of pyuria was required for the diagnosis of UTI in 13 of 47 (28%) studies, while both FDA and EMA guidelines [ 3–5 ] require pyuria in their definition of UTI. A cutoff for pyuria was specified in 12 studies, of which 10 (83%) applied a cutoff value of >10 leukocytes/µL or >10 leukocytes per high-power field (HPF). None of the included studies required the presence of nitrites for the diagnosis of UTI, although they were conditionally required in 3 studies (6%). A positive urine culture was mandatory for UTI diagnosis in 26 of 47 (55%) studies, of which 12 (55%) were conducted in the primary care or outpatient setting and 14 (56%) involved hospitalized patients. Of the 19 studies that mentioned a cutoff value for CFU/mL, 8 (42%) used a cutoff of 10 3 CFU/mL. Among all studies, 7 (15%) required a positive urine culture with at least 10 5 CFU/mL, complying with EMA and FDA guidelines [ 3–5 ].
Complicated UTI
We included 14 studies that defined complicated UTI. Three (21%) based their definition on complicating host factors only, 1 (7%) on systemic involvement only, and 9 (64%) on both host factors and systemic involvement. The various host factors included in the definition are provided in Table 4 . Male sex was considered a complicating factor in 2 studies (17%).
Definition of Complicated Urinary Tract Infection
| Complicated UTI (n = 14) . | No. (%) . |
|---|---|
| How is complicated UTI defined? | |
| Both host factors and systemic involvement | 9 (64) |
| Only host factors | 3 (21) |
| Only systemic involvement | 1 (7) |
| Complicated UTI not further defined | 1 (7) |
| Which host factors are part of complicated UTI criteria? | |
| Obstructive uropathy | 11 (92) |
| Functional or anatomical abnormalities of the urinary tract | 10 (83) |
| Indwelling catheter or nephrostomy tube | 9 (75) |
| Intrinsic renal disease | 8 (67) |
| Urinary retention in men due to BPH | 5 (42) |
| Urinary retention in general | 3 (25) |
| Male sex (regardless of urinary retention) | 2 (17) |
| Diabetes mellitus | 2 (17) |
| Systemic lupus erythematosus | 2 (17) |
| Pregnancy | 1 (8) |
| Immunocompromised state | 1 (8) |
| Kidney transplant recipient | 1 (8) |
| Complicated UTI (n = 14) . | No. (%) . |
|---|---|
| How is complicated UTI defined? | |
| Both host factors and systemic involvement | 9 (64) |
| Only host factors | 3 (21) |
| Only systemic involvement | 1 (7) |
| Complicated UTI not further defined | 1 (7) |
| Which host factors are part of complicated UTI criteria? | |
| Obstructive uropathy | 11 (92) |
| Functional or anatomical abnormalities of the urinary tract | 10 (83) |
| Indwelling catheter or nephrostomy tube | 9 (75) |
| Intrinsic renal disease | 8 (67) |
| Urinary retention in men due to BPH | 5 (42) |
| Urinary retention in general | 3 (25) |
| Male sex (regardless of urinary retention) | 2 (17) |
| Diabetes mellitus | 2 (17) |
| Systemic lupus erythematosus | 2 (17) |
| Pregnancy | 1 (8) |
| Immunocompromised state | 1 (8) |
| Kidney transplant recipient | 1 (8) |
For the purpose of this table, systemic involvement was defined as the presence of fever and/or rigors in the criteria for diagnosis of complicated UTI.
Abbreviations: BPH, benign prostatic hyperplasia; UTI, urinary tract infection.
Host factors were specified in 12 studies; this was used as the denominator for the proportions.
In this systematic review, we demonstrate that UTI definitions used in current research studies are highly heterogeneous in terms of clinical signs and diagnostic tests. In addition, few studies met symptom, pyuria, and urine culture criteria mentioned in existing research guidelines.
The presence of signs and symptoms was required in the majority of UTI definitions used in the included studies. As symptoms and signs remain the cornerstone of UTI diagnosis, it is noteworthy that 15% of studies did not require signs and symptoms for the diagnosis of UTI and an even greater number of studies did not specify which symptoms and signs needed to be present. Defining specific symptoms may help to mitigate the risk of misclassification. Symptom specification is especially relevant in studies involving older patients with UTI, given the high background prevalence of asymptomatic bacteriuria and pyuria [ 57–59 ]. Most of the studies that did clarify which symptoms were part of the UTI definition included classic UTI-associated symptoms such as dysuria, frequency, and urgency. However, we also found a broad variety of nonspecific manifestations, particularly in studies that did not define the UTI phenotype under investigation. Regardless of the unclear clinical relevance of nonspecific symptoms in UTI, this diversity of symptoms contributes to heterogeneity between studies, which is supported by our finding that few of the included studies used the same set of symptoms to define UTI. Furthermore, in over a third of the included reports, a minimum number of symptoms (for diagnosis) was not mentioned. Given the fact that even classic lower urinary tract symptoms are not 100% specific for UTI, and probability of UTI increases when a combination of symptoms is present, a minimum number of symptoms should be specified [ 60 ].
Pyuria and Bacteriuria
Interestingly, less than a third of included studies required the presence of pyuria in the definition of UTI. With the exception of patients with absolute neutropenia and complete obstructive uropathy, pyuria is present in virtually all symptomatic patients with bacteriuria, and its absence has a high negative predictive value for UTI [ 61–63 ]. In the included studies, pyuria was rarely quantified and thresholds for significant pyuria were low. A recent study has shown that low pyuria cutoffs should be avoided in older women, as the specificity for UTI is very low in this population [ 64 ]. Moreover, studies used different units of measurement interchangeably (ie, identical thresholds were applied for cells/µL and HPF), while results are influenced by different (pre)analytical procedures and previous studies have shown a µL-to-HPF ratio of 5:1 [ 65 ]. Be that as it may, quantification of pyuria in UTI studies should be encouraged, and pyuria should be included in the definition of UTI to reduce the risk of misclassification.
As growth of a uropathogen supports the diagnosis of UTI in a symptomatic patient, it is surprising that a positive urine culture was not part of the UTI definition in approximately half of the included studies. Even though urine cultures are not always required in a clinical setting (eg, in primary care), we believe that culture confirmation should at least be encouraged in a research setting. Furthermore, we found that studies used varying cutoffs for significant bacteriuria, ranging from 10 3 to 10 5 CFU/mL, while EMA and FDA guidelines both recommend a threshold of 10 5 CFU/mL. The question remains whether this is the optimal cutoff [ 66 ]; colony counts as low as 10 2 CFU/mL in midstream urine have been found in symptomatic premenopausal woman with Escherichia coli bacteriuria [ 61 , 62 ].
Studies differed widely in their definition of complicated UTI. Since the majority of studies defined complicated UTI based on both complicating host factors and systemic involvement, different clinical phenotypes were included in each study. This not only contributes further to disparities between studies, it also affects the applicability of study results. Moreover, the aforementioned heterogeneity is compounded by the fact that host factors are very diverse in themselves and there is no consensus about which host factors should be included in the definition of complicated UTI. As astutely phrased by James Johnson [ 67 ], “it may be time to find a different term than complicated UTI for UTIs that occur in patients with underlying predisposing factors, since this term seems hopelessly mired in ambiguity.” Johansen et al [ 68 ]. have proposed a UTI classification system for clinical and research purposes based on clinical phenotype, severity, host factors, and pathogen susceptibility. However, this classification system was not used by any of the included studies in our review. In the Netherlands, the primary care guidelines for UTI have already made a distinction between a UTI in a complicated host versus UTI with systemic involvement [ 69 ].
Existing Research Guidelines
We found that few studies met symptom, pyuria, and urine culture criteria mentioned in FDA and EMA guidelines [ 3–5 ]. In addition, we identified that studies more frequently based UTI definitions on clinical practice guidelines. The use of clinical practice guidelines in the place of research guidelines seems inappropriate, as clinical guidelines are less stringent than research guidelines and base empirical treatment recommendations on limited diagnostic information. Taken together, our findings imply that a widely accepted, consensus-based gold standard for the diagnosis of UTI is lacking and is much needed in the field of UTI research.
Strengths and Limitations
Strengths of this systematic review include our comprehensive search strategy, including multiple electronic databases, and extracting data from supplemental material , as UTI definitions were frequently only mentioned in a supplemental protocol. Our study has several limitations. For some of the included therapeutic studies, eligibility criteria served as a proxy for the UTI definition, if a definition was not mentioned separately. This might have contributed to additional heterogeneity. For instance, prophylactic studies including patients with recurrent UTI had more frequently provided separate UTI definitions, since these often served as outcome measures. Also, some heterogeneity might be explained by the fact that we included studies that investigated different UTI phenotypes. However, this effect was mitigated by evaluating different UTI phenotypes separately. Another limitation is that we filtered our search strategy on publication date and study type. While expanding the time period would have provided more data, it would not reflect the most recent consensus and would likely have contributed to further heterogeneity, as these studies were published before the FDA and EMA guidance documents. Furthermore, including more observational studies most likely would not have reduced heterogeneity, as these are presumably less likely to follow FDA and EMA guidelines for drug approval. Since we did not find any recent studies that were conducted in LTCFs, and we excluded studies regarding CA-UTI and UTI in spinal cord injury patients, it is unclear how heterogeneous definitions are in these areas. Defining UTI might be even more challenging for these populations and settings.
UTI definitions differ widely across recent therapeutic and interventional studies. An international consensus-based reference standard is needed to reduce misclassification bias within studies and heterogeneity between studies. To avoid ambiguity, such a reference standard should veer away from the term “complicated UTI” and instead categorize UTI based on systemic involvement, as these are different entities with different treatments. Based on results of this systematic review, our group has initiated an international consensus study to construct a UTI reference standard for research purposes.
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Author contributions. Conceptualization and methodology: M. P. B., R. M. H. J., S. P. C., L. G. V., and M. M. C. L. Screening and data extraction: M. P. B. and R. M. H. J. Data analysis: M. P. B. Writing–original draft preparation: M. P. B. and R. M. H. J. Writing–review and editing: M. P. B., R. M. H. J., C. S., T. N. P., C. N., L. M., J. M. C., S. E. G., B. K., F. W., S. P. C., L. G. V., and M. M. C. L. Supervision: M. M. C. L., S. P. C., and L. G. V. All authors have read and agreed to the final version of the manuscript.
Acknowledgments. The authors thank J. W. Schoones for his contribution to the search strategy.
Gupta K , Grigoryan L , Trautner B . Urinary tract infection . Ann Intern Med 2017 ; 167 : ITC49–64 .
Google Scholar
Foxman B . Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden . Infect Dis Clin North Am 2014 ; 28 : 1 – 13 .
European Medicines Agency . Evaluation of medicinal products indicated for treatment of bacterial infections . 2022 . Available at: https://www.ema.europa.eu/en/evaluation-medicinal-products-indicated-treatment-bacterial-infections . Accessed 25 February 2023.
US Food and Drug Administration . Uncomplicated urinary tract infections: developing drugs for treatment guidance for industry . 2019 . Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/uncomplicated-urinary-tract-infections-developing-drugs-treatment-guidance-industry . Accessed 25 February 2023.
US Food and Drug Administration . Complicated urinary tract infections: developing drugs for treatment . 2018 . Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/complicated-urinary-tract-infections-developing-drugs-treatment . Accessed 25 February 2023.
Stone ND , Ashraf MS , Calder J , et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria . Infect Control Hosp Epidemiol 2012 ; 33 : 965 – 77 .
Gupta K , Hooton TM , Naber KG , et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases . Clin Infect Dis 2011 ; 52 : e103 – 20 .
Bonkat G , Bartoletti F , Bruyere F , et al. EAU guidelines on urological infections. Available at : https://uroweb.org/guidelines/urological-infections . Accessed 25 February 2023.
Page MJ , McKenzie JE , Bossuyt PM , et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . BMJ 2021 ; 372 : n71 .
Aloush SM , Al-Awamreh K , Abu Sumaqa Y , Halabi M , Al Bashtawy M , Salama FB . Effectiveness of antibiotics versus ibuprofen in relieving symptoms of nosocomial urinary tract infection: a comparative study . J Am Assoc Nurse Pract 2019 ; 31 : 60 – 4 .
Arakawa S , Kawahara K , Kawahara M , et al. The efficacy and safety of tazobactam/ceftolozane in Japanese patients with uncomplicated pyelonephritis and complicated urinary tract infection . J Infect Chemother 2019 ; 25 : 104 – 10 .
Babar A , Moore L , Leblanc V , et al. High dose versus low dose standardized cranberry proanthocyanidin extract for the prevention of recurrent urinary tract infection in healthy women: a double-blind randomized controlled trial . BMC Urol 2021 ; 21 : 44 .
Boel JB , Antsupova V , Knudsen JD , Jarløv JO , Arpi M , Holzknecht BJ . Intravenous mecillinam compared with other β-lactams as targeted treatment for Escherichia coli or Klebsiella spp. bacteraemia with urinary tract focus . J Antimicrob Chemother 2021 ; 76 : 206 – 11 .
Botros C , Lozo S , Iyer S , et al. Methenamine hippurate compared with trimethoprim for the prevention of recurrent urinary tract infections: a randomized clinical trial . Int Urogynecol J 2022 ; 33 : 571 – 80 .
Bruyère F , Azzouzi AR , Lavigne JP , et al. A multicenter, randomized, placebo-controlled study evaluating the efficacy of a combination of propolis and cranberry ( Vaccinium macrocarpon ) (DUAB®) in preventing low urinary tract infection recurrence in women complaining of recurrent cystitis . Urol Int 2019 ; 103 : 41 – 8 .
Costache RC , Novac B , Bardan TR , Agapie DN , Edu A . Xyloglucan + gelose combination versus placebo as adjuvant therapy to first-line antimicrobials for uncomplicated urinary tract infection in adults . Urol Int 2019 ; 102 : 468 – 75 .
Diebold R , Schopf B , Stammer H , Mendling W . Vaginal treatment with lactic acid gel delays relapses in recurrent urinary tract infections: results from an open, multicentre observational study . Arch Gynecol Obstet 2021 ; 304 : 409 – 17 .
Drekonja DM , Trautner B , Amundson C , Kuskowski M , Johnson JR . Effect of 7 vs 14 days of antibiotic therapy on resolution of symptoms among afebrile men with urinary tract infection: a randomized clinical trial . JAMA 2021 ; 326 : 324 – 31 .
Eckburg PB , Muir L , Critchley IA , et al. Oral tebipenem pivoxil hydrobromide in complicated urinary tract infection . N Engl J Med 2022 ; 386 : 1327 – 38 .
Edlund C , Ternhag A , Skoog Ståhlgren G , et al. The clinical and microbiological efficacy of temocillin versus cefotaxime in adults with febrile urinary tract infection, and its effects on the intestinal microbiota: a randomised multicentre clinical trial in Sweden . Lancet Infect Dis 2022 ; 22 : 390 – 400 .
El Nekidy WS , Abdelsalam MM , Nusair AR , et al. Is cefoxitin a carbapenem sparing agent in the management of urinary tract infections caused by ESBL producing Enterobacterales? Hosp Pharm 2021 ; 57 : 568 – 74 ..
Ferrante KL , Wasenda EJ , Jung CE , Adams-Piper ER , Lukacz ES . Vaginal estrogen for the prevention of recurrent urinary tract infection in postmenopausal women: a randomized clinical trial . Female Pelvic Med Reconstr Surg 2021 ; 27 : 112 – 7 .
Gama CRB , Pombo MAG , Nunes CP , et al. Treatment of recurrent urinary tract infection symptoms with urinary antiseptics containing methenamine and methylene blue: analysis of etiology and treatment outcomes . Res Rep Urol 2020 ; 12 : 639 – 49 .
Gamble KC , Rose DT , Sapozhnikov J . Intravenous to oral antibiotics versus intravenous antibiotics: a step-up or a step-down for extended spectrum beta-lactamase (ESBL)-producing urinary tract infections without concomitant bacteraemia? Int J Antimicrob Agents 2022 ; 59 : 106541 .
Gágyor I , Hummers E , Schmiemann G , et al. Herbal treatment with uva ursi extract versus fosfomycin in women with uncomplicated urinary tract infection in primary care: a randomized controlled trial . Clin Microbiol Infect 2021 ; 27 : 1441 – 7 .
Harding C , Chadwick T , Homer T , et al. Methenamine hippurate compared with antibiotic prophylaxis to prevent recurrent urinary tract infections in women: the ALTAR non-inferiority RCT . Health Technol Assess 2022 ; 26 : 1 – 172 .
Jansaker F , Thonnings S , Hertz FB , et al. Three versus five days of pivmecillinam for community-acquired uncomplicated lower urinary tract infection: a randomised, double-blind, placebo-controlled superiority trial . EClinicalMedicine 2019 ; 12 : 62 – 9 .
Kaye KS , Rice LB , Dane AL , et al. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, A phase 2/3 randomized trial . Clin Infect Dis 2019 ; 69 : 2045 – 56 .
Kohno S , Bando H , Yoneyama F , et al. The safety and efficacy of relebactam/imipenem/cilastatin in Japanese patients with complicated intra-abdominal infection or complicated urinary tract infection: a multicenter, open-label, noncomparative phase 3 study . J Infect Chemother 2021 ; 27 : 262 – 70 .
Koradia P , Kapadia S , Trivedi Y , Chanchu G , Harper A . Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: a controlled pilot study . Expert Rev Anti Infect Ther 2019 ; 17 : 733 – 40 .
Li Y , Yin Y , Peng X , et al. A randomized, active-controlled, multicentre clinical trial to evaluate the efficacy and safety of oral sitafloxacin versus levofloxacin in Chinese adults with acute uncomplicated or complicated urinary tract infection . Ann Med 2021 ; 53 : 217 – 26 .
Lojanapiwat B , Nimitvilai S , Bamroongya M , et al. Oral sitafloxacin vs intravenous ceftriaxone followed by oral cefdinir for acute pyelonephritis and complicated urinary tract infection: a randomized controlled trial . Infect Drug Resist 2019 ; 12 : 173 – 81 .
Mir MA , Chaudhary S , Payasi A , Sood R , Mavuduru RS , Shameem M . Ceftriaxone + sulbactam + isodium EDTA versus meropenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: PLEA, a double-blind, randomized noninferiority trial . Open Forum Infect Dis 2019 ; 6 : ofz373 .
Mirzaei M , Daneshpajooh A , Farsinezhad A , et al. The therapeutic effect of intravesical instillation of platelet rich plasma on recurrent bacterial cystitis in women: a randomized clinical trial . Urol J 2019 ; 16 : 609 – 13 .
Montelin H , Forsman KJ , Tängdén T . Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men . PLoS One 2019 ; 14 : e0211098 .
Nestler S , Grune B , Schilchegger L , Suna A , Perez A , Neisius A . Efficacy of vaccination with StroVac for recurrent urinary tract infections in women: a comparative single-centre study . Int Urol Nephrol 2021 ; 53 : 2267 – 72 .
Overcash JS , Tiffany CA , Scangarella-Oman NE , et al. Phase 2a pharmacokinetic, safety, and exploratory efficacy evaluation of oral gepotidacin (GSK2140944) in female participants with uncomplicated urinary tract infection (acute uncomplicated cystitis) . Antimicrob Agents Chemother 2020 ; 64 : e00199–20 .
Overcash JS , Bhiwandi P , Garrity-Ryan L , et al. Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: results from a phase 1b study . Antimicrob Agents Chemother 2019 ; 63 : e02083–18 .
Pierrotti LC , Pérez-Nadales E , Fernández-Ruiz M , et al. Efficacy of beta-lactam/beta-lactamase inhibitors to treat extended-spectrum beta-lactamase-producing Enterobacterales bacteremia secondary to urinary tract infection in kidney transplant recipients (INCREMENT-SOT Project) . Transpl Infect Dis 2020 ; 23 : e13520 ..
Rădulescu D , David C , Turcu FL , Spătaru DM , Popescu P , Văcăroiu IA . Combination of cranberry extract and D-mannose—possible enhancer of uropathogen sensitivity to antibiotics in acute therapy of urinary tract infections: results of a pilot study . Exp Ther Med 2020 ; 20 : 3399 – 406 .
Ryanto S , Wong M , Czarniak P , et al. The use of initial dosing of gentamicin in the management of pyelonephritis/urosepsis: a retrospective study . PLoS One 2019 ; 14 : e0211094 .
Safwat AS , Hasanain A , Shahat A , et al. Cholecalciferol for the prophylaxis against recurrent urinary tract infection among patients with benign prostatic hyperplasia: a randomized, comparative study . World J Urol 2019 ; 37 : 1347 – 52 .
Sagan O , Yakubsevitch R , Yanev K , et al. Pharmacokinetics and tolerability of intravenous sulbactam-durlobactam with imipenem-cilastatin in hospitalized adults with complicated urinary tract infections, including acute pyelonephritis . Antimicrob Agents Chemother 2020 ; 64 : e01506–19 .
Sashidhar RB , Manoj KYM , Manisha S , Sree SRS , Kamala SH . Assessment of efficacy and safety of oral fosfomycin single dose in uncomplicated urinary tract infection at a tertiary care hospital in south India . Asian J Pharm Clin Res 2022 ; 15 : 60 – 3 .
Senard O , Lafaurie M , Lesprit P , et al. Efficacy of cefoxitin versus carbapenem in febrile male urinary tract infections caused by extended spectrum beta-lactamase-producing Escherichia coli : a multicenter retrospective cohort study with propensity score analysis . Eur J Clin Microbiol Infect Dis 2020 ; 39 : 121 – 9 .
Sharara SL , Amoah J , Pana ZD , Simner PJ , Cosgrove SE , Tamma PD . Is piperacillin-tazobactam effective for the treatment of pyelonephritis caused by extended-spectrum beta-lactamase-producing organisms? Clin Infect Dis 2020 ; 71 : e331 – 7 .
Sojo-Dorado J , López-Hernández I , Rosso-Fernandez C , et al. Effectiveness of fosfomycin for the treatment of multidrug-resistant Escherichia coli bacteremic urinary tract infections: a randomized clinical trial . JAMA Netw Open 2022 ; 5 : e2137277 .
Sorlí L , Luque S , Li J , et al. Colistin for the treatment of urinary tract infections caused by extremely drug-resistant Pseudomonas aeruginosa : dose is critical . J Infect 2019 ; 79 : 253 – 61 .
Stalenhoef JE , van Nieuwkoop C , Menken PH , Bernards ST , Elzevier HW , van Dissel JT . Intravesical gentamicin treatment for recurrent urinary tract infections caused by multidrug resistant bacteria . J Urol 2019 ; 201 : 549 – 55 .
Tehrani S , Elyasi F , Abolghasemi S . Levofloxacin versus ceftriaxone for the treatment of acute pyelonephritis in Iranian adults . Infect Disord Drug Targets 2021 ; 21 : 603 – 7 .
Ten Doesschate T , van Werkhoven H , Meijvis S , et al. Fosfomycin-trometamol for urinary tract infections in kidney transplant recipients . Transplantation 2019 ; 103 : 1272 – 6 .
Ten Doesschate T , Kuiper S , van Nieuwkoop C , et al. Fosfomycin versus ciprofloxacin as oral step-down treatment for Escherichia coli febrile urinary tract infections in women: a randomised, placebo-controlled, double-blind, multicenter trial . Clin Infect Dis 2021 ; 75 : 221 – 9 ..
Tseng CS , Chang SJ , Meng E , Chang HC , Lee YJ . The efficacy of pentosan polysulfate monotherapy for preventing recurrent urinary tract infections in women: a multicenter open-label randomized controlled trial . J Formos Med Assoc 2020 ; 119 : 1314 – 9 .
Tullos JB , Stoudenmire LL , Pouliot JD . Piperacillin-tazobactam versus carbapenems for the treatment of nonbacteremic urinary tract infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae . Hosp Pharm 2020 ; 55 : 44 – 9 .
Wagenlehner FME , Cloutier DJ , Komirenko AS , et al. Once-daily plazomicin for complicated urinary tract infections . N Engl J Med 2019 ; 380 : 729 – 40 .
Wald-Dickler N , Lee TC , Tangpraphaphorn S , et al. Fosfomycin vs ertapenem for outpatient treatment of complicated urinary tract infections: a multicenter, retrospective cohort study . Open Forum Infect Dis 2022 ; 9 : ofab620 .
Nicolle LE , Bradley S , Colgan R , et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults . Clin Infect Dis 2005 ; 40 : 643 – 54 .
Rodhe N , Lofgren S , Matussek A , et al. Asymptomatic bacteriuria in the elderly: high prevalence and high turnover of strains . Scand J Infect Dis 2008 ; 40 : 804 – 10 .
Ouslander JG , Schapira M , Fingold S , Schnelle J . Accuracy of rapid urine screening tests among incontinent nursing home residents with asymptomatic bacteriuria . J Am Geriatr Soc 1995 ; 43 : 772 – 5 .
Bent S , Nallamothu BK , Simel DL , Fihn SD , Saint S . Does this woman have an acute uncomplicated urinary tract infection? JAMA 2002 ; 287 : 2701 – 10 .
Hooton TM , Roberts PL , Cox ME , Stapleton AE . Voided midstream urine culture and acute cystitis in premenopausal women . N Engl J Med 2013 ; 369 : 1883 – 91 .
Stamm WE , Counts GW , Running KR , Fihn S , Turck M , Holmes KK . Diagnosis of coliform infection in acutely dysuric women . N Engl J Med 1982 ; 307 : 463 – 8 .
Klaassen IL , de Haas V , van Wijk JA , Kaspers GJ , Bijlsma M , Bokenkamp A . Pyuria is absent during urinary tract infections in neutropenic patients . Pediatr Blood Cancer 2011 ; 56 : 868 – 70 .
Bilsen MP , Aantjes MJ , van Andel E , et al. Current pyuria cut-offs promote inappropriate UTI diagnosis in older women . Clin Infect Dis 2023 ; 76 : 2070 – 6 .
van den Broek D , Keularts IM , Wielders JP , Kraaijenhagen RJ . Benefits of the iQ200 automated urine microscopy analyser in routine urinalysis . Clin Chem Lab Med 2008 ; 46 : 1635 – 40 .
Wilson ML , Gaido L . Laboratory diagnosis of urinary tract infections in adult patients . Clin Infect Dis 2004 ; 38 : 1150 – 8 .
Johnson JR . Definition of complicated urinary tract infection . Clin Infect Dis 2017 ; 64 : 529 .
Johansen TE , Botto H , Cek M , et al. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system . Int J Antimicrob Agents 2011 ; 38(Suppl) : 64 – 70 .
Bouma M , Geerlings SE , Klinkhamer S , et al. NHG standaard: urineweginfecties [Dutch primary care guideline: UTI] . 2020 . Available at: https://richtlijnen.nhg.org/standaarden/urineweginfecties . Accessed 15 March 2023.
Author notes
- urinary tract infections
- heterogeneity
- urine culture
- urinary tract infection, acute
Supplementary data
| Month: | Total Views: |
|---|---|
| June 2023 | 195 |
| July 2023 | 2,223 |
| August 2023 | 2,228 |
| September 2023 | 1,071 |
| October 2023 | 998 |
| November 2023 | 951 |
| December 2023 | 884 |
| January 2024 | 1,410 |
| February 2024 | 1,324 |
| March 2024 | 1,182 |
| April 2024 | 1,177 |
| May 2024 | 878 |
| June 2024 | 818 |
| July 2024 | 689 |
| August 2024 | 756 |
| September 2024 | 548 |
Email alerts
More on this topic, related articles in pubmed, citing articles via, looking for your next opportunity, affiliations.
- Online ISSN 2328-8957
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

ORIGINAL RESEARCH article
Bacterial profile and antibiotic susceptibility pattern of uropathogens causing urinary tract infection in the eastern part of northern india.

- 1 Amity Institute of Microbial Technology, Amity University Rajasthan, Jaipur, India
- 2 Department of Microbiology, Institute of Medical Sciences (IMS), Banaras Hindu University, Varanasi, India
- 3 Department of Medicine, Hayes Memorial Hospital, Sam Higginbottom University of Agriculture, Technology and Sciences (SHUATS), Allahabad, India
- 4 Amity Institute of Biotechnology, Amity University Rajasthan, Jaipur, India
Urinary tract infection (UTI) is a common infectious disease that affects men and women. It is a significant health concern due to multidrug-resistant (MDR) organisms. Therefore, it is necessary to have a current understanding of the antibiotic susceptibility (AS) pattern of uropathogens to manage UTI effectively. Since the bacterial pathogen causing UTI and its AS vary with time and place, the prevailing AS pattern of the causative agents are essential for empirical antibiotic therapy. This study aims to determine the prevalence and AS of uropathogens isolated from UTI patients in the eastern part of Northern India. The study was carried out between November 2018 and December 2019. Clean catch midstream urine samples were collected and processed using standard guidelines for microbiological procedures. Positive microbiological cultures were found in 333 of the 427 patients, where 287 were gram-negative bacteria (GNB), and 46 were gram-positive bacteria (GPB). Females had a higher prevalence of UTI (60.7%) than males (39.3%) ( p = 0.00024). The most susceptible age group in females was 18–50 years as compared to males, whereas at the age of 51–80 years and >80 years males were more susceptible than females ( p = 0.053). The most prevalent pathogen identified were Escherichia coli (55.0%), followed by Proteus sp. (6.9%), Klebsiella pneumoniae (6.6%), Pseudomonas aeruginosa (6.3%), of which 96.0% were MDR bacteria. The susceptibility pattern of our study also revealed that amikacin, gentamycin and imipenem were the most effective drugs against GNB. In contrast, nitrofurantoin, vancomycin, and chloramphenicol were the most effective drugs against GPB. According tothe findings, MDR pathogens are very much prevalent. Since UTI is one of the most frequent bacterial diseases, proper management necessitates extensive investigation and implementation of antibiotic policy based on AS patterns for a particular region.
Introduction
Urinary tract infections (UTIs) are inflammatory disorders caused by microorganisms that have proliferated abnormally in the urinary system ( Malik et al., 2021 ). UTIs are known to induce short-term morbidities such as fever, dysuria, lower abdominal pain, and may result in permanent kidney scarring ( Leung et al., 2019 ). UTIs are either community-acquired or hospital-acquired (HA). Infection of the urinary system originates in individuals either in the community (within 48 h of admission) or a hospital setting ( Revelas, 2012 ). HA-UTI emerges 48 h after hospitalization and is not incubating at the time of admission or within 3 days of discharge ( Iacovelli et al., 2014 ; Motbainor et al., 2020 ). UTIs can be asymptomatic or symptomatic, imposing a strain on public health care and lowering the quality of life ( Olowe et al., 2015 ).
Urinary tract infection is more common in women than in men because of the anatomical proximity of the urethra to gut opening ( Fazly Bazzaz et al., 2021 ). The most prevalent bacteria causing UTI is Escherichia coli , followed by Klebsiella pneumoniae , Staphylococcus sp., Proteus sp., Pseudomonas aeruginosa, Enterococcus sp., and Enterobacter sp. with variations in their sequence of prevalence ( Ahmed et al., 2019 ; Patel et al., 2019 ; Mukherjee et al., 2020 ). Approximately 150 million UTI cases per year are diagnosed globally, resulting in at least $6 billion in healthcare costs ( Kucheria et al., 2005 ; Flores-Mireles et al., 2015 ). Susceptibility data from local microbiological facilities assist in the empirical selection of antibiotics for UTI treatment; however, these data are confined to complicated UTIs because uncomplicated UTI specimens are rarely sent to laboratories ( Prakash and Saxena, 2013 ). Therefore, UTIs are currently treated empirically, particularly in rural and small-town settings where the facility of urine culture is unavailable, resulting in antibiotic misuse ( Al-Zahrani et al., 2019 ). The increasing incidence of drug resistance among uropathogens is a significant public health concern, necessitating constant antibiotic susceptibility (AS) screening for organisms causing UTI ( Kot, 2019 ). In addition, antimicrobial sensitivity for UTI-causing bacteria varies with time and location. Therefore, screening for susceptibility in each location is critical for producing up-to-date epidemiological data ( Ahmed et al., 2019 ; Daoud et al., 2020 ). Unfortunately, the resistance profile of community-acquired uropathogens in diverse geographical regions of India has not been adequately explored ( Sood and Gupta, 2012 ; Mohapatra et al., 2022 ). Since UTIs are frequently treated empirically in regions where microbiological facilities are either unavailable or prohibitively expensive for the majority of the Indian population, treatment is based on the anticipated pathogens with their AS pattern of that geographic area. We chose to conduct this study because we were unaware of the bacterial composition and AS pattern of uropathogens causing UTI in Prayagraj (Uttar Pradesh), India, which is situated in the eastern region of North India.
Materials and methods
Study area and population.
A cross-sectional study was conducted at Hayes Memorial Mission Hospital in Prayagraj, Uttar Pradesh, between November 2018 and December 2019 to investigate the prevalence and AS profile of uropathogens among patients presenting with UTI. Sample size was calculated by Kish (1965) formula, n = z 2 p(1-p)/d 2 , where z = Z score for 95% confidence interval = 1.96; p = prevalence (22.8%) and d = acceptable error (5%). The formula also included 1.5 times the design effect and a 5% non-response rate. A total of 427 samples were acquired based on a subjective symptom-based questionnaire [data not shown], of which 333 were later verified microbiologically as positive UTI cases.
Exclusion criteria
Patients under the age of five, those with polymicrobial infections involving more than two bacterial species, patients with Candida sp. as the sole pathogen or with bacteria, pregnant females with asymptomatic bacteriuria, and those who had previously been on antibiotic therapy were all excluded from the study.
Sample collection and processing
Each patient’s clean-catch midstream urine was collected in a sterile screw-capped universal container. All patients were instructed on collecting samples aseptically to avoid contamination. A urine sample is medical waste material voluntarily given by patients visiting OPD, without invasive sample collection procedures. However, patients’ oral and/or written consent was also collected before specimen collection and the study was approved by institutional committee. A sterile calibrated loopful of urine sample was plated on sheep blood agar (SBA) and MacConkey agar (MA) to isolate bacterial uropathogens and incubated at 37°C for 24 h.
Identification and biochemical characterization of pathogens
Bacterial isolates were identified based on their standard microbiological techniques, i.e., culture and biochemical characteristics. All the bacteria isolated from the sample were identified using catalase test (3% v/v H 2 O 2 ), coagulase test (0.85% v/v of normal saline), bile esculin test, oxidase test, indole test with H 2 S production (sulphide indole motility medium), citrate utilization test (Simmon’s citrate medium), urease test (Christensen’s urea agar), triple sugar iron agar test and fermentation using sugars (Glucose, Lactose, Sucrose, and Mannitol). Isolates identified were preserved at room temperature of 25°C in peptone soft agar that was wax sealed with a cork and sub-cultured for further processing.
Antimicrobial susceptibility
The antimicrobial susceptibility test was performed on Mueller–Hinton agar (HiMedia Laboratories, Mumbai, India) using the Kirby–Bauer disk diffusion method and interpreted according to Clinical Laboratory Standards Institute (CLSI) guidelines ( Table 1 ). Extended-spectrum beta-lactamase (ESBL) producing strains were confirmed by utilizing a double-disk synergy test with cephalosporin and cephalosporin/clavulanate combination disks (ceftazidime and ceftazidime-clavulanic acid) for E. coli and K. pneumoniae . Standard strains of E. coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Pseudomonas aeruginosa (ATCC 27853) were used in this study as quality control.

Table 1. Antibiotics used against different isolated uropathogens.
Statistical analysis
The data were analyzed using descriptive statistics for UTI prevalence, uropathogen frequency, AS profile, Chi-square test where applicable. All statistical tests were performed using SPSS software version 23 and Microsoft Excel 2016 (Microsoft Corporation, Redmond, Was, United States).
Of the whole study group of 427, 333 (77.9%) were excreting a significant number of bacteria in their urine. Our study shows that 39.3% (131/333) males and 60.7% (202/333) of females were suffering from UTIs (χ 2 = 13.495; degree of freedom = 1; p = 0.00024). The prevalence of UTI in females was significantly higher than the males ( p = 0.00024). The most susceptible age group for UTI was 18–50 years, followed by 51–80, 5–17, and >80 years ( Figure 1 ). However, at the age of 51–80 years (35.9%) and >80 years (3.8%), males had a higher prevalence of UTI than females (25.7 and 1.5%), but in the childhood and adolescent age, group females were more susceptible. The chi-square test showed a significant association between age group and gender (χ 2 = 7.69; degree of freedom = 3; p = 0.053). A total of 333 bacterial uropathogens comprising of 287 (67.2%) gram-negative and 46 (10.8%) gram-positive bacteria were isolated from positive urine samples. There were nine different uropathogens isolated, six of which were gram-negative bacteria, and three were gram-positive bacteria. E. coli was the most predominant gram-negative bacteria, accounting for 54.95% (183/333) of all isolates, followed by Proteus sp . 6.9%, K. pneumoniae 6.6%, P. aeruginosa 6.3%, Citrobacter sp. 6.3%, S. aureus 6.0%, Enterococcus sp. 5.4%, E. cloacae 5.1% and S. epidermidis 2.4%. Also, gender ( p = 0.620) had no significant association with the types of bacterial pathogens isolated ( Table 2 ). The highest number of E. coli was found in the age group of 18–50 years (58.17%, 121/208) followed by 51–80 years (51.5%, 51/99), 5–17 years (50%, 9/18), >80 years (25%, 2/8). The second most prevalent organism among the age group of 18–50 years was Proteus sp., 7.2% (15/208), followed by S. aureus , 6.7% (14/208); P. aeruginosa , 5.8% (12/208), Citrobacter sp., 5.8% (12/208); K. pneumoniae , 5.3% (11/208); E. cloacae , 4.3% (9/208); Enterococcus sp., 3.8% (8/208); S. epidermidis , 2.9% (6/208). For 51–80 years, the second most prevalent organism is K. pneumoniae , 8.1% (8/99), Proteus sp., 8.1% (8/99) followed by E. cloacae , 7.1% (7/99); Enterococcus sp., 6.1% (6/99), Citrobacter sp., 6.1% (6/99), S. aureus , 6.1% (6/99); P. aeruginosa , 5.1% (5/99), and S. epidermidis , 2.0% (2/99). Also, for 5–17 years, the second most prevalent organism is P. aeruginosa 16.7% (3/18), K. pneumonia e, 16.7% (3/18); followed by Enterococcus sp ., 11.1% (2/18) and E. cloacae , 5.6% (1/18). A significant association was found among the age group ( p = 0.039) with respect to bacterial isolate ( Table 3 ).
Figure 1. Distribution of male and female positive urinary tract infection (UTI) patients among different age groups.
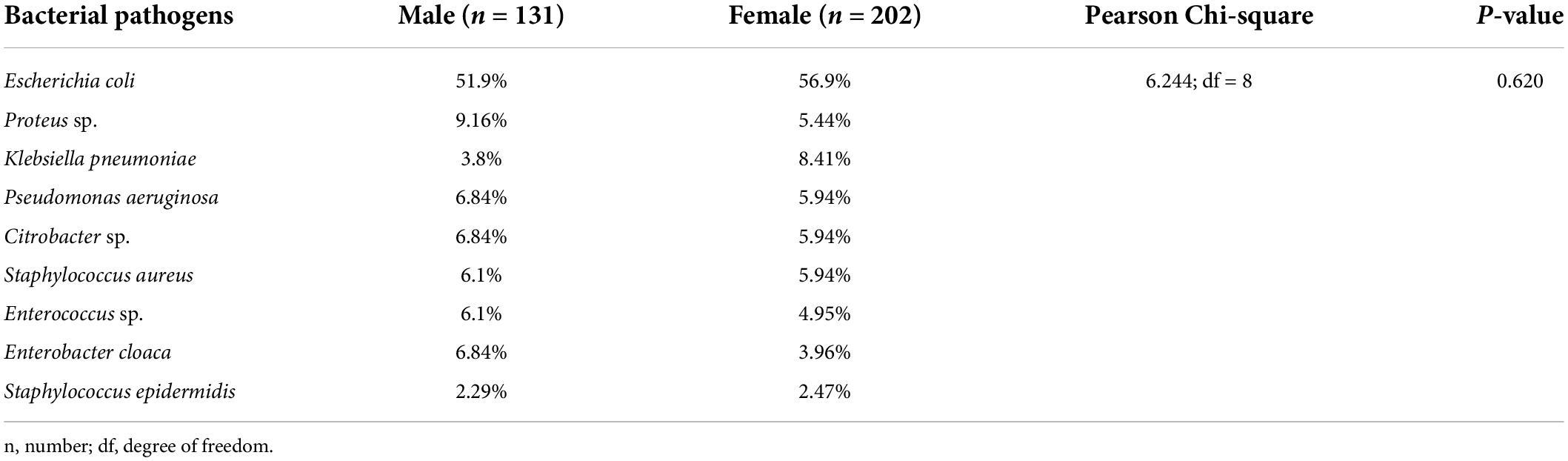
Table 2. Distribution of bacteria among gender in the study population.
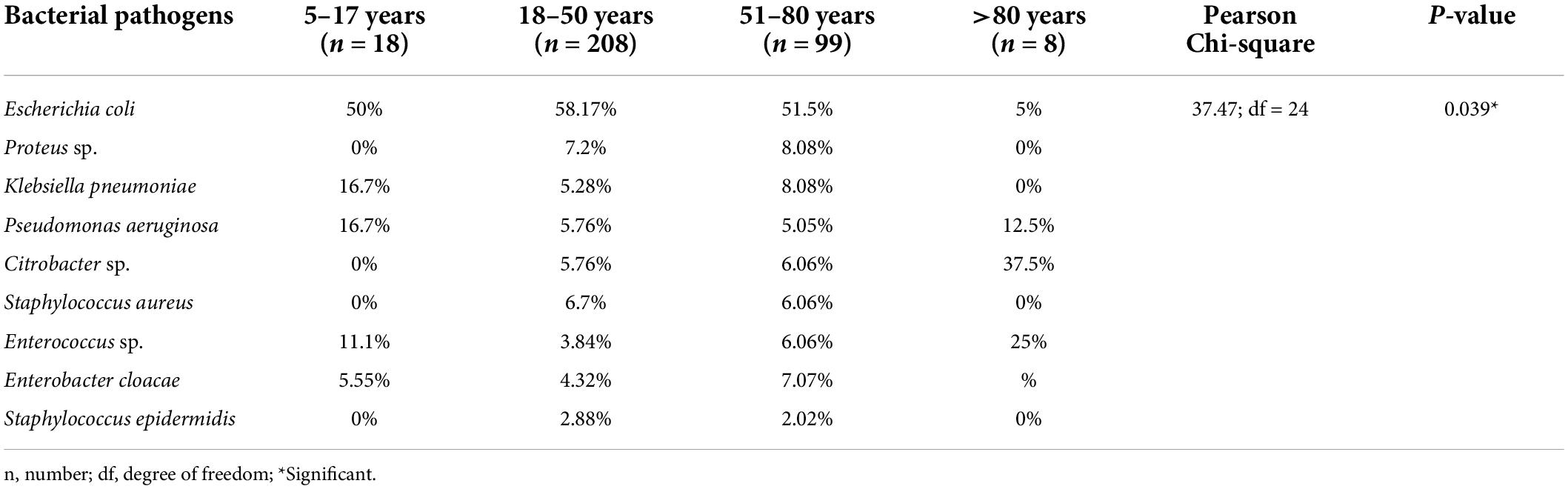
Table 3. Distribution of bacteria among the age groups of the study population.
Antibiotic susceptibility testing revealed that 96.0% (320/333) of the pathogenic bacteria isolated from urine samples were MDR organisms. It was found that 40.4% (74/183) of E. coli and none of the K. pneumoniae were ESBL producing organisms. Table 4 demonstrates that aminoglycoside antibiotics such as amikacin and gentamycin were the most effective drugs. Amikacin was effective against 77.0% of E. coli , 73.9% of Proteus sp., 81.8% of K. pneumoniae , 52.9% of E. cloacae , 90.5% of Citrobacter sp., and 76.2% of P. aeruginosa . Gentamycin demonstrated an almost similar level of efficacy with susceptibility rates of 49.7% for E. coli , 56.5% for Proteus sp., 86.4% for K. pneumoniae , 52.9% for E. cloacae , 81.0% for Citrobacter sp., and 81.0% for P. aeruginosa . Tobramycin was tested only against P. aeruginosa isolates and was effective against 71.4% of them. With the exception of piperacillin-tazobactam and ceftriaxone, the isolates were relatively resistant to the β-lactam group of antibiotics, penicillin, and cephalosporins. Piperacillin-tazobactam inhibited 52.2% of Proteus sp., 71.4% of Citrobacter sp. and 71.4% of P. aeruginosa , as shown in Table 4 , whereas, ceftriaxone was able to inhibit 42.9% of Citrobacter sp . isolates. The two carbapenem antibiotics also performed poorly, with meropenem showing efficacy against 52.4% of Citrobacter sp. but less than 50% of the other gram-negative isolates. However, imipenem outperformed meropenem by inhibiting 57.4% E. coli , 72.7% K. pneumoniae , 57.1% Citrobacter sp. and 90.5% P. aeruginosa. Nitrofurantoin, a nitrofuran antibiotic, was effective against a few bacteria, inhibiting 49.7% of E. coli and 61.9% of Citrobacter sp. Nitrofurantoin demonstrated poor susceptibility rates of 13.6 and 17.6% against K. pneumoniae , and E. cloacae , respectively ( Table 4 ).
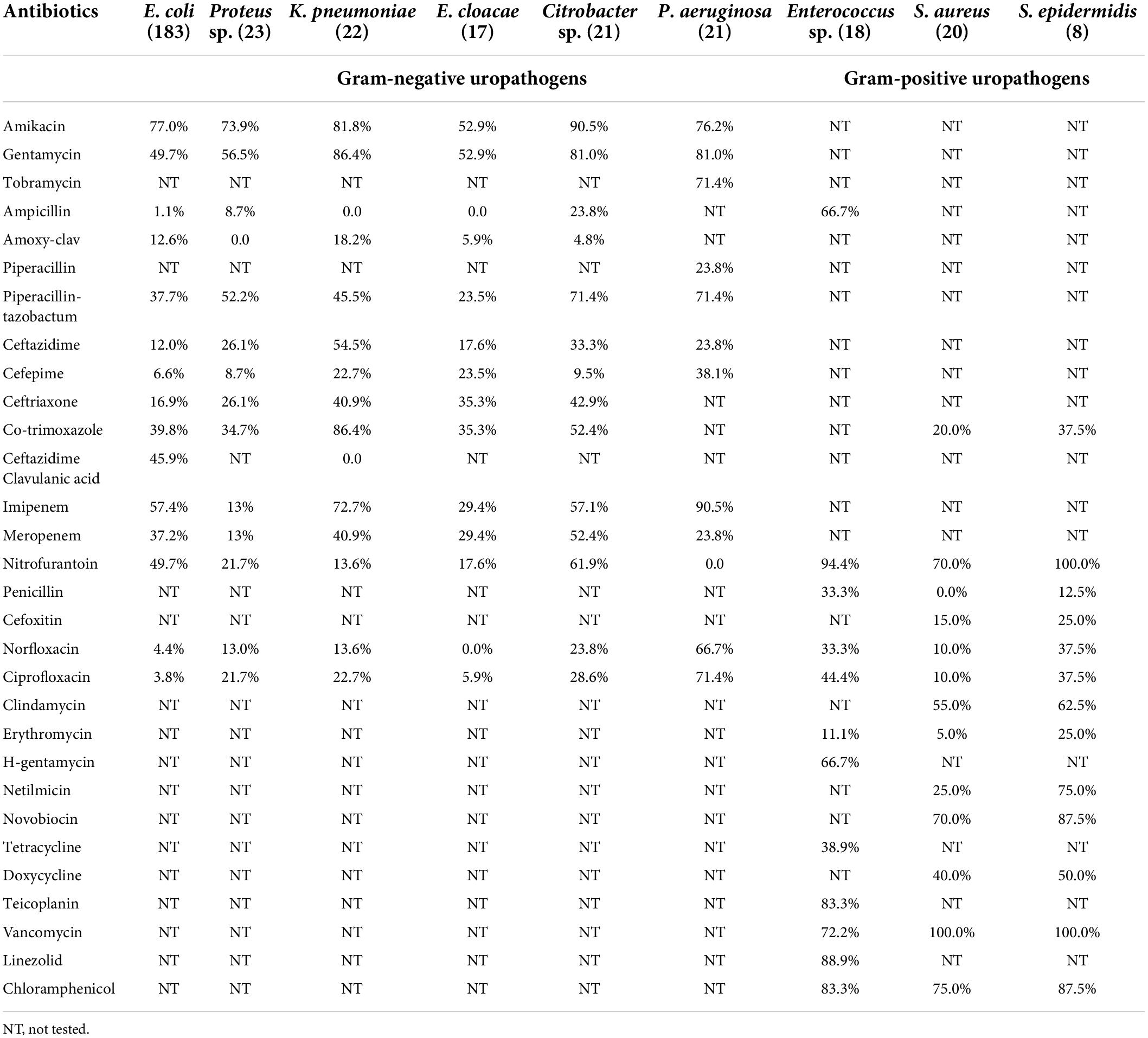
Table 4. Susceptibility of different antibiotics against isolated gram-negative and gram-positive uropathogens.
Nitrofurantoin, vancomycin, and chloramphenicol were particularly effective against gram-positive bacteria. Vancomycin, an antibiotic with restricted prescription, was found to inhibit 100% of Staphylococcus sp. and 72.2% of Enterococcus sp. Nitrofurantoin was also found to be effective against 94.4% of Enterococcus sp., 70.0% of S. aureus and 100% of S. epidermidis. Chloramphenicol, a rarely prescribed antibiotic, inhibited 83.3% of Enterococcus sp., 75.0% of S. aureus , and 87.5% of S. epidermidis . Ampicillin inhibited 66.7% of the Enterococcus sp. Furthermore, Enterococcus sp. showed an 88.9% susceptibility to linezolid and a 66.7% susceptibility to high concentration gentamycin.
The etiology, pathophysiology, and AS patterns of uropathogens have altered over time and place, which will continue to do so in the future ( Ahmed et al., 2019 ). Identification of the organism and its AS is crucial for managing UTI. It exemplifies the importance of close collaboration and cooperation between the clinician and the microbiologist ( Moue et al., 2015 ). This study aimed to assess the status of antimicrobial resistance among uropathogens and compare the situation in the Prayagraj region, the eastern part of North India. In our study, the prevalence of UTI was 79.9% since the inclusion criteria of patients was based on rigorous screening through a questionnaire [data not shown] by the clinicians. This prevalence rate is higher as compared to previous studies, which account for 45.7, 53.8, 65.4, and 37.3% in India, even though their inclusion criteria were symptom-based ( Prakash and Saxena, 2013 ; Critchley et al., 2019 ; Patel et al., 2019 ; Sharma et al., 2020 ). The prevalence of UTI in our investigation correlates to a study conducted in the Mexican population, where 97.3% of patients excreted significant uropathogens and Ethiopia, where 90.1% of patients showed significant growth of uropathogens ( García-Morúa et al., 2009 ; Seifu and Gebissa, 2018 ). According to several studies, the frequency of UTIs is higher in females than in males ( Prakash and Saxena, 2013 ; Odoki et al., 2019 ; Malik et al., 2021 ). In concordance with previous research, our findings also indicate a higher prevalence of UTI in females (60.7%) than in males (39.3%). The proximity of the urethral meatus to the anus, the shorter urethra, sexual intercourse, incontinence, and improper toilet habits may contribute to a higher rate of UTI in females than in males ( Prakash and Saxena, 2013 ). In our study, young females in the age of 18–50 years (reproductive age) showed a higher incidence of UTI, which is similar to the findings of the study in Meerut (26–36 years, 90.7%), Jaipur (21–50 years, 41.3%) and Ethiopia (20–29 years, 37.5%) as their anatomy makes them more vulnerable and prone to this disease ( Sood and Gupta, 2012 ; Prakash and Saxena, 2013 ; Seifu and Gebissa, 2018 ). However, our study also revealed that elderly males (51–80 y) had a higher incidence of UTI (35.9%) than elderly females (25.7%). These findings mirrored studies conducted in Jaipur (Rajasthan), 47.3%; Meerut (Uttar Pradesh), 71.2%; Sonipat (Haryana), 58.3% India ( Sood and Gupta, 2012 ; Prakash and Saxena, 2013 ; Malik et al., 2021 ). The leading causes of higher UTI incidence in elderly males might be attributed to the higher prevalence of benign prostate enlargement and neurogenic bladder ( Lee and Kuo, 2017 ). Other researchers backed up similar findings, claiming that prostate disease in elderly males is responsible for the higher incidence of UTI ( Rowe and Juthani-Mehta, 2013 ). The most common gram-negative bacteria isolated from samples in our investigation was E. coli (55.0%). These findings are consistent with those of several other published studies where the prevalence of E. coli was 97.0, 92.6, 74.0, 55.0, 49.3, 43.5, 41.9, and 40.0% ( Arora et al., 2016 ; Odoki et al., 2019 ; Chen et al., 2020 ; Daoud et al., 2020 ; Ali et al., 2022 ; Huang et al., 2022 ; Jagadeesan et al., 2022 ; Komagamine et al., 2022 ). In our study, Proteus sp. (6.9%) and K. pneumoniae (6.6%) was the second and third most frequent bacteria reported, followed by P. aeruginosa (6.3%) and Citrobacter sp. (6.3%). Proteus sp. colonizes in the gastrointestinal tract of humans and causes UTI by ascending from the rectum to the urethral tissue and the urinary bladder. The increased prevalence of gram-negative bacteria from the Enterobacteriaceae family causing UTI can be attributed to several factors, including adherence to the uroepithelium due to urogenital mucosa colonization via adhesins, pili, fimbriae, and P-1 blood group phenotypic receptor ( Terlizzi et al., 2017 ). P. aeruginosa is an unusual uropathogen that is primarily responsible for catheter-associated UTIs in adults. Its presence as the second commonest isolate (3/18, 16.7%) in the age group of 7–18 years needs further exploration. However, Bitsori et al. (2012) has suggested that with a history of previous UTI episodes, hospitalization, antibiotic use, malformations predisposing to UTIs, vesicourethral reflux, abnormal DMSA (dimercaptosuccinic acid) scan, longer hospitalization and surgery makes children more prone to P. aeruginosa UTI. The emergence of Citrobacter sp. as an uropathogen, especially in the age group >80 years, which is resistant to the majority of antibiotics, is alarming. Citrobacter sp. should no longer be ignored as commensal and proper surveillance in the antimicrobial sensitivity testing must be done ( Sami et al., 2017 ).
In our study, 96.0% of the pathogens were MDR, compared to 91.3% in Nepal, 85.5% in Somaliland, 83.0% in Haryana, 45.1% in Tunisia, and 42.6% in China ( Ben Ayed et al., 2019 ; Huang et al., 2022 ; Malik et al., 2021 ; Shilpakar et al., 2021 ; Ali et al., 2022 ). The inappropriate and indiscriminate use of broad-spectrum antibiotics and prolonged hospital stay are key etiological factors associated with MDR infections ( Prestinaci et al., 2015 ). In our study, 40.4% of E. coli produced ESBLs, whereas other publications reported 25.2%; 35.7, 46.0, and 52–67% ( Gharavi et al., 2021 ; Huang et al., 2022 ; Naushad et al., 2022 ; Sadeghi et al., 2022 ). ESBL producers hydrolyze and eliminate the majority of broad-spectrum beta-lactam antibiotics, increasing morbidity and mortality ( Mahmud et al., 2020 ). Because ESBL-producing bacteria do not easily hydrolyze carbapenems, they are routinely used as first-line therapy in clinical settings. However, abuse of carbapenems, on the other hand, may make treatment of this type of bacterium more difficult ( Gharavi et al., 2021 ). Antibiotic susceptibility revealed that amoxy-clav followed by ampicillin and cefepime were the most ineffective drugs against all identified gram-negative bacteria. In contrast, amikacin, gentamycin, and imipenem were the most susceptible drugs for gram-negative bacteria. These AS findings were consistent with prior research conducted in Sonipat (Haryana) and Meerut (UP) by other authors ( Prakash and Saxena, 2013 ; Malik et al., 2021 ). In our study, tobramycin showed promising sensitivity to P. aeruginosa ; however, according to a study conducted in Meerut, 60.0% of P. aeruginosa were resistant to tobramycin ( Prakash and Saxena, 2013 ). In our study, imipenem and meropenem exhibited poor antimicrobial activity against gram-negative bacteria, in contrast to previous investigations in which carbapenem susceptibility was greater than 80.0% ( Patel et al., 2019 ; Malik et al., 2021 ). Several studies have reported resistance to the β-lactam group of antibiotics, cephalosporins and fluoroquinolones, which is similar to that of our investigation, where a substantial decrease in sensitivity pattern was observed ( Sood and Gupta, 2012 ; Sharma et al., 2020 ; Malik et al., 2021 ). Furthermore, in our study, nitrofurantoin exhibited significant susceptibility to E. coli but not to other Enterobacteriaceae (except Citrobacter sp.), which is consistent with a study conducted in Jaipur ( Sood and Gupta, 2012 ). It is presumably due to irrational use of it in the past with insufficient dose and duration. Antibiotics showed considerably high sensitivity rates to gram-positive bacteria in our study, which was in concordance with the investigation conducted by other authors ( Sood and Gupta, 2012 ; Patel et al., 2019 ).
The main factor fueling AMR is improper usage of antibiotics that needs to be checked ( Duan et al., 2021 ). According to the Infectious Diseases Society of America’s proposed regulations, empirical antibiotic treatment for UTI should be based on regional susceptibility data, drug accessibility, and patient history ( Tamma et al., 2022 ). Resistance to bacterial uropathogens is becoming a public health issue in India. Many Indian cities and towns lack appropriate microbiological laboratories, leading to fewer microbiological assessments and increased empirical antibiotic use. Typically, urine samples are sent for microbiological testing only after treatment failure, recurrent or relapsing infection. Our findings emphasize the significance of local antibiotic resistance patterns, which may subsequently be used to develop hospital and regional antibiotic policies. To avoid/contain the emergence of antibiotic resistance in bacteria, the government must introduce laws requiring the prudent use of these antibiotics.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethical Committee for Human Research at Amity University Rajasthan, Jaipur, India (AUR/REG/2709). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
NJ, GN, and KB hypothesized and designed the research plan. AB and KB performed the data acquisition. KB and RK performed the experimental study. KB, NJ, AB, and GN did statistical analysis, interpretation of data, and manuscript preparation. GN, NJ, AB, and GA did final editing and reviewing. All the authors have reviewed the manuscript and approved the submitted version.
Acknowledgments
We are grateful to the hospital administration, Dean, OPD physicians, and technical staffs of Hayes Memorial Mission Hospital SHUATS, Prayagraj (U.P.), India, for granting permission to collect sample of UTI patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed, S. S., Shariq, A., Alsalloom, A. A., Babikir, I. H., and Alhomoud, B. N. (2019). Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int. J. Health Sci. 13, 48–55.
Google Scholar
Ali, A. H., Reda, D. Y., and Ormago, M. D. (2022). Prevalence and antimicrobial susceptibility pattern of urinary tract infection among pregnant women attending Hargeisa Group Hospital, Hargeisa, Somaliland. Sci. Rep. 12:1419.
Al-Zahrani, J., Al Dossari, K., Gabr, A. H., Ahmed, A. F., Al Shahrani, S. A., and Al-Ghamdi, S. (2019). Antimicrobial resistance patterns of Uropathogens isolated from adult women with acute uncomplicated cystitis. BMC Microbiol. 19:237. doi: 10.1186/s12866-019-1612-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Arora, G., Kaur, P., and Agrawal, D. (2016). Urinary tract infection in women of the rural population of Haryana: A rising problem. Int. J. Reprod. Contracept. Obstet. Gynecol. 5, 4470–4474. doi: 10.18203/2320-1770.ijrcog20164365
CrossRef Full Text | Google Scholar
Ben Ayed, H., Koubaa, M., Hammami, F., Marrakchi, C., Rekik, K., Ben Jemaa, T., et al. (2019). Performance of an Easy and Simple New Scoring Model in Predicting Multidrug-Resistant Enterobacteriaceae in Community-Acquired Urinary Tract Infections. Open Forum Infect. Dis. 6:ofz103.
Bitsori, M., Maraki, S., Koukouraki, S., and Galanakis, E. (2012). Pseudomonas aeruginosa urinary tract infection in children: Risk factors and outcomes. J. Urol. 187, 260–264. doi: 10.1016/j.juro.2011.09.035
Chen, H. E., Tain, Y. L., Kuo, H. C., and Hsu, C. N. (2020). Trends in Antimicrobial Susceptibility of Escherichia coli Isolates in a Taiwanese Child Cohort with Urinary Tract Infections between 2004 and 2018. Antibiotics 9:501.
Critchley, I. A., Cotroneo, N., Pucci, M. J., and Mendes, R. (2019). The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One 14:e0220265. doi: 10.1371/journal.pone.0220265
Daoud, N., Hamdoun, M., Hannachi, H., Gharsallah, C., Mallekh, W., and Bahri, O. (2020). Antimicrobial Susceptibility Patterns of Escherichia coli among Tunisian Outpatients with Community-Acquired Urinary Tract Infection (2012-2018). Curr. Urol. 14, 200–205. doi: 10.1159/000499238
Duan, L., Liu, C., and Wang, D. (2021). The General Population’s Inappropriate Behaviors and Misunderstanding of Antibiotic Use in China: A Systematic Review and Meta-Analysis. Antibiotics 10:497.
Fazly Bazzaz, B. S., Fork, S. D., Ahmadi, R., and Khameneh, B. (2021). Deep insights into urinary tract infections and effective natural remedies. Afr. J. Urol. 27, 1–13.
Flores-Mireles, A. L., Walker, J. N., Caparon, M., and Hultgren, S. J. (2015). Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284. doi: 10.1038/nrmicro3432
García-Morúa, A., Hernández-Torres, A., Salazar-de-Hoyos, J. L., Jaime-Dávila, R., and Gómez-Guerra, L. S. (2009). Community-acquired urinary tract infection etiology and antibiotic resistance in a Mexican population group. Rev. Mex. de Urol. 69, 45–48.
Gharavi, M. J., Zarei, J., Roshani-Asl, P., Yazdanyar, Z., Sharif, M., and Rashidi, N. (2021). Comprehensive study of antimicrobial susceptibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci. Rep. 11:578.
Huang, L., Huang, C., Yan, Y., Sun, L., and Li, H. (2022). Urinary Tract Infection Etiological Profiles and Antibiotic Resistance Patterns Varied Among Different Age Categories: A Retrospective Study from a Tertiary General Hospital During a 12-Year Period. Front. Microbiol. 12:813145. doi: 10.3389/fmicb.2021.813145
Iacovelli, V., Gaziev, G., Topazio, L., Bove, P., Vespasiani, G., and Finazzi Agrò, E. (2014). Nosocomial urinary tract infections: A review. Urologia 81, 222–227. doi: 10.5301/uro.5000092
Jagadeesan, S., Tripathi, B. K., Patel, P., and Muthathal, S. (2022). Urinary tract infection and Diabetes Mellitus—Etio-clinical profile and antibiogram: A North Indian perspective. J. Fam. Med. Prim. Care 11, 1902–1906. doi: 10.4103/jfmpc.jfmpc_2017_21
Kish, L. (1965). Survey Sampling . (New York, NY: John Wiley & Sons, Inc).
Komagamine, J., Yabuki, T., Noritomi, D., and Okabe, T. (2022). Prevalence of and factors associated with atypical presentation in bacteremic urinary tract infection. Sci. Rep. 12:5197.
Kot, B. (2019). Antibiotic Resistance Among Uropathogenic Escherichia coli . Pol. J. Microbiol. 68, 403–415. doi: 10.33073/pjm-2019-048
Kucheria, R., Dasgupta, P., Sacks, S. H., Khan, M. S., and Sheerin, N. S. (2005). Urinary tract infections: New insights into a common problem. Postgrad. Med. J. 81, 83–86. doi: 10.1136/pgmj.2004.023036
Lee, C. L., and Kuo, H. C. (2017). Pathophysiology of benign prostate enlargement and lower urinary tract symptoms: Current concepts. Ci Ji Yi Xue Za Zhi 29, 79–83.
Leung, A. K., Wong, A. H., Leung, A. A., and Hon, K. L. (2019). Urinary tract infection in children. Recent Pat. Inflamm. Allergy Drug Discov. 13, 2–18. doi: 10.2174/1872213X13666181228154940
Mahmud, Z. H., Kabir, M. H., Ali, S., Moniruzzaman, M., Imran, K. M., Nafiz, T. N., et al. (2020). Extended-spectrum beta-lactamase-producing Escherichia coli in drinking water samples from a forcibly displaced, densely populated community setting in Bangladesh. Front. Public Health 8:228. doi: 10.3389/fpubh.2020.00228
Malik, S., Rana, J. S., and Nehra, K. (2021). Prevalence and antibiotic susceptibility pattern of uropathogenic Escherichia coli strains in Sonipat region of Haryana in India. Biomed. Biotechnol. Res. J. 5, 80–87.
Mohapatra, S., Panigrahy, R., Tak, V., Shwetha, J. V., Sneha, K. C., Chaudhuri, S., et al. (2022). Prevalence and resistance pattern of uropathogens from community settings of different regions: An experience from India. Access. Microbiol. 4:000321.
Motbainor, H., Bereded, F., and Mulu, W. (2020). Multi-drug resistance of blood stream, urinary tract and surgical site nosocomial infections of Acinetobacter baumannii and Pseudomonas aeruginosa among patients hospitalized at Felegehiwot referral hospital, Northwest Ethiopia: A cross-sectional study. BMC Infect. Dis. 20:92. doi: 10.1186/s12879-020-4811-8
Moue, A., Aktaruzzaman, S. A., Ferdous, N., Karim, M. R., Khalil, M. M., and Das, A. K. (2015). Prevalence of urinary tract infection in both outpatient department and inpatient department at a medical college setting of Bangladesh. Int. J. Biosci. 7, 146–152. doi: 10.12692/ijb/7.5.146-152
Mukherjee, S., Mishra, S., and Tiwari, S. (2020). Aetiological Profile and Antibiogram of Urinary Isolates Causing UTI in Patients Attending a Tertiary Care Hospital of Western Odisha. J. Evol. Med. Dent. Sci. 9, 662–667. doi: 10.14260/jemds/2020/144
Naushad, V. A., Purayil, N. K., Wilson, G. J., Chandra, P., Joseph, P., Khalil, Z., et al. (2022). Epidemiology of urinary tract infection in adults caused by extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae - a case-control study from Qatar. IJID Reg. 3, 278–286. doi: 10.1016/j.ijregi.2022.05.001
Odoki, M., Almustapha, A. A., Tibyangye, J., Nyabayo, M. J., Wampande, E., Drago Kato, C., et al. (2019). Prevalence of Bacterial Urinary Tract Infections and Associated Factors among Patients Attending Hospitals in Bushenyi District, Uganda. Int. J. Microbiol. 2019:4246780.
Olowe, O. A., Ojo-Johnson, B. B., Makanjuola, O. B., Olowe, R. A., and Mabayoje, V. O. (2015). Detection of bacteriuria among human immunodeficiency virus seropositive individuals in Osogbo, south-western Nigeria. Eur. J. Microbiol. Immunol. 5, 126–130. doi: 10.1556/EuJMI-D-14-00036
Patel, H. B., Soni, S. T., Bhagyalaxmi, A., and Patel, N. M. (2019). Causative agents of urinary tract infections and their antimicrobial susceptibility patterns at a referral center in Western India: An audit to help clinicians prevent antibiotic misuse. J. Fam. Med. Prim. Care 8, 154–159. doi: 10.4103/jfmpc.jfmpc_203_18
Prakash, D., and Saxena, R. S. (2013). Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of Meerut city, India. ISRN Microbiol. 2013:749629.
Prestinaci, F., Pezzotti, P., and Pantosti, A. (2015). Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 109, 309–318. doi: 10.1179/2047773215Y.0000000030
Revelas, A. (2012). Healthcare - associated infections: A public health problem. Niger. Med. J. 53, 59–64. doi: 10.4103/0300-1652.103543
Rowe, T. A., and Juthani-Mehta, M. (2013). Urinary tract infection in older adults. Aging Health 9, 519–528 doi: 10.2217/ahe.13.38
Sadeghi, M., Ebrahim-Saraie, H. S., and Mojtahedi, A. (2022). Prevalence of ESBL and AmpC genes in E. coli isolates from urinary tract infections in the north of Iran. New Microbes New Infect. 45:100947.
Sami, H., Sultan, A., Rizvi, M., Khan, F., Ahmad, S., Shukla, I., et al. (2017). Citrobacter as a uropathogen, its prevalence and antibiotics susceptibility pattern. CHRISMED. J. Health Res. 4, 23–26. doi: 10.4103/2348-3334.196037
Seifu, W. D., and Gebissa, A. D. (2018). Prevalence and antibiotic susceptibility of Uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect. Dis. 18:30. doi: 10.1186/s12879-017-2911-x
Sharma, P., Netam, A. K., and Singh, R. (2020). Prevalence and in vitro antibiotic susceptibility pattern of bacterial strains isolated from tribal women suffering from urinary tract infections in District Anuppur, Madhya Pradesh, India. Biomed. Res. Ther. 7, 3944–3953. doi: 10.15419/bmrat.v7i8.625
Shilpakar, A., Ansari, M., Rai, K. R., Rai, G., and Rai, S. K. (2021). Prevalence of multidrug-resistant and extended-spectrum beta-lactamase producing Gram-negative isolates from clinical samples in a tertiary care hospital of Nepal. Trop. Med. Health 49:23.
Sood, S., and Gupta, R. (2012). Antibiotic resistance pattern of community acquired uropathogens at a tertiary care hospital in Jaipur, Rajasthan. Indian J. Community Med. 37, 39–44. doi: 10.4103/0970-0218.94023
Tamma, P. D., Aitken, S. L., Bonomo, R. A., Mathers, A. J., van Duin, D., and Clancy, C. J. (2022). Infectious diseases society of america guidance on the treatment of AmpC β-Lactamase-producing Enterobacterales, Carbapenem-resistant Acinetobacter baumannii , and Stenotrophomonas maltophilia infections. Clin. Infect. Dis . 74, 2089–2114.
Terlizzi, M. E., Gribaudo, G., and Maffei, M. E. (2017). UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 8:1566. doi: 10.3389/fmicb.2017.01566
Keywords : antibiotic susceptibility (AS), antimicrobial resistance (AMR), multidrug resistance (MDR), urinary tract infections, uropathogens, India
Citation: Bhargava K, Nath G, Bhargava A, Kumari R, Aseri GK and Jain N (2022) Bacterial profile and antibiotic susceptibility pattern of uropathogens causing urinary tract infection in the eastern part of Northern India. Front. Microbiol. 13:965053. doi: 10.3389/fmicb.2022.965053
Received: 09 June 2022; Accepted: 15 July 2022; Published: 09 August 2022.
Reviewed by:
Copyright © 2022 Bhargava, Nath, Bhargava, Kumari, Aseri and Jain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neelam Jain, [email protected] ; Gopal Nath, [email protected]
† ORCID: Gopal Nath, https://orcid.org/0000-0003-2722-1308 ; Neelam Jain, https://orcid.org/0000-0003-1471-7419
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

Urinary Tract Infection
Catherine M. Bettcher , MD, Lead, Elizabeth Campbell , MD, Lindsay A. Petty , MD, Karl T. Rew , MD, Jennifer C. Zelnik , MD, and Giulia I. Lane , MD, Consultant. Ambulatory Clinical Guidelines Oversight: R. Van Harrison , PhD, April L. Proudlock , RN, and Karl T. Rew , MD.
Affiliations
- Copyright and Permissions
Patient population: Adults with UTI
Objective: Implement a cost-effective and evidence-based strategy for UTI management in adults
- History. Diagnosis is primarily made by history. In women with dysuria and urinary frequency, in the absence of vaginitis, acute cystitis is the diagnosis 90% of the time [IC * ] . Complicated cystitis is associated with factors that either increase the risk of serious outcomes or decrease the efficacy of treatment ( Table 1 ). Diagnose pyelonephritis based on symptoms of cystitis plus acute onset of flank pain or tenderness, with a urinalysis indicating bacteriuria or pyuria. In men, consider if prostatitis might be present.
- Telephone triage. In nonpregnant women with prior history of uncomplicated cystitis, consider telephone triage [IIC] .
- Urinalysis. Urinalysis for detection of pyuria by dipstick or microscopy has a sensitivity of 80 - 90% and a specificity of 50% for predicting UTI [IB] .
- Urine culture . Urine culture is NOT indicated in most cases of acute cystitis in women [IIIC] but should be sent in men [IC] . At a threshold of > 100,000 organisms, urine culture is 50% sensitive for UTI; at a threshold of >100 organisms, it is 90% sensitive. Always perform a urine culture with sensitivities in suspected pyelonephritis. Consider urine culture in patients with recurrent UTI or in the presence of complicating factors. Screen pregnant women at the initial prenatal visit with a urine culture. Always obtain a urine culture in symptomatic pregnant women.
- Uncomplicated cystitis ( Table 2 ) : First line: nitrofurantoin for 5 days [IA] ; Second line : trimethoprim/sulfamethoxazole (TMP/SMX) for 3 days [IA] , or oral cephalexin for 3-7 days, or fosfomycin for 1 day.
- Complicated cystitis ( Table 2 ): First line: nitrofurantoin for 7 days [IA] ; Second line : TMP/SMX for 7 days [IA] , or oral cephalexin for 7 days, or fosfomycin every 48 hours for 3 doses.
- Pyelonephritis ( Table 4 ): Ceftriaxone 1 g IM or IV × 1 dose * , followed by an oral antibiotic: First line: TMP/SMX for 7-14 days [IB] ; Second line: ciprofloxacin for 7 days [IA] , or levofloxacin for 5 days; 1 Third Line: amoxicillin/clavulanate for 10-14 days [IB].
- Asymptomatic bacteriuria: Do not treat asymptomatic bacteriuria [IIIA] , except in pregnancy and prior to some urologic procedures.
- Prostatitis: Choose an antibiotic that penetrates the prostate, such as TMP/SMX, ciprofloxacin, or levofloxacin, when concerned about prostatitis [IC] .
- Patients with resolved symptoms require no routine laboratory follow-up [IIIB] .
- - In postmenopausal women, offer intravaginal estrogen [IB] .
- - Encourage premenopausal women to drink at least 1.5 L of water per day [IB] .
- - Consider antibiotic prophylaxis or self-initiated therapy if behavioral strategies are not effective [IIA].
- - Assess for urinary retention in men [IA] and postmenopausal women [IB].
- - Cystoscopy and urinary tract imaging are rarely indicated in patients with recurrent UTI [IIID].
- Avoid catheterization whenever possible. When needed, choose clean intermittent catheterization over an indwelling catheter.
Diagnosis and Management of UTI in Adult Non-Pregnant Women.
Complicating Factors.
Treatment of Acute Uncomplicated and Complicated Cystitis.
Management of Recurrent UTI.
Treatment of Pyelonephritis.
Strength of recommendation:
I = generally should be performed; II = may be reasonable to perform; III = generally should not be performed.
Level of evidence supporting a diagnostic method or an intervention:
A = Systematic review of randomized controlled trials; B = Randomized controlled trials; C = Systematic review of nonrandomized controlled trials; D = Individual observation descriptive studies; E = Expert opinion.
Urinary tract infections (UTIs) lead to over 10 million office visits per year, 2 at a cost of several billion dollars annually in the United States. 3 , 4
There are two key principles for cost-effective care of most UTIs. First, order laboratory tests only when the results are likely to alter management or outcome. Second, prescribe antibiotics only for as long as necessary to be effective.
Recurrent UTIs are managed by modifying risk factors and using intermittent or daily antibiotic prophylaxis.
This guideline addresses the following topics:
- Types of UTI and definitions
- Risk factors
- Microbial etiology
- Complicating factors and medical conditions
- Cystitis, uncomplicated and complicated
- Recurrent UTI
- Asymptomatic bacteriuria
- Pyelonephritis
- UTI in pregnancy
- UTI in older persons
Epidemiology
UTIs are common, with an annual US incidence of 12% among women and 3% among men. 5 UTIs are most common among sexually active women age 18-29 years. 6 Roughly 50% of women will develop acute cystitis at least once during their lives, 4 and about a quarter will experience recurrence. 7 The lifetime prevalence of UTI in men is about 12%. 8 Data from 1996-2001 reveal about 7 million patient visits per year for uncomplicated UTI. 9 Morbidity for most UTIs is low, but because UTIs are so common, the annual cost of UTIs in the US is about $2.3 billion. 6
Types of UTI and Definitions
UTI in adults is classified as cystitis or pyelonephritis. In both men and women, the differential diagnosis includes urethritis, a sexually transmitted infection. In men, the UTI spectrum encompasses acute and chronic bacterial prostatitis. Related infections in men include epididymitis, orchitis, and epididymo-orchitis.
Uncomplicated cystitis : A bladder infection in a healthy, nonpregnant, premenopausal female with a normal urinary tract.
Complicated cystitis: A bladder infection associated with factors that either increase the risk of serious outcomes or decrease the efficacy of treatment. This includes: cystitis plus a foreign body (such as a catheter or urinary tract stone), recent instrumentation, urinary tract abnormalities, or vesicoureteral reflux. It also includes cystitis in men, pregnant women, and patients with renal transplant or other causes of an immunocompromised state, and UTIs due to atypical organisms or multi-drug resistant bacteria.
Uncomplicated pyelonephritis : A kidney infection that occurs in a healthy nonpregnant patient with a normal urinary tract.
Complicated pyelonephritis : A kidney infection that occurs during pregnancy or in a patient with other complicating factors (as noted above, under complicated cystitis), typically requiring hospital admission.
Recurrent UTI : Defined as ≥ 2 UTIs in 6 months, or ≥ 3 UTIs in 12 months.
Asymptomatic bacteriuria (colonization): > 100,000 cfu/mL of bacteria are present on clean catch urine culture without signs or symptoms of illness.
Risk Factors
Females are at higher risk of UTI than males, likely due to the shorter distance from the urethral opening to the bladder, and the closer proximity of the urethral opening to the bacteria-rich vagina and rectum. For healthy premenopausal females, the risk of both acute cystitis and recurrent UTI is increased with recent or frequent sexual activity, or with use of spermicide, either of which increases the risk of periurethral E. coli colonization. 10 , 11
Older age (≥ 65 years, and particularly ≥ 80 years) increases the risk of UTI for both women and men. UTI is uncommon in men under age 60 years, but the rate increases substantially thereafter, such that by age 80, both men and women have similar rates of UTI. 12
Decreased estrogen levels in postmenopausal women is a risk factor.
Incontinence of urine quadruples the risk of UTI. 13 Fecal incontinence exposes the urethral opening to bacteria, but most are rapidly cleared from the urinary tract unless they are uropathogenic strains.
Family history and genetics also influence risk. A woman whose mother had UTIs is at a 2-4 fold increased risk. 14 There appears to be a genetic predisposition affecting UTI severity as well. 15 – 17
Catheterization markedly increases the risk of UTI, particularly with longer duration of catheterization. 18 , 19 Catheter-associated UTIs account for about 70% of UTIs in hospitalized patients. 20 Clean intermittent catheterization is safer than using an indwelling catheter. Problems requiring catheterization (such as incomplete bladder emptying, neurogenic bladder, and anatomic abnormalities of the genitourinary tract) all increase the risk of UTI.
Procedure. UTI risk increases in the days following a urinary tract procedure, such as flexible ureteroscopy for stone management or treatment of urinary tract carcinomas. A Cochrane review showed that antibiotic prophylaxis for patients undergoing cystoscopy may reduce the risk of symptomatic UTI. 21 Women undergoing urogynecologic surgery are at increased risk of UTI. Between 7% and 24% of women undergoing surgery for pelvic organ prolapse or stress urinary incontinence will develop a postoperative UTI. 22
Kidney or bladder stones can retain bacteria and cause recurrent UTI, usually with the same organism. The presence of ureteral stones in patients with pyelonephritis increases the risk of urosepsis.
Diabetes mellitus doubles the risk of UTI. 23 Obesity has been statistically associated with an increased risk of UTI, but it is unclear if obesity is the cause. 24
Immunocompromised status , particularly kidney transplant or other solid organ transplant, increases the risk of UTI. 25 In one study, UTI occurred in 28% of 417 patients within 13 days of kidney transplant. 26 Infection with human immunodeficiency virus (HIV) is not thought to affect the risk of symptomatic UTI, even in patients with low CD4 counts. 27
Microbial Etiology
The microbial etiology of UTI is fairly consistent across multiple studies, although there are differences between populations. 15 , 28 , 29 Uropathogenic strains of E. coli are the most common cause, found in 70-95% of outpatient UTIs. These strains have virulence factors that help them survive within the urinary tract and evade host immune responses.
Staphylococcus saprophyticus causes 5-15% of outpatient UTIs, but is more common in women. Non- E. coli Enterobacteriaceae, such as Klebsiella pneumoniae and Proteus spp , are also fairly common causes of UTI. Enterococcus faecalis and Streptococcus agalactiae (Group B Streptococci) are also found.
Pseudomonas aeruginosa rarely causes UTI, but it is associated with complicated UTIs with high morbidity and mortality, partly because of its virulence factors, biofilm formation, and tendency for antibiotic resistance. 30 , 31 Candida UTI is uncommon, and candiduria more often represents colonization. When it occurs, it is associated with diabetes, prolonged antibiotic therapy, or immunosuppression. 32 , 33 , 34
The prevalence of UTI due to drug-resistant (including fluoroquinolone-resistant) strains of E. coli and other Enterobacteriaceae, is increasing. 35 , 36 Risk factors for multi-drug resistant Enterobacteriaceae infections include: fluoroquinolone use within the past 3-6 months, catheter use, hospital stay or nursing home admission, and obstructive uropathy. 37 , 38 Risk factors for methicillin resistant Staphylococcus aureus (MRSA) bacteriuria include increased age, patient comorbidity, hospital exposure, and catheter use. 39 Multi-drug resistant UTI is a complicated UTI that may require hospital admission and IV antibiotic treatment.
Complicating Factors and Medical Conditions
The most severe complication of UTI is urosepsis, with a mortality rate of 20-40%. The underlying infection usually is a complicated UTI involving a urogenital organ, typically prostate or kidney. Obstructive pyelonephritis due to urolithiasis is the most common cause of urosepsis, but about 17% of cases are associated with urological procedures. The elderly, diabetics, and immunosuppressed are at highest risk. Management of urosepsis is beyond the scope of this guideline, but rapid diagnosis and prompt intensive care are essential. 40
Patients with complicating factors and medical conditions are at increased risk of developing pyelonephritis or infection with resistant organisms. Complicating factors are listed in Table 1 . It is necessary to differentiate these patients from those with uncomplicated UTI in terms both of evaluation and treatment. Unlike patients with uncomplicated UTI, care for those with complicating factors may include:
- Culture. Obtain pretreatment urine culture and sensitivity.
- Treatment. Initiate longer antibiotic treatment course.
- Possible structural evaluation. If there is concern for concurrent urolithiasis or urinary tract structural or functional abnormality, consider CT with and without IV contrast and urology consultation for cystoscopy. 41
- Acute Cystitis
Recommendations
- Diagnose acute cystitis by history.
- Do not routinely perform additional diagnostic testing.
- Consider initiating treatment without laboratory testing if the clinical presentation supports the diagnosis.
- Do not perform a urine culture for uncomplicated cystitis.
Symptoms. History alone is often sufficient to make a diagnosis of acute uncomplicated cystitis. Patients with dysuria as an isolated symptom have an approximately 50% probability of acute cystitis. The presence of both dysuria and urinary frequency, in the absence of vaginal discharge, raises the probability of cystitis to greater than 90%. 42 When the history is typical for cystitis, do not perform additional diagnostic testing; it is unlikely to be of additional benefit or to alter management. In a patient with a strongly suggestive history, for example, a negative dipstick urinalysis does not rule out UTI. Testing is not required prior to initiating treatment, given the high probability of UTI with classic symptoms.
UTI symptoms typically have an abrupt onset (< 3 days); longer or intermittent symptoms increase the likelihood of other etiologies. The presence of vaginal discharge or irritation reduces the probability of cystitis and may suggest vaginitis or cervicitis. Consider a diagnosis of interstitial cystitis or painful bladder syndrome in patients who have recurrent symptoms without identifiable cause, with negative laboratory testing.
Physical examination. Physical exam is generally not necessary to diagnose UTI, unless symptoms suggest pyelonephritis (see Pyelonephritis section). Perform a pelvic exam if vaginal symptoms are present.
Laboratory tests. If laboratory testing is determined to be necessary, urine dipstick and microscopy are usually sufficient. Dipstick urinalysis is quick and inexpensive. The presence of leukocyte esterase or nitrite is consistent with inflammation or bacteriuria, and in the context of urinary signs and symptoms (urinary urgency, frequency, dysuria, suprapubic pain, new onset hematuria), can further confirm the diagnosis of UTI. Several studies found nitrites to be the most predictive of bacteriuria, but nitrites only turn positive in the presence of bacteria that produce nitrate reductase. 43 , 44
Do not routinely perform a urine culture in uncomplicated cystitis. Consider a urine culture if dipstick urinalysis is negative but clinical suspicion remains high, or in the setting of complicating factors. Previous diagnosis thresholds for UTI were 100,000 cfu/mL of a single organism. Lower colony counts of 100 to 10,000 cfu/mL in voided urine from symptomatic patients may constitute UTI versus contamination. 15 Symptomatic patients with low colony counts may respond as well to antibiotic treatment as those with high colony counts.
- Consider acetaminophen or ibuprofen for pain, and phenazopyridine for symptom relief in patients with cystitis.
- Prescribe nitrofurantoin as first-line treatment for both uncomplicated and complicated cystitis.
- Consider trimethoprim/sulfamethoxazole (TMP/SMX), cephalexin, and fosfomycin as second-line treatment options.
- Treatment duration for uncomplicated cystitis depends on the medication ( Table 2 ).
- Treat most cases of complicated cystitis for 7 days.
- Do not use fluoroquinolones for the treatment of cystitis if a first or second-line treatment option exists.
Non-antibiotic treatment of cystitis. Acetaminophen or ibuprofen may be used for symptom relief in cystitis, but should not be recommended as the primary treatment of UTI. In randomized trials, women given only NSAIDs may be at increased risk of pyelonephritis. Delayed antibiotic use with close follow-up may be reasonable for a subset of women with mild cystitis. 45 , 46
Phenazopyridine, a urinary analgesic, also relieves dysuria. Dysuria typically diminishes within a few hours of initiation of antimicrobials, but some women with severe dysuria may benefit from up to 2 days of phenazopyridine.
Uncomplicated Cystitis: Antibiotic Selection and Duration
Nitrofurantoin is the first line treatment recommendation for acute uncomplicated cystitis. Nitrofurantoin achieves good concentration in the urine, has low resistance rates for E. coli , and can be used in patients with CrCl > 30 mL/min/1.73 m 2 . 47 Alternatives include TMP/SMX, cephalexin, and fosfomycin ( Table 2 ).
The rate of resistance to TMP/SMX is > 30% in some areas. In contrast, nitrofurantoin resistance remains < 5%. The 2010 resistance rates to TMP/SMX for E. coli in the US were 28%, which led to TMP/SMX no longer being recommended as first line therapy for uncomplicated cystitis. However, it remains a reasonable alternative. Since TMP/SMX is concentrated in the urine, in vitro resistance does not necessarily translate into therapeutic failures. Reported TMP/SMX resistance rates may be misleadingly high as they represent patients receiving urine cultures, whereas most acute uncomplicated cystitis is not assessed with a urine culture.
In uncomplicated cystitis, consider shorter courses of oral antibiotics, with specific duration dependent on the specific antibiotic. In trials, shorter courses were as effective as longer courses, with fewer adverse events. 48 , 49 Advantages of shorter therapy include decreased prescription costs, improved adherence, and decreased adverse effects of antibiotic treatment. 48 , 49 No benefit is apparent in increasing the duration of TMP/SMX treatment. Cystitis cure rates of about 85% have been achieved with 3-day therapy, while adverse effects increase markedly if treatment is continued longer than 3 days. 49 , 50
When beta-lactam therapy, such as cephalexin, is chosen due to allergies or other factors, little evidence guides treatment duration for cystitis. Three to 7 days are recommended, depending on symptom severity.
Single-dose cystitis treatment regimens are less efficient than 3-5 day regimens at eradicating bacteriuria (23-81% versus 77-91% long-term cure, respectively). A 2018 randomized controlled trial demonstrated decreased efficacy of a single dose of fosfomycin compared to 5 days of nitrofurantoin. 51
Avoid fluoroquinolones for uncomplicated cystitis due to high rates of E. coli resistance and risk of collateral damage (resistance, C. difficile infection). Fluoroquinolones may cause permanent injury to tendons, muscles, joints, nerves, and the central nervous system. For uncomplicated cystitis, the FDA recommends fluoroquinolones be reserved for patients with no other treatment options. 52
Complicated Cystitis: Antibiotic Selection and Duration
Prescribe longer courses of antibiotics for male patients with cystitis and for patients who have complicating factors, including: uncontrolled diabetes, pregnancy, nephrolithiasis, catheter use, anatomic or functional abnormalities, or immunosuppression. (See text sections below for management of UTI in pregnancy and UTI in men.) As a general rule, 7 days of oral antibiotics are recommended for complicated cystitis.
Follow-up. Follow up urinalysis and urine cultures (so-called “test-of-cure”) are not indicated for patients with uncomplicated cystitis. Approximately 5-10% of women treated for uncomplicated cystitis will have persistent bacteriuria after therapy completion. The vast majority of these women will be symptomatic and return for medical attention. Those who are asymptomatic require no treatment except in pregnant patients, or patients undergoing urologic procedures. (See section on asymptomatic bacteriuria .)
Telephone triage-nurse managed evaluation. Most UTIs in women are uncomplicated and resolve readily with a short course of antibiotics. Therefore, many women can be assessed and safely managed without an office visit or laboratory evaluation. Studies have found that use of a telephone triage guideline decreased cost and increased appropriate antibiotic use with no increase in adverse outcomes. 53 , 54
Consider telephone triage and treatment without an office visit or laboratory testing for nonpregnant women who previously had acute cystitis that responded to antibiotics. Management without an office visit is not recommended for men, patients with symptoms of pyelonephritis, or those with complicating factors. Patients who do not respond promptly should be evaluated in the office.
- Diagnose recurrent UTI based on 2 culture-proven UTIs in the past 6 months or ≥ 3 in 1 year.
- Obtain urine culture with sensitivities prior to initiating treatment for recurrent UTI.
- Consider measuring bladder postvoid residual volume in men and postmenopausal women with recurrent UTI.
- Do not routinely perform cystoscopy or imaging in patients with recurrent UTI.
Recurrent urinary tract infections are defined as 2 culture-proven UTIs in the past 6 months, or ≥ 3 in 1 year. In sexually active college women, the risk of a second UTI was 24% in 6 months. 55 A large study of US women, ages 18 to 64 years, showed that about 1 in 1,000 women per year develop an uncomplicated recurrent UTI. The incidence of recurrent UTI was highest in postmenopausal women, approximately double that of younger women. 56 Recurrent UTI also is more common in older men; see section on UTI in men .
Reinfection is the cause for most women with recurrent UTIs, while a minority have relapse. Reinfection typically occurs at least 2 weeks after UTI treatment and is caused by a different organism. In relapse, the same strain of bacteria causes recurrent UTI. 57 , 58 The bacteriuria persists during treatment or recurs soon (1-2 weeks) after treatment is completed. Symptomatic recurrent UTI due to relapse tends to occur much sooner than does reinfection. Relapse typically implies an unresolved infection and may be associated with an underlying urologic abnormality.
Risk factors for recurrent UTI in premenopausal women include frequent intercourse, a new sexual partner, a maternal history of UTI, and a history of UTI before age 15 years. In postmenopausal women, risk factors include urinary incontinence and increased bladder postvoid residual volume. 57 , 59 In men, urinary retention is the main risk factor.
There is no proven association between recurrent UTI in women and pre- or postcoital voiding patterns, frequency of urination, wiping patterns, douching, tight underwear, delayed voiding habits, or hot tub use. There have been no prospective randomized studies of these factors. 14
Physicians should confirm the diagnosis of recurrent UTI by urine culture. Cystoscopy and urinary tract imaging are not routinely recommended, but may be performed in certain circumstances, such as suspected stone or obstruction. 57 , 59 Physicians should measure bladder postvoid residual volume in men and postmenopausal women with recurrent UTI to evaluate for retention. 57
- Treat recurrent UTI with the recommended antibiotic regimens for acute cystitis, taking into account prior culture and sensitivity results.
- Counsel patients on risk factors for recurrent UTI.
- Consider lower threshold for imaging and urology referral.
Most patients with recurrent UTIs respond to recommended antibiotic regimens ( Table 3 ). Persistent bacteriuria or early clinical recurrence (within 2 weeks) should raise the concern for possible relapse. In cases of relapse, physicians should have a lower threshold for imaging and urology referral.
Most uncomplicated recurrent UTI represents reinfection rather than relapse. Use prior urine culture and sensitivity results to guide empiric treatment. 59 These patients rarely have a urologic structural abnormality causing the reinfection, so imaging is not indicated. Counsel them about modifying risk factors for recurrent UTIs, and consider use of prophylactic or self-initiated antibiotic therapy.
In patients with recurrent typical symptoms of cystitis, yet persistently negative urine cultures and negative STI testing, consider a referral to urology for evaluation of interstitial cystitis, or painful bladder syndrome. Asymptomatic patients with recurrent positive urine cultures should not be diagnosed with recurrent UTI, as they have asymptomatic bacteriuria.
Prophylaxis of Recurrent UTIs
- Offer intravaginal estrogen to reduce recurrent UTIs in postmenopausal women.
- Encourage premenopausal women to drink 1.5 L of water per day to prevent recurrent UTI.
- Consider intermittent single-dose post-coital or daily antibiotic prophylaxis to reduce risk of recurrent UTI in nonpregnant women if behavioral strategies are not effective.
- Assess for urinary retention in men with recurrent UTI.
Strategies for preventing recurrent UTI include using antibiotics or nonpharmacologic therapies. Whether to use antibiotic prophylaxis, and which agent, should be a shared decision between the physician and patient. Before prescribing antibiotics, clinicians should counsel patients about behavioral strategies to prevent recurrent UTIs given the increasing resistance to antibiotics, adverse effects of antibiotics on normal flora, and potential for serious adverse effects. 59
In sexually active women, prophylactic antibiotic use reduces the frequency of recurrent UTIs compared to placebo (NNT = 2). 59 Intermittent single-dose antibiotic prophylaxis after coitus appears as effective as daily prophylaxis, with fewer adverse effects. 60 Single-dose prophylaxis is the preferred option for women who develop cystitis related to sexual intercourse. 59 The reduction in recurrent UTI only lasts as long as the woman takes the antibiotic. Once antibiotics are discontinued, UTIs occur at the same rate as in placebo-treated sexually active women. Adverse events from antibiotic use are generally mild, although women vary in their evaluation of the impact of various side effects (ie, vaginal candidiasis may be perceived as a severe side effect by some, mild by others). 59
For recurrent UTI prevention, nitrofurantoin, TMP/SMX, beta-lactams, and fluoroquinolones are equally effective. 59 Review prior urine culture and sensitivity results to guide antibiotic choice. If prescribing prophylactic antibiotics, use nitrofurantoin 50 mg daily (first-line as long as CrCl > 30 mL/min/1.73 m 2 ) or TMP/SMX SS 80/400 mg daily. Avoid fluoroquinolones due to high rates of E. coli resistance and other potential complications ( C. difficile infection, tendinopathy). Consider prescribing prophylactic antibiotics for up to 6 months. 59
Advise patients to increase their daily consumption of water. According to one randomized controlled trial, nonpregnant premenopausal women who drank 1.5 L of additional water per day decreased their risk of recurrent UTI compared to women who consumed their usual fluid intake. 61
Recurrent UTIs are more common in older patients. In postmenopausal women, one possible cause is low levels of estrogen leading to increased pH in the vagina, enabling its colonization by uropathogens. 3 Randomized controlled trials showed that a vaginal estrogen ring or cream prevented recurrent UTIs in postmenopausal women (NNT range 2-9). 3 , 59 Women using vaginal estrogens had more vaginal irritation compared to those taking oral antibiotics. 59 In older men, urinary retention can cause recurrent UTI (see section on UTI in men ).
Meta-analyses have produced conflicting results regarding whether cranberry products help prevent recurrent UTIs in nonpregnant women, and the studies comprise low quality evidence. The type of cranberry product and frequency of administration varied across the studies. 59 Most women cannot tolerate drinking large quantities of cranberry juice but may be amenable to cranberry tablets or capsules. Cranberry products do not reduce the risk of recurrent UTIs in pregnant women, elderly women, or men. 59
One small randomized controlled trial showed that oral D-mannose (200 mL of 1% solution daily) prevents recurrent UTIs in nonpregnant women compared to no treatment, with significantly fewer adverse effects compared to antibiotics. 62
Other interventions, such as lactobacillus probiotics and methenamine hippurate lack good quality evidence to show whether they reduce the risk of recurrent UTI. 59 , 63 , 64
- Asymptomatic Bacteriuria
Asymptomatic bacteriuria is the presence of significant numbers of bacteria in the urine in a person without symptoms. The presence of one organism per high-power field in a clean-catch, midstream, unspun urine sample, is considered significant bacteriuria (equivalent to > 100,000 cfu/mL).
Asymptomatic bacteriuria occurs in 30-50% of elderly adults, especially in nursing homes. In controlled studies that address issues of underlying illness, asymptomatic bacteriuria does not increase risk of death. 65 , 66
Patients with chronic indwelling catheters are at particular risk for developing asymptomatic bacteriuria. The risk of UTI can be decreased by using a catheter only when necessary, inserting the catheter under aseptic technique, using a closed drainage system, and avoiding irrigation (unless clinically necessary). Intermittent catheterization and external (condom type) catheters are associated with fewer infections than indwelling catheters.
Screening and Treatment
Recommendation.
- Do not screen for, or treat asymptomatic bacteriuria, except in pregnancy and patients undergoing urologic procedures.
Screening and treatment of asymptomatic bacteriuria in most settings is not recommended because of unproved efficacy, risk of adverse effects from antibiotics, development of antibiotic resistance, and excess costs.
Treatment of asymptomatic bacteriuria is recommended in the following situations:
- Pregnancy. See pregnancy section.
- Before transurethral resection of the prostate or other urologic procedure in which mucosal bleeding is anticipated. Postoperative complications, including bacteremia, are reduced by treating bacteriuria prior to such procedures.
- Diagnose pyelonephritis based on acute onset of flank pain or tenderness plus a urinalysis indicating bacteriuria or pyuria.
- Obtain a urine culture with sensitivities prior to starting antibiotics in suspected acute pyelonephritis.
- Imaging is not indicated for the diagnosis of acute uncomplicated pyelonephritis.
- If complicated pyelonephritis is suspected, or when the infection does not respond to antibiotics within 48-72 hours, order CT imaging of the abdomen and pelvis with and without IV contrast.
- Consider MR with diffusion-weighted imaging or Doppler ultrasound of the kidneys and bladder for the diagnosis of pyelonephritis in pregnant women.
Suspect acute pyelonephritis in patients presenting with sudden onset of typical lower urinary tract symptoms (dysuria, frequency, urgency) with associated fever, chills, back or flank pain, nausea, vomiting, or costovertebral angle tenderness. Specific diagnostic criteria for pyelonephritis do not exist. 67 Always perform a urine culture with sensitivities in suspected pyelonephritis. Antibiotic therapy should be tailored to the ensuing sensitivity results. 68 , 69
Most acute pyelonephritis is uncomplicated, responds quickly to treatment, and results in no residual kidney damage. 68 Imaging is not indicated for the diagnosis of uncomplicated pyelonephritis. 70 Almost all patients with uncomplicated pyelonephritis diagnosed clinically become afebrile within 72 hours of appropriate antibiotic therapy. 70
Complicated pyelonephritis occurs in individuals with a structural or functional abnormality of the urinary tract (indwelling urinary catheter, urinary obstruction, renal stones), or an underlying disease (pregnancy, diabetes, immunocompromised status) that increases the risk of treatment failure. Complicated pyelonephritis includes patients who do not respond to initial antibiotics. 70 Bacteria are more likely to be resistant to antibiotics in complicated infections. 68 Consider CT of the abdomen and pelvis with and without IV contrast to diagnose pyelonephritis in patients with complex clinical presentations and in those who do not improve with antibiotics within 48-72 hours. 70
- Consider a 7-day course of antibiotics to treat acute pyelonephritis in a female patient less than 65 years old without comorbidities.
- Use a 14-day course of antibiotics to treat complicated pyelonephritis. (Seven days of a fluoroquinolone may be considered if the patient is not immunosuppressed, has no urinary anatomic abnormalities, is not pregnant, and responds well.)
- Decompress urinary tract obstruction in patients with pyelonephritis related to urinary tract obstruction.
Most patients with pyelonephritis but without risk factors for developing complications can be safely managed on an outpatient basis with oral antibiotics ( Table 4 ). Hospital admission with intravenous antibiotics is indicated for acutely toxic, pregnant, or immunocompromised patients; those who are dehydrated or unable to take oral fluids; or when compliance is a problem. 68
If the local prevalence of fluoroquinolone or TMP/SMX resistance exceeds 10%, acute pyelonephritis should be treated empirically with an initial one-time dose of a broad-spectrum, long-acting parenteral antibiotic. 67 , 69 Following that initial dose (eg, ceftriaxone 1 g IM or IV), prescribe TMP/SMX 160/800 mg by mouth twice daily for 7-14 days; OR ciprofloxacin 500 mg by mouth twice daily for 7 days; OR levofloxacin 750 mg by mouth daily for 5-7 days; OR amoxicillin/clavulanate 875/125 mg by mouth twice daily for 10-14 days. 67 , 69 ( Table 4 ). A meta-analysis showed that ciprofloxacin for 7 days is as effective as a longer 14-day course. 71 One study of nonpregnant women with E. coli pyelonephritis showed that oral TMP/SMX for 7 days was as effective as oral ciprofloxacin for 7 days. 72
Physicians may use their clinical judgment based on the severity of the patient’s pyelonephritis and underlying medical conditions to defer ceftriaxone and monitor closely. For example, an otherwise healthy woman with mild illness may be solely treated with oral ciprofloxacin or TMP/SMX. 67
Adjust the initial antibiotic choice if indicated, based on the urine culture and sensitivity results. 73 Adequate response to therapy is defined as clear improvement in clinical condition over 48-72 hours; it does not necessarily include becoming afebrile. Do not perform a follow-up urine culture if symptoms have resolved. 74 Routine structural evaluation is rarely indicated.
For pyelonephritis related to urinary tract obstruction, start antibiotics as above, but also promptly decompress the obstruction and consult urology. Use a urinary catheter for obstruction due to prostatic hyperplasia. Immediate consultation with urology for consideration of ureteral stent insertion or percutaneous nephrostomy for pyelonephritis associated with ureteral stones can be lifesaving. 73
- Special Populations
UTI in Pregnancy
- Screen pregnant women for bacteriuria at the initial prenatal visit with a urine culture. 75
- Treat asymptomatic bacteriuria during pregnancy with nitrofurantoin, amoxicillin, or cephalexin, depending on culture results. 75
Etiology. Pregnancy results in dilation of the ureters and renal pelvises, increased urinary pH, and glycosuria. These changes predispose pregnant women with bacteriuria to an increased risk of pyelonephritis and preterm birth.
Asymptomatic Bacteriuria in Pregnancy
Asymptomatic bacteriuria occurs in up to 10% of pregnant patients. Unlike in nonpregnant patients, asymptomatic bacteriuria in pregnancy should be treated. Treatment decreases the risk of pyelonephritis and associated maternal and neonatal complications, including septicemia, respiratory distress, preterm birth, and low birth weight. Controlled trials found treatment of asymptomatic bacteriuria in pregnancy was associated with a reduced incidence of persistent bacteriuria (NNT = 2), pyelonephritis (NNT = 7), and preterm delivery (NNT = 7). 59
Treat asymptomatic bacteriuria during pregnancy with antibiotics selected based on culture and susceptibility testing. Recommended options are nitrofurantoin for 5 days, cephalexin for 7 days, or fosfomycin as a single dose. Avoid using nitrofurantoin after 37 weeks gestation due to the risk of neonatal jaundice. Avoid the use of fluoroquinolones in pregnancy unless other options are contraindicated. Follow up with urine cultures; persistent bacteriuria requires repeat treatment guided by sensitivities, and then consideration of daily suppressive therapy, usually with nitrofurantoin.
Symptomatic Cystitis in Pregnancy
- Treat cystitis during pregnancy with nitrofurantoin for 7 days (first line) or cephalexin for 7 days. 75
The diagnosis of cystitis in pregnancy is made based on the presence of lower urinary tract symptoms and laboratory testing. Unlike in nonpregnant patients, urine culture should be routinely obtained to confirm the diagnosis and guide treatment.
Treat cystitis during pregnancy with nitrofurantoin for 7 days (preferred) or cephalexin for 7 days. Avoid use of nitrofurantoin after 37 weeks gestation because it may increase neonatal jaundice.
Pyelonephritis in Pregnancy
- Admit pregnant patients with pyelonephritis for treatment with IV antibiotics.
The diagnosis of pyelonephritis should be based on symptoms and exam findings (see Pyelonephritis section). Urine culture should be obtained prior to initiation of treatment.
Pregnant patients with pyelonephritis should be admitted to the hospital for treatment with IV antibiotics, due to the increased risks of septicemia, acute respiratory distress syndrome, and obstetric complications. Choice of antibiotics is dependent on urine culture and susceptibility results. A parenteral, broad-spectrum beta-lactam antibiotic (such as ceftriaxone) should be initially given, followed by a course of oral TMP-SMX or a beta-lactam. Fluoroquinolones should be avoided in pregnancy if possible.
Rates of UTI are low in younger males, but increase significantly in older men. The incidence of UTI in men < 55 years is 0.9 to 2.4 cases per 1000, but in men > 85 years the incidence is 7.7/1000, similar to that of women in the same age group. 12 UTI in men is less well studied than in women. There are relatively few studies of UTI incidence in men; most studies in males focus on UTI in infancy and early childhood, when the risk is highest. Based on an outpatient study in veterans, the overall prevalence of UTI as a primary diagnosis was 2.3 times greater in women than in men. 76 About 12% of all men will have at least one UTI in their lifetimes, compared to about half of all women. 8
Etiology. The causes of UTI in men vary with age and risk factors for sexually transmitted infections. In younger males (age 14-35 years), UTI symptoms are more likely to be due to pathogens causing urethritis, such as Neisseria gonorrhea or Chlamydia trachomatis , but in older males they are more likely to be due to E. coli or other enterobacteriaceae. Other less common causes of male urethritis or epididymitis include Mycoplasma genitalium , Ureaplasma urealyticum , and Trichomonas vaginalis.
Risk Factors in Men
Urinary Retention. Acute urinary retention is defined as the inability to pass urine, despite having a full bladder, which on exam may be painfully distended or palpable. 77 In contrast, men with chronic urinary retention can pass some urine, but have a chronically elevated postvoid residual, with slow urinary flow and sensation of incomplete bladder emptying. 77 Traditionally, chronic urinary retention has been defined as postvoid residual ≥ 300 mL, 78 but current literature has shifted away from arbitrary volume cut-offs towards patient symptoms. 79 Both acute and chronic urinary retention are much more common in men than in women. 80 Prostatic hyperplasia increases the risk of recurrent UTI in men and is an important cause of urinary retention.
Chronic bladder outlet obstruction with an increased volume of urine retention appears to increase the risk of UTI in men, but studies of this have produced inconsistent results, and it is unclear how much retention is too much. In some studies, a bladder postvoid residual volume over 180 mL increased the risk of UTI, but this cutoff was only 28% sensitive and 94% specific for predicting bacteriuria. Other studies used a higher volume cutoff of 300 mL to define retention in men who were voiding, or even 1000 mL in men who were unable to void. Retention of urine is a complex problem in men, and postvoid residual volume is not clearly correlated with age, prostate volume, or PSA. 79 , 81 , 82 Numerous medications have been associated with increased urinary retention; many of these are drugs with anticholinergic effects. 80 Older men are at higher risk for drug-related urinary retention.
Anatomic Factors. Adult males with a history of hypospadias, whether repaired or unrepaired, are at increased risk for urethral stricture, UTI, and other urologic problems. 83
Circumcision reduces the risk of UTI, but this is most relevant in infants. A meta-analysis of 22 studies showed that lifetime UTI risk was 32% in uncircumcised males and 8.8% in circumcised males. 84
Other Risk Factors. Many of the risk factors mentioned above for female UTI also apply to men. However, men with catheter-associated bacteriuria have a higher risk of bacteremia than women. 85
Men who undergo transrectal ultrasound-guided prostate biopsy are at risk for UTI, bacteremia, and urosepsis. In a study of 1529 men, about 1.4% developed UTI after such a biopsy. 86 A 2011 Cochrane review confirmed that antibiotic prophylaxis is effective at preventing infection due to transrectal prostate biopsy. 87 Transperineal prostate biopsy is an alternate approach that is increasingly used; it appears to be associated with an almost zero rate of urosepsis. 88
Diagnosis of UTI in Men
- First obtain initial void urine for STI testing, if indicated.
- Obtain a clean-catch urine sample for urinalysis and culture.
- Measure bladder postvoid residual volume with ultrasound.
Symptoms of cystitis and pyelonephritis are similar in men and women. However, acute UTI in men can also include acute bacterial prostatitis, epididymitis, and urethritis. Suspect urethritis in any sexually active male with dysuria. UTI in men is generally considered to be complicated because of the risk of acute bacterial prostatitis, which can be a severe and potentially life-threatening systemic infection. Chronic bacterial prostatitis presents as recurrent UTI, typically with the same bacterial strain each time.
Symptoms unique to men include slow urinary stream, a sense of incomplete emptying, penile discharge, suprapubic or groin pain, and testicular pain. Signs of acute UTI in men may include an enlarged and tender boggy prostate, or a tender epididymis or testis.
If STI is a consideration, first collect an initial void urine sample (without cleansing), before collecting a clean catch midstream sample. Send the initial void urine sample for gonorrhea and Chlamydia testing in symptomatic males age 14-35 years, as well as in those who have STI exposure risks, a new sexual partner, penile discharge, or signs and symptoms of epididymitis or orchitis. STI testing can also be done on a urethral swab.
Obtain a clean catch urinalysis. The presence of bacteriuria along with symptoms supports a diagnosis of UTI. Pyuria is not diagnostic of UTI, but the absence of pyuria can be used to rule out UTI with a negative predictive value of 95%. 89
Obtain a urine culture in all men with UTI symptoms, prior to antibiotic initiation. In a patient with symptoms, a culture result showing > 100,000 cfu/mL of a single organism is diagnostic. A clean catch urine culture with > 100,000 cfu/mL of two or more organisms, or a culture with at least 1000 cfu/mL of a single organism may indicate UTI or contamination, but these need to be interpreted in clinical context. The urine sample for culture should be a midstream collection, with retraction of the foreskin (if present) and cleansing prior to collection to minimize risk of contamination. 12 , 90
If the cause of symptoms is unclear, consider testing for less common causes of urethritis and epididymitis, such as Mycoplasma genitalium, Mycoplasma hominis , and Ureaplasma urealyticum . Be aware that asymptomatic carriage of these organisms is common, and the treatment cure rate for mycoplasma is low. 91 – 93 Trichomonas vaginalis has been associated with persistent urethritis in some populations of men, so if initial treatment for the more common causes of urethritis is unsuccessful, consider nucleic acid amplification testing of an initial-void urine (or a urethral swab) for Trichomonas. 93
Use ultrasound to measure bladder postvoid residual volume and assess for urinary retention in symptomatic men. In many healthy men the bladder can empty completely and the postvoid residual will be near zero. Although residual volumes over 100 mL are considered abnormal, the normal range varies widely, and the clinical significance of mild urinary retention is unclear. Physical examination of the bladder is not a reliable way to estimate urinary retention.
Checking PSA is usually neither necessary nor helpful in most men with UTI, but can be considered when prostate cancer is suspected or when the diagnosis is not clear. In more than 90% of men with a febrile UTI, there will be a transient increase in the PSA. Although PSA typically decreases after 1 month, it may not fully normalize for 6 or more months after a febrile UTI. 94
In men with bacteremia or urosepsis, or who are not responding to antibiotics, transrectal ultrasound can assess for prostate abscess. 95 Otherwise, prostate imaging is generally not useful in men with UTI.
Differential Diagnosis of UTI in Men
Chronic bacterial prostatitis causes recurrent UTIs, and usually the same bacterial strain is found with each episode. However, only about 10% of men with chronic prostatitis symptoms actually have chronic bacterial prostatitis, with E. coli being the most common organism. 96
Urologic chronic pelvic pain syndrome in men (also known as chronic nonbacterial prostatitis) may cause lower urinary tract symptoms (LUTS). 97 Urologic chronic pelvic pain syndrome encompasses the related diagnosis that occurs in both men and women, called painful bladder syndrome or interstitial cystitis. Typical symptoms include chronic pelvic pain, often with urinary urgency and frequency. If no infection is found, routine use of antibiotics is not recommended. 98
Kidney stones and bladder stones can cause UTI-like symptoms, usually with gross or microscopic hematuria. If stone disease is suspected, imaging with a renal stone protocol (noncontrast) abdominal CT is recommended.
Prostate cancer is typically asymptomatic in its early stages, but can cause lower urinary tract symptoms or urinary retention in some men. A full discussion is beyond the scope of this guideline, but an elevated PSA or abnormal prostate on digital rectal exam are indications for a urology consultation.
Epididymitis, orchitis, and epididymo-orchitis may be due to a bacterial infection or an STI such as Neisseria gonorrhea or Chlamydia trachomatis . (See Diagnosis of UTI in Men, above.) Viral causes are also possible, so when orchitis is present, consider testing for mumps using a buccal swab, especially if parotitis is also present.
Treatment of UTI in Men
- Treat acute uncomplicated cystitis with nitrofurantoin (first line) or alternative antibiotics, as per Table 2 .
- Choose an antibiotic that penetrates the prostate, such as TMP/SMX, ciprofloxacin, or levofloxacin, when concerned about prostatitis.
- Treat chronic bacterial prostatitis for 4-6 weeks and consider using a shorter duration of 2 weeks for acute bacterial prostatitis.
- Manage acute or chronic urinary retention with an alpha blocker.
Antibiotic Selection and Duration. Before prescribing, review any prior urine culture results and consider if the patient is at risk for a drug-resistant infection. Send a urine culture to guide treatment.
When treating acute uncomplicated cystitis in men, use the recommended antibiotics per Table 2 , with nitrofurantoin being the first-line choice. When concerned about prostatitis, choose an antibiotic that penetrates the prostate, such as TMP/SMX, ciprofloxacin, or levofloxacin. Beta lactam antibiotics such as penicillins and cephalosporins can be used when indicated by culture results, but tend to be less effective in men due to poor prostate penetration. 94 Because nitrofurantoin achieves therapeutic concentrations only in the bladder, it is not effective for pyelonephritis or prostatitis. 99
For presumed acute bacterial prostatitis, the duration of antibiotic treatment is traditionally 4-6 weeks based on limited observational studies, 100 but 2 weeks may be adequate. 101 , 102 Acute prostatitis following a urologic procedure, such as transrectal biopsy of the prostate or cystoscopy, should prompt urologic consult.
Chronic bacterial prostatitis may be difficult to eradicate. TMP/SMX or a fluoroquinolone is the treatment of choice due to better penetration of prostate tissue. A 4-6 week course of treatment resolves chronic bacterial prostatitis in about 60–80% of patients with E. coli and other Enterobacteriaceae infections. 96
Managing Urinary Retention. Men with UTI and acute retention require decompression with either clean intermittent self-catheterization or an indwelling catheter. When there is acute or symptomatic chronic urinary retention in a man with a UTI, consider starting an alpha blocker (eg, tamsulosin 0.4 mg at bedtime) at the same time as the antibiotics. 77 If symptomatic chronic urinary retention persists, despite appropriate antibiotic treatment and use of an alpha blocker, consider teaching the patient to do clean intermittent self-catheterization, and refer to urology. In the case of very high volume postvoid residuals (ie, >1000 mL) start an alpha blocker, minimize anticholinergic medications, teach intermittent self-catheterization or place an indwelling catheter, consider assessing renal function and obtaining renal ultrasound, and refer to urology.
- Avoid or limit use of anticholinergic medications that can cause or exacerbate urinary retention.
Avoid catheterization whenever possible. When it is needed, limit the duration of catheterization, and use clean intermittent catheterization instead of an indwelling catheter. Assess for urinary retention in men using a bladder scan (ultrasound), rather than catheterization. 103 Patient education for intermittent self-catheterization for females and males can be found at these hyperlinks.
Prophylactic antibiotics are mandatory for patients undergoing transrectal prostate biopsy or other high-risk, contaminated urinary tract procedures. 104
Avoid or limit use of medications that can cause or exacerbate urinary retention, particularly those with anticholinergic effects (eg, diphenhydramine, oxybutynin, scopolamine, and many others). Treat acute or chronic urinary retention with alpha blockers such as tamsulosin. These medicines improve voiding by decreasing smooth muscle sympathetic tone in the bladder neck and prostate;retrograde ejaculation is a common side effect. If urinary retention persists despite use of an alpha blocker, and prostate cancer is not suspected, consider adding a 5-alpha reductase inhibitor (finasteride or dutasteride). The 5-alpha reductase inhibitors decrease prostate volume and will artificially reduce the PSA by about half over 6-12 months. While the effects of alpha blockers can be seen within several weeks, 5-alpha reductase inhibitors take at least 3 months for improvement in symptoms. Refer the patient to a urologist if pharmacotherapy is not effective.
Complications of UTI in Men . Bacteremia, urosepsis, and struvite stones are potential complications for both women and men.
In men, prostate abscess is a potential complication of acute prostatitis that increases the risk of urosepsis and requires surgical or needle drainage. Abscess formation is more common in men who are immunocompromised, have diabetes, or require catheterization. 95
Acute and chronic urinary retention are common in men with prostatic hyperplasia, but acute urinary retention can be precipitated by a urinary tract infection and may require catheterization and initiation of alpha blocker treatment. 105 Urinary retention can in turn cause hydronephrosis and acute kidney injury, and when prolonged can lead to chronic kidney disease. 106
Follow up . Do not perform a follow-up urine culture in men whose symptoms resolve with treatment. 12
Refer men with UTI to urology if they have urinary retention not improved with medication, a persistently elevated PSA or abnormal prostate exam, an unclear diagnosis, recurrent UTI, or are not responding to treatment. Men who have gross hematuria or persistent microscopic hematuria but have a negative urine culture should also be referred to urology.
- UTI in Older Persons
- Diagnose UTI in patients older than 65 years in the same way as in younger patients.
The approach to diagnosis of UTI in patients > 65 years old is the same as it is for younger adults. Diagnosis is based on urinary symptoms, abnormal urinalysis, and culture. Older age does not inherently suggest need for diagnosis of a complicated UTI, unless other factors are present (eg, immunosuppression, indwelling catheter, etc.).
Give special attention to the diagnosis of acute cystitis in older patients who have cognitive impairment. Diagnosis in these patients can be challenging as they may have chronic lower urinary tract symptoms and difficulty reporting complaints.
Caregivers often indicate a concern for cystitis in older patients who have a change in mental status or function, such as confusion, agitation, falls, or fatigue. Since asymptomatic bacteriuria is common in this population, finding bacteriuria may lead to an overdiagnosis of cystitis and resulting overtreatment with antibiotics. 107
Nonspecific symptoms do not predictably correlate to UTIs. A 2011 cross-sectional study of 421 nursing home residents showed that patients with and without nonspecific symptoms had similar rates of positive urine cultures. 108 A 2019 systematic literature review looked for links between confusion and UTI in the elderly, but found that evidence was insufficient to reach a conclusion. 109 Treatment of bacteriuria in this setting could cause harm by exposing patients unnecessarily to antibiotics. 110
Criteria exist to define acute cystitis in nursing home residents. In noncatheterized patients, diagnosis requires localizing genitourinary or infectious symptoms along with bacteriuria. 111 Consider an alternate diagnosis in an older patient with nonspecific functional changes without any specific urinary or infectious symptoms, even if he or she has bacteriuria. 111
- Treat UTI in patients older than > 65 years the same as for younger patients.
- Do not treat asymptomatic bacteriuria.
Treatment of acute uncomplicated cystitis in older patients is the same as for younger adults. A 2008 meta-analysis demonstrated that shorter courses of antibiotics are as effective as longer courses in women over age 65 years. 112 Despite prior recommendations to avoid it, nitrofurantoin is a reasonable first-line option for treatment of acute cystitis regardless of age, as long as CrCl is greater than 30 mL/min/1.73 m 2 . 113 , 114 For elderly patients with uncomplicated cystitis living in long-term care facilities, the usual antibiotic guidelines can be used as long as the patient has no history of resistant cystitis. 115 Treatment of asymptomatic bacteriuria is not recommended and can cause harm. 66
Follow up. Do not perform a follow-up urinalysis or urine culture when symptoms have resolved. 66 Patients with ongoing symptoms after 2-3 days of treatment should have urinalysis and culture to verify ongoing evidence of infection and to test for antibiotic susceptibility.
- Strategy for Literature Search
Within the Medline (Ovid) database, the following search strategy was used, searching from 2009 to 2019.
- Urinary tract infections
- exp urethritis/or exp cystitis
- UTI.ti. or UTIS.ti or urethritis.ti or cystitis.ti
- exp pyelonephritis/or pyelonephritis.ti,ab.
- Bacteriuria
- Asymptomatic or subclinical and bacteriuria,ti, ab, or bacteria urine.ti, ab.
- Pregnancy or pregnant women/or pregnan*ab,ti.
- exp male/or men or male or males.ab, ti.
- exp aged/or elderly or mature or aging or geriatric*. ti, ab.
The Main search retrieved 11472 references. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follows:
- Urinary tract infection - Guidelines, total results were 1070
- Urinary tract infection - Clinical Trials, total results were 3070
- Urinary tract infection - Cohort Studies, total results were 7332
Within the Cochrane Database of Systematic Reviews, 57 reviews were found using the strategy in the search strategies document.
The results were limited to Humans, English, adults and 2009 to current.
The search was conducted in components each keyed to a specific causal link in a formal problem structure (available upon request). The search was supplemented with very recent clinical trials known to expert members of the panel. Negative trials were specifically sought. The search was a single cycle.
- A = systematic reviews of randomized controlled trials
- B = randomized controlled trials
- C = systematic review of nonrandomized controlled trials or observational studies, nonrandomized controlled trials, group observation studies (eg, cohort, cross-sectional, case control)
- D = individual observation studies (case or case series)
- E = opinion of expert panel.
- Related National Guidelines
The UMHHC Clinical Guideline on Urinary Tract Infection is consistent with:
- National Institute for Health and Care Excellence (NICE): Urinary tract infection (lower): antimicrobial prescribing, Oct 31, 2018. 75
- National Institute for health and Care Excellence (NICE): Urinary tract infection (recurrent): antimicrobial prescribing, Oct 31, 2018. 59
- National Institute for Health and Care Excellence (NICE): Pyelonephritis (acute): antimicrobial prescribing, Oct 31, 2018. 68
- Disclosures
The University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
View in own window
| Team Member | Company | Relationship |
|---|---|---|
| Catherine M. Bettcher, MD | (None) | |
| Elizabeth Campbell, MD | (None) | |
| Giulia I. Lane, MD | (None) | |
| Lindsay A. Petty, MD | (None) | |
| Karl T. Rew, MD | (None) | |
| Jennifer C. Zelnik, MD | (None) | |
| R. Van Harrison, PhD | (None) |
- Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Medical School to which the content is most relevant: Family Medicine, General Medicine, General Obstetrics & Gynecology, and Infectious Diseases. The Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers endorsed the final version.
- Acknowledgments
The following individuals are acknowledged for their contributions to previous versions of this guideline.
1999: Steven E. Gradwohl, MD, General Medicine; Carol E. Chenoweth, MD, Infectious Diseases; Karen R. Fonde, MD, Family Medicine; R. Van Harrison, PhD, Medical Education; Kathy Munger, MS, BSN, RN, Ambulatory Care Nursing; Lauren B. Zoschnick, MD, Obstetrics and Gynecology.
2005: Steven E. Gradwohl, MD, General Medicine; Carol E. Chenoweth, MD, Infectious Diseases; Karen R. Fonde, MD, Family Medicine; R. Van Harrison, PhD, Medical Education; Lauren B. Zoschnick, MD, Obstetrics and Gynecology.
2011: Steven E. Gradwohl, MD, General Medicine; Carol E. Chenoweth, MD, Infectious Diseases; Lauren B Zoschnick, MD, Obstetrics & Gynecology.
| Antimicrobial Committee | Date: 08/10/2020 |
| P&T | Date: 03/16/2021 |
| ACOC | Date: 03/25/2021 |
| CPC | Date: 04/01/2021 |
| ECCA | Date: 05/11/2021 |
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Created: May 2021.
Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
- Cite this Page Bettcher CM, Campbell E, Petty LA, et al. Urinary Tract Infection [Internet]. Ann Arbor (MI): Michigan Medicine University of Michigan; 2021 May.
- PDF version of this title (514K)
In this Page
Other titles in this collection.
- Michigan Medicine Clinical Care Guidelines
Related information
- NLM Catalog Related NLM Catalog Entries
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Pain Management [ 2021] Review Pain Management Fenske JN, Berland DW, Chandran S, Van Harrison R, Schneiderhan J, Hilliard PE, Bialik KC, Clauw DJ, Lowe DA, Mehari KS, et al. 2021 Jan
- Review [National S3 guideline on uncomplicated urinary tract infection: recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients]. [Urologe A. 2011] Review [National S3 guideline on uncomplicated urinary tract infection: recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients]. Wagenlehner FM, Schmiemann G, Hoyme U, Fünfstück R, Hummers-Pradier E, Kaase M, Kniehl E, Selbach I, Sester U, Vahlensieck W, et al. Urologe A. 2011 Feb; 50(2):153-69.
- Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. [Pediatrics. 1999] Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. . Pediatrics. 1999 Apr; 103(4 Pt 1):843-52.
- Age, nursing home residence, and presentation of urinary tract infection in U.S. emergency departments, 2001-2008. [Acad Emerg Med. 2012] Age, nursing home residence, and presentation of urinary tract infection in U.S. emergency departments, 2001-2008. Caterino JM, Ting SA, Sisbarro SG, Espinola JA, Camargo CA Jr. Acad Emerg Med. 2012 Oct; 19(10):1173-80.
- Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement. [Pediatrics. 1999] Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement. Downs SM. Pediatrics. 1999 Apr; 103(4):e54.
Recent Activity
- Urinary Tract Infection Urinary Tract Infection
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Urinary tract infections (UTIs) are caused by a wide range of. pathogens, including Gram-negative and Gram-positive bacteria, as well as fungi. Uncomplicated UTIs typically affect. women, children ...
invade urinary tract and cause infection to this urine drainage system. The urinary tract in humans consists of kidneys, ureters, urine bladder, and urethra. Infections can happen at any parts of the urinary system. However, bladder infection (cystitis) is the most common type (CDC, 2021). Classification of UTI may differ.
Diagram showing contribution o f various microbes for causing the UTI: E. coli 79%, S. Saprophyticus 11%, Klebsiella 3%, Mixed 3%, Proteus 2%, Enterococcus 2%, others 2%. SYMPTOMS. Common urinary ...
Urinary tract infections (UTIs) remain the most common bacterial infection in human population and is also one of the most frequently occurring nosocomial infec tions (Gastmeier et a l., 1998).
A THESIS SUBMITTED IN FULFILLMENT FOR THE AWARD OF DEGREE OF MASTER OF SCIENCE IN INFECTIOUS DISEASES (BACTERIOLOGY) IN THE SCHOOL OF MEDICINE, ... Urinary Tract Infections (UTI) is known to affect millions of people annually and can lead to serious health problem issues (Tajbakhsh, 2015. Globally, seven million patients
Urinary tract infections (UTIs) are some of the most common bacterial infections, affecting 150 million people each year worldwide 1.In 2007, in the United States alone, there were an estimated 10.5 million office visits for UTI symptoms (constituting 0.9% of all ambulatory visits) and 2-3 million emergency department visits 2-4.Currently, the societal costs of these infections, including ...
Urinary tract infections; etiological profile and antimicrobial susceptibility patterns of uropathogens. Professional Med J 2016;23(1):010-014. DOI: 10.17957/TPMJ/16.3044 INTRODUCTION Urinary tract infections (UTIs) constitute important bacterial disease which contributes to significant morbidity in both out-patient and in-patients.1,2
Urinary tract infection (UTI) is a common bacterial infection in women of all ages but the incidence and prevalence increase with age. Despite the high incidence of UTI, little is known about its impact on morale or subjective wellbeing and daily life in old women.
Isolated infections of the bladder and lower urinary tract without signs or symptoms of upper urinary tract or sys-temic infection are referred to as ' stitis ' or 'simple cystitis' (TaBle ...
1Department of Medicine, University of Miami Miller School of Medicine, Miami, FL 33136. UTI may involve the lower or upper urinary tract. ABSTRACT. and may be uncomplicated or complicated. The emphasis of this chapter is uncomplicated UTI. The diagnosis of uncomplicated cystitis (bladder infection) and pyelonephritis (kidney infection) is ...
3 ABSTRACT Master thesis, Programme in Medicine. Urinary tract infections - Etiology, antibiotic susceptibility and treatment in surgical patients in Nepal. Adam Oscarson, University of Gothenburg, Sweden 2014. Background: Urinary tract infections (UTI) are among the most common postoperative nosocomial infections and the second most common reason for empirical antibiotic treatment.
Urinary tract infections (UTIs) are common, recurrent infections that can be mild to life-threatening. The continued emergence of antibiotic resistance, together with our increasing understanding of the detrimental effects conferred by broad-spectrum antibiotic use on the health of the beneficial microbiota of the host, has underscored the weaknesses in our current treatment paradigm for UTIs.
Urinary tract infections are common infections of urinary tract which includes urethra, kidneys and bladder that. irritates the lining of urinary tract and it becomes inflamed. Males are less ...
Urinary tract infection (UTI) refers to a plethora of clinical phenotypes, including cystitis, pyelonephritis, prostatitis, urosepsis, and catheter-associated UTI (CA-UTI) [1, 2]. In both clinical practice and in research, the diagnosis of UTI is based on a multitude of clinical signs and symptoms and diagnostic tests.
Urinary tract infections (UTIs) are among the most common bacterial infections worldwide, occurring in both community and healthcare settings. Although the clinical symptoms of UTIs are heterogeneous and range from uncomplicated (uUTIs) to complicated (cUTIs), most UTIs are usually treated empirically. Bacteria are the main causative agents of ...
UTIs are the most common outpatient infections in the United States (US). With the exception of a spike in young women aged 14−24 years old, the prevalence of UTIs increases with age.2 The preva-lence in women over 65 years of age is approxi-mately 20%, compared with approximately 11% in.
Introduction. Urinary tract infections (UTIs) are inflammatory disorders caused by microorganisms that have proliferated abnormally in the urinary system (Malik et al., 2021).UTIs are known to induce short-term morbidities such as fever, dysuria, lower abdominal pain, and may result in permanent kidney scarring (Leung et al., 2019).UTIs are either community-acquired or hospital-acquired (HA).
Urinary Tract Infections (UTIs) are the second most common bacterial infections worldwide, affecting about 150 million people each year. They are frequent disease in both ambulantory and. acute ...
Mechanisms of Catheter-Associated Urinary Tract Infection. UTIs generally occur through urethral contamination with rectal flora, followed by microbial migration to the bladder, adhesion, and colonization.2 Invasion of the bladder is then mediated by pili and adhesins, and neutrophil infiltration commences. Bacteria then multiply and form biofilms, and bacterial proteases and toxins trigger ...
Abstract. The report revealed that in human beings the ten most commonly isolated bacteria from UTI cases from most frequent to less frequent belonged to Escherichia, Staphylococcus, Streptococcus ...
1. Introduction. Urinary tract infections (UTIs) are the inflammatory disorders of the urinary tract caused by the abnormal growth of pathogens [1, 2].Urinary tract infection is known to cause short-term morbidity in terms of fever, dysuria, and lower abdominal pain (LAP) and may result in permanent scarring of the kidney [3, 4].Urinary tract infections can be community acquired or nosocomial.
The infection emanates from bacterial infestation of the upper or lower urinary tracts, the common isolates being Escherichia coli, Staphylococcus aureus, and Klebsiella spp. [2], [3].It remains a ...
UTI in Men. Rates of UTI are low in younger males, but increase significantly in older men. The incidence of UTI in men < 55 years is 0.9 to 2.4 cases per 1000, but in men > 85 years the incidence is 7.7/1000, similar to that of women in the same age group. 12 UTI in men is less well studied than in women.