- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Coronavirus disease (COVID-19) /

Global research on coronavirus disease (COVID-19)
Section navigation.
- WHO COVID-19 Solidarity Therapeutics Trial
- "Solidarity II" global serologic study for COVID-19
- Accelerating a safe and effective COVID-19 vaccine
- Solidarity Trial Vaccines
WHO is bringing the world’s scientists and global health professionals together to accelerate the research and development process, and develop new norms and standards to contain the spread of the coronavirus pandemic and help care for those affected.
The R&D Blueprint has been activated to accelerate diagnostics, vaccines and therapeutics for this novel coronavirus.
The solidarity of all countries will be essential to ensure equitable access to COVID-19 health products.
WHO COVID-19 Research Database
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO Library & Digital Information Networks and was funded by COVID-19 emergency funds. The database was built by BIREME, the Specialized Center of PAHO/AMRO. Its content spanned the time period March 2020 to June 2023. It has now been archived, and no longer searchable since January 2024.
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
17k Accesses
30 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
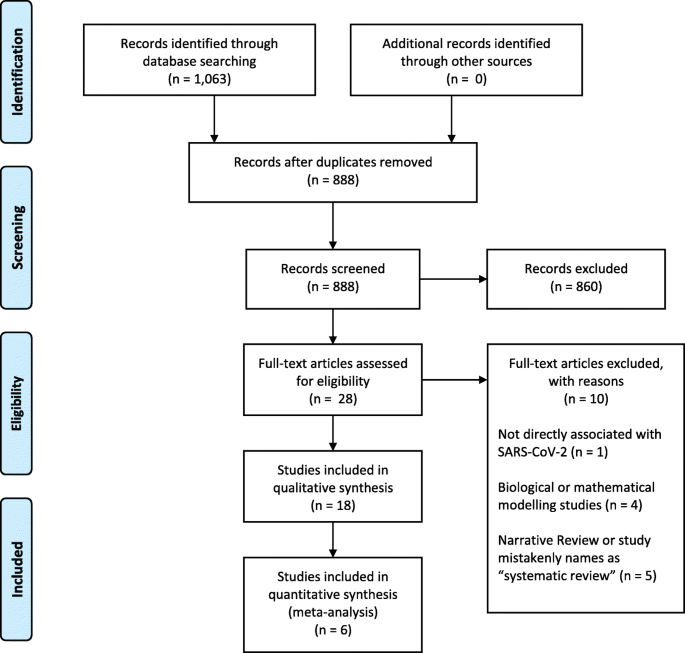
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
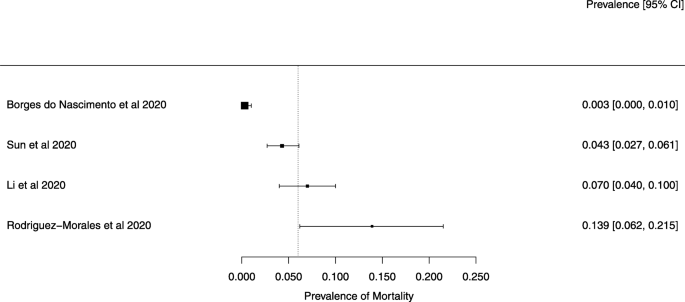
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
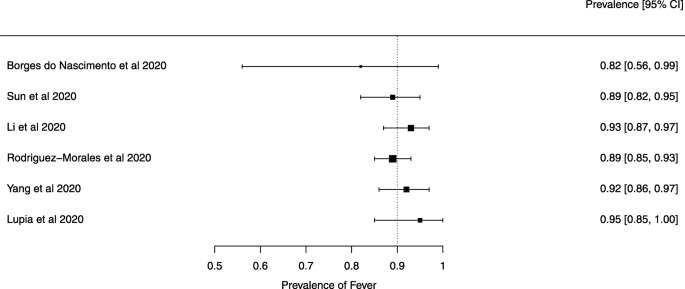
A meta-analysis of the prevalence of fever
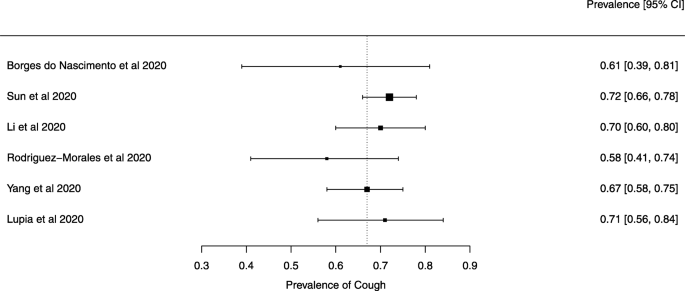
A meta-analysis of the prevalence of cough
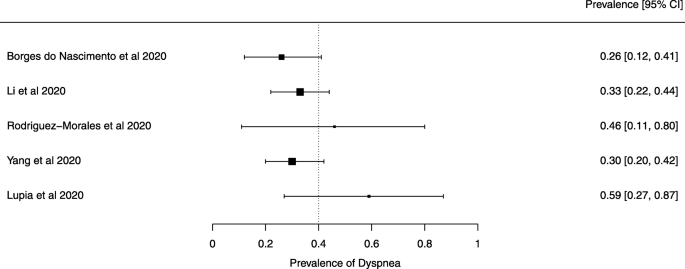
A meta-analysis of the prevalence of dyspnea
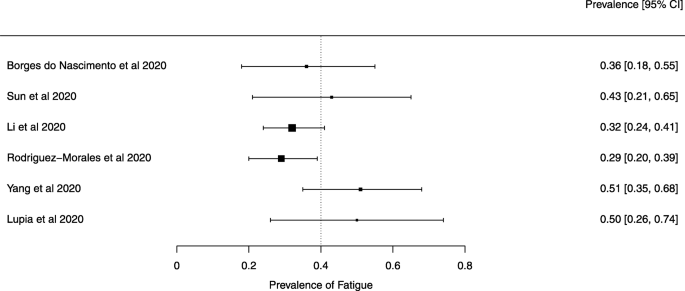
A meta-analysis of the prevalence of fatigue or myalgia
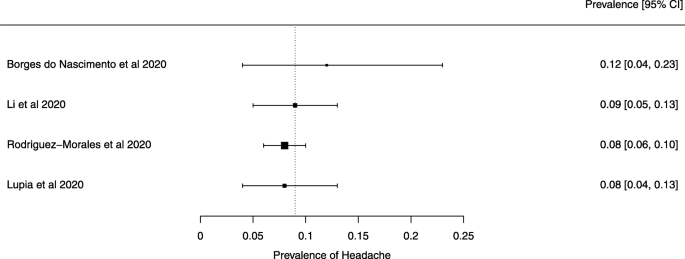
A meta-analysis of the prevalence of headache
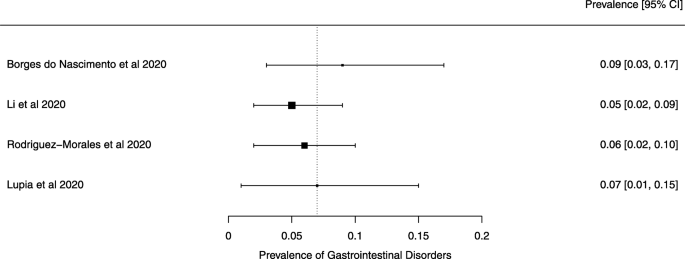
A meta-analysis of the prevalence of gastrointestinal disorders
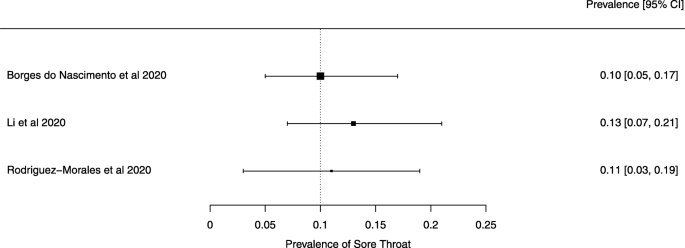
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
BMJ's Coronavirus (covid-19) Hub
BMJ's covid-19 hub supports health professionals and researchers with practical guidance and online CPD courses, as well as free access to the latest news, comment, and research from The BMJ. Covid-related research content from the rest of our portfolio of journals is also freely available for a year.
BMJ is committed to providing access to covid-related materials to those who need them. If you require access to an article that is no longer freely available, please contact [email protected] with the subject line "Covid Article Request".
What you need to know
Highlights from the bmj, what do we know about covid-19’s effects on the gut.
01 May 2024
NHS hospital capacity during covid-19: overstretched staff, space, systems, and stuff
03 April 2024
UK workers during the pandemic: inadequate protection and, consequently, increased risk
01 March 2024
US public health after covid-19: learning from the failures of the hollow state and racial capitalism
05 February 2024
Essential workers during covid-19: how quickly we forget
25 January 2024
Learning to live with covid-19: testing, vaccination, and mask wearing still play a key part in managing the pandemic
14 December 2023
Embedding implementation research to cross the quality of care chasm during the covid-19 pandemic and beyond
11 December 2023
Communicating scientific advice: lessons from the UK covid-19 inquiry
07 December 2023
Covid-19 vaccine effectiveness against post-covid-19 condition among 589 722 individuals in Sweden: population based cohort study
22 November 2023
Equity and technology in the pandemic treaty
03 November 2023
Clinical guidance
A living who guideline on drugs to prevent covid-19.
24 March 2023
A living WHO guideline on drugs for covid-19
14 July 2022
Covid-19 diagnosis and management
BMJ Best Practice provides regularly updated information on prevention, diagnosis, management, follow up
Work and vocational rehabilitation for people living with long covid
10 May 2024
Cognitive dysfunction after covid-19
01 February 2024
Diagnosis of venous thromboembolic diseases in people with covid-19: summary of updated NICE guidance
12 October 2023
Pathophysiology, diagnosis, and management of neuroinflammation in covid-19
18 August 2023
Breathing difficulties after covid-19: a guide for primary care
14 June 2023
Orthostatic tachycardia after covid-19
24 February 2023
Update to living WHO guideline on drugs for covid-19
12 January 2023
Long covid—an update for primary care
22 September 2022
Covid-19 vaccination in pregnancy
10 August 2022
Diagnosis and management of covid-19 in pregnancy
26 April 2022
From The BMJ
Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (regain study): multicentre randomised controlled trial.
07 February 2024
Efficacy and safety of an inactivated virus-particle vaccine for SARS-CoV-2, BIV1-CovIran: randomised, placebo controlled, double blind, multicentre, phase 3 clinical trial
21 September 2023
Long term risk of death and readmission after hospital admission with covid-19 among older adults: retrospective cohort study
09 August 2023
Safety of BA.4-5 or BA.1 bivalent mRNA booster vaccines: nationwide cohort study
25 July 2023
Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: nationwide cohort study
Comparative effectiveness of heterologous third dose vaccine schedules against severe covid-19 during omicron predominance in nordic countries: population based cohort analyses.
24 July 2023
From BMJ Journals
Lessons from covid-19 syndromic surveillance through emergency department activity: a prospective time series study from western switzerland, covid-19, body weight and the neighbourhood: food system dimensions and consumption associated with changes in body weight of peruvian adults during first wave lockdowns, impact of physical activity on covid-19-related symptoms and perception of physical performance, fatigue and exhaustion during stay-at-home orders, effectiveness of ehteraz digital contact tracing app versus conventional contact tracing in managing the outbreak of covid-19 in the state of qatar, vaccine rollout.
Protecting infants through covid-19 vaccination during pregnancy
Maternal mrna covid-19 vaccination during pregnancy and delta or omicron infection or hospital admission in infants: test negative design study, investing in robust surveillance of the effects of covid-19 and future emerging infections in pregnancy should be prioritised, analgesia for non-specific low back pain, ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses, trends in cardiovascular disease incidence among 22 million people in the uk over 20 years: population based study, effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials, colchicine in patients with acute ischaemic stroke or transient ischaemic attack (chance-3): multicentre, double blind, randomised, placebo controlled trial, sglt-2 inhibitors, glp-1 receptor agonists, and dpp-4 inhibitors and risk of hyperkalemia among people with type 2 diabetes in clinical practice: population based cohort study, covid-19: study sheds light on how some people avoid the disease.
21 June 2024
Fauci is defiant as congressional hearing into covid origins fails to produce smoking gun
04 June 2024
Pandemic treaty: panel calls for “reset” to talks as negotiators miss deadline
30 May 2024
Covid-19: South African disclosures reveal secretive world of nations’ vaccine contracts
28 May 2024
Covid inquiry: What have we learnt about Northern Ireland’s response?
24 May 2024
Covid-19: Charities question inquiry’s “refusal” to fully examine mental health impact
12 April 2024
Covid inquiry: What we have learnt about Wales’s response?
11 March 2024
Covid-19 taskforce called for a new national vaccine agency to prepare for future pandemics
29 February 2024
Covid inquiry: What did we learn this week?
26 January 2024
Covid-19: Doctors instruct law firm in bid for compensation after developing long covid
Covid-19: vaccines have saved at least 1.4 million lives in europe, who reports.
17 January 2024
Covid-19: Undervaccination was associated with higher risk of hospital admission or death, study finds
16 January 2024
Covid-19: NICE plans to expand Paxlovid eligibility to 1.4 million more people
11 January 2024

Covid-19: Some US states and hospitals recommend masks again
05 January 2024
Covid-19: WHO adds JN.1 as new variant of interest
21 December 2023
Covid-19: Bereaved families group collects BMJ award for holding ministers to account
15 December 2023
Covid inquiry: Sunak defends his pandemic policies made as chancellor
13 December 2023
Covid-19: Preliminary figures show 1.2% of people in England had infection at end of November
Pfizer sues poland over unclaimed covid vaccines.
28 November 2023
Covid-19: Whitehall chaos and misplaced confidence undermined UK’s response, inquiry hears
Prisons and pandemic preparedness.
24 April 2023
How pandemic publishing struck a blow to the visibility of women’s expertise
06 April 2023
Closing the gap in childhood immunisation after the pandemic
21 March 2023
WHO keeps covid-19 a public health emergency of international concern
06 March 2023
Financing covid-19 mRNA vaccines
01 March 2023
08 February 2023
Labour’s proposed covid corruption commissioner: an important signal, but it won’t be easy to get the money back
25 October 2023
Hancock’s covid inquiry evidence offers few clues as to why long covid was sidelined
Helen salisbury: covid booster chaos.
05 September 2023
Outsourcing covid-19 vaccination to the private sector will increase health inequalities
04 September 2023
Why older adults can continue to benefit from covid-19 boosters
Weakened by a decade of austerity: why the uk’s covid-19 inquiry is right to look at policies since 2010.
06 June 2023
The covid public health emergency is ending: it now joins the ordinary emergency that is American health
26 April 2023
Employers must provide better support to workers with long covid
Helen salisbury: unexplained symptoms aren’t always long covid, latest covid-19 blogs from bmj journals.
A centralised hub of all the latest covid-19 blogs and podcasts posts from BMJ's 70 specialty journals. All posts are freely available and you can search by subject area or journal.
Treat your patients with confidence
BMJ Learning offers our most relevant CPD online courses to update and refresh clinical knowledge for those supporting our healthcare systems in the diagnosis and treatment of covid-19
(16 COURSES)
Infographics
Updated: 1 Dec 2021
Updated: 22 June 2021
Updated: 11 August 2020
Covid-19 jobs
Follow us on, content links.
- Collections
- Health in South Asia
- Women’s, children’s & adolescents’ health
- News and views
- BMJ Opinion
- Rapid responses
- Editorial staff
- BMJ in the USA
- BMJ in South Asia
- Submit your paper
- BMA members
- Subscribers
- Advertisers and sponsors
Explore BMJ
- Our company
- BMJ Careers
- BMJ Learning
- BMJ Masterclasses
- BMJ Journals
- BMJ Student
- Academic edition of The BMJ
- BMJ Best Practice
- The BMJ Awards
- Email alerts
- Activate subscription
Information

Decades in the Making: mRNA COVID-19 Vaccines
Recent News & Stories
March 20, 2024 | News Release
Severe Lung Infection During COVID-19 Can Cause Heart Damage
March 12, 2024 | News Release
NIH Opens Long COVID Treatment Trials For Nervous System Dysfunction

February 14, 2024 | News Release
COVID-19 Vaccination, Boosters During Pregnancy Protect Infants for 6 Months
Explore the Impact of COVID-19 Research
Home Test to Treat Program Expands Nationwide
Shining a Light on Long COVID Brain Fog
Common Cold Virus May Increase Risk for Long COVID
SARS-CoV-2 Infection May Increase Risk of Heart Disease, Stroke
Severe COVID-19 May Cause Long-Term Immune System Changes
SARS-CoV-2 Antibodies From Vaccination During Pregnancy May Transfer to Fetuses
Featured COVID-19 Topics
Questions and answers about COVID-19 vaccine guidelines, development, and safety.

Information about research and updates on Long COVID.
Symptoms of Long COVID
Investigating the most promising treatments and therapies for COVID-19.
Antiviral Treatment Reduces Likelihood of Severe Illness From Omicron

COVID-19 Datasets for Researchers
Find COVID-19 datasets, data tools, and publications to use in research.
- High Contrast
- Increase Font
- Decrease Font
- Default Font
- Turn Off Animations
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The impact of the COVID-19 pandemic on scientific research in the life sciences
Roles Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing
Affiliation AXES, IMT School for Advanced Studies Lucca, Lucca, Italy
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Chair of Systems Design D-MTEC, ETH Zürich, Zurich, Switzerland
- Massimo Riccaboni,
- Luca Verginer

- Published: February 9, 2022
- https://doi.org/10.1371/journal.pone.0263001
- Reader Comments
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on world scientific production in the life sciences and find indications that the usage of medical subject headings (MeSH) has changed following the outbreak. We estimate through a difference-in-differences approach the impact of the start of the COVID-19 pandemic on scientific production using the PubMed database (3.6 Million research papers). We find that COVID-19-related MeSH terms have experienced a 6.5 fold increase in output on average, while publications on unrelated MeSH terms dropped by 10 to 12%. The publication weighted impact has an even more pronounced negative effect (-16% to -19%). Moreover, COVID-19 has displaced clinical trial publications (-24%) and diverted grants from research areas not closely related to COVID-19. Note that since COVID-19 publications may have been fast-tracked, the sudden surge in COVID-19 publications might be driven by editorial policy.
Citation: Riccaboni M, Verginer L (2022) The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE 17(2): e0263001. https://doi.org/10.1371/journal.pone.0263001
Editor: Florian Naudet, University of Rennes 1, FRANCE
Received: April 28, 2021; Accepted: January 10, 2022; Published: February 9, 2022
Copyright: © 2022 Riccaboni, Verginer. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The processed data, instructions on how to process the raw PubMed dataset as well as all code are available via Zenodo at https://doi.org/10.5281/zenodo.5121216 .
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The COVID-19 pandemic has mobilized the world scientific community in 2020, especially in the life sciences [ 1 , 2 ]. In the first three months after the pandemic, the number of scientific papers about COVID-19 was fivefold the number of articles on H1N1 swine influenza [ 3 ]. Similarly, the number of clinical trials related to COVID-19 prophylaxis and treatments skyrocketed [ 4 ]. Thanks to the rapid mobilization of the world scientific community, COVID-19 vaccines have been developed in record time. Despite this undeniable success, there is a rising concern about the negative consequences of COVID-19 on clinical trial research, with many projects being postponed [ 5 – 7 ]. According to Evaluate Pharma, clinical trials were one of the pandemic’s first casualties, with a record number of 160 studies suspended for reasons related to COVID-19 in April 2020 [ 8 , 9 ] reporting a total of 1,200 trials suspended as of July 2020. As a consequence, clinical researchers have been impaired by reduced access to healthcare research infrastructures. Particularly, the COVID-19 outbreak took a tall on women and early-career scientists [ 10 – 13 ]. On a different ground, Shan and colleagues found that non-COVID-19-related articles decreased as COVID-19-related articles increased in top clinical research journals [ 14 ]. Fraser and coworker found that COVID-19 preprints received more attention and citations than non-COVID-19 preprints [ 1 ]. More recently, Hook and Porter have found some early evidence of ‘covidisation’ of academic research, with research grants and output diverted to COVID-19 research in 2020 [ 15 ]. How much should scientists switch their efforts toward SARS-CoV-2 prevention, treatment, or mitigation? There is a growing consensus that the current level of ‘covidisation’ of research can be wasteful [ 4 , 5 , 16 ].
Against this background, in this paper, we investigate if the COVID-19 pandemic has induced a shift in biomedical publications toward COVID-19-related scientific production. The objective of the study is to show that scientific articles listing covid-related Medical Subject Headings (MeSH) when compared against covid-unrelated MeSH have been partially displaced. Specifically, we look at several indicators of scientific production in the life sciences before and after the start of the COVID-19 pandemic: (1) number of papers published, (2) impact factor weighted number of papers, (3) opens access, (4) number of publications related to clinical trials, (5) number of papers listing grants, (6) number of papers listing grants existing before the pandemic. Through a natural experiment approach, we analyze the impact of the pandemic on scientific production in the life sciences. We consider COVID-19 an unexpected and unprecedented exogenous source of variation with heterogeneous effects across biomedical research fields (i.e., MeSH terms).
Based on the difference in difference results, we document the displacement effect that the pandemic has had on several aspects of scientific publishing. The overall picture that emerges from this analysis is that there has been a profound realignment of priorities and research efforts. This shift has displaced biomedical research in fields not related to COVID-19.
The rest of the paper is structured as follows. First, we describe the data and our measure of relatedness to COVID-19. Next, we illustrate the difference-in-differences specification we rely on to identify the impact of the pandemic on scientific output. In the results section, we present the results of the difference-in-differences and network analyses. We document the sudden shift in publications, grants and trials towards COVID-19-related MeSH terms. Finally, we discuss the findings and highlight several policy implications.
Materials and methods
The present analysis is based primarily on PubMed and the Medical Subject Headings (MeSH) terminology. This data is used to estimate the effect of the start of the COVID 19 pandemic via a difference in difference approach. This section is structured as follows. We first introduce the data and then the econometric methodology. This analysis is not based on a pre-registered protocol.
Selection of biomedical publications.
We rely on PubMed, a repository with more than 34 million biomedical citations, for the analysis. Specifically, we analyze the daily updated files up to 31/06/2021, extracting all publications of type ‘Journal Article’. For the principal analysis, we consider 3,638,584 papers published from January 2019 to December 2020. We also analyze 11,122,017 papers published from 2010 onwards to identify the earliest usage of a grant and infer if it was new in 2020. We use the SCImago journal ranking statistics to compute the impact factor weighted number (IFWN) of papers in a given field of research. To assign the publication date, we use the ‘electronically published’ dates and, if missing, the ‘print published’ dates.
Medical subject headings.
We rely on the Medical Subject Headings (MeSH) terminology to approximate narrowly defined biomedical research fields. This terminology is a curated medical vocabulary, which is manually added to papers in the PubMed corpus. The fact that MeSH terms are manually annotated makes this terminology ideal for classification purposes. However, there is a delay between publication and annotation, on the order of several months. To address this delay and have the most recent classification, we search for all 28 425 MeSH terms using PubMed’s ESearch utility and classify paper by the results. The specific API endpoint is https://eutils.ncbi.nlm.nih.gov/entrez/eutils/esearch.fcgi , the relevant scripts are available with the code. For example, we assign the term ‘Ageusia’ (MeSH ID D000370) to all papers listed in the results of the ESearch API. We apply this method to the whole period (January 2019—December 2020) and obtain a mapping from papers to the MeSH terms. For every MeSH term, we keep track of the year they have been established. For instance, COVID-19 terms were established in 2020 (see Table 1 ): in January 2020, the WHO recommended 2019-nCoV and 2019-nCoV acute respiratory disease as provisional names for the virus and disease. The WHO issued the official terms COVID-19 and SARS-CoV-2 at the beginning of February 2020. By manually annotating publications, all publications referring to COVID-19 and SARS-CoV-2 since January 2020 have been labelled with the related MeSH terms. Other MeSH terms related to COVID-19, such as coronavirus, for instance, have been established years before the pandemic (see Table 2 ). We proxy MeSH term usage via search terms using the PubMed EUtilities API; this means that we are not using the hand-labelled MeSH terms but rather the PubMed search results. This means that the accuracy of the MeSH term we assign to a given paper is not perfect. In practice, this means that we have assigned more MeSH terms to a given term than a human annotator would have.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0263001.t001
The list contains only terms with at least 100 publications in 2020.
https://doi.org/10.1371/journal.pone.0263001.t002
Clinical trials and publication types.
We classify publications using PubMed’s ‘PublicationType’ field in the XML baseline files (There are 187 publication types, see https://www.nlm.nih.gov/mesh/pubtypes.html ). We consider a publication to be related to a clinical trial if it lists any of the following descriptors:
- D016430: Clinical Trial
- D017426: Clinical Trial, Phase I
- D017427: Clinical Trial, Phase II
- D017428: Clinical Trial, Phase III
- D017429: Clinical Trial, Phase IV
- D018848: Controlled Clinical Trial
- D065007: Pragmatic Clinical Trial
- D000076362: Adaptive Clinical Trial
- D000077522: Clinical Trial, Veterinary
In our analysis of the impact of COVID-19 on publications related to clinical trials, we only consider MeSH terms that are associated at least once with a clinical trial publication over the two years. We apply this restriction to filter out MeSH terms that are very unlikely to be relevant for clinical trial types of research.
Open access.
We proxy the availability of a journal article to the public, i.e., open access, if it is available from PubMed Central. PubMed Central archives full-text journal articles and provides free access to the public. Note that the copyright license may vary across participating publishers. However, the text of the paper is for all effects and purposes freely available without requiring subscriptions or special affiliation.
We infer if a publication has been funded by checking if it lists any grants. We classify grants as either ‘old’, i.e. existed before 2019, or ‘new’, i.e. first observed afterwards. To do so, we collect all grant IDs for 11,122,017 papers from 2010 on-wards and record their first appearance. This procedure is an indirect inference of the year the grant has been granted. The basic assumption is that if a grant number has not been listed in any publication since 2010, it is very likely a new grant. Specifically, an old grant is a grant listed since 2019 observed at least once from 2010 to 2018.
Note that this procedure is only approximate and has a few shortcomings. Mistyped grant numbers (e.g. ‘1234-M JPN’ and ‘1234-M-JPN’) could appear as new grants, even though they existed before, or new grants might be classified as old grants if they have a common ID (e.g. ‘Grant 1’). Unfortunately, there is no central repository of grant numbers and the associated metadata; however, there are plans to assign DOI numbers to grants to alleviate this problem (See https://gitlab.com/crossref/open_funder_registry for the project).
Impact factor weighted publication numbers (IFWN).
In our analysis, we consider two measures of scientific output. First, we simply count the number of publications by MeSH term. However, since journals vary considerably in terms of impact factor, we also weigh the number of publications by the impact factor of the venue (e.g., journal) where it was published. Specifically, we use the SCImago journal ranking statistics to weigh a paper by the impact factor of the journal it appears in. We use the ‘citation per document in the past two years’ for 45,230 ISSNs. Note that a journal may and often has more than one ISSN, i.e., one for the printed edition and one for the online edition. SCImago applies the same score for a venue across linked ISSNs.
For the impact factor weighted number (IFWN) of publication per MeSH terms, this means that all publications are replaced by the impact score of the journal they appear in and summed up.
COVID-19-relatedness.
To measure how closely related to COVID-19 is a MeSH term, we introduce an index of relatedness to COVID-19. First, we identify the focal COVID-19 terms, which appeared in the literature in 2020 (see Table 1 ). Next, for all other pre-existing MeSH terms, we measure how closely related to COVID-19 they end up being.
Our aim is to show that MeSH terms that existed before and are related have experienced a sudden increase in the number of (impact factor weighted) papers.
Intuitively we can read this measure as: what is the probability in 2020 that a COVID-19 MeSH term is present given that we chose a paper with MeSH term i ? For example, given that in 2020 we choose a paper dealing with “Ageusia” (i.e., Complete or severe loss of the subjective sense of taste), there is a 96% probability that this paper also lists COVID-19, see Table 1 .
Note that a paper listing a related MeSH term does not imply that that paper is doing COVID-19 research, but it implies that one of the MeSH terms listed is often used in COVID-19 research.
In sum, in our analysis, we use the following variables:
- Papers: Number of papers by MeSH term;
- Impact: Impact factor weighted number of papers by MeSH term;
- PMC: Papers listed in PubMed central by MeSH term, as a measure of Open Access publications;
- Trials: number of publications of type “Clinical Trial” by MeSH term;
- Grants: number of papers with at least one grant by MeSH term;
- Old Grants: number of papers listing a grant that has been observed between 2010 and 2018, by MeSH term;
Difference-in-differences
The difference-in-differences (DiD) method is an econometric technique to imitate an experimental research design from observation data, sometimes referred to as a quasi-experimental setup. In a randomized controlled trial, subjects are randomly assigned either to the treated or the control group. Analogously, in this natural experiment, we assume that medical subject headings (MeSH) have been randomly assigned to be either treated (related) or not treated (unrelated) by the pandemic crisis.
Before the COVID, for a future health crisis, the set of potentially impacted medical knowledge was not predictable since it depended on the specifics of the emergency. For instance, ageusia (loss of taste), a medical concept existing since 1991, became known to be a specific symptom of COVID-19 only after the pandemic.
Specifically, we exploit the COVID-19 as an unpredictable and exogenous shock that has deeply affected the publication priorities for biomedical scientific production, as compared to the situation before the pandemic. In this setting, COVID-19 is the treatment, and the identification of this new human coronavirus is the event. We claim that treated MeSH terms, i.e., MeSH terms related to COVID-19, have experienced a sudden increase in terms of scientific production and attention. In contrast, research on untreated MeSH terms, i.e., MeSH terms not related to COVID-19, has been displaced by COVID-19. Our analysis compares the scientific output of COVID-19 related and unrelated MeSH terms before and after January 2020.
In our case, some of the terms turn out to be related to COVID-19 in 2020, whereas most of the MeSH terms are not closely related to COVID-19.
Thus β 1 identifies the overall effect on the control group after the event, β 2 the difference across treated and control groups before the event (i.e. the first difference in DiD) and finally the effect on the treated group after the event, net of the first difference, β 3 . This last parameter identifies the treatment effect on the treated group netting out the pre-treatment difference.
For the DiD to have a causal interpretation, it must be noted that pre-event, the trends of the two groups should be parallel, i.e., the common trend assumption (CTA) must be satisfied. We will show that the CTA holds in the results section.
To specify the DiD model, we need to define a period before and after the event and assign a treatment status or level of exposure to each term.
Before and after.
The pre-treatment period is defined as January 2019 to December 2019. The post-treatment period is defined as the months from January 2020 to December 2020. We argue that the state of biomedical research was similar in those two years, apart from the effect of the pandemic.
Treatment status and exposure.
The treatment is determined by the COVID-19 relatedness index σ i introduced earlier. Specifically, this number indicates the likelihood that COVID-19 will be a listed MeSH term, given that we observe the focal MeSH term i . To show that the effect becomes even stronger the closer related the subject is, and for ease of interpretation, we also discretize the relatedness value into three levels of treatment. Namely, we group MeSH terms with a σ between, 0% to 20%, 20% to 80% and 80% to 100%. The choice of alternative grouping strategies does not significantly affect our results. Results for alternative thresholds of relatedness can be computed using the available source code. We complement the dichotomized analysis by using the treatment intensity (relatedness measure σ ) to show that the result persists.
Panel regression.
In this work, we estimate a random effects panel regression where the units of analysis are 28 318 biomedical research fields (i.e. MeSH terms) observed over time before and after the COVID-19 pandemic. The time resolution is at the monthly level, meaning that for each MeSH term, we have 24 observations from January 2019 to December 2020.
The outcome variable Y it identifies the outcome at time t (i.e., month), for MeSH term i . As before, P t identifies the period with P t = 0 if the month is before January 2020 and P t = 1 if it is on or after this date. In (3) , the treatment level is measure by the relatedness to COVID-19 ( σ i ), where again the γ 1 identifies pre-trend (constant) differences and δ 1 the overall effect.
In total, we estimate six coefficients. As before, the δ l coefficient identifies the DiD effect.
Verifying the Common Trend Assumption (CTA).
We show that the CTA holds for this model by comparing the pre-event trends of the control group to the treated groups (COVID-19 related MeSH terms). Namely, we show that the pre-event trends of the control group are the same as the pre-event trends of the treated group.
Co-occurrence analysis
To investigate if the pandemic has caused a reconfiguration of research priorities, we look at the MeSH term co-occurrence network. Precisely, we extract the co-occurrence network of all 28,318 MeSH terms as they appear in the 3.3 million papers. We considered the co-occurrence networks of 2018, 2019 and 2020. Each node represents a MeSH term in these networks, and a link between them indicates that they have been observed at least once together. The weight of the edge between the MeSH terms is given by the number of times those terms have been jointly observed in the same publications.
Medical language is hugely complicated, and this simple representation does not capture the intricacies, subtle nuances and, in fact, meaning of the terms. Therefore, we do not claim that we can identify how the actual usage of MeSH terms has changed from this object, but rather that it has. Nevertheless, the co-occurrence graph captures rudimentary relations between concepts. We argue that absent a shock to the system, their basic usage patterns, change in importance (within the network) would essentially be the same from year to year. However, if we find that the importance of terms changes more than expected in 2020, it stands to reason that there have been some significant changes.
To show that that MeSH usage has been affected, we compute for each term in the years 2018, 2019 and 2020 their PageRank centrality [ 17 ]. The PageRank centrality tells us how likely a random walker traversing a network would be found at a given node if she follows the weights of the empirical edges (i.e., co-usage probability). Specifically, for the case of the MeSH co-occurrence network, this number represents how often an annotator at the National Library of Medicine would assign that MeSH term following the observed general usage patterns. It is a simplistic measure to capture the complexities of biomedical research. Nevertheless, it captures far-reaching interdependence across MeSH terms as the measure uses the whole network to determine the centrality of every MeSH term. A sudden change in the rankings and thus the position of MeSH terms in this network suggests that a given research subject has risen as it is used more often with other important MeSH terms (or vice versa).
We then compare the growth for each MeSH i term in g i (2019), i.e. before the the COVID-19 pandemic, with the growth after the event ( g i (2020)).
Publication growth
Changes in output and COVID-19 relatedness
Before we show the regression results, we provide descriptive evidence that publications from 2019 to 2020 have drastically increased. By showing that this growth correlates strongly with a MeSH term’s COVID-19 relatedness ( σ ), we demonstrate that (1) σ captures an essential aspect of the growth dynamics and (2) highlight the meteoric rise of highly related terms.
We look at the year over year growth in the number of the impact weighted number of publications per MeSH term from 2018 to 2019 and 2019 to 2020 as defined in the methods section.
Fig 1 shows the yearly growth of the impact weighted number of publications per MeSH term. By comparing the growth of the number of publications from the years 2018, 2019 and 2020, we find that the impact factor weighted number of publications has increased by up to a factor of 100 compared to the previous year for Betacoronavirus, one of the most closely related to COVID-19 MeSH term.
Each dot represents, a MeSH term. The y axis (growth) is in symmetric log scale. The x axis shows the COVID-19 relatedness, σ . Note that the position of the dots on the x-axis is the same in the two plots. Below: MeSH term importance gain (PageRank) and their COVID-19 relatedness.
https://doi.org/10.1371/journal.pone.0263001.g001
Fig 1 , first row, reveals how strongly correlated the growth in the IFWN of publication is to the term’s COVID-19 relatedness. For instance, we see that the term ‘Betacoronavirus’ skyrocketed from 2019 to 2020, which is expected given that SARS-CoV-2 is a species of the genus. Conversely, the term ‘Alphacoronavirus’ has not experienced any growth given that it is twin a genus of the Coronaviridae family, but SARS-CoV-2 is not one of its species. Note also the fast growth in the number of publications dealing with ‘Quarantine’. Moreover, MeSH terms that grew significantly from 2018 to 2019 and were not closely related to COVID-19, like ‘Vaping’, slowed down in 2020. From the graph, the picture emerges that publication growth is correlated with COVID-19 relatedness σ and that the growth for less related terms slowed down.
To show that the usage pattern of MeSH terms has changed following the pandemic, we compute the PageRank centrality using graph-tool [ 18 ] as discussed in the Methods section.
Fig 1 , second row, shows the change in the PageRank centrality of the MeSH terms after the pandemic (2019 to 2020, right plot) and before (2018 to 2019, left plot). If there were no change in the general usage pattern, we would expect the variance in PageRank changes to be narrow across the two periods, see (left plot). However, PageRank scores changed significantly more from 2019 to 2020 than from 2018 to 2019, suggesting that there has been a reconfiguration of the network.
To further support this argument, we carry out a DiD regression analysis.
Common trends assumption
As discussed in the Methods section, we need to show that the CTA assumption holds for the DiD to be defined appropriately. We do this by estimating for each month the number of publications and comparing it across treatment groups. This exercise also serves the purpose of a placebo test. By assuming that each month could have potentially been the event’s timing (i.e., the outbreak), we show that January 2020 is the most likely timing of the event. The regression table, as noted earlier, contains over 70 estimated coefficients, hence for ease of reading, we will only show the predicted outcome per month by group (see Fig 2 ). The full regression table with all coefficients is available in the S1 Table .
The y axis is in log scale. The dashed vertical line identifies January 2020. The dashed horizontal line shows the publications in January 2019 for the 0–20% group before the event. This line highlights that the drop happens after the event. The bands around the lines indicate the 95% confidence interval of the predicted values. The results are the output of the Stata margins command.
https://doi.org/10.1371/journal.pone.0263001.g002
Fig 2 shows the predicted number per outcome variable obtained from the panel regression model. These predictions correspond to the predicted value per relatedness group using the regression parameters estimated via the linear panel regression. The bands around the curves are the 95% confidence intervals.
All outcome measures depict a similar trend per month. Before the event (i.e., January 2020), there is a common trend across all groups. In contrast, after the event, we observe a sudden rise for the outcomes of the COVID-19 related treated groups (green and red lines) and a decline in the outcomes for the unrelated group (blue line). Therefore, we can conclude that the CTA assumption holds.
Regression results
Table 3 shows the DiD regression results (see Eq (3) ) for the selected outcome measures: number of publications (Papers), impact factor weighted number of publications (Impact), open access (OA) publications, clinical trial related publications, and publications with existing grants.
https://doi.org/10.1371/journal.pone.0263001.t003
Table 3 shows results for the discrete treatment level version of the DiD model (see Eq (4) ).
Note that the outcome variable is in natural log scale; hence to get the effect of the independent variable, we need to exponentiate the coefficient. For values close to 0, the effect is well approximated by the percentage change of that magnitude.
In both specifications we see that the least related group, drops in the number of publications between 10% and 13%, respectively (first row of Tables 3 and 4 , exp(−0.102) ≈ 0.87). In line with our expectations, the increase in the number of papers published by MeSH term is positively affected by the relatedness to COVID-19. In the discrete model (row 2), we note that the number of documents with MeSH terms with a COVID-19 relatedness between 20 and 80% grows by 18% and highly related terms by a factor of approximately 6.6 (exp(1.88)). The same general pattern can be observed for the impact weighted publication number, i.e., Model (2). Note, however, that the drop in the impact factor weighted output is more significant, reaching -19% for COVID-19 unrelated publications, and related publications growing by a factor of 8.7. This difference suggests that there might be a bias to publish papers on COVID-19 related subjects in high impact factor journals.
https://doi.org/10.1371/journal.pone.0263001.t004
By looking at the number of open access publications (PMC), we note that the least related group has not been affected negatively by the pandemic. However, the number of COVID-19 related publications has drastically increased for the most COVID-19 related group by a factor of 6.2. Note that the substantial increase in the number of papers available through open access is in large part due to journal and editorial policies to make preferentially COVID research immediately available to the public.
Regarding the number of clinical trial publications, we note that the least related group has been affected negatively, with the number of publications on clinical trials dropping by a staggering 24%. At the same time, publications on clinical trials for COVID-19-related MeSH have increased by a factor of 2.1. Note, however, that the effect on clinical trials is not significant in the continuous regression. The discrepancy across Tables 3 and 4 highlights that, especially for trials, the effect is not linear, where only the publications on clinical trials closely related to COVID-19 experiencing a boost.
It has been reported [ 19 ] that while the number of clinical trials registered to treat or prevent COVID-19 has surged with 179 new registrations in the second week of April 2020 alone. Only a few of these have led to publishable results in the 12 months since [ 20 ]. On the other hand, we find that clinical trial publications, considering related MeSH (but not COVID-19 directly), have had significant growth from the beginning of the pandemic. These results are not contradictory. Indeed counting the number of clinical trial publications listing the exact COVID-19 MeSH term (D000086382), we find 212 publications. While this might seem like a small number, consider that in 2020 only 8,485 publications were classified as clinical trials; thus, targeted trials still made up 2.5% of all clinical trials in 2020 . So while one might doubt the effectiveness of these research efforts, it is still the case that by sheer number, they represent a significant proportion of all publications on clinical trials in 2020. Moreover, COVID-19 specific Clinical trial publications in 2020, being a delayed signal of the actual trials, are a lower bound estimate on the true number of such clinical trials being conducted. This is because COVID-19 studies could only have commenced in 2020, whereas other studies had a head start. Thus our reported estimates are conservative, meaning that the true effect on actual clinical trials is likely larger, not smaller.
Research funding, as proxied by the number of publications with grants, follows a similar pattern, but notably, COVID-19-related MeSH terms list the same proportion of grants established before 2019 as other unrelated MeSH terms, suggesting that grants which were not designated for COVID-19 research have been used to support COVID-19 related research. Overall, the number of publications listing a grant has dropped. Note that this should be because the number of publications overall in the unrelated group has dropped. However, we note that the drop in publications is 10% while the decline in publications with at least one grant is 15%. This difference suggests that publications listing grants, which should have more funding, are disproportionately COVID-19 related papers. To further investigate this aspect, we look at whether the grant was old (pre-2019) or appeared for the first time in or after 2019. It stands to reason that an old grant (pre-2019) would not have been granted for a project dealing with the pandemic. Hence we would expect that COVID-19 related MeSH terms to have a lower proportion of old grants than the unrelated group. In models (6) in Table 4 we show that the number of old grants for the unrelated group drops by 13%. At the same time, the number of papers listing old grants (i.e., pre-2019) among the most related group increased by a factor of 3.1. Overall, these results suggest that COVID-19 related research has been funded largely by pre-existing grants, even though a specific mandate tied to the grants for this use is unlikely.
The scientific community has swiftly reallocated research efforts to cope with the COVID-19 pandemic, mobilizing knowledge across disciplines to find innovative solutions in record time. We document this both in terms of changing trends in the biomedical scientific output and the usage of MeSH terms by the scientific community. The flip side of this sudden and energetic prioritization of effort to fight COVID-19 has been a sudden contraction of scientific production in other relevant research areas. All in all, we find strong support to the hypotheses that the COVID-19 crisis has induced a sudden increase of research output in COVID-19 related areas of biomedical research. Conversely, research in areas not related to COVID-19 has experienced a significant drop in overall publishing rates and funding.
Our paper contributes to the literature on the impact of COVID-19 on scientific research: we corroborate previous findings about the surge of COVID-19 related publications [ 1 – 3 ], partially displacing research in COVID-19 unrelated fields of research [ 4 , 14 ], particularly research related to clinical trials [ 5 – 7 ]. The drop in trial research might have severe consequences for patients affected by life-threatening diseases since it will delay access to new and better treatments. We also confirm the impact of COVID-19 on open access publication output [ 1 ]; also, this is milder than traditional outlets. On top of this, we provide more robust evidence on the impact weighted effect of COVID-19 and grant financed research, highlighting the strong displacement effect of COVID-19 on the allocation of financial resources [ 15 ]. We document a substantial change in the usage patterns of MeSH terms, suggesting that there has been a reconfiguration in the way research terms are being combined. MeSH terms highly related to COVID-19 were peripheral in the MeSH usage networks before the pandemic but have become central since 2020. We conclude that the usage patterns have changed, with COVID-19 related MeSH terms occupying a much more prominent role in 2020 than they did in the previous years.
We also contribute to the literature by estimating the effect of COVID-19 on biomedical research in a natural experiment framework, isolating the specific effects of the COVID-19 pandemic on the biomedical scientific landscape. This is crucial to identify areas of public intervention to sustain areas of biomedical research which have been neglected during the COVID-19 crisis. Moreover, the exploratory analysis on the changes in usage patterns of MeSH terms, points to an increase in the importance of covid-related topics in the broader biomedical research landscape.
Our results provide compelling evidence that research related to COVID-19 has indeed displaced scientific production in other biomedical fields of research not related to COVID-19, with a significant drop in (impact weighted) scientific output related to non-COVID-19 and a marked reduction of financial support for publications not related to COVID-19 [ 4 , 5 , 16 ]. The displacement effect is persistent to the end of 2020. As vaccination progresses, we highlight the urgent need for science policy to re-balance support for research activity that was put on pause because of the COVID-19 pandemic.
We find that COVID-19 dramatically impacted clinical research. Reactivation of clinical trials activities that have been postponed or suspended for reasons related to COVID-19 is a priority that should be considered in the national vaccination plans. Moreover, since grants have been diverted and financial incentives have been targeted to sustain COVID-19 research leading to an excessive entry in COVID-19-related clinical trials and the ‘covidisation’ of research, there is a need to reorient incentives to basic research and otherwise neglected or temporally abandoned areas of biomedical research. Without dedicated support in the recovery plans for neglected research of the COVID-19 era, there is a risk that more medical needs will be unmet in the future, possibly exacerbating the shortage of scientific research for orphan and neglected diseases, which do not belong to COVID-19-related research areas.
Limitations
Our empirical approach has some limits. First, we proxy MeSH term usage via search terms using the PubMed EUtilities API. This means that the accuracy of the MeSH term we assign to a given paper is not fully validated. More time is needed for the completion of manually annotated MeSH terms. Second, the timing of publication is not the moment the research has been carried out. There is a lead time between inception, analysis, write-up, review, revision, and final publication. This delay varies across disciplines. Nevertheless, given that the surge in publications happens around the alleged event date, January 2020, we are confident that the publication date is a reasonable yet imperfect estimate of the timing of the research. Third, several journals have publicly declared to fast-track COVID-19 research. This discrepancy in the speed of publication of COVID-19 related research and other research could affect our results. Specifically, a surge or displacement could be overestimated due to a lag in the publication of COVID-19 unrelated research. We alleviate this bias by estimating the effect considering a considerable time after the event (January 2020 to December 2020). Forth, on the one hand, clinical Trials may lead to multiple publications. Therefore we might overestimate the impact of COVID-19 on the number of clinical trials. On the other hand, COVID-19 publications on clinical trials lag behind, so the number of papers related COVID-19 trials is likely underestimated. Therefore, we note that the focus of this paper is scientific publications on clinical trials rather than on actual clinical trials. Fifth, regarding grants, unfortunately, there is no unique centralized repository mapping grant numbers to years, so we have to proxy old grants with grants that appeared in publications from 2010 to 2018. Besides, grant numbers are free-form entries, meaning that PubMed has no validation step to disambiguate or verify that the grant number has been entered correctly. This has the effect of classifying a grant as new even though it has appeared under a different name. We mitigate this problem by using a long period to collect grant numbers and catch many spellings of the same grant, thereby reducing the likelihood of miss-identifying a grant as new when it existed before. Still, unless unique identifiers are widely used, there is no way to verify this.
So far, there is no conclusive evidence on whether entry into COVID-19 has been excessive. However, there is a growing consensus that COVID-19 has displaced, at least temporally, scientific research in COVID-19 unrelated biomedical research areas. Even though it is certainly expected that more attention will be devoted to the emergency during a pandemic, the displacement of biomedical research in other fields is concerning. Future research is needed to investigate the long-run structural consequences of the COVID-19 crisis on biomedical research.
Supporting information
S1 table. common trend assumption (cta) regression table..
Full regression table with all controls and interactions.
https://doi.org/10.1371/journal.pone.0263001.s001
- View Article
- Google Scholar
- PubMed/NCBI
- 8. Brown A, Edwin E, Fagg J. Evaluate Pharma 2021 Preview; 2020. https://www.evaluate.com/thought-leadership/vantage/evaluate-vantage-2021-preview .
- 15. Hook D, Porter S. The COVID Brain Drain; 2020. https://www.natureindex.com/news-blog/covid-brain-drain-scientific-research .
- 17. Page L, Brin S, Motwani R, Winograd T. The PageRank citation ranking: Bringing order to the web. Stanford InfoLab; 1999.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

COVID-19 Research Articles Downloadable Database
March 19, 2020
Updated January 12, 2024
COVID-19 Research Guide Home
- Research Articles Downloadable Database
- COVID-19 Science Updates
- Databases and Journals
- Secondary Data and Statistics
Important announcement:
The CDC Database of COVID-19 Research Articles became a collaboration with the WHO to create the WHO COVID-19 database during the pandemic to make it easier for results to be searched, downloaded, and used by researchers worldwide.
The last version of the CDC COVID-19 database was archived and remain available on this website. Please note that it has stopped updating as of October 9, 2020 and all new articles were integrated into the WHO COVID-19 database . The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
If you have any questions, concerns, or problems accessing the WHO COVID-19 Database please email the CDC Library for assistance.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
Below are options to download the archive of COVID-19 research articles. You can search the database of citations by author, keyword (in title, author, abstract, subject headings fields), journal, or abstract when available. DOI, PMID, and URL links are included when available.
This database was last updated on October 9, 2020 .
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide. The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
- To help inform CDC’s COVID-19 Response, as well as to help CDC staff stay up to date on the latest COVID-19 research, the Response’s Office of the Chief Medical Officer has collaborated with the CDC Office of Library Science to create a series called COVID-19 Science Update . This series, the first of its kind for a CDC emergency response, provides brief summaries of new COVID-19-related studies on many topics, including epidemiology, clinical treatment and management, laboratory science, and modeling. As of December 18, 2021, CDC has stopped production of the weekly COVID-19 Science Update.
Excel download:
- Articles from August until October 9 2020 [XLS – 29 MB]
- Articles from December 2019 through July 2020 [XLS – 45 MB]
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide.
- October 8 in Excel [XLS – 1 MB]
- October 7 in Excel [XLS – 1 MB]
- October 6 in Excel [XLS – 1 MB]
- Note the main Excel file can also be sorted by date added.
Citation Management Software (EndNote, Mendeley, Zotero, Refman, etc.) download:
- Part 1 [ZIP – 38 MB]
- Part 2 [ZIP – 43 MB]
- October 8 in citation management software format [RIS – 2 MB]
- October 7 in citation management software format [RIS – 2 MB]
- October 6 in citation management software format [RIS – 2 MB]
- Note the main RIS file can also be sorted by date added.
The COVID-19 pandemic is a rapidly changing situation. Some of the research included above is preliminary. Materials listed in this database are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
To access the full text, click on the DOI, PMID, or URL links. While most publishers are making their COVID-19 content Open Access, some articles are accessible only to those with a CDC user id and password. Find a library near you that may be able to help you get access to articles by clicking the following links: https://www.worldcat.org/libraries OR https://www.usa.gov/libraries .
CDC users can use EndNote’s Find Full Text feature to attach the full text PDFs within their EndNote Library. CDC users, please email Martha Knuth for an EndNote file of all citations. Once you have your EndNote file downloaded, to get the full-text of journal articles listed in the search results you can do the following steps:
- First, try using EndNote’s “Find Full-Text” feature to attach full-text articles to your EndNote Library.
- Next, check for full-text availability, via the E-Journals list, at: http://sfxhosted.exlibrisgroup.com/cdc/az .
- If you can’t find full-text online, you can request articles via DocExpress, at: https://docexpress.cdc.gov/illiad/
The following databases were searched from Dec. 2019-Oct. 9 2020 for articles related to COVID-19: Medline (Ovid and PubMed), PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, and LitCovid. Selected grey literature sources were searched as well, including the WHO COVID-19 website, CDC COVID-19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv (preprints), medRxiv (preprints), chemRxiv (preprints), and SSRN (preprints).
Detailed search strings with synonyms used for COVID-19 are below.
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF – 135 KB]
Note on preprints: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
To receive the COVID-19 Science Update, please enter your email address to subscribe today.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
News and views for the UB community
- Stories >
Researchers use big data to establish long COVID subtypes
research news

By ELLEN GOLDBAUM
Published June 25, 2024

The announcement earlier this month from the National Academies of Science, Engineering and Medicine of a consensus definition of long COVID mentioned that it was not the final word on this condition but that the definition would be revised as new findings are published.
Earlier this year, researchers at UB and the Department of Veterans Affairs who were using big data to work on a data-driven long COVID definition were invited to report their findings in testimony before the National Academies.
In April they published those findings in JMIR Public Health Surveillance based on more than 250,000 patients in the Veterans Health Administration who had tested positive for COVID.
Finding different subtypes
“Because we had such large numbers of patients at the VA, it was a wonderful place to do this study,” says Peter L. Elkin, corresponding author and chair of the Department of Biomedical Informatics in the Jacobs School of Medicine and Biomedical Sciences at UB. “We looked at the full electronic health records of patients so that we could tease apart all the different symptoms of this complex disease to find all the different subtypes that exist.”
One of the goals is to help clinicians better recognize cases of long COVID. “The idea is to make more clinicians aware of who has long COVID and who is maybe not yet out of the woods from long COVID,” he explains. “Now that we have a definition, you can tell who in your specialty has long COVID and who doesn’t.”
The findings could help researchers develop a more scientific approach to finding out how COVID infection causes the specific conditions seen in long COVID.
The study was done using the electronic health records of more than 2.3 million patients who were seen at a VA health facility between Jan. 1, 2020, and Aug. 18, 2022. There were 367,148 patients who tested positive for COVID-19 at a VA facility; of those, 268,320 were considered to have long COVID if they had a novel diagnosis between one and seven months following a positive COVID-19 test.
Based on the symptoms experienced by patients with long COVID, the researchers assigned a total of 324 ICD (International Classification of Disease) codes. They identified 180 clinical scenarios and 17 clinical subtypes that were upregulated in people who had long COVID.
Highest case counts in cardiology
The highest long COVID case counts were in cardiology, with diagnoses including low blood pressure, heart failure, arrythmias and atrial fibrillation, followed by neurology (symptoms included low back pain, severe muscle weakness and cognitive impairment), ophthalmology and pulmonology.
Among the most commonly cited symptoms were fatigue and acute respiratory distress. Respiratory issues are among the most common in long COVID, including chronic cough, respiratory failure and dependence on supplemental oxygen. Cognitive difficulties, including brain fog, were also frequently cited.
Factors that put patients at higher risk for developing long COVID included older age, other health conditions prior to becoming infected, a more severe case of COVID and low oxygen saturation during COVID. Patients who had not received a COVID-19 vaccine were 1.3 times more likely to develop long COVID.
The study provides definitions of each of the long COVID subtypes and odds ratios defining the risk for each of them.
“These data will allow us to better identify patients with long COVID and it can also help support public health research and policy initiatives going forward,” Elkin says.
The authors note that a limitation of the study was that the study population was 84% male.
Co-authors with Elkin are Skyler Resendez, postdoctoral fellow; Hugo Sebastian Ruiz Ayala; and Prahalad Rangan, all of the Department of Biomedical Informatics in the Jacobs School; and Steven H. Brown, Jonathan Nebeker and Diana Montella of the Office of Health Informatics of the Department of Veterans Affairs.
The work was supported by the National Library of Medicine, the National Institute on Alcohol Abuse and Alcoholism, and the National Center for Advancing Translational Science, all of the National Institutes of Health, as well as the Department of Veterans Affairs.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

A Review of Coronavirus Disease-2019 (COVID-19)
Tanu singhal.
Department of Pediatrics and Infectious Disease, Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Mumbai, India
There is a new public health crises threatening the world with the emergence and spread of 2019 novel coronavirus (2019-nCoV) or the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus originated in bats and was transmitted to humans through yet unknown intermediary animals in Wuhan, Hubei province, China in December 2019. There have been around 96,000 reported cases of coronavirus disease 2019 (COVID-2019) and 3300 reported deaths to date (05/03/2020). The disease is transmitted by inhalation or contact with infected droplets and the incubation period ranges from 2 to 14 d. The symptoms are usually fever, cough, sore throat, breathlessness, fatigue, malaise among others. The disease is mild in most people; in some (usually the elderly and those with comorbidities), it may progress to pneumonia, acute respiratory distress syndrome (ARDS) and multi organ dysfunction. Many people are asymptomatic. The case fatality rate is estimated to range from 2 to 3%. Diagnosis is by demonstration of the virus in respiratory secretions by special molecular tests. Common laboratory findings include normal/ low white cell counts with elevated C-reactive protein (CRP). The computerized tomographic chest scan is usually abnormal even in those with no symptoms or mild disease. Treatment is essentially supportive; role of antiviral agents is yet to be established. Prevention entails home isolation of suspected cases and those with mild illnesses and strict infection control measures at hospitals that include contact and droplet precautions. The virus spreads faster than its two ancestors the SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), but has lower fatality. The global impact of this new epidemic is yet uncertain.
Introduction
The 2019 novel coronavirus (2019-nCoV) or the severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) as it is now called, is rapidly spreading from its origin in Wuhan City of Hubei Province of China to the rest of the world [ 1 ]. Till 05/03/2020 around 96,000 cases of coronavirus disease 2019 (COVID-19) and 3300 deaths have been reported [ 2 ]. India has reported 29 cases till date. Fortunately so far, children have been infrequently affected with no deaths. But the future course of this virus is unknown. This article gives a bird’s eye view about this new virus. Since knowledge about this virus is rapidly evolving, readers are urged to update themselves regularly.
Coronaviruses are enveloped positive sense RNA viruses ranging from 60 nm to 140 nm in diameter with spike like projections on its surface giving it a crown like appearance under the electron microscope; hence the name coronavirus [ 3 ]. Four corona viruses namely HKU1, NL63, 229E and OC43 have been in circulation in humans, and generally cause mild respiratory disease.
There have been two events in the past two decades wherein crossover of animal betacorona viruses to humans has resulted in severe disease. The first such instance was in 2002–2003 when a new coronavirus of the β genera and with origin in bats crossed over to humans via the intermediary host of palm civet cats in the Guangdong province of China. This virus, designated as severe acute respiratory syndrome coronavirus affected 8422 people mostly in China and Hong Kong and caused 916 deaths (mortality rate 11%) before being contained [ 4 ]. Almost a decade later in 2012, the Middle East respiratory syndrome coronavirus (MERS-CoV), also of bat origin, emerged in Saudi Arabia with dromedary camels as the intermediate host and affected 2494 people and caused 858 deaths (fatality rate 34%) [ 5 ].
Origin and Spread of COVID-19 [ 1 , 2 , 6 ]
In December 2019, adults in Wuhan, capital city of Hubei province and a major transportation hub of China started presenting to local hospitals with severe pneumonia of unknown cause. Many of the initial cases had a common exposure to the Huanan wholesale seafood market that also traded live animals. The surveillance system (put into place after the SARS outbreak) was activated and respiratory samples of patients were sent to reference labs for etiologic investigations. On December 31st 2019, China notified the outbreak to the World Health Organization and on 1st January the Huanan sea food market was closed. On 7th January the virus was identified as a coronavirus that had >95% homology with the bat coronavirus and > 70% similarity with the SARS- CoV. Environmental samples from the Huanan sea food market also tested positive, signifying that the virus originated from there [ 7 ]. The number of cases started increasing exponentially, some of which did not have exposure to the live animal market, suggestive of the fact that human-to-human transmission was occurring [ 8 ]. The first fatal case was reported on 11th Jan 2020. The massive migration of Chinese during the Chinese New Year fuelled the epidemic. Cases in other provinces of China, other countries (Thailand, Japan and South Korea in quick succession) were reported in people who were returning from Wuhan. Transmission to healthcare workers caring for patients was described on 20th Jan, 2020. By 23rd January, the 11 million population of Wuhan was placed under lock down with restrictions of entry and exit from the region. Soon this lock down was extended to other cities of Hubei province. Cases of COVID-19 in countries outside China were reported in those with no history of travel to China suggesting that local human-to-human transmission was occurring in these countries [ 9 ]. Airports in different countries including India put in screening mechanisms to detect symptomatic people returning from China and placed them in isolation and testing them for COVID-19. Soon it was apparent that the infection could be transmitted from asymptomatic people and also before onset of symptoms. Therefore, countries including India who evacuated their citizens from Wuhan through special flights or had travellers returning from China, placed all people symptomatic or otherwise in isolation for 14 d and tested them for the virus.
Cases continued to increase exponentially and modelling studies reported an epidemic doubling time of 1.8 d [ 10 ]. In fact on the 12th of February, China changed its definition of confirmed cases to include patients with negative/ pending molecular tests but with clinical, radiologic and epidemiologic features of COVID-19 leading to an increase in cases by 15,000 in a single day [ 6 ]. As of 05/03/2020 96,000 cases worldwide (80,000 in China) and 87 other countries and 1 international conveyance (696, in the cruise ship Diamond Princess parked off the coast of Japan) have been reported [ 2 ]. It is important to note that while the number of new cases has reduced in China lately, they have increased exponentially in other countries including South Korea, Italy and Iran. Of those infected, 20% are in critical condition, 25% have recovered, and 3310 (3013 in China and 297 in other countries) have died [ 2 ]. India, which had reported only 3 cases till 2/3/2020, has also seen a sudden spurt in cases. By 5/3/2020, 29 cases had been reported; mostly in Delhi, Jaipur and Agra in Italian tourists and their contacts. One case was reported in an Indian who traveled back from Vienna and exposed a large number of school children in a birthday party at a city hotel. Many of the contacts of these cases have been quarantined.
These numbers are possibly an underestimate of the infected and dead due to limitations of surveillance and testing. Though the SARS-CoV-2 originated from bats, the intermediary animal through which it crossed over to humans is uncertain. Pangolins and snakes are the current suspects.
Epidemiology and Pathogenesis [ 10 , 11 ]
All ages are susceptible. Infection is transmitted through large droplets generated during coughing and sneezing by symptomatic patients but can also occur from asymptomatic people and before onset of symptoms [ 9 ]. Studies have shown higher viral loads in the nasal cavity as compared to the throat with no difference in viral burden between symptomatic and asymptomatic people [ 12 ]. Patients can be infectious for as long as the symptoms last and even on clinical recovery. Some people may act as super spreaders; a UK citizen who attended a conference in Singapore infected 11 other people while staying in a resort in the French Alps and upon return to the UK [ 6 ]. These infected droplets can spread 1–2 m and deposit on surfaces. The virus can remain viable on surfaces for days in favourable atmospheric conditions but are destroyed in less than a minute by common disinfectants like sodium hypochlorite, hydrogen peroxide etc. [ 13 ]. Infection is acquired either by inhalation of these droplets or touching surfaces contaminated by them and then touching the nose, mouth and eyes. The virus is also present in the stool and contamination of the water supply and subsequent transmission via aerosolization/feco oral route is also hypothesized [ 6 ]. As per current information, transplacental transmission from pregnant women to their fetus has not been described [ 14 ]. However, neonatal disease due to post natal transmission is described [ 14 ]. The incubation period varies from 2 to 14 d [median 5 d]. Studies have identified angiotensin receptor 2 (ACE 2 ) as the receptor through which the virus enters the respiratory mucosa [ 11 ].
The basic case reproduction rate (BCR) is estimated to range from 2 to 6.47 in various modelling studies [ 11 ]. In comparison, the BCR of SARS was 2 and 1.3 for pandemic flu H1N1 2009 [ 2 ].
Clinical Features [ 8 , 15 – 18 ]
The clinical features of COVID-19 are varied, ranging from asymptomatic state to acute respiratory distress syndrome and multi organ dysfunction. The common clinical features include fever (not in all), cough, sore throat, headache, fatigue, headache, myalgia and breathlessness. Conjunctivitis has also been described. Thus, they are indistinguishable from other respiratory infections. In a subset of patients, by the end of the first week the disease can progress to pneumonia, respiratory failure and death. This progression is associated with extreme rise in inflammatory cytokines including IL2, IL7, IL10, GCSF, IP10, MCP1, MIP1A, and TNFα [ 15 ]. The median time from onset of symptoms to dyspnea was 5 d, hospitalization 7 d and acute respiratory distress syndrome (ARDS) 8 d. The need for intensive care admission was in 25–30% of affected patients in published series. Complications witnessed included acute lung injury, ARDS, shock and acute kidney injury. Recovery started in the 2nd or 3rd wk. The median duration of hospital stay in those who recovered was 10 d. Adverse outcomes and death are more common in the elderly and those with underlying co-morbidities (50–75% of fatal cases). Fatality rate in hospitalized adult patients ranged from 4 to 11%. The overall case fatality rate is estimated to range between 2 and 3% [ 2 ].
Interestingly, disease in patients outside Hubei province has been reported to be milder than those from Wuhan [ 17 ]. Similarly, the severity and case fatality rate in patients outside China has been reported to be milder [ 6 ]. This may either be due to selection bias wherein the cases reporting from Wuhan included only the severe cases or due to predisposition of the Asian population to the virus due to higher expression of ACE 2 receptors on the respiratory mucosa [ 11 ].
Disease in neonates, infants and children has been also reported to be significantly milder than their adult counterparts. In a series of 34 children admitted to a hospital in Shenzhen, China between January 19th and February 7th, there were 14 males and 20 females. The median age was 8 y 11 mo and in 28 children the infection was linked to a family member and 26 children had history of travel/residence to Hubei province in China. All the patients were either asymptomatic (9%) or had mild disease. No severe or critical cases were seen. The most common symptoms were fever (50%) and cough (38%). All patients recovered with symptomatic therapy and there were no deaths. One case of severe pneumonia and multiorgan dysfunction in a child has also been reported [ 19 ]. Similarly the neonatal cases that have been reported have been mild [ 20 ].
Diagnosis [ 21 ]
A suspect case is defined as one with fever, sore throat and cough who has history of travel to China or other areas of persistent local transmission or contact with patients with similar travel history or those with confirmed COVID-19 infection. However cases may be asymptomatic or even without fever. A confirmed case is a suspect case with a positive molecular test.
Specific diagnosis is by specific molecular tests on respiratory samples (throat swab/ nasopharyngeal swab/ sputum/ endotracheal aspirates and bronchoalveolar lavage). Virus may also be detected in the stool and in severe cases, the blood. It must be remembered that the multiplex PCR panels currently available do not include the COVID-19. Commercial tests are also not available at present. In a suspect case in India, the appropriate sample has to be sent to designated reference labs in India or the National Institute of Virology in Pune. As the epidemic progresses, commercial tests will become available.
Other laboratory investigations are usually non specific. The white cell count is usually normal or low. There may be lymphopenia; a lymphocyte count <1000 has been associated with severe disease. The platelet count is usually normal or mildly low. The CRP and ESR are generally elevated but procalcitonin levels are usually normal. A high procalcitonin level may indicate a bacterial co-infection. The ALT/AST, prothrombin time, creatinine, D-dimer, CPK and LDH may be elevated and high levels are associated with severe disease.
The chest X-ray (CXR) usually shows bilateral infiltrates but may be normal in early disease. The CT is more sensitive and specific. CT imaging generally shows infiltrates, ground glass opacities and sub segmental consolidation. It is also abnormal in asymptomatic patients/ patients with no clinical evidence of lower respiratory tract involvement. In fact, abnormal CT scans have been used to diagnose COVID-19 in suspect cases with negative molecular diagnosis; many of these patients had positive molecular tests on repeat testing [ 22 ].
Differential Diagnosis [ 21 ]
The differential diagnosis includes all types of respiratory viral infections [influenza, parainfluenza, respiratory syncytial virus (RSV), adenovirus, human metapneumovirus, non COVID-19 coronavirus], atypical organisms (mycoplasma, chlamydia) and bacterial infections. It is not possible to differentiate COVID-19 from these infections clinically or through routine lab tests. Therefore travel history becomes important. However, as the epidemic spreads, the travel history will become irrelevant.
Treatment [ 21 , 23 ]
Treatment is essentially supportive and symptomatic.
The first step is to ensure adequate isolation (discussed later) to prevent transmission to other contacts, patients and healthcare workers. Mild illness should be managed at home with counseling about danger signs. The usual principles are maintaining hydration and nutrition and controlling fever and cough. Routine use of antibiotics and antivirals such as oseltamivir should be avoided in confirmed cases. In hypoxic patients, provision of oxygen through nasal prongs, face mask, high flow nasal cannula (HFNC) or non-invasive ventilation is indicated. Mechanical ventilation and even extra corporeal membrane oxygen support may be needed. Renal replacement therapy may be needed in some. Antibiotics and antifungals are required if co-infections are suspected or proven. The role of corticosteroids is unproven; while current international consensus and WHO advocate against their use, Chinese guidelines do recommend short term therapy with low-to-moderate dose corticosteroids in COVID-19 ARDS [ 24 , 25 ]. Detailed guidelines for critical care management for COVID-19 have been published by the WHO [ 26 ]. There is, as of now, no approved treatment for COVID-19. Antiviral drugs such as ribavirin, lopinavir-ritonavir have been used based on the experience with SARS and MERS. In a historical control study in patients with SARS, patients treated with lopinavir-ritonavir with ribavirin had better outcomes as compared to those given ribavirin alone [ 15 ].
In the case series of 99 hospitalized patients with COVID-19 infection from Wuhan, oxygen was given to 76%, non-invasive ventilation in 13%, mechanical ventilation in 4%, extracorporeal membrane oxygenation (ECMO) in 3%, continuous renal replacement therapy (CRRT) in 9%, antibiotics in 71%, antifungals in 15%, glucocorticoids in 19% and intravenous immunoglobulin therapy in 27% [ 15 ]. Antiviral therapy consisting of oseltamivir, ganciclovir and lopinavir-ritonavir was given to 75% of the patients. The duration of non-invasive ventilation was 4–22 d [median 9 d] and mechanical ventilation for 3–20 d [median 17 d]. In the case series of children discussed earlier, all children recovered with basic treatment and did not need intensive care [ 17 ].
There is anecdotal experience with use of remdeswir, a broad spectrum anti RNA drug developed for Ebola in management of COVID-19 [ 27 ]. More evidence is needed before these drugs are recommended. Other drugs proposed for therapy are arbidol (an antiviral drug available in Russia and China), intravenous immunoglobulin, interferons, chloroquine and plasma of patients recovered from COVID-19 [ 21 , 28 , 29 ]. Additionally, recommendations about using traditional Chinese herbs find place in the Chinese guidelines [ 21 ].
Prevention [ 21 , 30 ]
Since at this time there are no approved treatments for this infection, prevention is crucial. Several properties of this virus make prevention difficult namely, non-specific features of the disease, the infectivity even before onset of symptoms in the incubation period, transmission from asymptomatic people, long incubation period, tropism for mucosal surfaces such as the conjunctiva, prolonged duration of the illness and transmission even after clinical recovery.
Isolation of confirmed or suspected cases with mild illness at home is recommended. The ventilation at home should be good with sunlight to allow for destruction of virus. Patients should be asked to wear a simple surgical mask and practice cough hygiene. Caregivers should be asked to wear a surgical mask when in the same room as patient and use hand hygiene every 15–20 min.
The greatest risk in COVID-19 is transmission to healthcare workers. In the SARS outbreak of 2002, 21% of those affected were healthcare workers [ 31 ]. Till date, almost 1500 healthcare workers in China have been infected with 6 deaths. The doctor who first warned about the virus has died too. It is important to protect healthcare workers to ensure continuity of care and to prevent transmission of infection to other patients. While COVID-19 transmits as a droplet pathogen and is placed in Category B of infectious agents (highly pathogenic H5N1 and SARS), by the China National Health Commission, infection control measures recommended are those for category A agents (cholera, plague). Patients should be placed in separate rooms or cohorted together. Negative pressure rooms are not generally needed. The rooms and surfaces and equipment should undergo regular decontamination preferably with sodium hypochlorite. Healthcare workers should be provided with fit tested N95 respirators and protective suits and goggles. Airborne transmission precautions should be taken during aerosol generating procedures such as intubation, suction and tracheostomies. All contacts including healthcare workers should be monitored for development of symptoms of COVID-19. Patients can be discharged from isolation once they are afebrile for atleast 3 d and have two consecutive negative molecular tests at 1 d sampling interval. This recommendation is different from pandemic flu where patients were asked to resume work/school once afebrile for 24 h or by day 7 of illness. Negative molecular tests were not a prerequisite for discharge.
At the community level, people should be asked to avoid crowded areas and postpone non-essential travel to places with ongoing transmission. They should be asked to practice cough hygiene by coughing in sleeve/ tissue rather than hands and practice hand hygiene frequently every 15–20 min. Patients with respiratory symptoms should be asked to use surgical masks. The use of mask by healthy people in public places has not shown to protect against respiratory viral infections and is currently not recommended by WHO. However, in China, the public has been asked to wear masks in public and especially in crowded places and large scale gatherings are prohibited (entertainment parks etc). China is also considering introducing legislation to prohibit selling and trading of wild animals [ 32 ].
The international response has been dramatic. Initially, there were massive travel restrictions to China and people returning from China/ evacuated from China are being evaluated for clinical symptoms, isolated and tested for COVID-19 for 2 wks even if asymptomatic. However, now with rapid world wide spread of the virus these travel restrictions have extended to other countries. Whether these efforts will lead to slowing of viral spread is not known.
A candidate vaccine is under development.
Practice Points from an Indian Perspective
At the time of writing this article, the risk of coronavirus in India is extremely low. But that may change in the next few weeks. Hence the following is recommended:
- Healthcare providers should take travel history of all patients with respiratory symptoms, and any international travel in the past 2 wks as well as contact with sick people who have travelled internationally.
- They should set up a system of triage of patients with respiratory illness in the outpatient department and give them a simple surgical mask to wear. They should use surgical masks themselves while examining such patients and practice hand hygiene frequently.
- Suspected cases should be referred to government designated centres for isolation and testing (in Mumbai, at this time, it is Kasturba hospital). Commercial kits for testing are not yet available in India.
- Patients admitted with severe pneumonia and acute respiratory distress syndrome should be evaluated for travel history and placed under contact and droplet isolation. Regular decontamination of surfaces should be done. They should be tested for etiology using multiplex PCR panels if logistics permit and if no pathogen is identified, refer the samples for testing for SARS-CoV-2.
- All clinicians should keep themselves updated about recent developments including global spread of the disease.
- Non-essential international travel should be avoided at this time.
- People should stop spreading myths and false information about the disease and try to allay panic and anxiety of the public.
Conclusions
This new virus outbreak has challenged the economic, medical and public health infrastructure of China and to some extent, of other countries especially, its neighbours. Time alone will tell how the virus will impact our lives here in India. More so, future outbreaks of viruses and pathogens of zoonotic origin are likely to continue. Therefore, apart from curbing this outbreak, efforts should be made to devise comprehensive measures to prevent future outbreaks of zoonotic origin.
Compliance with Ethical Standards
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
Long Covid feels like a gun to my head
By Rachel Hall-Clifford June 18, 2024

I have spent my career studying infectious diseases that fall under the heading of neglected tropical diseases . Now I have a neglected disease — long Covid — an incurable (for now and for me) disease.
As a medical anthropologist working in global health, I thought I understood the despair of poor health. I didn’t. I join 7% of the U.S. adult population — or about 18 million Americans — who have experienced long Covid. Diagnosis of long Covid remains uncertain and contested , and treatments, ranging from repurposed drugs to hyperbaric oxygen, are even more so.
advertisement
I was infected with SARS-CoV2 during the Omicron wave of January 2022. It crashed through my kid’s kindergarten class and swept our household along with it. We had none of the “ underlying conditions ” that may indicate increased risks of poor outcomes from Covid (and which have been used throughout the pandemic to allay fears that dangerous outcomes would only happen to “others”). My acute infection wasn’t scary: I had fever, aches, and chills for about four days. My initial Covid aches and pains were nothing in comparison to when I had dengue fever, known as “bone break fever,” while working in Guatemala’s remote mountains.
And then I just never got better . It took a couple of months for me to realize that. I developed crushing chest pain and a heart rate that would rival a hummingbird’s. I couldn’t walk around my block without stopping to catch my breath. I was often dizzy, and my arms and legs felt like leaden sausages that had grown too big for their casings.
Related: Listen: Why Long Covid can feel scarier than a gun to the head
Like many of my global health colleagues, I love a good adventure and don’t mind flirting with danger a little. I’ll go anywhere and talk to anyone. I once talked a Guatemalan street gang out of harming my small research team as they held a Kalashnikov to our heads during a robbery. It was scary, but I didn’t fear for my life. I knew it wasn’t the end of my story. But I have thought that long Covid might be: At its worst, I wrote letters to my children in fear that I wouldn’t survive the night.
More than two years in, I’m among the luckiest of those living with long Covid. My symptoms are managed, though imperfectly. I have the academic background to follow the latest research findings and access to brilliant colleagues doing some of that work. I have the money, insurance, and health care providers that have enabled me to try several treatments.
Here’s a bit of what I’ve tried so far, all shots in the dark: A beta blocker controls my chest pain and high heart rate. A 3-month course of powerful blood thinners improved numbness and pain in my limbs. Constant use of electrolyte fluids like Gatorade and Pedialyte (ironically what I studied in graduate school) improves my dizziness and is essential for propping myself up to teach a class in a lecture hall or get through a day of Zoom meetings. My iliac vein has completely collapsed in my left leg, and my cardiologist wants me to get a stent.
I wouldn’t be able to hold down the jobs in warehouses, factories, and farms that many in my family have had.
Though my world has gotten small, and I’m not able to travel for my work as I once did, most days I feel like I just got off a long-haul flight and live in a permanent state of jet lag . I have one of those pill organizers stuffed full of medications and supplements that I hope will help at least a little. (I still struggle to reconcile my self-identity with this new reality.)
Related: NIH documents show how $1.6 billion long Covid initiative has failed so far to meet its goals
I was able to take a 15-day course of the antiviral Paxlovid , and it was the best I’ve felt in two years. For many people, the side effects of this medicine are terrible, but I never wanted its hallmark metallic tang to end. About two days after my course of Paxlovid ended, though, my symptoms crept back. Recent findings of viral persistence came as no surprise to me, and new results from a clinical trial investigating a 15-day course of Paxlovid in long Covid patients has shown no benefit .
I am now taking (at great cost) maraviroc , an antiviral used to treat HIV, which helps partially control my symptoms. I recently slid into the whirring tomb of an MRI machine to try to find an explanation for persistent post-Covid migraines in my brain, but that was a dead end. Nothing was found, and I don’t know whether to be disappointed or relieved.
I admit I am scared. This is not a funny story I will tell colleagues over drinks later. There’s no gangland drug lord to negotiate with this time. Instead, I spend a lot of my time lying in the dark (I’m here now, even as I type this) negotiating with god and science to make me — and all of us suffering with long Covid and other post-viral illnesses — better. It’s surprisingly been the short periods when I have felt better that are the most upsetting, as they highlight how terrible I feel most of the time.
So I fake it. I need the pretense of being my old, fearless self. I need to discuss interesting things with colleagues and teach and run my lab. I need to take the snacks to soccer and help my kids with homework. That’s what makes me who I am, even as I playact a poor facsimile of my healthy self that requires hours (sometimes days) of recovery time afterwards.
I will continue to bargain with the universe to get to live the life I have worked to build for myself. I want that for everyone. My work in global health has shown me both the fragility of life but also the value of fighting for everyone’s right to a full and healthy life.
Sign up for First Opinion
The smartest thinkers in life sciences on what's happening — and what's to come
I understand that no one cares much about Covid anymore. It’s been a long haul for all of us, even those who aren’t “long haulers.” I hope everyone who hasn’t experienced long Covid never really understand what I’m talking about — what others with chronic illness and disability have tried to teach us — that our abled bodies are only temporary. Long Covid and the SARS-CoV-2 infections that cause it are harsh teachers.
I am inspired by the work of the long Covid Patient-Led Research Collaborative and the research being done to uncover the causes of and cures for long Covid. But it’s not enough. Given the widespread burden of disease and the losses to the economy and social fabric it is causing in the U.S. and around the globe, the U.S. government must act quickly and decisively to curb long Covid. The Long Covid Moonshot is a collective advocating for $1 billion in annual research funding for long Covid, akin to the Operation Warp Speed that enabled the first generation of Covid-19 vaccines. U.S. Senator Bernie Sanders (D-Vt.) recently released a Long Covid Moonshot legislative proposal . Bipartisan support for long Covid is essential so that someday no one needs to care about Covid and its lasting effects.
Long Covid feels like living with a gun to my head. Please pull the trigger on the moonshot.
Rachel Hall-Clifford, Ph.D., is an assistant professor of global health, human health, and sociology at Emory University in Atlanta.
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the author reprints, rachel hall-clifford.
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

To get basic standard addiction treatment, Americans should move to Canada

Eating rocks: The case for early integration of medical ethics into AI product development

STAT Plus: Top FDA official Peter Marks overruled staff, review team to approve Sarepta gene therapy

STAT Plus: What the Sarepta decision means for Duchenne patients, the company, and the FDA

STAT Plus: A primer on HELIOS-B, an enormous stock-moving event for Alnylam Pharmaceuticals
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS AND VIEWS
- 19 June 2024
First encounter with SARS-CoV-2: immune portraits of COVID susceptibility
- Benjamin Israelow ORCID: http://orcid.org/0000-0002-1308-8246 0 &
- Akiko Iwasaki ORCID: http://orcid.org/0000-0002-7824-9856 1
Benjamin Israelow is in the Department of Internal Medicine, Section of Infectious Diseases, Yale School of Medicine, Yale University, New Haven, Connecticut 06510, USA, and the Center for Infection and Immunity, Yale School of Medicine.
You can also search for this author in PubMed Google Scholar
Akiko Iwasaki is at the Center for Infection and Immunity, Yale School of Medicine, Yale University, New Haven, Connecticut 06510, USA, the Department of Immunobiology, Yale School of Medicine and at the Howard Hughes Medical Institute, Chevy Chase, Maryland, USA.
More than four years after the start of the COVID-19 pandemic, and the mass mobilization of the scientific community, key questions remain about why some people get infected and others don’t. What early immune responses are required to control infection, and which responses are associated with uncontrolled infection and disease? Writing in Nature , Lindeboom et al . 1 tackle these queries head-on, using a controlled human infection model in which unvaccinated individuals who hadn’t previously encountered the SARS-CoV-2 virus were exposed to it. This work provides unprecedented insights into the dynamics of immune responses to exposure of the virus.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-024-01644-x
Lindeboom, R. G. H. et al. Nature https://doi . org/10.1038/s41586-024-07575-x (2024).
Article Google Scholar
Zhang, Q. et al. Science 370 , eabd4570 (2020).
Article PubMed Google Scholar
Bastard, P. et al. Science 370 , eabd4585 (2020).
Monk, P. D. et al. ERJ Open Res. 9 , 00605-02022 (2023).
Kalil, A. C. et al. Lancet Respir. Med. 9 , 1365–1376 (2021).
Davoudi-Monfared, E. et al. Antimicrob. Agents Chemother. 64 , e01061-20 (2020).
Policard, M., Jain, S., Rego, S. & Dakshanamurthy, S. Virus Res. 301 , 198464 (2021).
Saichi, M. et al. Nature Cell Biol. 23 , 538–551 (2021).
Rudy, G. B. & Lew, A. M. J. Immunol. 158 , 2116–2125 (1997).
Rowe, J. R., Neme de Gimenez, M. H., Emler, C. A. & Sheehy, M. J. Hum. Immunol. 29 , 256–262 (1990).
Pathi, A. et al. J. Rheumatol. 48 , 985–991 (2021).
Hill-Burns, E. M., Factor, S. A., Zabetian, C. P., Thomson, G. & Payami, H. PLoS ONE 6 , e27109 (2011).
Download references
Reprints and permissions
Competing Interests
The authors declare no competing interests.
Related Articles

See all News & Views
- Medical research
What causes long COVID? Case builds for rogue antibodies
News 13 JUN 24

‘Preposterous’: Anthony Fauci denies cover-up of COVID origins during tense hearing
News 03 JUN 24

Hope for global pandemic treaty rises — despite missed deadline
Gut microbiome discovery provides roadmap for life-saving cancer therapies
News 20 JUN 24

Could rats and dogs detect disease better than the finest lab equipment?
Outlook 19 JUN 24

Human neuroscience is entering a new era — it mustn’t forget its human dimension
Editorial 19 JUN 24

Oxylipins and metabolites from pyroptotic cells act as promoters of tissue repair
Article 26 JUN 24

Mini saunas save endangered frogs from fungal disease
News & Views 26 JUN 24

Hotspot shelters stimulate frog resistance to chytridiomycosis
Postdoctoral fellow – iPSC generation for modeling genetic disorders
Postdoctoral fellow – iPSC generation for modeling genetic disorders – Mayo Clinic
Rochester, Minnesota (US)
Mayo Clinic
Staff Scientist - Proteomics
Houston, Texas (US)
Baylor College of Medicine (BCM)
Staff Scientist - Immunology
Three multidisciplinary postdoctoral scholarships (2 years).
The Integrated Science Lab (IceLab) offers three postdoctoral scholarships that will be affiliated with one of six possible multidisciplinary projects
Umeå, Sweden
Umeå University (KBC)
Postdoctoral Researcher in the field of Computer Simulation of Magnetic Materials
Elucidation of magnetic properties such as coercivity mechanism by employing statistical physical approaches. Theoretical research on permanent ma...
Tsukuba city, Japan (JP)
National Institute for Materials Science (NIMS)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Explore a collection of articles and other resources on the Coronavirus (Covid-19) outbreak, including clinical reports, management guidelines, and commentary.
SARS-CoV-2 is a positive-sense single-stranded RNA virus. It is contagious in humans and is the cause of the coronavirus disease 2019 (COVID-19). Controlled infection with SARS-CoV-2 of people who ...
During the early stages of the Covid-19 pandemic, reports emerged that persons who had been infected with SARS-CoV-2 were having lingering health problems. Such long-term issues were collectively r...
Long COVID is an often debilitating illness that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms have been identified with ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
Coronavirus (COVID-19) research. Medical, social, and behavioral science articles from Sage Sage believes in the power of the social and behavioral sciences to convert the best medical research into policies, practices, and procedures to improve - and even save - lives. This collection includes the latest medical research from Sage related ...
Long Covid may affect 10% or more of people infected with SARS-CoV-2. ... and others reflective of multi-organ-system involvement. 4,5 Although this categorization is helpful for research and ...
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO ...
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a zoonotic infection, is responsible for COVID-19 pandemic and also is known as a public health concern. However, so far, the origin of the causative virus and its intermediate hosts is yet to be fully determined. ... Articles from Journal of Research in Medical Sciences : ...
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 , and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data .
In their study in The Lancet Infectious Diseases, Wenting Zuo and colleagues collected tissue samples from 225 patients who had recovered from mild COVID-19 and found that SARS-CoV-2 viral RNA was distributed across ten distinct solid tissues, plasma, and blood cells up to 4 months after infection. Importantly, detection of viral RNA, and higher virus copy numbers, were significantly ...
The number of articles on COVID-19 steadily increased before 6 February 2020. However, they lack diversity and are almost non-existent in some study fields, such as clinical research. ... Furthermore, our large sample size is sufficient to illustrate the state of research and identify research gaps related to COVID-19 at the onset of the ...
To mitigate research issues arising from the pandemic, research institutions strongly reinforced techniques allowing online work and collaboration by video-conferencing. For example, new portals for sharing scientific data, such as the European COVID-19 Data Portal, emerged, and conferences and workshops were held online (Korbel and Stegle 2020 ...
COVID-19 was—and to a large extent remains—the most meaningful health event in recent global history ().Unlike the 2003 Severe Acute Respiratory Syndrome (SARS) epidemic, it spread globally; unlike Zika, everyone is at risk of infection with COVID-19; and unlike recent swine flu pandemics, the disease severity and mortality from COVID-19 were so high it led to life expectancy reversals in ...
A centralised hub of all the latest covid-19 blogs and podcasts posts from BMJ's 70 specialty journals. All posts are freely available and you can search by subject area or journal. BMJ has created this coronavirus hub to support healthcare professionals and researchers dealing with covid-19. It includes practical guidance, latest news and ...
Browse our 25 most downloaded COVID-19 articles published in 2021. ... These papers highlight valuable research into the biology of coronavirus infection, its detection, treatment as well as into ...
Find COVID-19 datasets, data tools, and publications to use in research. EXPLORE COVID-19 DATA. Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines.
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on ...
The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database. Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide. The WHO Covid-19 Research Database was a resource created in response to the Public Health ...
COVID-19 Resource Centre. As of January 2024, changes have been made to our COVID-19 Resource Centre. A COVID-19 Collection is available where you can continue to explore and access The Lancet Group's COVID-19 research, reviews, commentary, news, and analysis as it is published.
The announcement earlier this month from the National Academies of Science, Engineering and Medicine of a consensus definition of long COVID mentioned that it was not the final word on this condition but that the definition would be revised as new findings are published.. Earlier this year, researchers at UB and the Department of Veterans Affairs who were using big data to work on a data ...
The federal government sent nearly $200 billion to U.S. schools in the past few years to help address Covid-era learning challenges. Now the first studies are out showing what the money ...
There have been around 96,000 reported cases of coronavirus disease 2019 (COVID-2019) and 3300 reported deaths to date (05/03/2020). The disease is transmitted by inhalation or contact with infected droplets and the incubation period ranges from 2 to 14 d. The symptoms are usually fever, cough, sore throat, breathlessness, fatigue, malaise ...
11 January — Traitorous antibodies are linked to COVID death. Antibodies normally attack pathogens, but, sometimes, rogue antibodies instead besiege bodily components such as immune cells. Now ...
Several new COVID variants are likely contributing to the summer spike in cases, Dr. Dan Barouch, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center ...
The Long Covid Moonshot is a collective advocating for $1 billion in annual research funding for long Covid, akin to the Operation Warp Speed that enabled the first generation of Covid-19 vaccines.
Greene accused Fauci of presiding over science that was "disgusting and evil". She questioned Fauci over the royalties received by National Institutes of Health scientists for their research. Fauci had earlier denied receiving any such royalties for his work on COVID-19.
The advent of the open-access movement in science publishing in the past 20 years was meant to make the results of scientific work more widely accessible, and to a substantial degree that goal has ...
By unravelling the mysteries of early immune responses, the study offers promising avenues for future research and therapeutic development in the ongoing fight against COVID-19.