Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 February 2023

Deep learning ensemble 2D CNN approach towards the detection of lung cancer
- Asghar Ali Shah 1 ,
- Hafiz Abid Mahmood Malik 2 ,
- AbdulHafeez Muhammad 1 ,
- Abdullah Alourani 3 &
- Zaeem Arif Butt 1
Scientific Reports volume 13 , Article number: 2987 ( 2023 ) Cite this article
8337 Accesses
28 Citations
1 Altmetric
Metrics details
- Biotechnology
- Computational biology and bioinformatics
- Health care
In recent times, deep learning has emerged as a great resource to help research in medical sciences. A lot of work has been done with the help of computer science to expose and predict different diseases in human beings. This research uses the Deep Learning algorithm Convolutional Neural Network (CNN) to detect a Lung Nodule, which can be cancerous, from different CT Scan images given to the model. For this work, an Ensemble approach has been developed to address the issue of Lung Nodule Detection. Instead of using only one Deep Learning model, we combined the performance of two or more CNNs so they could perform and predict the outcome with more accuracy. The LUNA 16 Grand challenge dataset has been utilized, which is available online on their website. The dataset consists of a CT scan with annotations that better understand the data and information about each CT scan. Deep Learning works the same way our brain neurons work; therefore, deep learning is based on Artificial Neural Networks. An extensive CT scan dataset is collected to train the deep learning model. CNNs are prepared using the data set to classify cancerous and non-cancerous images. A set of training, validation, and testing datasets is developed, which is used by our Deep Ensemble 2D CNN. Deep Ensemble 2D CNN consists of three different CNNs with different layers, kernels, and pooling techniques. Our Deep Ensemble 2D CNN gave us a great result with 95% combined accuracy, which is higher than the baseline method.
Similar content being viewed by others
A whole-slide foundation model for digital pathology from real-world data
A guide to artificial intelligence for cancer researchers

Mapping the landscape of histomorphological cancer phenotypes using self-supervised learning on unannotated pathology slides
Introduction.
Deep learning and machine learning algorithms provide state-of-the-art results in almost every field of life, including wireless sensor networks, order management systems, semantic segmentation, etc 1 , 2 , 3 . It hugely impacts bioinformatics, specifically cancer detection 4 , 5 . Cancer is a disease with the most death toll. It is the most dangerous disease ever known to humans. Cancer is still not curable as the people suffering from it come to know about it in the later stages. It is complicated to detect it at an early stage, and more cancer-related deaths are mostly lung cancer. Therefore, significant research has been conducted to develop a system that can detect lung cancer from CT scan images 6 . It is challenging to prevent cancer as it shows signs in the later stages where it is impossible to come out of it. So, people can only do a regular checkup every six months, especially those who drink and smoke. This study aims to develop a state-of-the-art system for the early detection of lung nodules using the latest proposed ensemble deep learning framework.
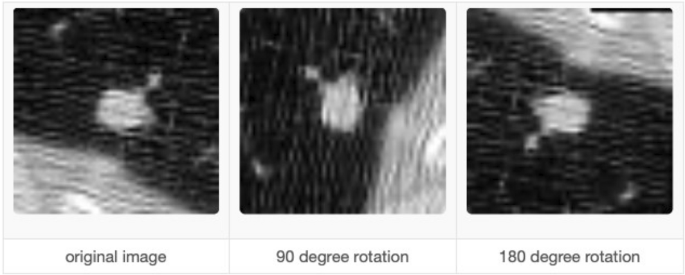
According to the latest report of the World Health Organization, death caused by Lung Cancer has moved from the top 9 to the top 6 in the list of diseases that cause the most significant number of deaths 7 . Lung Cancer has different types: small cell lung cancer and non-small cell Lung Cancer 8 . Figure 1 explains the CT Scan images used to detect the presence of a Lung Nodule, a cancer tumor. All tumors are not cancerous; the primary tumor types are Benign, Premalignant, and Malignant 6 .
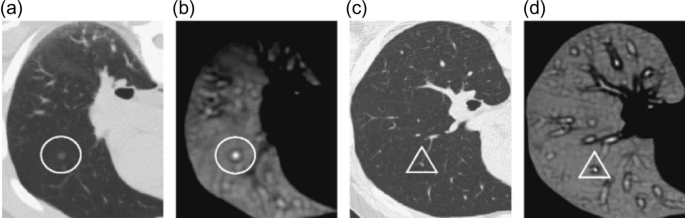
CT scan images show lung nodules with different locations and shapes in CT.
In this research, we have used a supervised deep learning model CNN because we need to classify the result as cancerous or non-cancerous. The simplest definition of understanding deep learning is, It learns from examples. It works like our brain works to learn from examples. For the concerns mentioned above related to Lung Nodule detection for early diagnosis of Lung Cancer, an ensemble of 2D CNN approaches has been developed to detect Lung Nodules. The data set used in this research is LUNA 16 Grand Challenge. Medical Sciences is one of the industries becoming an active part of practicing different machine learning and deep learning-based computerized automated software to balance the workload. With high-performance computing coming into the big picture, deep learning is becoming an active part of the research industry. The most critical role any deep learning model can play is to increase the system's efficiency, quality, and diagnosis to detect certain diseases more accurately and way before time to improve treatments and clinical results. Medicine and Health care are witnessing more implications if these deep learning and machine learning-based systems increase the accuracy of prediction and detection of diseases. Cancer is one of the most important parts of clinical research now a day's due to its high death rate and fewer chances of cure. Early detection of different cancer types can help in reducing the number of deaths all over the world. For the concerns mentioned earlier related to Lung Nodule detection for early diagnosis of Lung Cancer, an ensemble of 2D CNN approach has been developed to detect Lung Nodules. The data set used in this research is LUNA 16 Grand Challenge.
An Ensemble approach has been developed to help detect Lung nodules because it is tough to differentiate between a Lung Nodule and a Lung Tissue. For this purpose, a more accurate model should be developed to distinguish between the Lung Nodule Candidate and the actual Lung Nodule. Primarily the main issue faced by any researcher is the acquisition of relevant annotations/labeled image data instead of the availability of image data. All Free-text reports based on radiologists' findings are stored in the format of the PACS system. So, converting all these reports into more appropriate and accurate labeling of data and structural results can be a daunting task and requires text-mining methods. These text-mining methods themselves are an essential field of study. Deep learning nowadays is also widely used with text mining. In this regard, developing a structured reporting system will benefit Machine and Deep Learning objectives. This development can lead to the improvement of radiologic findings, and the patient care CAD system can help radiologists take the responsibility of more than one doctor. The Lung Nodule detection process includes a detailed inspection of Nodule Candidates and True Nodules. Lung Nodule candidates consist of true and false nodules resembling true ones. So, a classification system should be developed to select true nodules among all possible candidate nodules. Two challenges need to be addressed with more attention to establishing such nodules to detect true nodules.
Non-Nodules are highlighted, and some nodules are ignored in the CT scan, which is the radiological heterogeneity. It can lead to increased difficulty in differentiating between nodules and non-nodules. Nodules are in different sizes and different shapes. Larger nodules have a better tendency to be detected by the system, whereas small nodules have fewer chances, adding more to the challenges. Different shapes of a nodule are another factor that needs to be addressed by the model.
Related work
Many studies used deep learning and ensemble learning processes for classification problems 9 . The current CAD applications for Lung Cancer classifying lung nodules are very close to this paper's objective. Therefore, we researched the recently developed and state-of-the-art lung nodule classification techniques.
2D convolutional neural network
A two-dimensional CNN has been used to detect lung nodule presence in the CT scan. In 2D CNN, CNN only takes two dimensions. Around the image to get the main features and learn these features, CNN with a transfer learning approach was developed by Wangxia Zuo, Fuqiang Zhou, and Zuoxin Li 10 with MultiResolution CNN and Knowledge Transfer for Candidate Classification in Lung Nodule Detection. Image-wise calculation with CNN and different depth layers applied for Lung Nodule classification on Luna 16 Data Set to improve the accuracy of Lung Nodule Detection with 0.9733 Accuracy. Sanchez and Bram van Ginneken 11 developed CAD system for' pulmonary nodules using multi-view convolutional networks for False Positive Reduction. MultiView-KBC was developed for Lung Nodule Detection by Yutong Xie, Yong Xia, Jianpeng Zhang, Yang Song, Dagan Feng, Michael Fulham, and Weidong Cai 12 , which is based on Knowledge-based Collaborative Deep Learning for Benign-Malignant Lung Nodule Classification on Chest. Siddharth Bhatia, Yash Sinha, and Lavika Goel present a deep residual learning approach using CT Scan for cancer detection 13 . ResNet 14 and UNet models are used for feature extraction in this method. Machine learning algorithms XGBoost and RF (Random forest used to classify cancerous images. The accuracy of this model was 84%. The research proposed by Muhammad Imran Faisal, Saba Bashir, Zain Sikandar Khan, and Farhan Hassan Khan uses machine learning and ensamble learning methods to predict lung cancer through early symptoms. This study use different machine learning algorithms, including MLP (multilayer perceptron) 15 , SVM (Support vector machine) 16 , Naïve Bayes, and Neural network for the classification of lung cancer. The dataset used for this study is extracted from UCI repository. The accuracy of the ensemble learning method for the proposed study was 90% 17 .
3D convolutional neural network
Same as 2D CNN, but in this 3-Dimensional CNN, CNN considers three dimensions while learning the features like x, y, and z. Two sides are considered at once, like x and y, y and z, and z and x. False-Positive Reduction in Lung Nodules Detection using Chest Radiographs by an Ensemble of CNN was developed by Chaofeng Li, Guoce Zhu, Xiaojun Wu, and Yuanquan Wang 18 . For false positive reduction on Chest Radiographs with a fivefold cross-validation Multilevel contextual Encoding to detect the variable size and nodule shapes developed by Qi Dou, Hao Chen, Lequan Yu, Jing Qin, and Pheng-Ann Heng 19 . An Architecture developed to reduce the number of False Positives achieved 87% sensitivity with four false positives/scans. Qing Wu and Wenbing Zhao proposed a novel approach to detecting Small Cell Lung Cancer, and they suggested the entropy degradation method (EDM) for detecting Small Cell Lung Cancer. Due to the data set limitations, they developed their novel neural network, which they referred to as (EDM). They used 12 data sets: 6 were healthy, and six were cancerous. Their approach gave 77.8% accurate results in detecting Small Cell Lung Cancer. Wasudeo Rahane, Himali Dalvi, Yamini Magar Anjali Kalane, and Satyajeet Jondhale 20 used Machine Learning techniques to detect Lung Cancer with the help of Image Processing . Data were pre-processed with different image processing techniques so the machine learning algorithm could use it; a Support Vector Machine for the classification was used. Allison M Rossetto and Wenjin Zhou 21 give an approach to Convolution Neural Networks (CNN) with the help of multiple pre-processing methods. Deep learning played a significant role in this research. The implementation of CNNs did the accuracy of automated labeling of the scans. The results showed consistently high accuracy and a low percentage of false positives.
As discussed in the above section, none of the studies use an ensemble learning approach of machine learning or deep learning to identify the lung nodule. The main issue of the previous results was the improper or small dataset for the detection taken from minimum subjects. The above section clearly shows that the accuracy of detection with more machine learning or deep learning algorithms is very low. The current proposed study is going to cover these loopholes of the studies.
Proposed method
The previously presented studies had an issue with the ensemble learning approach. All the studies presented in the past did not use an ensemble learning approach of deep learning algorithms for lung cancer identification. As the ensemble learning approach gives the best average accuracies, this study will cover the loophole of the previous studies by using the ensemble learning approach on CNN algorithms using CT images taken from LUNA 16 dataset. A final solution Deep Ensemble 2D CNN is developed with the help of the Deep Learning Algorithm 22 to detect Lung Nodules from CT Scan images. It is imperative to select which model should be used to detect Lung Cancer with the help of Deep Learning. Here, the Supervised Deep Learning Algorithm 2D CNN is used to detect lung nodules. This section explains every step of the Deep Ensemble 2D CNN model that performs to get the best results and help develop a CAD system for Lung Nodule Detection. The idea of this Ensemble CNN with different CNN blocks is to get the correct features, which are very important to classify a true nodule among candidate nodules. In the end, we have calculated Accuracy, Precision, and recall using the formula below 23 , 24 .
In these equations, TPV is the true positive value, TVN is the True negative value, FPV is the False positive value, and FNV is the False-negative value 25 , 26 .
The step-by-step working of the model is explained as.
Access the dataset from Luna 16.
Data pre-processing (Data Balancing, Plotting, Data Augmentation, Feature extraction)
Splitting the dataset into training and testing data.
Applying Deep 2D Neural Network to the training and testing dataset.
Combine the prediction of Deep 2DNN.
Final Prediction of Lung cancer.
Figure 2 describes the research paradigm for the proposed model.
The architecture of the proposed methodology.
Data collection
The crucial step in every research is the collection of data, as collecting the correct data helps get better results. The first step is organizing the enormous data set of CT Scan images. A Data set of CT Scan images were collected from LUNA 16 Data set which has helped to get the research completed 27 . It is essential to collect high-quality data so that the machines can understand the data easily. All CT Scan images are the same quality in showing the reports to any doctor. Images in the LUNA Data set were formatted as (.mhd) and (.raw) files. The .mhd files contained the header data, and the raw files had multidimensional image data. We used the SimpleITK python library to pre-process all these images to read all .mhd files.
Data pre-processing
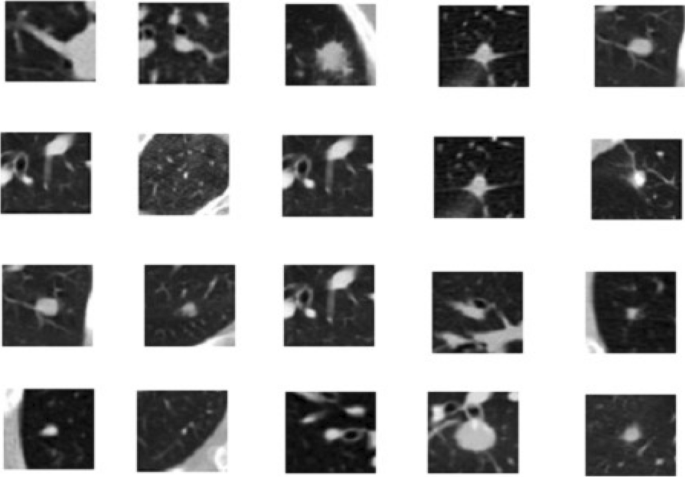
The next step in the proposed solution is data pre-processing. It is a critical step in which data is converted into a more understandable form, making it very easy to understand and process by the machines 28 , 29 . It is the most vital step to transform data into the desired format so that devices can better understand it. All the CT scans in LUNA 16 Data set consisted of n 512 × 512 axial scans with 200 images in each CT scan. Only 1351 were positive nodules in these annotations, and all others were negative. There was an imbalance between the two classes, so we need to augment the data to overcome this issue. We can train the CNN model on all original pixels, increasing the computational load with training time. Instead, we decided to crop all images around the coordinates provided in the annotations. Figure 3 explains the dropped CT scan image from the dataset.
Cropped CT scan images.
Furthermore, all the annotations provided in LUNA 16 Data set were in Cartesian coordinates. All these were converted to voxel coordinates. The image intensity in the dataset was defined in the Hounsfield scale. All these must be changed and rescaled for image processing purposes. All the images in the dataset belong to two classes which are positive and negative. Nodule Candidates with categories marked as 1 were positive and those with types marked as 0 were negative. Image Labels were created according to positive and negative. So finally, these label data can be used for training and testing.
It is usually in the format of Dicom images or MHD/Raw files. Before feeding data into any machine learning or deep learning model, it is crucial to converting the data into the required format so that machines can use it to understand and learn from it. Figure 4 shows the plotted image for the proposed system.
Input images to the proposed methodology.
Converting data into JPEG images
The next step is to convert all the pre-processed data into Jpeg format so that computers can understand it. Jpeg format is human readable, and humans can verify whether all the images are in the desired format, which can be seen and viewed easily in Jpeg format. Furthermore, the data was converted into small 50 × 50 images so that it would reduce the size of the data and it will consume less computing power. Hue data size consumes a lot of computing power, so to overcome this issue, images were reduced to 50 × 50.
Data augmentation
It is imperative to augment the data when there is an imbalance issue. Manual data augmentation is done because data was not balanced. Data augmentation 30 helps in this regard so that it rotates the images in all possible directions and makes a copy of them. This way, you can create more copies of the same data from a different angle, which helps solve the data imbalance issue. We also used Keras Image Data Generator for image pre-processing and data augmentation. Keras Image Augmentation will zoom in and out to learn more about image data shear range to flip an image. These are critical steps so that the data is possibly processed in all possible ways so machines can learn the data in each possible way.
Split the data set into training and testing
The next important thing is splitting the data into testing and training or training and validation data. In this way, we can give machines the data to train and then provide the validation data to check the accuracy of our model. Reading the candidate's data from the CSV file and then splitting the cancerous and non-cancerous data so it can be correctly labeled. Making separate folders of cancer and non-cancer files is essential so that machines can learn what these files are and train. Training data is the data the artificial neural network and CNN will understand so they can learn more about the data and learn from it. It is a significant step to split the data so some portion of the data can be used for training. The next important thing is to give the test data to the artificial neural network and CNNs so the results can be generated and detect Lung Cancer form the CT Scan images can be done with the test data. Test Data is the actual data on which the algorithm's accuracy will be checked. If the result's accuracy is as required, then the results will be noted. If the results are not up to the mark, then some changes will be made to the layers in artificial neural networks and CNN to get more accurate results.
Deep ensemble 2D convolutional neural network
Figure 5 explains the different layers of the CNN Model. A final solution Deep Ensemble 2D CNN is developed with the help of the Deep Learning Algorithm to detect Lung Nodules from CT Scan images. It is essential to select which model should be used to detect Lung Cancer with the help of Deep Learning. This section explains every step our Deep Ensemble 2D CNN model will perform to get the best results and help develop a CAD system for Lung Nodule Detection. The idea of this Ensemble CNN with different CNN blocks is to get the correct features, which are very important to classify a true nodule among candidate nodules.
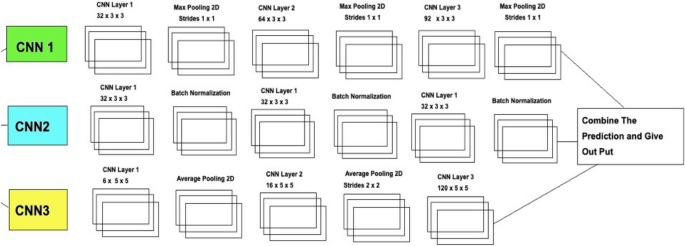
Deep ensemble 2D CNN architecture.
A Deep Ensemble 2D CNN Architecture was designed for an effective Lung Nodule Detection CAD system. A total of 3 2D CNNs have been designed and developed with different layers and pooling techniques. Each CNN in Deep Ensemble 2D CNN architecture has a different number of feature maps kernels with Max Pooling, Average Pooling, and Batch Normalization. Convolutional Layers in CNN Architecture do the feature extraction work. Each kernel convolves on the input and extracts the main features, which will help make output features later used for learning.
Keeping that in mind, we designed a Deep CNN model with more depth layers with a different number of feature maps, which will help extract true nodules among nodule candidates. The first layer in this Deep Ensemble, 2D CNN architecture, has 32 feature maps and 6 in the third CNN to learn the features of nodules with 3 × 3 and 5 × 5 kernel sizes. As the layers go deeper, we increase the number of feature maps with the same kernel size. As the neural network grows with more layers, more memory blocks are created to store the information, which helps to decide the nodule. Each CNN in this Deep Ensemble 2D CNN has a different number of layers and kernels.
Furthermore, in CNN, Maxpooling 31 is used to get the maximum value from the pooling layer filter. In the 2nd CNN, batch normalization is used, and in the third CNN, Average Pooling is utilized to get the average of all values. Furthermore, more depth layers were added to increase the accuracy and tuning of the architecture to overcome the over-fitting issues. More layers were introduced into this architecture to increase the efficiency of this model. This Deep Ensemble 2D CNN will help get more accurate modular features and minimize the false positives in the true nodules. In the end, the predictions of all three CNN will be combined to make a more accurate model. Using these predictions final confusion matrix was developed, which gave good results. Figure 6 illustrates the CNN architecture.
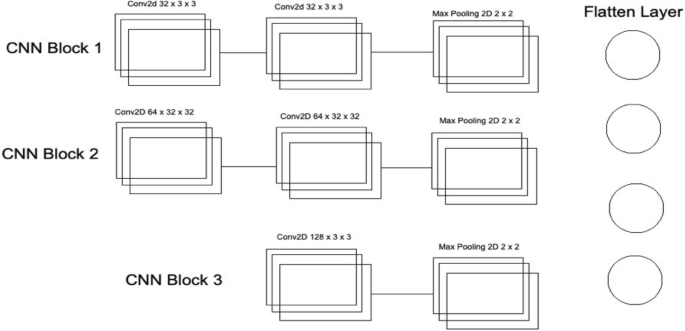
Convolutional neural network one architecture.
As mentioned in Deep Ensemble 2D CNN, this architecture is developed by developing and combining three different CNNs. This section explains the architecture of each CNN with several layers of each CNN. In CNN1 Architecture, three blocks of CNN are developed with a different number of layers and feature maps. The first CNN block has the 1st input layer of CNN, which uses a 3 × 3 kernel with 32 feature maps. In the first layer, RELU 32 is used as an activation function. The input size is given in the first layer, and we have used the same image size, 50 × 50, and RGB channels as 3. Moving into the further hidden layers, in this first block of CNN, the next CNN layer has the same 32 feature maps with the same kernel size of 3 × 3. At the end of this 1st block, a Max Pooling 2D function will sub-sample the data. Our Max pooling filter size is 2 × 2, which will convolve on the data extracted by the feature maps, and it will use a 2 × 2 filter and get the maximum value from the data. This first CNN block is essential to get the features of the nodule and non-nodules. It will extract the main features that help to distinguish between the nodule candidate and the true nodule. Moving into the 2nd of CNN, the number of feature maps increased with 64 feature maps and kept the kernel size same to 3 × 3. The activation function is the same as the above layers, RELU, and the next CNN layer has the same number of feature maps and kernel size. Moving forward toward the Max Pooling layer in this 2nd block, there is the same Max Pooling as above, which is 2 × 2. In the last and third block of CNN, only one layer of CNN has 128 feature maps with the same kernel size, which is 3 × 3, and the Max Pooling layer is the same as above, which has a 2 × 2 size. In this 3rd block of CNN, we have a dropout rate of 0.1, meaning 10% of the neurons will be dropped in this layer to increase accuracy and avoid over and under-fitting issues.
After the above CNN blocks, a Flatter layer will convert our CNN model into a one-dimensional layer, converting it into a pure ANN form. Then dense layers are added to make the architecture into a complete ANN form. In this layer, there is a dropout rate of 20. In the last layer, we have output dim as one because we need to predict only one result as nodule or non-nodule. Sigmoid is used as the activation function because we need binary output, not categorical, so Sigmoid is the best choice to predict the binary result 33 . Figure 7 illustrates the conversion of the CNN model array to flatten the layer.
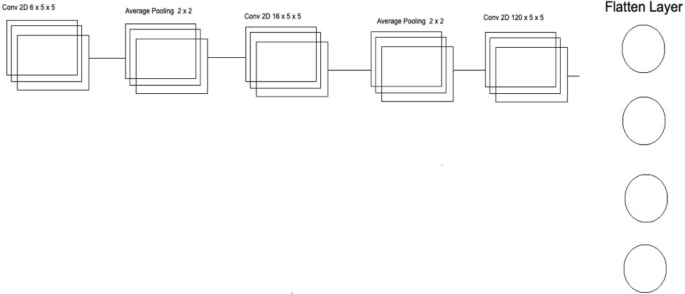
Convolutional neural network two architecture.
The second CNN in Deep Ensemble 2D CNN Architecture is different in structure as a different number of layers and batch normalization is used instead of Max Pooling to sub-sample the data. In the very first layer of CNN, we used 5 × 5 size of kernels with six feature maps only instead of 3 × 3 because we tried to develop a different CNN as compared to the very first so we could know which feature map size could help get a more accurate result.
With strides of 1 × 1, the kernel filter will move one by one, and for activation, we have used RELU like the first CNN model. The exact size of 50 × 50 is used with three RGB channels for input shape. After the first layer, Average Pooling is utilized instead of Max Pooling. Average pooling works the same way as Max Pooling, but the calculation differs. In Max Pooling, we get the maximum value, and in Average Pooling, an average of data is calculated inside the feature kernel used to subsample the data. This Average Pooling uses a 2 × 2 size of kernel and strides of 1 to move the filter one by one. After the Average Pooling layer, there are some more hidden layers of CNN. The second Layer of CNN has 16 Feature maps with the same filter size of 5 × 5, keeping the stride the same to move one by one.
After this layer, there is another layer of Average Pooling. In this pooling layer, the same filter size 2 × 2 is there, but this time there is the strides of 2, which means our filter will move two steps instead of the traditional one-step movement. We need to get the features in every possible way and help our network to get the components in every possible way and learn from them. What features can it get by moving only one step, what features will it get by moving two steps each time, and how much will it help to understand the data better. In the last and third layers of CNN, we have used the same kernel size, which is 5 × 5 with 120 feature maps, and keeping the strides to 1. After this last layer, we have a flattened layer that will convert the CNN layers into one-dimensional ANN Architecture. After that, the traditional ANN is used to learn from CNN and classify the data. In the last layer, the Sigmoid activation function is utilized. Our results are binary, as we need to predict only nodules and non-nodule. If there is a need to predict more than two for any categorical data, SoftMax is a good option, as explained in Fig. 8 .
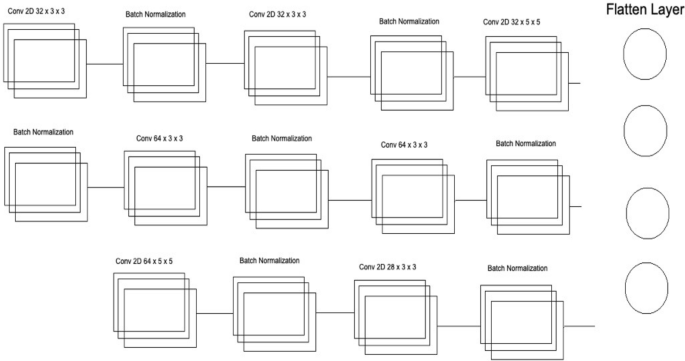
Convolutional neural network three architecture.
Several layers and feature maps have been used in the last and third CNN Models of this Deep Ensemble 2D CNN Architecture. This CNN model uses three layers with 32 feature maps and a kernel size of 3 × 3. In the first layer, the input shape of the data is the same as the image size, which is 50 × 50, and the activation function used is RELU. 2 Layers of CNN have a 3 × 3 filter size, and the third layer has a 5 × 5 size. The dropout rate in this set of CNN layers is set to 0.4 after three layers of CNN, meaning 40% of neurons will be dropped. In this architecture of CNN, no average or max pooling is used. Instead, batch normalization has been used to increase the learning rate of the mode. In the hidden layers of this CNN model, three layers of CNN with 64 feature maps and 3 × 3 kernel size have been used. In the last layer of this CNN block, a 5 × 5 kernel size is used. After this block of CNN, the dropout rate is added to 0.4, which means 40% of the neurons will be dropped. Moving forwards in the third section of this CNN model, there is one layer of CNN with feature maps of 128 and kernel size of 3 × 3. After filtering this last layer, we have a flattened layer, which will convert the CNN layers into one-dimensional ANN Architecture. Later, the traditional ANN is used to learn from the CNN and classify the data.
Our Deep Ensemble 2D CNN used RELU as the activation function. Rectified Linear Units (RELUs) are a well-known and mostly used activation function in our proposed CNN model. A study from Krizhevsky et al. 34 showed that RELUs enable the network to train several times faster than using the units in deep CNN. RELU is used for Input Layers and other multi-hidden layers in our Deep Ensemble 2D CNN.
As mentioned earlier, we used the Sigmoid activation function in the last layer 35 . Our results are binary, as we need to predict only nodules and non-nodule. If there is a need to predict more than two for any categorical data, then SoftMax is a good choice. Nonlinear Activation Functions make it easy for the model to adapt or generalize with a different type of data and differentiate between the output. Our classification task is the binary classification between nodule and non-nodule, so Sigmoid is the best choice for binary classification.
Moreover, we mainly use the Sigmoid function because it exists between 0 and 1. Therefore, it is primarily used for tasks where we must predict the probability as an output. Since the probability of anything exists between 0 and 1, Sigmoid is the right choice. The function is differentiable. That means we can find the slope of the Sigmoid curve at any two points. Figure 9 shows the working of the sigmoid function.
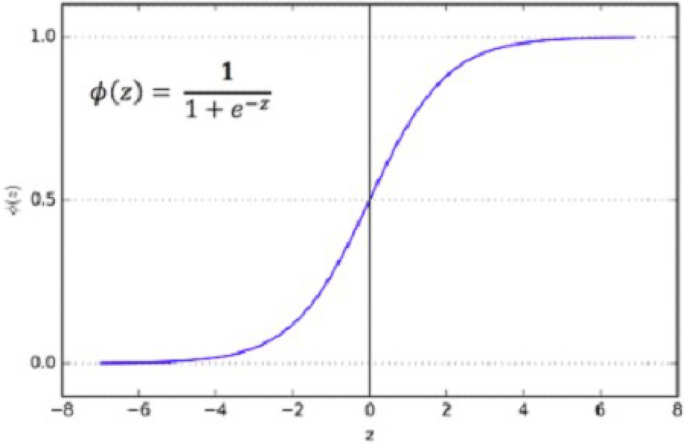
The Sigmoid curve at any two points.
Experimental results and analysis
After pre-processing the data in the correct format, the very next important is to check the data on our Deep Ensemble 2D CNN Architecture. In this regard, the whole data was segmented into training and validation data. Both data segments have cancer and non-cancer lung nodule files, so the CNN Model can get to know both data types while training.
This section uses Deep Ensemble 2D CNN architecture and a validation split of 10%, which will help to use 90% of the data as training and the remaining as validation. It helps to make models train and test at the same time. With 70 epochs set in the model fit generator, it will iterate the dataset 70 times.
Result of CNN1
This section explains how each CNN has performed on the data. In the first CNN model, we first ran it on training and validation data. After the results, the test data was given to the model to predict the outcome of the CNN. The first iterative model of CNN provides an accuracy of 94.5%, which would be considered excellent results according to AUC accuracy values 36 . Figure 10 explains the results.
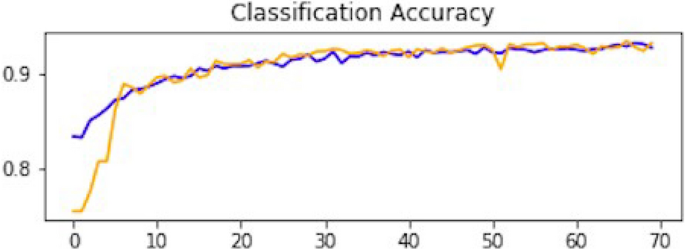
Accuracy curve of CNN1.
As mentioned, the model was compiled with 70 epochs 37 . Each epoch validation split divides 80% of the data into training and 20% of the data into validation. The training progress and epochs also show that the classification accuracy is increasing. At the same time, the loss of the model decreases rapidly at each iteration. The loss curve gives the result of 0.14 at the first iteration of CNN. The results of the Loss curve are described in Fig. 11 .
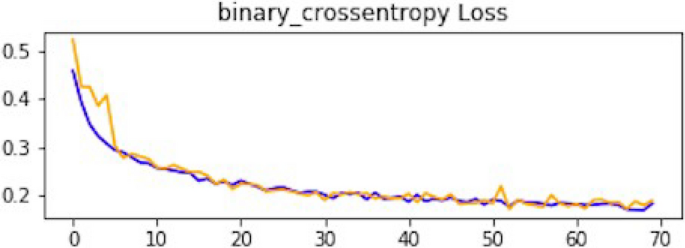
Loss curve of CNN1.
Table 1 explains the training accuracy and loss for the first CNN model.
It gradually decreased as the model got more and more training in each epoch, and in the end, only a fraction of 0.1891 was recorded. According to the above results, accuracy is not enough to judge the model's performance. Later, we gave our model some data to predict the results and had around 1600 images to predict. After the prediction was made, the next step was to check the accuracy of the predictions, and for this purpose, we made a confusion matrix 38 , 39 . Below are the confusion matrix results for the data used for the first CNN layer. Here Nodules and Non-Nodules are the values of the detected and non-detected lung cancer images explained in Fig. 12 . Figure 13 explains the ROC curve for the CNN model for the training dataset.
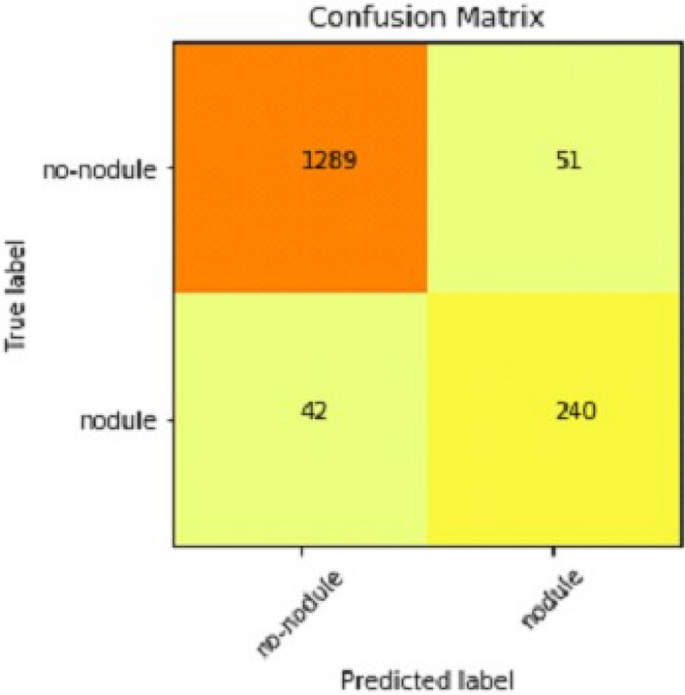
Confusion matrix of CNN1.
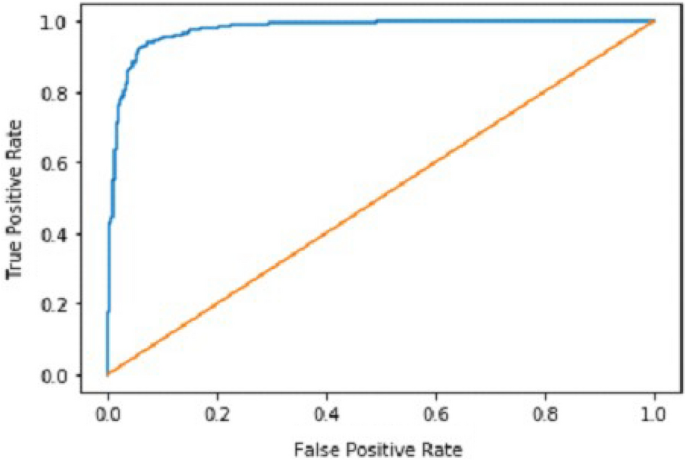
ROC curve of CNN1.
Result of CNN2
After the performance evaluation explanation of CNN1, moving forward in this section, it is explained how CNN2 has performed on the testing data. In the second CNN model, we first ran it on training and validation data. After getting the results, we gave this model the test data to predict the outcome of the CNN. The second model of CNN gave us some good results, which are stated below. The result also shows an accuracy of 0.93. The accuracy is gradually increasing from the first iteration to the last. Figure 14 explains the accuracy of CNN2.
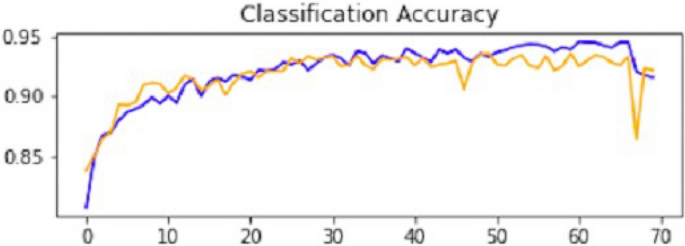
Classification accuracy of CNN2.
Table 2 shows that accuracy is insufficient to evaluate the model's performance. Afterward, we provided our model with information to forecast the outcomes and had roughly 1600 photos. The next step after making a prediction is to assess its accuracy, and a confusion matrix was created for this reason. The results of CCN2 for the testing images are explained in Fig. 15 . The ROC curve for the testing dataset is presented in Fig. 16 .
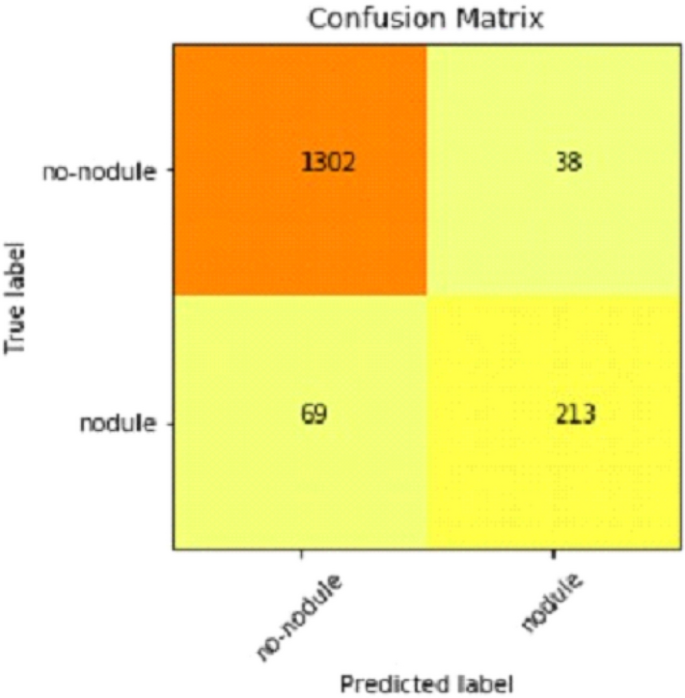
Confusion matrix of CNN2.
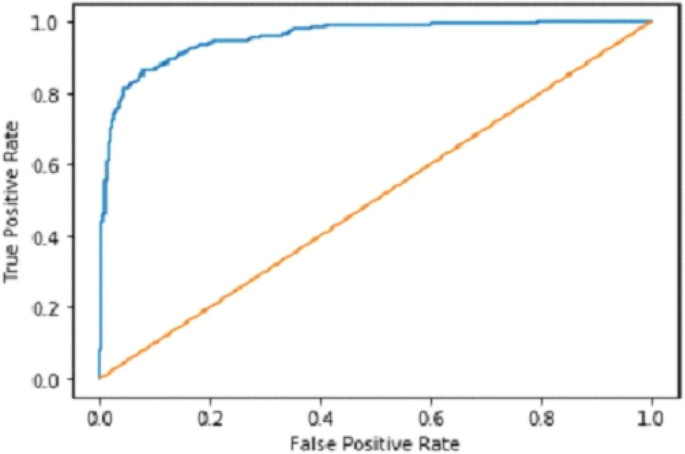
ROC curve of CNN2.
Result of CNN3
We explain how CNN3 fared on the testing data in the sections that follow the discussion of CNN2's performance evaluation. We first tested the third CNN model using training and validation data. After the outcomes, we provided this model with test data to forecast how the CNN would turn out. As a result, the CNN third model produced some promising results, which we have included in Table 3 . Figures 17 and 18 present the confusion Matrix and ROC curve for CNN3.
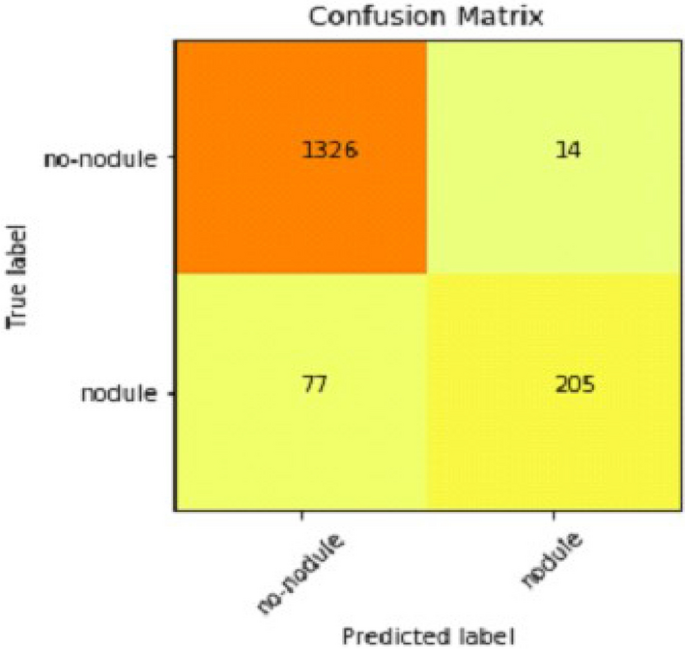
Confusion matrix of CNN 3.
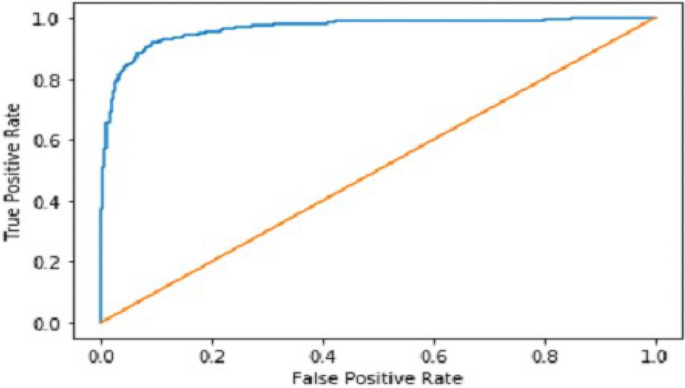
ROC curve of CNN 3.
After the above results, accuracy is not enough to judge the model performance. After this, we gave our model some data to predict the results, and we had around 1600 images to predict. After the prediction was made, the next step was to check the accuracy of the predictions, and for this purpose, we made a confusion matrix. Below are the results.
Combine results of all CNN (deep ensemble 2D CNN architecture)
After combining the prediction of all three CNNs, which we have designed especially for this Lung Nodule issue. We clearly can see from the confusion matrix that there is a difference in TP, TN, FP, and FN, which tells that combining all three CNN was an excellent choice to increase the accuracy and reduce the False Positives. The CNN architecture results are combined using the averaging method of deep ensemble learning 40 .
Our Deep Ensemble 2D CNN has three different CNNs, which achieve an accuracy of 90% and above. Our CNN1 attained an accuracy of 94.07%, CNN2 achieved an accuracy of 94.44%, and CNN3 attained an accuracy of 94.23%. Now we shall calculate the overall accuracy, precision, and recall of our Deep Ensemble 2D CNN from the confusion matrix. Table 4 illustrates the overall results of the CNN model. Figures 19 and 20 explain the combined confusion matrix and ROC curve results for the CNN model.
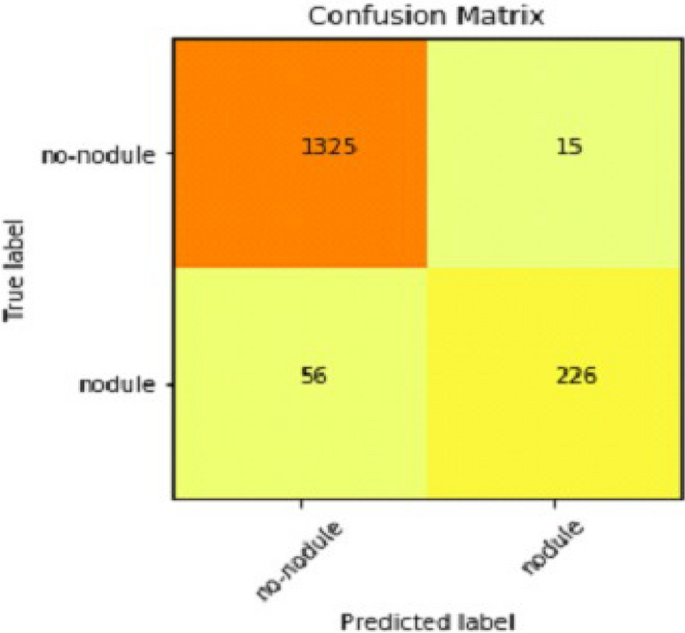
Confusion matrix of deep ensemble 2D CNN.
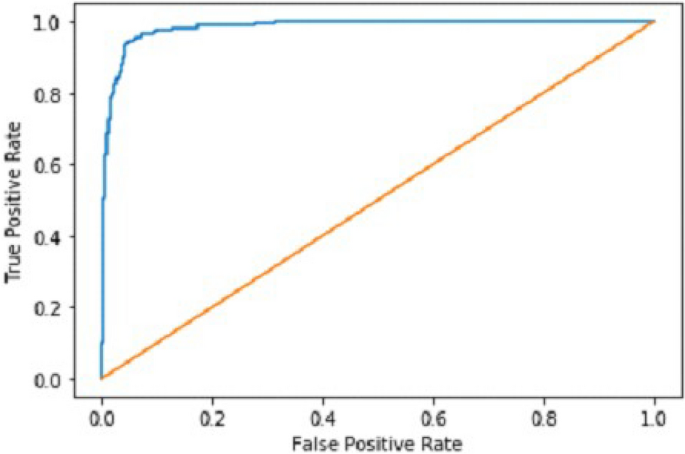
ROC curve of deep ensemble 2D CNN.
Comparison with other methodologies
Below we have stated the comparison between our proposed deep ensemble 2D CNN methodology and baseline methodology, which we considered in this approach to improve the accuracy and performance of the model. The comparison of the model with the base papers of the study is illustrated in Table 5 .
Table 5 compares the proposed study with the previously presented studies. Knowledge-based Collaborative Deep Learning 11 obtained the highest accuracy of 94% from the previous studies. An accuracy of 90% was obtained from the Ensemble learning method with SVM, GNB, MLP, and NN 17 . This was the base paper for the study. The study uses the ensemble learning approach for deep learning CNN model for the early identification of Lung cancer from LUNA 16. The proposed study gives an accuracy of 95% with an ensemble learning model considered the highest accuracy in deep learning and ensemble learning algorithms presented to date.
Gaps and future direction
Our Multilayer CNN is developed by focusing on a 2D Convolution Neural Network. In future work, 3D CNN should be used as 3D can get more spatial information. More data should be gathered to make the model more mature and accurate. An extensive data set will help train the model on a new data set, which will help make the model more accurate. More diverse data will enhance the performance of the model.
There is always room for improvement in any research conducted. There is no final product that has been developed for the detection of any cancer. There has been no international standard developed that will be followed for the detection and prediction of cancers. So, there is always considerable room to increase the accuracy of predictions and detections. More work for detecting and forecasting different cancers will lead to new openings and solutions for detecting cancer in the early stages.
Cancer is a hazardous disease related to a massive number of deaths yearly. Billions of dollars have been spent till now on the research of cancer. Still, no final product has been developed for this purpose. It shows the need for more work to understand the cause and make early predictions. This opens a new opportunity for researchers to develop a system or conduct research that will be very helpful in early cancer detection. If this Is made possible to detect cancer in the very beginning, it can help millions of people out there. There has not been a standard set or final output product which will be used for cancer detections. So, all the researchers should collect current and fresh data and then apply different deep learning and machine learning algorithms to detect and predict cancer. It is essential to use new and existing data, which will help us know whether these Deep Learning and Machine Learning models still give the same accuracy.
Every year a massive number of deaths are related to cancer which is increasing daily. Billions of dollars have been spent on the research of cancer. It is still an unanswered mystery that needs to be solved. Cancer research is still going on and will be going on and on because no final product has been developed. No specific standards set are used for the detection and prediction of cancer. Cancer research is an open question that needs to get more attention. The latest research on the current data set will open gateways for new research by giving some latest stats and inside stories of what we have achieved till now for the detection and prediction of cancer. It will help to understand some latest causes or signs of cancer.
Many previous studies were presented by the researchers for identifying lung cancer, as discussed in the related work section. The problem of their researchers was low accuracy, lass algorithms, and an inefficient dataset. The proposed study was developed to overcome the loophole of the previous study by using the Deep 2D CNN approach. Three CNN models are used for the proposed study CNN1, CNN2, and CNN3. The results of these three models are deeply explained in Tables 1 , 2 , and 3 . After that, the ensemble 2D approach of deep learning combines all these three deep learning methods. The ensemble deep learning method gives an accuracy of 95%, which is the recorded maximum value of any deep learning algorithm for identifying lung cancer to date. This study shows state-of-the-art results of an ensemble learning approach for identifying lung cancer from the image dataset. In the future, a system may be developed that uses many algorithms in ensemble learning with another extensive and efficient dataset for identifying lung cancer.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Hojjatollah Esmaeili, Vesal Hakami, Behrouz Minaei Bidgoli, M. S. Application-specific clustering in wireless sensor networks using combined fuzzy firefly algorithm and random forest. Expert Syst. Appl. Volume 210 , (2022).
Sohail, A. et al. A systematic literature review on machine learning and deep learning methods for semantic segmentation. IEEE Access https://doi.org/10.1109/ACCESS.2022.3230983 (2022).
Article Google Scholar
Ilyas, S., Shah, A. A. & Sohail, A. Order management system for time and quantity saving of recipes ingredients using GPS tracking systems. IEEE Access 9 , 100490–100497 (2021).
Shah, A. A., Ehsan, M. K., Sohail, A. & Ilyas, S. Analysis of machine learning techniques for identification of post translation modification in protein sequencing: A review. in 4th International Conference on Innovative Computing, ICIC 2021 1–6 (IEEE, 2021). doi: https://doi.org/10.1109/ICIC53490.2021.9693020 .
Shah, A. A., Alturise, F., Alkhalifah, T. & Khan, Y. D. Evaluation of deep learning techniques for identification of sarcoma-causing carcinogenic mutations. Digit. Heal. 8 , (2022).
Rahane, W., Dalvi, H., Magar, Y., Kalane, A. & Jondhale, S. Lung cancer detection using image processing and machine learning healthcare. In Proceedings of the 2018 Interanational Conference on Current Trends Towards Converging Technology. ICCTCT 2018 1–5 (2018) doi: https://doi.org/10.1109/ICCTCT.2018.8551008 .
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA. Cancer J. Clin. 71 , 7–33 (2021).
Article PubMed Google Scholar
Gilad, S. et al. Classification of the four main types of lung cancer using a microRNA-based diagnostic assay. J. Mol. Diagnostics 14 , 510–517 (2012).
Article CAS Google Scholar
Ghasemi Darehnaei, Z., Shokouhifar, M., Yazdanjouei, H. & Rastegar Fatemi, S. M. J. SI-EDTL: Swarm intelligence ensemble deep transfer learning for multiple vehicle detection in UAV images. Int. J. Commun. Syst. https://doi.org/10.1002/cpe.6726 (2022).
Zuo, W., Zhou, F., Li, Z. & Wang, L. Multi-resolution cnn and knowledge transfer for candidate classification in lung nodule detection. IEEE Access 7 , 32510–32521 (2019).
Setio, A. A. A. et al. Pulmonary nodule detection in CT images: false positive reduction using multi-view convolutional networks. IEEE Trans. Med. Imaging 35 , 1160–1169 (2016).
Xie, Y. et al. Knowledge-based collaborative deep learning for benign-malignant lung nodule classification on chest CT. IEEE Trans. Med. Imaging 38 , 991–1004 (2019).
Rao, G. S., Kumari, G. V., & Rao, B. P. Network for biomedical applications . vol. 2 (Springer Singapore, 2019).
Wang, W. et al. Exploring cross-image pixel contrast for semantic segmentation. In Proceedings of the. IEEE Int. Conf. Comput. Vis. 7283–7293 (2021) doi: https://doi.org/10.1109/ICCV48922.2021.00721 .
Ramchoun, H., Amine, M., Idrissi, J., Ghanou, Y. & Ettaouil, M. Multilayer perceptron: Architecture optimization and training. Int. J. Interact. Multimed. Artif. Intell. 4 , 26 (2016).
Google Scholar
Berwick, R. An Idiot's Guide to Support vector machines (SVMs): A New Generation of Learning Algorithms Key Ideas. Village Idiot 1–28 (2003).
Faisal, M. I., Bashir, S., Khan, Z. S. & Hassan Khan, F. An evaluation of machine learning classifiers and ensembles for early stage prediction of lung cancer. In 2018 3rd International Conference on Emerging Trends Engineering Science Technology. ICEEST 2018 1–4 (2019). https://doi.org/10.1109/ICEEST.2018.8643311 .
Li, C., Zhu, G., Wu, X. & Wang, Y. False-positive reduction on lung nodules detection in chest radiographs by ensemble of convolutional neural networks. IEEE Access 6 , 16060–16067 (2018).
Dou, Q. et al. 3D deeply supervised network for automatic liver segmentation from CT volumes. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 9901 LNCS , 149–157 (2016).
Al-Tawalbeh, J. et al. Classification of lung cancer by using machine learning algorithms. In IICETA 2022 - 5th Interantional Conference on Engineering Technology Its Applications 528–531 (2022). https://doi.org/10.1109/IICETA54559.2022.9888332 .
Gulhane, M. & P.S, M. Intelligent Fatigue Detection and Automatic Vehicle Control System. Int. J. Comput. Sci. Inf. Technol. 6 , 87–92 (2014).
Shrestha, A. & Mahmood, A. Review of deep learning algorithms and architectures. IEEE Access 7 , 53040–53065 (2019).
Yu, L. et al. Prediction of pathologic stage in non-small cell lung cancer using machine learning algorithm based on CT image feature analysis. BMC Cancer 19 , 1–12 (2019).
Shah, A. A., Alturise, F., Alkhalifah, T. & Khan, Y. D. Deep Learning Approaches for Detection of Breast Adenocarcinoma Causing Carcinogenic Mutations. Int. J. Mol. Sci. 23 , (2022).
Shah, A. A. & Khan, Y. D. Identification of 4-carboxyglutamate residue sites based on position based statistical feature and multiple classification. Sci. Rep. 10 , 2–11 (2020).
Article ADS Google Scholar
Mohammed, S. A., Darrab, S., Noaman, S. A. & Saake, G. Analysis of breast cancer detection using different machine learning techniques . Communications in Computer and Information Science vol. 1234 CCIS (Springer Singapore, 2020).
Chon, A. & Balachandar, N. Deep convolutional neural networks for lung cancer detection. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 9887 LNCS , 533–534 (2016).
Shamim, H. I., Shamim, H. S. & Shah, A. A. Automated vulnerability detection for software using NLP techniques. 48–57.
Guyon, I., Gunn, S., Nikravesh, M. & Zadeh, L. Feature extraction foundations. 1–8 (2006).
Chlap, P. et al. A review of medical image data augmentation techniques for deep learning applications. J. Med. Imaging Radiat. Oncol. 65 , 545–563 (2021).
Badrinarayanan, V., Kendall, A. & Cipolla, R. SegNet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 39 , 2481–2495 (2017).
Agarap, A. F. Deep learning using rectified linear units (ReLU). at http://arxiv.org/abs/1803.08375 (2018).
Naz, N., Ehsan, M. K., Qureshi, M. A., Ali, A. & Rizwan, M. Prediction of covid-19 daily infected cases ( worldwide & united states ) using regression models and Neural Network. 9 , 36–43 (2021).
Gonzalez, T. F. Handbook of approximation algorithms and metaheuristics. Handb. Approx. Algorithms Metaheuristics 1–1432 (2007) doi: https://doi.org/10.1201/9781420010749 .
Han, J. & Moraga, C. The influence of the sigmoid function parameters on the speed of backpropagation learning. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 930 , 195–201 (1995).
Cortes, C. & Mohri, M. AUC optimization vs. error rate minimization. Adv. Neural Inf. Process. Syst. (2004).
Marius-Constantin, P., Balas, V. E., Perescu-Popescu, L. & Mastorakis, N. Multilayer perceptron and neural networks. WSEAS Trans. Circuits Syst. 8 , 579–588 (2009).
Chicco, D. & Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21 , 1–13 (2020).
Visa Sofia, D. Confusion matrix-based feature selection sofia visa. Confusion Matrix-based Featur. Sel. Sofia 710 , 8 (2011).
Murray, I. Averaging predictions. 1–4 (2016).
Download references
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Majmaah University, Saudi Arabia, for supporting this work under Project number R-2023-16.
Author information
Authors and affiliations.
Department of Computer Sciences, Bahria University, Islamabad, Pakistan
Asghar Ali Shah, AbdulHafeez Muhammad & Zaeem Arif Butt
Faculty of Computer Studies, Arab Open University Bahrain, A’ali, Bahrain
Hafiz Abid Mahmood Malik
Department of Computer Science and Information, College of Science in Zulfi, Majmaah University, Al-Majmaah, Saudi Arabia
Abdullah Alourani
You can also search for this author in PubMed Google Scholar
Contributions
A.A.S. and H.A.M.M. envisioned the idea for research designed, wrote and discussed the results. A.M., Z.A.B., and A.A. worked on the literature and discussion section. All authors provided critical feedback, reviewed the paper, and approved the manuscript.
Corresponding author
Correspondence to Hafiz Abid Mahmood Malik .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shah, A.A., Malik, H.A.M., Muhammad, A. et al. Deep learning ensemble 2D CNN approach towards the detection of lung cancer. Sci Rep 13 , 2987 (2023). https://doi.org/10.1038/s41598-023-29656-z
Download citation
Received : 04 August 2022
Accepted : 08 February 2023
Published : 20 February 2023
DOI : https://doi.org/10.1038/s41598-023-29656-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Optimizing double-layered convolutional neural networks for efficient lung cancer classification through hyperparameter optimization and advanced image pre-processing techniques.
- M. Mohamed Musthafa
- I. Manimozhi
- Suresh Guluwadi
BMC Medical Informatics and Decision Making (2024)
Effective lung nodule detection using deep CNN with dual attention mechanisms
- Zia UrRehman
- Juanjuan Zhao
Scientific Reports (2024)
Assessing the efficacy of 2D and 3D CNN algorithms in OCT-based glaucoma detection
- Rafiul Karim Rasel
- Xiaoyi Raymond Gao
Blockchain security enhancement: an approach towards hybrid consensus algorithms and machine learning techniques
- K. Venkatesan
- Syarifah Bahiyah Rahayu
Are deep learning classification results obtained on CT scans fair and interpretable?
- Mohamad M. A. Ashames
- Ahmet Demir
- Cuneyt Calisir
Physical and Engineering Sciences in Medicine (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Standalone deep learning versus experts for diagnosis lung cancer on chest computed tomography: a systematic review
Affiliations.
- 1 Institute of Biophotonics, National Yang-Ming Chiao Tung University, Taipei, Taiwan.
- 2 School of Medicine, National Yang-Ming Chiao Tung University, Taipei, Taiwan.
- 3 Department of Chest Medicine, Taipei Veteran General Hospital, Taipei, Taiwan.
- 4 Institute of Biophotonics, National Yang-Ming Chiao Tung University, Taipei, Taiwan. [email protected].
- PMID: 38777902
- DOI: 10.1007/s00330-024-10804-6
Purpose: To compare the diagnostic performance of standalone deep learning (DL) algorithms and human experts in lung cancer detection on chest computed tomography (CT) scans.
Materials and methods: This study searched for studies on PubMed, Embase, and Web of Science from their inception until November 2023. We focused on adult lung cancer patients and compared the efficacy of DL algorithms and expert radiologists in disease diagnosis on CT scans. Quality assessment was performed using QUADAS-2, QUADAS-C, and CLAIM. Bivariate random-effects and subgroup analyses were performed for tasks (malignancy classification vs invasiveness classification), imaging modalities (CT vs low-dose CT [LDCT] vs high-resolution CT), study region, software used, and publication year.
Results: We included 20 studies on various aspects of lung cancer diagnosis on CT scans. Quantitatively, DL algorithms exhibited superior sensitivity (82%) and specificity (75%) compared to human experts (sensitivity 81%, specificity 69%). However, the difference in specificity was statistically significant, whereas the difference in sensitivity was not statistically significant. The DL algorithms' performance varied across different imaging modalities and tasks, demonstrating the need for tailored optimization of DL algorithms. Notably, DL algorithms matched experts in sensitivity on standard CT, surpassing them in specificity, but showed higher sensitivity with lower specificity on LDCT scans.
Conclusion: DL algorithms demonstrated improved accuracy over human readers in malignancy and invasiveness classification on CT scans. However, their performance varies by imaging modality, underlining the importance of continued research to fully assess DL algorithms' diagnostic effectiveness in lung cancer.
Clinical relevance statement: DL algorithms have the potential to refine lung cancer diagnosis on CT, matching human sensitivity and surpassing in specificity. These findings call for further DL optimization across imaging modalities, aiming to advance clinical diagnostics and patient outcomes.
Key points: Lung cancer diagnosis by CT is challenging and can be improved with AI integration. DL shows higher accuracy in lung cancer detection on CT than human experts. Enhanced DL accuracy could lead to improved lung cancer diagnosis and outcomes.
Keywords: Comparative study; Computed tomography (CT); Deep learning; Lung neoplasms; Meta-analysis.
© 2024. The Author(s).
PubMed Disclaimer
- Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48 - DOI - PubMed
- de Koning HJ, van der Aalst CM, de Jong PA et al (2020) Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503–513 - DOI - PubMed
- Dyer SC, Bartholmai BJ, Koo CW (2020) Implications of the updated lung CT screening reporting and data system (lung-RADS version 1.1) for lung cancer screening. J Thorac Dis 12:6966–6977 - DOI - PubMed - PMC
- Setio AAA, Traverso A, de Bel T et al (2017) Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: the LUNA16 challenge. Med Image Anal 42:1–13 - DOI - PubMed
- Hua KL, Hsu CH, Hidayati SC, Cheng WH, Chen YJ (2015) Computer-aided classification of lung nodules on computed tomography images via deep learning technique. Onco Targets Ther 8:2015–2022 - PubMed - PMC
Related information
Linkout - more resources, full text sources, miscellaneous.
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 23 March 2023
A review and comparative study of cancer detection using machine learning: SBERT and SimCSE application
- Mpho Mokoatle 1 ,
- Vukosi Marivate 1 ,
- Darlington Mapiye 2 ,
- Riana Bornman 4 &
- Vanessa. M. Hayes 3 , 4
BMC Bioinformatics volume 24 , Article number: 112 ( 2023 ) Cite this article
9717 Accesses
14 Citations
2 Altmetric
Metrics details
Using visual, biological, and electronic health records data as the sole input source, pretrained convolutional neural networks and conventional machine learning methods have been heavily employed for the identification of various malignancies. Initially, a series of preprocessing steps and image segmentation steps are performed to extract region of interest features from noisy features. Then, the extracted features are applied to several machine learning and deep learning methods for the detection of cancer.
In this work, a review of all the methods that have been applied to develop machine learning algorithms that detect cancer is provided. With more than 100 types of cancer, this study only examines research on the four most common and prevalent cancers worldwide: lung, breast, prostate, and colorectal cancer. Next, by using state-of-the-art sentence transformers namely: SBERT (2019) and the unsupervised SimCSE (2021), this study proposes a new methodology for detecting cancer. This method requires raw DNA sequences of matched tumor/normal pair as the only input. The learnt DNA representations retrieved from SBERT and SimCSE will then be sent to machine learning algorithms (XGBoost, Random Forest, LightGBM, and CNNs) for classification. As far as we are aware, SBERT and SimCSE transformers have not been applied to represent DNA sequences in cancer detection settings.
The XGBoost model, which had the highest overall accuracy of 73 ± 0.13 % using SBERT embeddings and 75 ± 0.12 % using SimCSE embeddings, was the best performing classifier. In light of these findings, it can be concluded that incorporating sentence representations from SimCSE’s sentence transformer only marginally improved the performance of machine learning models.
Peer Review reports
Introduction
Cancer is a disease where some cells in the body grow destructively and may spread to other body organs [ 1 ]. Typically, cells grow and expand through a cell division process to create new cells that can be used to repair old and damaged ones. However, this phenomenon can be interrupted resulting in abnormal cells growing uncontrollably to form tumors that can be malignant (harmful) or benign (harmless) [ 2 , 3 , 4 ].
With the introduction of genomic data that allows physicians and healthcare decision-makers to learn more about their patients and their response to the therapy they provide to them, this has facilitated the use of machine learning and deep learning to solve challenging cancer problems. These kinds of problems involve various tasks such as designing cancer risk-prediction models that try to identify patients that are at a higher risk of developing cancer than the general population, studying the progression of the disease to improve survival rates, and building methods that trace the effectiveness of treatment to improve treatment options [ 5 , 6 , 7 ].
Generally, the first step in analyzing genomic data to address cancer-related problems is selecting a data representation algorithm that will be used to estimate contiguous representations of the data. Examples of such algorithms include Word2vec [ 8 ], GloVe [ 9 ], and fastText [ 10 ]. The more recent and advanced versions of these algorithms are sentence transformers which are used to compute dense vector representations for sentences, paragraphs, and images. Similar texts are found close together in a vector space and dissimilar texts are far apart [ 11 ]. In this work, two such sentence transformers (SBERT and SimCSE) are proposed for detecting cancer in tumor/normal pairs of colorectal cancer patients. In this new approach, the classification algorithm relies on raw DNA sequences as the only input source. Moreover, this work provides a review of the most recent developments in cancers of the human body using machine learning and deep learning methods. While these kinds of similar reviews already exist in the literature, this study solely focuses on work that investigates four cancer types that have high prevalence rates worldwide [ 12 ] (lung, breast, prostate, and colorectal cancer) that have been published in the last five years (2018–2022).
Detection of cancer using machine learning
Lung cancer.
Lung cancer is the type of cancer that begins in the lungs and may spread to other organs in the body. This kind of cancer occurs when malignant cells develop in the tissue of the lung. There are two types of lung cancer: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). These cancers develop differently and thus their treatment therapies are different. Smoking (tobacco) is the leading cause of lung cancer. However, non-smokers can also develop lung cancer [ 13 , 14 ].
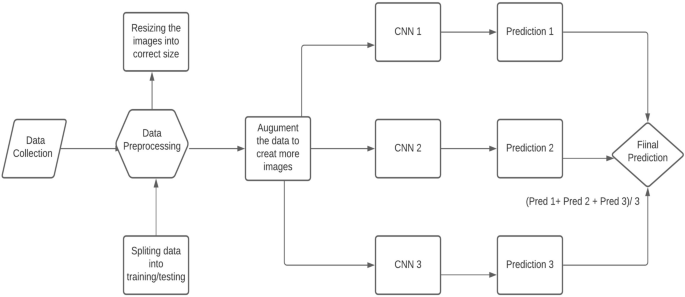
When it comes to the detection of lung cancer using machine learning (Fig. 1 ), a considerable amount of work has been done, a summary is provided (Table 1 ). Typically, a series of pre-processing steps using statistical methods and pretrained CNNs for feature extraction are carried out from several input sources (mostly images) to delineate the cancer region. Then, the extracted features are fed as input to several machine learning algorithms for classification of various lung cancer tasks such as the detection of malignant lung nodules from benign ones [ 15 , 16 , 17 ], the separation of a set of normalized biological data points into cancerous and non cancerous groups [ 18 ], and a basic comparative analysis of powerful machine learning algorithms for lung cancer detection [ 19 ].
Generalized machine learning framework for lung cancer prediction [ 33 ]
The lowest classification accuracy reported in Table 1 was 74.4% by work in [ 20 ]. In this work, a pretrained CNN model (DenseNet) was used to develop a lung cancer detection model. First, the model was fine-tuned to identify lung nodules from chest X-rays using the ChestX-ray14 dataset [ 21 ]. Second, the model was fine-tuned to identify lung cancer from images in the JSRT (Japanese Society of Radiological Technology) dataset [ 22 ].
The highest classification accuracy of 99.7% for lung cancer classification was reported by work in [ 18 ]. This study developed the Discrete AdaBoost Optimized Ensemble Learning Generalized Neural Network (DAELGNN) framework that uses a set of normalized biological data points to create a neural network that separates normal lung features from non-normal (cancerous) features.
Popular datasets used in lung cancer research using machine learning include the Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI) (LIDC-IDRI) database [ 23 ] initiated by the National Cancer Institute (NCI), and the histopathological images of lung and colon cancer (LC2500) database [ 24 ].
Breast cancer
Breast Cancer is a malignant tumor or growth that develops in the cells of the breast [ 34 ]. Similar to lung cancer, breast cancer also has the ability to metastasize to near by lymph nodes or to other body organs. Towards the end of 2020, there were approximately 7.8 million women who have been diagnosed with breast cancer, making this type of cancer the most prevalent cancer in the world. Risk factors of breast cancer include age, obesity, abuse of alcohol, and family history [ 35 , 36 , 37 ].
Currently, there is no identified prevention procedure for breast cancer. However, maintaining a healthy living habit such as physical exercise and less alcohol intake can reduce the risk of developing breast cancer [ 38 ]. It has also been said that early detection methods that rely on machine learning can improve the prognosis. As such, this type of cancer has been extensively studied using machine learning and deep learning [ 39 , 40 ].
As with lung cancer (Sect. 2.1 ), a great deal of work has been executed in developing breast cancer detection models, a generalized approach that illustrates the process using machine learning is provided (Fig. 2 ).
Generalized machine learning framework for breast cancer prediction [ 45 ]
Several classification problems have been studied that mainly focuses on the detection of breast cancer from thermogram images [ 41 ], handrafted features [ 42 ], mammograms [ 43 ], and whole slide images [ 44 ]. To develop a breast cancer detection model, initially, a pre-processing step is implemented that aims to extract features of interest. Then, the extracted features are provided as input to machine learning models for classification. This framework is implemented by several works such as [ 45 , 46 , 47 , 48 ].
One of the most popular datasets used for breast cancer detection using machine learning is the Wisconsin breast cancer dataset [ 42 ]. This dataset consists of features that describe the characteristics of the cell nuclei that is present in the image such as the diagnosis features (malignant or benign), radius, symmetry, and texture. Studies that used this dataset are [ 49 , 50 ]. In [ 49 ], the authors scaled the Wisconsin breast cancer features to be in the range between 0 and 1, then used a CNN for classification into benign or malignant. As opposed to using a CNN for classification, the authors [ 50 ] used traditional machine learning classifiers (Linear Regression, Multilayer Perceptron (MLP), Nearest Neighbor search, Softmax Regression, Gated recurrent Unit (GRU)-SVM, and SVM). For data pre-processing, the study used the Standard Scaler technique that standardizes data points by removing the mean and scaling the data to unit variance. The MLP model outperformed the other models by producing the highest accuracy of 99.04% which is almost similar to the accuracy of 99.6% that was reported by [ 49 ].
Different form binary classification of benign or malignant classes, a study [ 46 ] proposed a two-step approach to design a breast cancer multi-class classification model that predicts eight categories of breast cancer. In the first approach, the study used handcrafted features that are generated from histopathology images. These features were then fed as input to classical machine learning algorithms (RF, SVM, Linear Discriminant Analysis (LDA)). In the second approach, the study applied a transfer learning method to develop the multi-classification deep learning framework where pretained CNNs (ResNet50, VGG16 and VGG19) were used as feature extractors and baseline models. It was then found that the VGG16 pretrained CNN with the linear SVM provided the best accuracy in the range of 91.23% \(-\) 93.97%. This study also found that using pretrained CNNs as feature extractors improved the classification performance of the models.
The Table 2 provides a summary of the work that has been done to detect breast cancer using machine learning.
Prostate cancer
Prostate cancer is a type of cancer that develops when cells in the prostate gland start to grow uncontrollably (malignant). Prostate cancer often presents with no symptoms and grows at a slow rate. As a result, some men may die of other diseases before the cancer starts to cause notable problems. Comparably, prostate cancer can also be aggressive and metastasize to other body organs that are outside the confines of the prostate gland. Risk factors that are associated with this type of cancer include age, specifically, men that are above the age of 50. Other risk factors include ethnicity, family history of prostate cancer, breast or ovarian cancer, and obesity [ 61 , 62 , 63 ].
Transfer learning, which is defined as the reuse of a pretrained model on a new problem, was frequently applied to develop prostate cancer detection models using machine learning (Fig. 3 ). For example, a study [ 64 ] applied a transfer learning approach to detect prostate cancer on magnetic resonance images (MRI) by using a pretrained GoogleNet. A series of features such as texture, entropy, morphological, scale invariant feature transform (SIFT), and Elliptic Fourier Descriptors (EFDs) were extracted from the images as described by [ 65 , 66 ]. Other traditional machine learning classifiers were also evaluated such as Decision trees, and SVM Gaussian however, the GoogleNet model outperformed the other models.
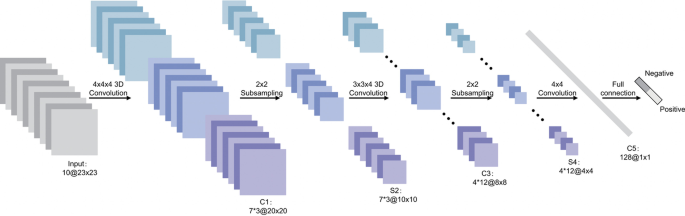
Generalized machine learning framework for prostate cancer prediction using 3-d CNNs, pooling layers, and a fully connected layer for classification [ 69 ]
Also using transfer learning, a study [ 67 ] developed a prostate cancer detection model by using MRI images and ultrasound (US) images. The model was developed in two stages: first, pretrained CNNs were used for classification of the US and MRI images into benign or malignant. While the pretrained CNNs performed well on the US images (accuracy 97%), the performance on the MRI images was not adequate. As a result, the best-performing pretrained CNN(VGG16) was selected and used as a feature extractor. The extracted features were then provided as input to traditional machine learning classifiers.
Another study [ 68 ] also used the same dataset as in [ 64 ] to create a prostate cancer detection model. However, instead of using GoogleNet as seen previously by [ 64 ], this study used a ResNet-101 and an autoencoder for feature reduction. Other machine learning models were also evaluated but, the study concluded that the pretrained ResNet-101 outperformed the other models with an accuracy of 100%. These results are similar to a previous study [ 64 ] that showed how pretrained CNNs outperform traditional machine learning models for cancer detection.
Table 3 , gives a summary of recent work that has been executed to create prostate cancer detection models.
Colorectal cancer
Colorectal cancer is a type of cancer that starts in the colon or rectum. The colon and rectum are parts of the human body that make up the large intestine that is part of the digestive system. A large part of the large intestine is made up of the colon which is divided into a few parts namely: ascending colon, transverse colon, descending colon, and sigmoid colon. The main function of the colon is to absorb water and salt from the remaining food waste after it has passed through the small intestine. Then, the waste that is left after passing through the colon goes into the rectum and is stored there until it is passed through the anus. Some colorectal cancers called polyps first develop as growth that can be found in the inner lining of the colon or rectum. Overtime, these polyps can develop into cancer, however, not all of them can be cancerous. Some of the risk factors of colorectal cancer include obesity, lack of exercise, diets that are rich in red meat, smoking, and alcohol [ 82 , 83 , 84 ].
In relation to the advancements made in colorectal cancer research using machine learning (Fig. 4 ), various tasks have been investigated such as predicting high-risk colorectal cancer from images, predicting five-year disease-specific survival, colorectal cancer tissue multi-class classification, and identifying the risk factors for lymph node metastasis (LNM) in colorectal cancer patients [ 85 , 86 , 87 , 88 ]. As with prostate cancer, transfer learning was mostly applied to extract features from various input sources such as colonoscopic images, tissue microarrays (TMA), and H &E slide images. Then, the extracted features were fed as input to machine learning algorithms for classification.
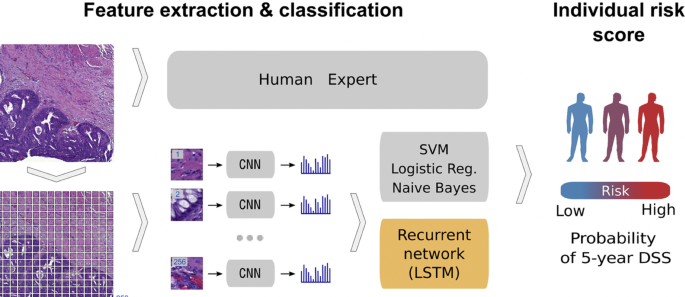
Using a deep CNN network to predict colorectal cancer outcome using images [ 86 ]
One common observation with regards to colorectal cancer models, is that the predictions made from the models were compared to those of experts. For example, a study [ 85 ] developed a deep learning model that detects high risk colorectal cancer from whole slide images that were collected from colon biopsies. The deep learning model was created in two stages: first, a segmentation procedure was executed to extract high risk regions from whole slide images. This segmentation procedure applied Faster-Region Based Convolutional Neural Network (Faster-RCNN) that uses a ResNet-101 model as a backbone for feature extraction. The second stage of implementing the model applied a gradient-boosted decision tree on the output of the Faster-RCNN deep learning model to classify the slides into either high or low risk colorectal cancer, and achieved an AUC of 91.7%. The study then found that the predictions made from the validation set were in agreement with annotations made by expert pathologists.
Work in [ 89 ] also compared predictions made by the Microsatellite instability (MSI)-predictor model with those of expert pathologists and found that experts achieved a mean AUROC of 61% while the model achieved an AUROC of 93% on a hold-out set and 87% on a reader experiment.
A previous study [ 90 ] developed a model named CRCNet, based a pretrained dense CNN, that automatically detects colorecal cancer from colonoscopic images and found that the model exceeded the avarage performance of expert endoscopists on a recall rate of 91.3% versus 83.8%.
In Table 4 , a summary is provided that describes the work that has been executed in colorectal cancer research using machine learning.
In summary of the literature survey (Sect. 2 ), a series of machine learning approaches for the detection of cancer were analysed. Imaging datasets, biological and clinical data, and EHRs were primarily employed as the initial input source when developing cancer detection algorithms. This procedure involved a few preprocessing steps. First, the input source was typically preprocessed at the beginning stages of the experiment to extract regions or features of interest. Next, the retrieved set of features were then applied to downstream machine learning classifiers for cancer prediction. In this work, as opposed to using imaging datasets, clinical and biological data or, EHRs as the starting input source, this work proposes to use raw DNA sequences as the only input source. Moreover, contrary to using statistical methods or advanced CNNs for data extraction and representation, this work proposes to use state-of-the-art sentence transformers namely: SBERT and SimCSE. As far as we are aware, these two sentence transformer models have not been applied for learning representations in cancer research. The learned representations will then be fed as input to machine learning algorithms for cancer prediction.
Data description
In this study, 95 samples from colorectal cancer patients and matched-normal samples from previous work [ 104 ] were analysed. Exon sequences from two key genes: APC and ATM were used. The full details of the exons that were used in this study is shown Tables 5 and 6 . Table 7 shows the data distribution among the normal/tumor DNA sequences. Ethics approval was granted by the University of Pretoria EBIT Research Ethics Committee (EBIT/139/2020).
Data encoding
To encode the DNA sequences, state-of-the-art sentence transformers: Sentence-BERT [ 105 ] and SimCSE [ 105 ] were used. These transformers are explained in the next subsection.
Sentence-BERT
Sentence-BERT (SBERT) (Fig. 5 ) adapts the pretrained BERT [ 106 ] and RoBERTa [ 107 ] transformer network and modifies it to use a siamese and triplet network architectures to compute fixed-sized vectors for more than 100 languages. The sentence embeddings can then be contrasted using the cosine-similarity. SBERT was trained on the combination of SNLI data [ 108 ] and the Multi-Genre NLI dataset [ 109 ].
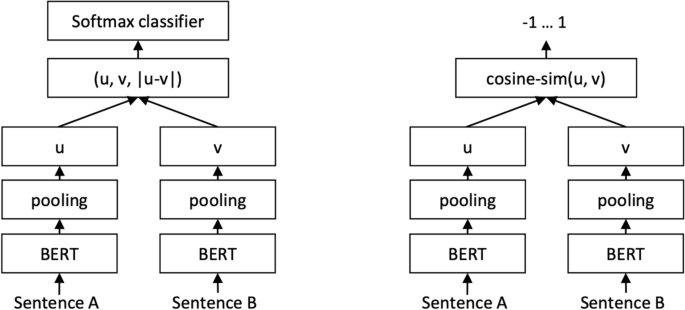
SBERT architecture with classification objective function (left) and the regression objective function (right) [ 105 ]
In its architecture, SBERT adds a default mean-pooling procedure on the output of the BERT or RoBERTa network to compute sentence embeddings. SBERT implements the following objective functions: classification objective function, regression objective function, and the triplet objective function. In the classification objective function, the sentence embeddings of two sentence pairs u and v are concatenated using the element-wise difference \(\mid u-v \mid\) and multiplied with the trainable weight \(W_{t} \epsilon {\mathbb {R}}^{3n *k}\) :
where n is the length or dimension of the sentence embeddings and k is the value of the target labels.
The regression objective function makes use of mean-squared-error loss as the objective function to compute the cosine-similarity between two sentence embeddings u and v .
The triplet objective function fine-tunes the network such that the distance between an anchor sentence a and a positive sentence p is smaller than the distance between sentence a and the negative sentence n .
Using the pretrained SBERT model: all-MiniLM-L6-v2 , each DNA sequence was represented by a 384-dimensional vector.
As with SBERT, Simple Contrastive Sentence Embedding (SimCSE) [ 110 ] (Fig. 6 is a transformer based model that modifies the BERT/RoberTa encoder to generate sentence embeddings. It uses a contrastive learning approach that aims to learn sentence representations by pulling close neighbours together and propelling non-neighbours. SimCSE comes in two learning forms: unsupervised and supervised SimCSE. In unsupervised SimCSE, the network is fine-tuned to predict the input sentence itself using dropout as noise then, the other sentences that are in the mini-batch are taken as negatives. In this case, dropout acts as a data augmentation method while previous [ 111 , 112 ] methods have used word deletion, reordering, and substitution as a way of generating positive instances. In unsupervised SimCSE, an input sentence is fed twice to the encoder then, two embeddings with different dropout masks z , \(z'\) are generated as output. The training objective for SimCSE is:
where z is the standard dropout mask that are found in Transformers and no additional dropout mask is added [ 110 ].
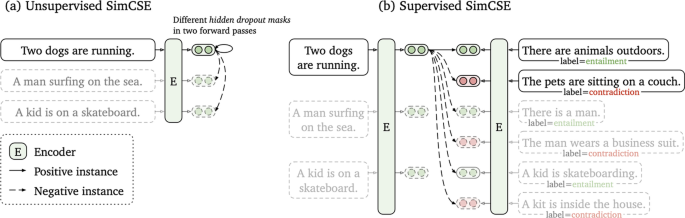
Unsupervised SimCSE ( a ) and supervised SimCSE ( b ) [ 110 ]
In supervised SimCSE, positive pairs are taken from the natural language inference (NLI) datasets and used to optimise the following equation:
where \(\tau\) is a temperature hyperparamter and \(sim(h_{1},h_{2})\) is the cosine similarity.
Using the unsupervised pretrained SimCSE model: unsup-simcse-bert-base-uncased , each DNA sequence was represented by a 768-dimensional vector.
K -means clustering
The k -means clustering algorithm was used to visualize the sentence representations generated from SBERT and SimCSE in an unsupervised approach. The k -means algorithm divides the data points into k clusters where each data point is said to belong to the cluster centroid closest to it. Since the data consists of two types of documents (tumor vs. normal), the k -means algorithm was asked to find 2 clusters n and assign each DNA sequence to its closest centroid [ 113 ].
Machine learning experiments
A total of three machine learning algorithms were used for classification: Light Gradient Boosting (LightGBM), eXtreme Gradient Boosting (XGBoost), and Random Forest (RF).
eXtreme gradient boosting (XGBoost)
eXtreme Gradient Boosting (XGBoost), is an efficient implementation of the gradient boosting algorithm. Gradient boosting belongs to a group of ensemble machine learning algorithms that be used to solve classification or regression problems. The ensembles are created from decision trees that are added one at a time to the ensemble, and fit to correct the classification error that were made by prior trees [ 114 ].
Light gradient boosting (LightGBM)
Light Gradient Boosting (LightGBM) machine is also a gradient boosting model that is used for ranking, classification, and regression. In contrast to XGBoost, LightGBM splits the tree vertically as opposed to horizontally. This method of growing the tree leaf vertically results in more loss reduction and provides higher accuracy while also being faster. LightGBM uses the Gradient-based One-Side Sampling (GOSS) method to filter out data instances for obtaining the best split value while XGBoost uses a pre-sorted and Histogram-based algorithm for calculating the best split value [ 115 ].
Random forest (RF)
Random forest (RF) is a supervised machine learning that is used in classification and regression tasks. It creates decision tress based on different samples and takes the majority vote for classification or average for regression. While XGBoost and LightGBM use a gradient boosting method, Random Forest uses a bagging method. The bagging method builds a different training subset from the training data with replacement. Each model is trained separately and the final result is based on a majority voting after consolidating the results of all the models [ 116 ].
Convolutional neural network (CNN)
Convolutional neural networks (CNNs) are a subset of neural networks that are frequently used to process speech, audio, and visual input signals. Convolutional, pooling, and fully connected (FC) layers are the three types of layers that are generally present in CNNs. The convolutional layer is the fundamental component of a CNN and is in charge of performing convolutional operations on the input before passing the outcome to the following layer. Then, the input is subjected to dimensionality reduction using pooling layers that reduces the number of parameters in the input. The FC layer uses a variety of activation functions, including the softmax activation function and the sigmoid activation function, to carry out the classification task using the features retrieved from the network’s prior layers [ 117 , 118 ]. In this work, a three-layer CNN model with a sigmoid activation function will be supplied with the embedding features that were retrieved by SBERT and SimCSE sentence transformers. Due to computational limitations, the network will be trained over 10 epochs using the RMSprop optimizer and cross-validated over five folds.
Performance evaluation metrics
To measure the performance of the machine learning models, the average performance of the models were reported using 5-fold cross validation and the following metrics were used: accuracy, precision, recall and F1 score. In Table 8 , the definition of these metrics is provided.
This section described the datasets used in the study as well as data representation methods and machine learning algorithms that were applied in this work. In the next section, the results of the applied methods are described.
Visualizations
In this subsection, unlabeled data from SBERT and SimCSE representations were explored and visualized with the k -means clustering algorithm. The representations of the SBERT algorithm (Fig. 7 ) revealed more overlap between the data points in comparison to the representations of the SimCSE algorithm (Fig. 8 ). In the next subsection, machine learning models are evaluated to reveal if there is sufficient signal in the representations of the two sentence transformers that can discriminate between tumor and normal DNA sequences.
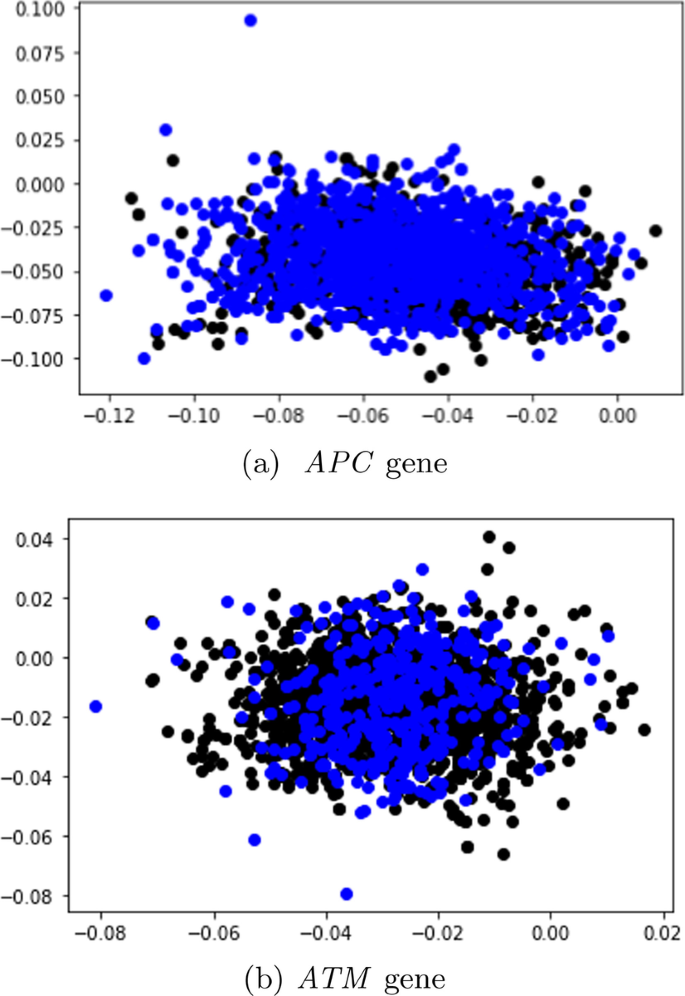
Visualisation of the SBERT documents with k -means clustering
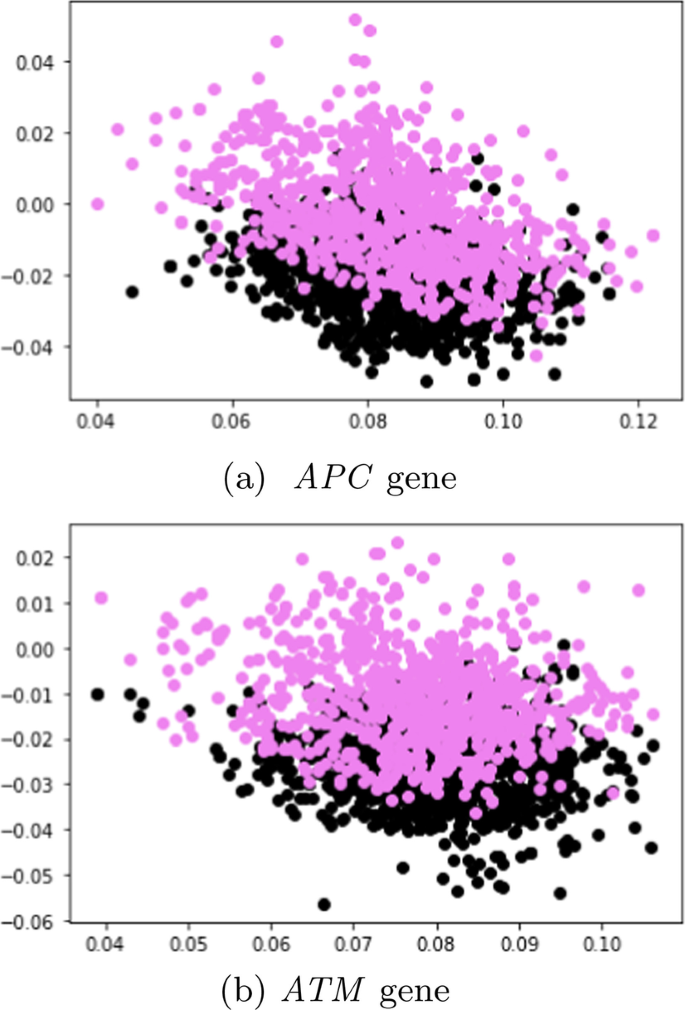
Visualisation of the SimCSE documents with k -means clustering
Comparative performance of the machine learning results
Sbert before smote.
Table 9 presents the performance of the machine learning models on the dev set in terms of the average accuracy, averaged over the five folds using the SBERT representations. More performance metrics such as F1 score, recall, and precision are reported in the Additional file 1 (Appendix A ).
Considering that the tumor DNA sequences belonging to the APC gene comprised of \(\approx\) 64% of the data before SMOTE sampling, the machine learning models classified most sequences as positive (tumor); with the CNN achieving the best overall with the highest accuracy of 67.3 ± 0.04%.
In contrast to the data distribution of the APC gene before SMOTE sampling, the original data distribution of sequences from the ATM gene were relatively balanced as the tumor sequences comprised of 53% of the total data, and normal DNA sequences made up 47%. Moreover, as opposed to predicting nearly all sequences as positive, the machine learning models demonstrated an unbiased above-average performance as the highest performing model (XGBoost) achieved an accuracy of 73. ± 0.13 %.
SBERT after SMOTE
The performance of the majority of the machine learning classifiers after applying SMOTE remained consistent in that very little improvement or decline was observed. Moreover, while the CNN model previously obtained the highest overall accuracy before SMOTE oversampling, it performed the worst after applying SMOTE with a reported accuracy of 47. ± 17.4 %. Although biased, the LightGBM classifier reached the highest accuracy of 64.9 ± 0.29 %. Its confusion matrix is shown (Fig. 9 ).
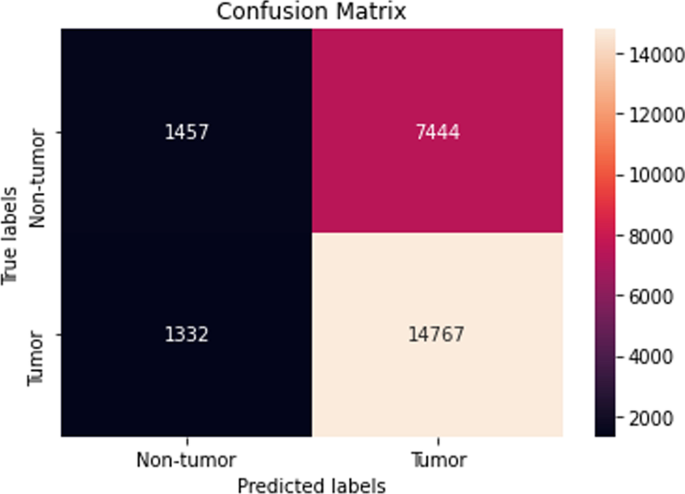
Confusion matrix of the LightGBM model using SBERT representations after SMOTE (dev set)
The same trend as seen in the previous Sect. 4.2.2 was also observed in this section with sequences from the ATM gene. Here, the performance of the machine learning models after SMOTE sampling was relatively similar to the performance of the machine learning models before SMOTE sampling as the XGBoost still maintained the best overall accuracy of 73. ± 0.13 % (Fig. 10 ).
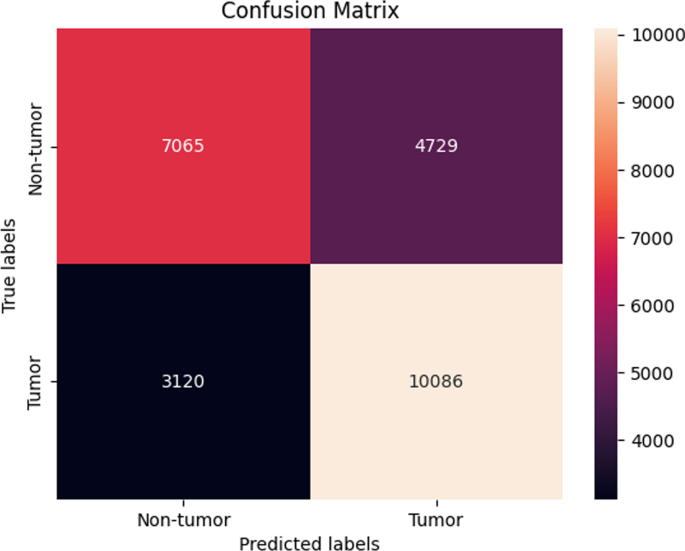
Confusion matrix of the XGBoost model using SBERT representations after SMOTE (dev set)
SimCSE before SMOTE
Table 9 also presents the performance of the machine learning models in terms of the average accuracy, averaged over the five folds using the SimCSE representations. Supplementary performance metrics are reported (Additional file 1 : Appendix A).
In this experimental setting, the performance of the machine learning models with SBERT representations before SMOTE sampling was similar to the performance of the models with SimCSE representations before SMOTE sampling. Here, the CNN achieved the best accuracy of 67. ± 0.0 %.
A similar pattern as in the previous Sect. ( APC , SimCSE before SMOTE) was also detected in this setting when using sequences from the ATM gene in that the performance of the SimCSE models were almost similar to the performance of the SBERT models (before SMOTE) with slight improvement. The LightGBM model achieved the highest accuracy of 74. ± 0.18 % which was an improvement in accuracy of approximately 4 %.
SimCSE after SMOTE
The LightGBM model achieved the highest accuracy of 64.7 ± 0.29 (Fig. 11 ), which was indistinguishable to the performance reported before SMOTE oversampling.
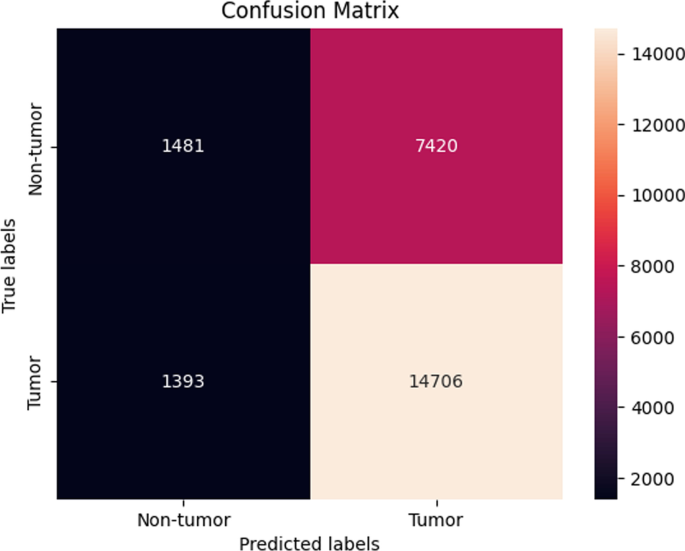
Confusion matrix of the LightGBM model using SimCSE representations after SMOTE (dev set)
ATM In this final experimental setting, the results demonstrated a consistent performance before SMOTE sampling and after SMOTE sampling. The highest performing model was the Random forest model as it achieved an average accuracy of 71.6 ± 1.47 % (Fig. 12 ).
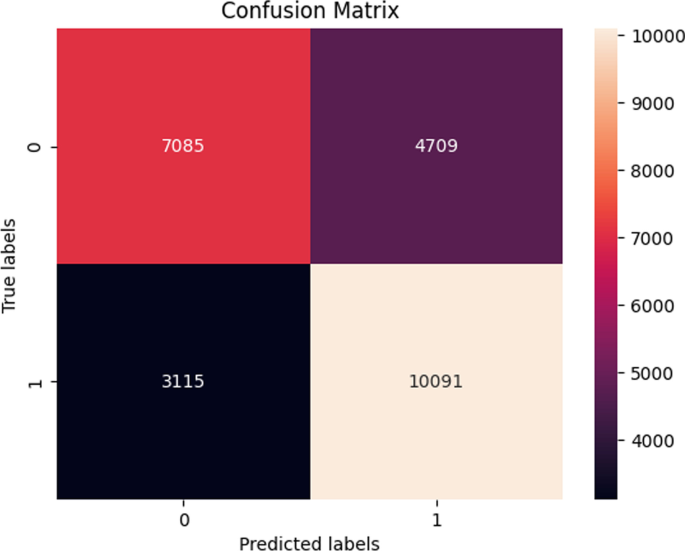
Confusion matrix of the Random forest model using SimCSE representations after SMOTE (dev set)
In Table 10 , the experiments were repeated on an additional unseen test set. Overall, the machine learning models demonstrated a slight increase in the accuracy as the highest performing model, XGBoost, achieved an average accuracy of 75. ± 0.12 % using SimCSE representations from the ATM gene.
This paper provided a literature review of how cancer has been detected using various machine learning methods. Additionally, this work developed machine learning models that detect cancer using raw DNA sequences as the only input source. The DNA sequences were retrieved from matched tumor/normal pairs of colorectal cancer patients as described by previous work [ 104 ]. For data representation, two state-of-the-art sentence transformers were proposed: SBERT and SimCSE. To the best of our knowledge, these two methods have not been used to represent DNA sequences in cancer detection problems using machine learning. In summary of the results, we note that using SimCSE representations only marginally improved the performance of the machine learning models.
The ability to detect cancer by relying on human DNA as the only input source to a learning algorithm was one of the significant contributions of this work. We acknowledge that similar research investigating the role that the DNA plays in various cancer types has been conducted in the past. In contrary, the way the DNA was represented for the learning algorithms in our work is different from that in earlier research. An example would be work performed by [ 120 ] that used cell-free DNA (cfDNA) data from shallow whole-genome sequencing to uncover patterns associated with a number of different cancers including Hodgkin lymphoma, diffuse large B-cell lymphoma, and multiple myeloma. This study used PCA transformed genome-wide coverage features and applied them as input to a support vector algorithm to predict cancer status rather than employing sentence transforms for data representation as was done in our study. Another study [ 121 ] also used cfDNA sequences to predict cancer tissue sequences from healthy ones. In this work, reads from hepatocellular carcinoma (HCC) patients and healthy individuals were integrated with methylation information and then, a deep learning model was created to predict the reads that originated from a cancer tissue. The deep learning model consisted of a 1-d CNN followed by a maxpooling layer, a bi-directional LSTM, a 1-d CNN, and three dense layers. To represent the cfDNA sequences and methylation information, the variables were encoded into a one-hot encoded matrix that was then provided as input to the deep learning model for classification. Different from relying on raw DNA or cfDNA data to develop cancer detection frameworks, a study [ 122 ] consolidated methods from variant calling and machine learning to develop a model that detects cancers of unknown primary (CUP) origin which account for approximately 3% of all cancer diagnoses. This work employed whole-genome-sequencing-based mutation features derived from structural variants that were generated through variant calling and fed them as input to an ensemble of random forest binary classifiers for the detection of 35 different cancers.
Limitations of the study
The machine learning experiments were only performed on two key genes: APC and APC , therefore it would have been interesting to see how the models generalize across various genes. The common disadvantage of conducting the experiments on multiple genes or whole genome sequencing data is that they require more computational resources which have a direct impact on cost. Another limitation of this work is that only two pretrained models were used for generating the sentence representations. Since there are several other pretrained models that are publicly available to choose from, some pretrained models were slower to execute than others hence a decision was made to focus on pretrained models that provided fast execution.
This article reviewed the literature and demonstrated how various machine learning techniques have been used to identify cancer. Given that they are the most common malignancies worldwide, this work placed a special emphasis on four cancer types: lung, breast, prostate, and colorectal cancer. Then, a new method for the identification of colorectal cancer employing SBERT and SimCSE sentence representations was presented. Raw DNA sequences from matched tumor/normal pairs of colorectal cancer served as the sole input for this approach. The learned representations were then provided as input to machine learning classifiers for classification. In light of the performance of the machine learning classifiers, XGBoost was found to be the best performing classifier overall. Moreover, using SimCSE representations only marginally improved the classification performance of the machine learning models.
Availability of data and materials
The data can be accessed at the host database (The European Genome-phenome Archive at the European Bioinformatics Institute, accession number: EGAD00001004582 Data access ).
Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92.
Article CAS PubMed PubMed Central Google Scholar
What Is Cancer? National Cancer Institute. https://www.cancer.gov/about-cancer/understanding/what-is-cancer
Zheng R, Sun K, Zhang S, Zeng H, Zou X, Chen R, Gu X, Wei W, He J. Report of cancer epidemiology in china, 2015. Zhonghua zhong liu za zhi. 2019;41(1):19–28.
CAS PubMed Google Scholar
Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35.
Article CAS PubMed Google Scholar
Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8–17.
Iqbal MJ, Javed Z, Sadia H, Qureshi IA, Irshad A, Ahmed R, Malik K, Raza S, Abbas A, Pezzani R, et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: looking into the future. Cancer Cell Int. 2021;21(1):1–11.
Article Google Scholar
Loud JT, Murphy J. Cancer screening and early detection in the 21st century. Semin Oncol Nurs. 2017;33:121–8.
Article PubMed PubMed Central Google Scholar
Goldberg Y, Levy O. word2vec explained: deriving mikolov et al.’s negative-sampling word-embedding method. 2014; arXiv preprint arXiv:1402.3722
Pennington J, Socher R, Manning CD. Glove: global vectors for word representation. In: Proceedings of the 2014 conference on empirical methods in natural language processing (EMNLP). 2014. p. 1532–43.
Bojanowski P, Grave E, Joulin A, Mikolov T. Enriching word vectors with subword information. Trans Assoc Comput Linguist. 2017;5:135–46.
Church KW. Word2vec. Natl Lang Eng. 2017;23(1):155–62.
Cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancer
Bade BC, Cruz CSD. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24.
Article PubMed Google Scholar
Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Global Health. 2019;85:1.
de Carvalho Filho AO, Silva AC, de Paiva AC, Nunes RA, Gattass M. Classification of patterns of benignity and malignancy based on ct using topology-based phylogenetic diversity index and convolutional neural network. Pattern Recogn. 2018;81:200–12.
Rodrigues MB, Da Nobrega RVM, Alves SSA, Reboucas Filho PP, Duarte JBF, Sangaiah AK, De Albuquerque VHC. Health of things algorithms for malignancy level classification of lung nodules. IEEE Access. 2018;6:18592–601.
Asuntha A, Srinivasan A. Deep learning for lung cancer detection and classification. Multim Tools Appl. 2020;79(11):7731–62.
Shakeel PM, Tolba A, Al-Makhadmeh Z, Jaber MM. Automatic detection of lung cancer from biomedical data set using discrete adaboost optimized ensemble learning generalized neural networks. Neural Comput Appl. 2020;32(3):777–90.
Abdullah DM, Abdulazeez AM, Sallow AB. Lung cancer prediction and classification based on correlation selection method using machine learning techniques. Qubahan Acad J. 2021;1(2):141–9.
Ausawalaithong W, Thirach A, Marukatat S, Wilaiprasitporn T. Automatic lung cancer prediction from chest x-ray images using the deep learning approach. In: 2018 11th biomedical engineering international conference (BMEiCON). 2018; pp. 1–5. IEEE
Wang X, Peng Y, Lu L, Lu Z, Bagheri M, Summers RM. Chestx-ray8: Hospital-scale chest x-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In: Proceedings of the IEEE conference on computer vision and pattern recognition. 2017; pp. 2097–106
Shiraishi J, Katsuragawa S, Ikezoe J, Matsumoto T, Kobayashi T, Komatsu K-I, Matsui M, Fujita H, Kodera Y, Doi K. Development of a digital image database for chest radiographs with and without a lung nodule: receiver operating characteristic analysis of radiologists’ detection of pulmonary nodules. Am J Roentgenol. 2000;174(1):71–4.
Article CAS Google Scholar
Armato SG III, McLennan G, Bidaut L, McNitt-Gray MF, Meyer CR, Reeves AP, Zhao B, Aberle DR, Henschke CI, Hoffman EA, et al. The lung image database consortium (lidc) and image database resource initiative (idri): a completed reference database of lung nodules on ct scans. Med Phys. 2011;38(2):915–31.
Kaggle: Lung and Colon Cancer Histopathological Images. https://www.kaggle.com/andrewmvd/lung-and-colon-cancer-histopathological-images Accessed 16 July 2020.
Radhika P, Nair RA, Veena G. A comparative study of lung cancer detection using machine learning algorithms. In: 2019 IEEE international conference on electrical, computer and communication technologies (ICECCT). 2019; pp. 1–4. IEEE
Salaken SM, Khosravi A, Khatami A, Nahavandi S, Hosen MA. Lung cancer classification using deep learned features on low population dataset. In: 2017 IEEE 30th Canadian conference on electrical and computer engineering (CCECE). 2017; pp. 1–5. IEEE.
Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et al. Classification of human lung carcinomas by mrna expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci. 2001;98(24):13790–5.
Bhatia S, Sinha Y, Goel L. Lung cancer detection: a deep learning approach. In: Soft computing for problem solving. 2019; p. 699–705. Springer.
Shin H, Oh S, Hong S, Kang M, Kang D, Ji Y-G, Choi BH, Kang K-W, Jeong H, Park Y, et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. 2020;14(5):5435–44.
Masud M, Sikder N, Nahid A-A, Bairagi AK, AlZain MA. A machine learning approach to diagnosing lung and colon cancer using a deep learning-based classification framework. Sensors. 2021;21(3):748.
Naseer I, Akram S, Masood T, Jaffar A, Khan MA, Mosavi A. Performance analysis of state-of-the-art cnn architectures for luna16. Sensors. 2022;22(12):4426.
Setio AAA, Traverso A, De Bel T, Berens MS, Van Den Bogaard C, Cerello P, Chen H, Dou Q, Fantacci ME, Geurts B, et al. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: the luna16 challenge. Med Image Anal. 2017;42:1–13.
Saba T. Recent advancement in cancer detection using machine learning: systematic survey of decades, comparisons and challenges. J Infect Pub Health. 2020;13(9):1274–89.
Sun Y-S, Zhao Z, Yang Z-N, Xu F, Lu H-J, Zhu Z-Y, Shi W, Jiang J, Yao P-P, Zhu H-P. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387.
Breast cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/breast-cancer
Kelsey JL, Gammon MD. The epidemiology of breast cancer. CA Cancer J Clin. 1991;41(3):146–65.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Prim. 2019;5(1):1–31.
Google Scholar
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300.
Tahmooresi M, Afshar A, Rad BB, Nowshath K, Bamiah M. Early detection of breast cancer using machine learning techniques. J Telecommun Electr Comput Eng. 2018;10(3):21–7.
Sharma S, Aggarwal A, Choudhury T. Breast cancer detection using machine learning algorithms. In: 2018 international conference on computational techniques, electronics and mechanical systems (CTEMS). 2018; p. 114–8 . IEEE.
VisualLab: A Methodology for Breast Disease Computer-Aided Diagnosis Using Dynamic Thermography. http://visual.ic.uff.br/en/proeng/thiagoelias/
Wolberg WH, Street WN, Mangasarian OL. Breast cancer wisconsin (diagnostic) data set. UCI machine learning repository. http://archive.ics.uci.edu/ml/ ; 1992.
Suckling JP. The mammographic image analysis society digital mammogram database. Digital Mammo. 1994; pp. 375–86.
Roy A. Deep convolutional neural networks for breast cancer detection. In: 2019 IEEE 10th annual ubiquitous computing, electronics & mobile communication conference (UEMCON). 2019; pp. 0169–71 . IEEE.
Mambou SJ, Maresova P, Krejcar O, Selamat A, Kuca K. Breast cancer detection using infrared thermal imaging and a deep learning model. Sensors. 2018;18(9):2799.
Sharma S, Mehra R. Conventional machine learning and deep learning approach for multi-classification of breast cancer histopathology images-a comparative insight. J Digit Imag. 2020;33(3):632–54.
Remya R, Rajini NH. Transfer learning based breast cancer detection and classification using mammogram images. In: 2022 international conference on electronics and renewable systems (ICEARS). 2022; pp. 1060–5 . IEEE.
Vaka AR, Soni B, Reddy S. Breast cancer detection by leveraging machine learning. ICT Express. 2020;6(4):320–4.
Khuriwal N, Mishra N. Breast cancer detection from histopathological images using deep learning. In: 2018 3rd international conference and workshops on recent advances and innovations in engineering (ICRAIE). 2018; pp. 1–4 . IEEE.
Agarap AFM. On breast cancer detection: an application of machine learning algorithms on the wisconsin diagnostic dataset. In: proceedings of the 2nd international conference on machine learning and soft computing. 2018; pp. 5–9.
Shen L, Margolies LR, Rothstein JH, Fluder E, McBride R, Sieh W. Deep learning to improve breast cancer detection on screening mammography. Sci Rep. 2019;9(1):1–12.
Sawyer Lee R, Gimenez F, Hoogi A, Rubin D. Curated Breast Imaging Subset of DDSM. The cancer imaging archive, 2016.
Moreira IC, Amaral I, Domingues I, Cardoso A, Cardoso MJ, Cardoso JS. Inbreast: toward a full-field digital mammographic database. Acad Radiol. 2012;19(2):236–48.
VRI: Breast Cancer Histopathological Database (BreakHis). https://web.inf.ufpr.br/vri/databases/breast-cancer-histopathological-database-breakhis/
Alanazi SA, Kamruzzaman M, Islam Sarker MN, Alruwaili M, Alhwaiti Y, Alshammari N, Siddiqi MH. Boosting breast cancer detection using convolutional neural network. J Healthc Eng 2021;2021.
Janowczyk, A.: Use case 6: invasive ductal carcinoma (IDC) segmentation. http://www.andrewjanowczyk.com/use-case-6-invasive-ductal-carcinoma-idc-segmentation/
Arooj S, et al.: Breast cancer detection and classification empowered with transfer learning. Front Pub Health. 2022;10.
Nasir MU, Ghazal TM, Khan MA, Zubair M, Rahman A-u, Ahmed R, Hamadi HA, Yeun CY. Breast cancer prediction empowered with fine-tuning. Comput Intell Neurosci. 2022;2022.
Breast cancer patients mris. Kaggle. https://www.kaggle.com/uzairkhan45/breast-cancer-patients-mris
Khan MBS, Nawaz MS, Ahmed R, Khan MA, Mosavi A, et al. Intelligent breast cancer diagnostic system empowered by deep extreme gradient descent optimization. Mathem Biosci Eng. 2022;19(8):7978–8002.
What is Prostate Cancer. UCLA Health. https://www.uclahealth.org/urology/prostate-cancer/what-is-prostate-cancer
Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, Gill IS. Trends in incidence of metastatic prostate cancer in the us. JAMA Netw Open. 2022;5(3):222246.
Cackowski FC, Heath EI. Prostate cancer dormancy and recurrence. Cancer Lett. 2022;524:103–8.
Abbasi AA, Hussain L, Awan IA, Abbasi I, Majid A, Nadeem MSA, Chaudhary Q-A. Detecting prostate cancer using deep learning convolution neural network with transfer learning approach. Cogn Neurodyn. 2020;14(4):523–33.
Hussain L, Ahmed A, Saeed S, Rathore S, Awan IA, Shah SA, Majid A, Idris A, Awan AA. Prostate cancer detection using machine learning techniques by employing combination of features extracting strategies. Cancer Biomark. 2018;21(2):393–413.
Hussain L, et al. Detecting brain tumor using machines learning techniques based on different features extracting strategies. Curr Med Imag. 2019;15(6):595–606.
Hassan MR, Islam MF, Uddin MZ, Ghoshal G, Hassan MM, Huda S, Fortino G. Prostate cancer classification from ultrasound and mri images using deep learning based explainable artificial intelligence. Fut Gener Comput Syst. 2022;127:462–72.
Iqbal S, Siddiqui GF, Rehman A, Hussain L, Saba T, Tariq U, Abbasi AA. Prostate cancer detection using deep learning and traditional techniques. IEEE Access. 2021;9:27085–100.
Feng Y, Yang F, Zhou X, Guo Y, Tang F, Ren F, Guo J, Ji S. A deep learning approach for targeted contrast-enhanced ultrasound based prostate cancer detection. IEEE/ACM transactions on computational biology and bioinformatics. 2018;16(6):1794–801.
Reda I, Khalil A, Elmogy M, Abou El-Fetouh A, Shalaby A, Abou El-Ghar M, Elmaghraby A, Ghazal M, El-Baz A. Deep learning role in early diagnosis of prostate cancer. Technol Cancer Res Treat. 2018;17:1533034618775530.
Barlow H, Mao S, Khushi M. Predicting high-risk prostate cancer using machine learning methods. Data. 2019;4(3):129.
Yoo S, Gujrathi I, Haider MA, Khalvati F. Prostate cancer detection using deep convolutional neural networks. Sci Rep. 2019;9(1):1–10.
Tolkach Y, Dohmgörgen T, Toma M, Kristiansen G. High-accuracy prostate cancer pathology using deep learning. Nat Mach Intell. 2020;2(7):411–8.
Genomic Data Commons Data Portal. National Cancer Institute (NIH) GDC Data Portal. http://portal.gdc.cancer.gov
Zenodo. Zenodo. https://zenodo.org/deposit/3825933
Hosseinzadeh M, Saha A, Brand P, Slootweg I, de Rooij M, Huisman H. Deep learning–assisted prostate cancer detection on bi-parametric mri: minimum training data size requirements and effect of prior knowledge. Eur Radiol. 2021; 1–11.
Natarajan S, Priester A, Margolis D, Huang J, Marks L. Prostate mri and ultrasound with pathology and coordinates of tracked biopsy (prostate-mri-us-biopsy). 2020.
Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, Moore S, Phillips S, Maffitt D, Pringle M, et al. The cancer imaging archive (tcia): maintaining and operating a public information repository. J Dig Imaging. 2013;26(6):1045–57.
Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, Dorey FJ, Marks LS. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189(1):86–92.
Tsuneki M, Abe M, Kanavati F. A deep learning model for prostate adenocarcinoma classification in needle biopsy whole-slide images using transfer learning. Diagnostics. 2022;12(3):768.
Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9(1):62–6.
What Is Colorectal Cancer? American Cancer Society. https://www.cancer.org/cancer/colon-rectal-cancer/about/what-is-colorectal-cancer.html
Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–78.
Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365(9454):153–65.
Ho C, Zhao Z, Chen XF, Sauer J, Saraf SA, Jialdasani R, Taghipour K, Sathe A, Khor L-Y, Lim K-H, et al. A promising deep learning-assistive algorithm for histopathological screening of colorectal cancer. Sci Rep. 2022;12(1):1–9.
Bychkov D, Linder N, Turkki R, Nordling S, Kovanen PE, Verrill C, Walliander M, Lundin M, Haglund C, Lundin J. Deep learning based tissue analysis predicts outcome in colorectal cancer. Sci Rep. 2018;8(1):1–11.
Damkliang K, Wongsirichot T, Thongsuksai P. Tissue classification for colorectal cancer utilizing techniques of deep learning and machine learning. Biomed Eng Appl Basis Commun. 2021;33(03):2150022.
Brockmoeller S, Echle A, Ghaffari Laleh N, Eiholm S, Malmstrøm ML, Plato Kuhlmann T, Levic K, Grabsch HI, West NP, Saldanha OL, et al. Deep learning identifies inflamed fat as a risk factor for lymph node metastasis in early colorectal cancer. J Pathol. 2022;256(3):269–81.
Yamashita R, Long J, Longacre T, Peng L, Berry G, Martin B, Higgins J, Rubin DL, Shen J. Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol. 2021;22(1):132–41.
Zhou D, Tian F, Tian X, Sun L, Huang X, Zhao F, Zhou N, Chen Z, Zhang Q, Yang M, et al. Diagnostic evaluation of a deep learning model for optical diagnosis of colorectal cancer. Nat Commun. 2020;11(1):1–9.
CAS Google Scholar
Wang Y-H, Nguyen PA, Islam MM, Li Y-C, Yang H-C, et al. Development of deep learning algorithm for detection of colorectal cancer in ehr data. In: MedInfo. 2019; pp. 438–41
Echle A, Grabsch HI, Quirke P, van den Brandt PA, West NP, Hutchins GG, Heij LR, Tan X, Richman SD, Krause J, et al. Clinical-grade detection of microsatellite instability in colorectal tumors by deep learning. Gastroenterology. 2020;159(4):1406–16.
Macenko M, et al. A method for normalizing histology slides for quantitative analysis. In: 2009 IEEE international symposium on biomedical imaging: from Nano to Macro, pp. 1107–10 (2009). IEEE.
Amitay EL, Carr PR, Jansen L, Walter V, Roth W, Herpel E, Kloor M, Bläker H, Chang-Claude J, Brenner H, et al. Association of aspirin and nonsteroidal anti-inflammatory drugs with colorectal cancer risk by molecular subtypes. JNCI J Natl Cancer Inst. 2019;111(5):475–83.
Group QC, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–9.
van den Brandt PA, Goldbohm RA, Veer PV, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in the netherlands. Journal of clinical epidemiology. 1990;43(3):285–95.
Taylor J, Wright P, Rossington H, Mara J, Glover A, West N, Morris E, Quirke P. Regional multidisciplinary team intervention programme to improve colorectal cancer outcomes: study protocol for the yorkshire cancer research bowel cancer improvement programme (ycr bcip). BMJ Open. 2019;9(11): 030618.
Histological images for MSI vs. MSS classification in gastrointestinal cancer, FFPE samples. Zenodo. https://zenodo.org/record/2530835#.Ypib9C8RpQI
Sarwinda D, Paradisa RH, Bustamam A, Anggia P. Deep learning in image classification using residual network (resnet) variants for detection of colorectal cancer. Proc Comput Sci. 2021;179:423–31.
Tissue Image Analytics (TIA) Centre. warwick. https://warwick.ac.uk/fac/cross_fac/tia/data/glascontest/download
Lorenzovici N, Dulf E-H, Mocan T, Mocan L. Artificial intelligence in colorectal cancer diagnosis using clinical data: non-invasive approach. Diagnostics. 2021;11(3):514.
Kather JN, Weis C-A, Bianconi F, Melchers SM, Schad LR, Gaiser T, Marx A, Zöllner FG. Multi-class texture analysis in colorectal cancer histology. Sci Rep. 2016;6(1):1–11.
Muti H, Loeffler C, Echle A, Heij L, Buelow R, Krause J, et al. The aachen protocol for deep learning histopathology: a hands-on guide for data preprocessing. Zenodo Aachen. 2020;10
Poulos RC, Perera D, Packham D, Shah A, Janitz C, Pimanda JE, Hawkins N, Ward RL, Hesson LB, Wong JW. Scarcity of recurrent regulatory driver mutations in colorectal cancer revealed by targeted deep sequencing. JNCI Cancer spectr. 2019;3(2):012.
Reimers N, Gurevych I. Sentence-bert: Sentence embeddings using siamese bert-networks. 2019; arXiv preprint arXiv:1908.10084
Devlin J, Chang M-W, Lee K, Toutanova K. Bert: pre-training of deep bidirectional transformers for language understanding. arXiv preprint arXiv:1810.04805 (2018)
Liu Y, Ott M, Goyal N, Du J, Joshi M, Chen D, Levy O, Lewis M, Zettlemoyer L, Stoyanov V. Roberta: a robustly optimized bert pretraining approach. 2019; arXiv preprint arXiv:1907.11692
Bowman, S.R., Angeli, G., Potts, C., Manning, C.D.: A large annotated corpus for learning natural language inference. arXiv preprint arXiv:1508.05326 (2015)
Williams A, Nangia N, Bowman SR. A broad-coverage challenge corpus for sentence understanding through inference. arXiv preprint arXiv:1704.05426 (2017)
Gao T, Yao X, Chen D. Simcse: simple contrastive learning of sentence embeddings. 2021;arXiv preprint arXiv:2104.08821
Wu, Z., Wang, S., Gu, J., Khabsa, M., Sun, F., Ma, H.: Clear: Contrastive learning for sentence representation. arXiv preprint arXiv:2012.15466 (2020)
Meng Y, Xiong C, Bajaj P, Bennett P, Han J, Song X, et al. Coco-lm: correcting and contrasting text sequences for language model pretraining. Advances in Neural Information Processing Systems. 2021;34
Hartigan JA, Wong MA. Algorithm as 136: a k-means clustering algorithm. J R Stat Soc. 1979;28(1):100–8.
Chen T, Guestrin C. Xgboost: a scalable tree boosting system. In: Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining. 2016; pp. 785–94
Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, Ye Q, Liu T-Y. Lightgbm: A highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. 2017;30
Breiman L. Random forests. Mach Learn. 2001;45(1):5–32.
Albawi S, Mohammed TA, Al-Zawi S. Understanding of a convolutional neural network. In: 2017 International conference on engineering and technology (ICET). 2017; pp. 1–6. IEEE
O’Shea K, Nash R. An introduction to convolutional neural networks. 2015; arXiv preprint arXiv:1511.08458
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay E. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Che H, Jatsenko T, Lenaerts L, Dehaspe L, Vancoillie L, Brison N, Parijs I, Van Den Bogaert K, Fischerova D, Heremans R, et al. Pan-cancer detection and typing by mining patterns in large genome-wide cell-free dna sequencing datasets. Clin Chem. 2022;68(9):1164–76.
Li J, Wei L, Zhang X, Zhang W, Wang H, Zhong B, Xie Z, Lv H, Wang X. Dismir: D eep learning-based noninvasive cancer detection by i ntegrating dna s equence and methylation information of i ndividual cell-free dna r eads. Brief Bioinf. 2021;22(6):250.
Nguyen L, Van Hoeck A, Cuppen E. Machine learning-based tissue of origin classification for cancer of unknown primary diagnostics using genome-wide mutation features. Nat Commun. 2022;13(1):4013.
Download references
Acknowledgements
The authors would like to thank the DAC for MCO colorectal cancer genomics at The University of New South Wales, for providing the data used in the study. The authors would also like to thank Prof. Jason Wong, for facilitating the data access requests and approvals.
The work reported herein was made possible through funding by the South African Medical Research Council (SAMRC) through its Division of Research Capacity Development under the Internship Scholarship Program from funding received from the South African National Treasury. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders.
Author information
Authors and affiliations.
Department of Computer Science, University of Pretoria, Pretoria, South Africa
Mpho Mokoatle & Vukosi Marivate
CapeBio TM Technologies, Centurion, South Africa
Darlington Mapiye
School of Medical Sciences, The University of Sydney, Sydney, Australia
Vanessa. M. Hayes
School of Health Systems and Public Health, University of Pretoria, Pretoria, South Africa
Riana Bornman & Vanessa. M. Hayes
You can also search for this author in PubMed Google Scholar
Contributions
MM conceptualised the work and wrote the main manuscript. VM and DM co-supervised and validated the results of experiments reported on the paper. RB and VMH provided expert advice on the topic and also reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mpho Mokoatle .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was granted by the University of Pretoria EBIT Research Ethics Committee (EBIT/139/2020). Data approval was granted by the DAC for MCO colorectal cancer genomics at UNSW.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Appendix A.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mokoatle, M., Marivate, V., Mapiye, D. et al. A review and comparative study of cancer detection using machine learning: SBERT and SimCSE application. BMC Bioinformatics 24 , 112 (2023). https://doi.org/10.1186/s12859-023-05235-x
Download citation
Received : 28 November 2022
Accepted : 17 March 2023
Published : 23 March 2023
DOI : https://doi.org/10.1186/s12859-023-05235-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cancer detection
- Machine learning
- SentenceBert
BMC Bioinformatics
ISSN: 1471-2105
- General enquiries: [email protected]
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
A comprehensive assessment of artificial intelligence applications for cancer diagnosis
- Open access
- Published: 20 June 2024
- Volume 57 , article number 179 , ( 2024 )
Cite this article
You have full access to this open access article

- Gaurav Singh 1 ,
- Anushka Kamalja 1 ,
- Rohit Patil 1 ,
- Ashutosh Karwa 1 ,
- Akansha Tripathi 1 &
- Pallavi Chavan 1
Artificial intelligence (AI) is being used increasingly to detect fatal diseases such as cancer. The potential reduction in human error, rapid diagnosis, and consistency of judgment are the primary motives for using these applications. Artificial Neural Networks and Convolution Neural Networks are popular AI techniques being increasingly used in diagnosis. Numerous academics have explored and evaluated AI methods used in the detection of various cancer types for comparison and analysis. This study presents a thorough evaluation of the AI techniques used in cancer detection based on extensively researched studies and research trials published on the subject. The manuscript offers a thorough evaluation and comparison of the AI methods applied to the detection of five primary cancer types: breast cancer, lung cancer, colorectal cancer, prostate cancer, skin cancer, and digestive cancer. To determine how well these models compare with medical professionals’ judgments, the opinions of developed models and of experts are compared and provided in this paper.
Avoid common mistakes on your manuscript.
1 Introduction
The recent advancement of AI applications in the medical sector has demonstrated success in rapid and precise diagnosis. The assessment of AI approaches used in cancer detection is considered essential since the disease is one of the most deadly and has a significant need for diagnosis. Many academics have conducted studies and developed methods to assess the effectiveness of a particular cancer detection technology.
By analyzing and contrasting the six primary cancer types provided in this paper, the primary objective of this study is to provide a broader view of various methodologies and malignancies in a single manuscript. This article is helpful to readers to get a comprehensive idea of cutting-edge AI methodologies, which further stimulates constructive research.
AI has made advances in many medical fields, including diagnosis, treatment, drug development, patient care, etc. Several studies on AI in cancer diagnosis have been undertaken. Cancer is still a leading cause of worldwide fatalities, and hence the error-free diagnosis of cancer is crucial. AI has proven to be significantly accurate in the imaging diagnosis of tumors (i.e., pathological diagnosis and endoscopic diagnosis). AI assists doctors in providing more accurate and effective medical care to patients (Dong et al. 2020 ).
Correct identification necessitates the mining of quantitative information for an informed diagnosis. However, human error is possible, and intricate faults make the issue worse. To effectively treat patients, AI must be used in therapeutic imaging assessment. Image segmentation, image registration, and image visualization are its three major pillars. In advanced countries, the Multidisciplinary Group (MDT), a team of specialists and medical professionals, is used to determine the most effective course of therapy for a condition. AI gathers data from numerous sources for a comprehensive analysis and treatment plan.
It is often expected (Shastry and Sanjay 2022 ) that a health professional’s diagnosis will be more precise with more information they have about the patient. The quantity of information that is currently available to health practitioners, however, is sometimes seen as overwhelming. Because it may be used to find pertinent patterns in complicated data, machine learning provides a solution to this issue. ML is applicable to “precision cancer treatment” for treating cancer patients. Based on the unique genetic profiles of each patient’s tumors, “precision cancer treatment” seeks to reliably forecast the appropriate drug treatments for a given patient (Injadat et al. 2021 ).
Deep learning (DL), a branch of machine learning, is employed in drug discovery, identification, and diagnostics to simulate human intelligence. It makes use of an Artificial Neural Network (ANN) to mimic how input is processed by artificial neurons, how output is generated, and the working of other hidden layers in the network. DL has been used in mechanical surgical treatments for gynecological disorders and heart valve replacement and is expected to play a significant role in the fight against cancer (Shastry and Sanjay 2022 ).
The literature is primarily derived from relevant past studies conducted on AI in cancer diagnosis. This survey consist of 36 research and review-based papers detailing AI applications and their comparisons across the diagnosis of major cancers. This review is created by thoroughly examining the relevant prior research and compiling the data in an organized manner. The primary objectives are to collect information about currently used and leading AI applications, their performance analysis, comparison of different study experiments, and understanding of the future scope in a single manuscript. This paper reviews heterogeneous set of studies for diverse cancer types to further analyze AI approaches, indicating a clear conclusion on the usage and influence of AI techniques in cancer detection. Furthermore, the utilization of numerous studies enables a clear comparison of methodologies to identify the best-performing models hence stimulating future research.
The studies conducted in the past on the most recent developments in AI for cancer detection primarily emphasized the evaluation of a particular method or experiment that led to the development of a more effective model. Other research experiments compared various approaches to see which ones are more effective against specific malignancies. This review adopts a different strategy by offering a thorough analysis of 6 significant tumors. The comparison of research is described, tabulated and potential new methods resulting from study experiments have been discussed. The primary medical modalities and AI techniques discussed in the paper are as shown in the Fig. 1 .

Medical and AI techniques discussed in the paper for six major cancer types
In the fight against cancer, AI techniques are powerful tools. AI is gradually being utilized for cancer screening. Automated approaches for detecting cancer are being implemented with the help of AI. Computer algorithms are being used to analyze magnetic resonance imaging (MRI) images, leaving little room for error. AI is also showing an immense contribution to drug discovery. Better cancer surveillance is taking place due to the advanced technology offered by the development of AI (Saba 2020 ). There are a variety of AI models for each cancer type discussed in this paper. Every method uses a different dataset to train and test the model. The training process with each AI technique may use biased input data. In this case, the generalized approach of AI for cancer detection needs to be presented. Data Preprocessing is an important strategy that needs to be performed in such a way that the training data fits the diverse groups. Another strategy is use bias detection techniques during the development of the model and deployment of the model. This important strategy is analysis of the data and the output of the model to identify potential biases.
This paper is organized as follows: First section gives the introduction. This section presents an extensive overview of AI applications in five primary malignancies. Section 2 presents a thorough analysis of the artificial intelligence methods utilized in the detection of breast cancer. Section 3 describes lung cancer detection techniques with detailed analysis. Section 4 presents AI techniques and their analysis for colorectal cancer type. Section 5 presents prostate cancer detection techniques using AI. Section 6 describes skin cancer and Sect. 7 describes digestive cancer detection techniques. Section 8 presents describes the ML approaches used for personalized cancer treatment using genotypic features. Section 9 presents a discussion on the all the techniques studied. The authors discusses here the problems with AI techniques and the confidentiality of data. Section 10 presents conclusion and future research directions.
2 AI techniques for breast cancer
AI-based breast cancer detection techniques leverage the power of artificial intelligence and machine learning algorithms to analyze medical data including mammograms, MRIs, and ultrasounds for early detection and diagnosis of breast cancer. These algorithms recognize the patterns and anomalies within the images that may indicate the presence of tumors or suspicious lesions, often with high accuracy. By continuously learning from vast amounts of data, AI systems can improve their performance over time. AI-based solutions help radiotherapists to become more skilled investigators and aid in the early diagnosis of breast cancer. Mammography, tomography, breast ultrasound, MRI, CT scans, and PET scans are the most frequently utilized tools for the diagnosis of breast cancer (Shastry and Sanjay 2022 ). Mammography is also known as breast screening. It is highly possible to detect breast cancer during screening requiring a minimal amount of time. Ultrasound technique delivers sound waves inside the body to examine the internal structure of the body. A transducer is used in this technique. This provides sound waves. It is located on the skin. They treat tissues as obstacles and react to them and echo. These echoes are recorded. The echo values are then transformed to grayscale for digital analysis. The positron emission tomography technique employs F-fluorodeoxyglucose. Imaging the body allows physicians to locate a tumor. The technique has its basis in detecting radiolabel cancer cells. These cells are specific tracers. MRIs are employed for detection. Elastography is a method that makes it possible to remove breast cancer tissue larger than normal parenchyma. Elastography is used for imaging by sound waves bouncing off the tissues. These waves are recorded, and the values are converted to grayscale for digital analysis. Elastography can differentiate between malignant and benign tumors (Sharif 2021 ). Active microwave imaging is a newly discovered technique for early detection in breast detection. When microwaves are bombarded with cancer tissues, they exhibit properties very different from healthy tissues (Bindu et al. 2006 ).
Mammography has been proven as one of the most useful screening tools for breast cancer. In previous investigations, half of the mammographically identified breast tumors were evident. Double reading of mammograms have been proven more potent in comparison to a single reading. In double reading, the sensitivity rate is enhanced by 5–15% (Watanabe et al. 2019 ). 10–30% of breast cancers are missed on mammography, which is ascribed to thick parenchyma hiding lesions, inadequate placement, perception mistakes, and interpretation errors.
The goal of the study by researchers was to create an artificial intelligence algorithm for mammography breast cancer diagnosis and observe its improved diagnostic accuracy. An AI algorithm was developed and validated. This experiment was carried out using 170,230 mammography examinations from five institutions in the United States, South Korea, and the United Kingdom. For the multicentre, observer-blinded, reader study, 320 mammograms were obtained independently from two institutions, with 160 being cancer-positive, 64 benign, and 96 normal. 14 radiologists acted as readers, assessing each mammogram for the probability of malignancy, likelihood of malignancy (LOM), location of malignancy, and the need to remember the patient, first without and then with the AI algorithm’s assistance. The performance of AI and radiologists was evaluated using the LOM-based area under the receiver operating characteristic curve (AUROC), as well as recall-based specificity and sensitivity.
To diagnose breast cancer, an AI system was built that employs the biggest breast cancer data set among existing AI algorithms. The algorithm was able to exhibit comparable performance in validation data sets from different nations since it was trained with data from multiple institutions. With the use of large-scale mammography data, the AI program outperformed doctors in terms of diagnosis, notably in early-stage invasive breast tumors. Mammographic characteristics of tumors found by the AI system were analyzed through a comparative study with radiologists to gain a better understanding of AI behavior. This comparison is shown in Fig. 2 .

Comparison of radiologist observations and AI performance
When compared to radiologists, AI algorithm constructed using large-scale mammography data performed better in breast cancer detection. The considerable increase in radiologists’ performance was observed when assisted by AI justifies using AI as a diagnostic assistance tool for mammography. According to this study, AI has the potential to improve early-stage breast cancer diagnosis in mammography. The performance of radiologists was greatly enhanced when assisted by AI, particularly in thick breast regions on a mammogram, which represents one of the primary challenges in screening. Such advancements leads to an increase in screen-detected malignancies and a decrease in interval cancers, improving mammography screening effectiveness. (Kim 2020 )
The radiologists’ accuracy is compared with AI systems using a non-inferiority null hypothesis (margin = 0.05) based on differences in the area under the receiver operating characteristic (AUROC) curve. The sensitivities of the radiologist and the AI system are compared with HMRC using standard analysis of variance (ANOVA) of two multi-reader multi-case (MRMC) modalities with the same level of specificity. The AUC of the AI system (0.840) is statistically non-inferior to that of the 101 radiologists (0.814) with a difference of 0.026 resulting in slightly higher for the AI system for low and mid-specificity.
Although the AI system has shown better results than the collective performance of the radiologists, it consistently performs low compared to the best radiologist. An ideal AI system should be able to function to the limits of the imaging modality itself, in other words, not be able to detect mammographic occult cancers while minimizing false positives. These systems are suitable for mass population screening to provide better and consistent results in a short time. It is beneficial in countries that lack experienced breast radiologists. The tested AI system based on deep learning algorithms has a similar performance to an average radiologist for detecting breast cancer in mammography (Rodriguez-Ruiz et al. 2019 ).
From previous mammograms, the radiologists studied and classified the retrospective data into three categories:
Actionable: This mammography was found in 155 previous mammograms from 90 individuals.
Non-actionable: In this category, because the lesion that was subsequently biopsied fell below the recollection threshold, it is not possible to be utilized.
Excluded: In this category,prior lumpectomy on the ipsilateral side and synthetic or three-dimensional tomography pictures are excluded (Watanabe et al. 2019 ).
cmAssist is a newly invented AI-CAD for mammography that uses deep learning, a prominent kind of artificial intelligence. The cmAssist method uses a combination of unique deep learning-based networks to accomplish great sensitivity without losing specificity. In addition, to enrich the varied appearances of cancer and benign structures in training dataset, the algorithms are trained using a proprietary, patent-pending data enrichment approach. The dataset consisted of 2D Full-Field Digital Mammograms (FFDM) collected from a community healthcare facility in Southern California for a retrospective study. All patients in the collected dataset were females, aged 40–90 years, who had a biopsy performed between October 2011 and March 2017. Of 1393 patients, 499 had a cancer biopsy, 973 had a benign biopsy, and 79 had both cancer and benign biopsies. The training set is made up of photos taken using a variety of mammography machines of different kinds.
Radiologist accuracy enhanced substantially as a result of the usage of cmAssist, as shown by a 7.2% improvement in the area-under-the-curve (AUC) of the receiver operating characteristic (ROC) curve for the reader group with a two-sided p-value of 0.01. With the usage of cmAssist, all radiologists observed substantial increase in cancer detection rate (CDR) with two-sided p-value of 0.030 and a confidence interval of 95%.
When radiologists employed cmAssist, their accuracy and sensitivity for identifying cancers that had previously gone undetected improved significantly. After utilizing cmAssist, the percentage improved drastically in CDR for the radiologists on the reader panel which went from 6 to 64% (mean 27%), while there is no increase in the number of false-positive recalls.
On this data set of missed malignancies, cmAssist has a maximum feasible sensitivity of 98% when used in stand-alone mode. Future work in AI-assisted false-positive reduction is underway, leading to even greater gains in cancer diagnosis accuracy (Watanabe et al. 2019 ).
2.1 Breast cancer detection using artificial neural networks
Basic data mining problems like classification and regression are effective in solving the breast cancer detection problems. While Artificial Neural Networks are frequently used to identify breast cancer with good amount of accuracy. ANN based design consists of a collection of linked neurons organized into three layers: input, hidden, and output. This kind of network learns to perform assigned tasks by taking into consideration a sufficient number of instances. The input layer consists of several neurons which is equal to the number of characteristics in the dataset. The hidden layer is another component of ANN with all hidden layers counted as a single one. In the past experiments with ANN, 31 neurons are connected to 9 of the hidden layers. There is a 9–9 mapping of connection between the first two hidden layers. As the problem is of the binary classification there is only a single neuron at the output.
The cross-validation was executed using a ten-fold technique, which meant that the dataset was divided into ten equal groups. The dataset used is the breast cancer dataset acquired from the University of California Irvine (UCI) machine learning repository. This dataset consists of 699 instances classified as benign or malignant, with 458 instances (65.50%) being benign and 241 instances (34.50%) being malignant. The model was calibrated for 100 epochs with five batch sizes, and the activation function is employed in the hidden layers while the sigmoid is employed at the output layer. The ANNs outperformed and computed 98.24% accuracy. The model produced by ANN is more robust and accurate than any other method, and it has the scope to make major advancements in breast cancer prediction.
However, ANN does not account for the training time and False Negative (FN) outcome when training the model. The researchers proposes a new strategy and the usage of the convolutional auto-encoder for breast cancer detection. The proposed hybrid convolutional network uses two models of convolutional autoencoders. First for picking up features and the second for categorizing those features. The fully connected layer is used for the output of the convolutional layers for categorizing input pictures as benign or malignant only after acquiring the most critical attributes. With a shorter training time, the suggested model achieves superior performance. The model’s sensitivity is found as 93.50% which is better than the previous studies (Sharif 2021 ).
Research has proven ANN to be highly accurate in case the of a breast cancer diagnosis. However, this method has some limitations:
ANN has some parameters to be tuned at the beginning of the training process such as hidden layers and hidden nodes, learning rates, and activation function.
It takes a long time for the training process due to the complex architecture and parameters update process in each iteration that needs expensive computational costs.
It can be trapped to local minima so that the optimal performance cannot be guaranteed.
Several attempts have been made to find answers to the constraints of neural networks. Huang and Babri showed that Single Hidden Layer Neural Networks (SFLN) can tackle those problems using a three-step extreme learning procedure termed ELM. ELM was compared to traditional Gradient-Based Back Propagation Artificial Neural Networks in terms of performance (BP-ANN). Performance is measured using sensitivity, specificity, and accuracy. It is observed that Extreme Learning Machine Neural Networks (ELM ANN) gives superior results in general than BP-ANN. Doctors may benefit from intelligent classification algorithms, particularly in reducing errors caused by inexperienced practitioners.
Three main differences between BP-ANN and ELM ANN are observed based on the definition:
We need to adjust several parameters like the number of hidden nodes, momentum, rate of learning, and termination criteria for BP ANN. Whereas, ELM ANN is an easy algorithm that only requires defining the number of nodes and no tuning.
In hidden and output nodes, ELM ANN can utilize both differentiable and non-differentiable activation functions whereas BP ANN can only employ differentiable activation functions.
The BP ANN is trained with a model which has a low training error to terminate at a local minimum. Whereas, ELM ANN is trained with a model that has minimal training error and weight norm, allowing it to develop better-generalized models and reach global minima.
The performance measures used for classification problems were accuracy, specificity, and sensitivity. Similar experiments were conducted with BP ANN for comparison. Results showed that generally ELM ANN was better than BP ANN.
ELM ANN surpassed BP ANN in breast cancer diagnosis. Even though the specificity rate found slightly lower, sensitivity and accuracy rates are significantly better in ELM ANN. The researchers concluded that ELM ANN has an enhanced generalization model than BP ANN based on these outcomes. The dataset used for these findings is the Breast Cancer Wisconsin Dataset obtained from the University of Wisconsin Hospital, Madison by Dr. William H. Wolberg. The dataset consists of 699 instances with 10 attributes plus the class attributes, with a class distribution of 65.5% (458 instances) for benign and 34.5% (241 instances) for malignant (Prasetyo et al. 2014 ).
2.2 Breast cancer detection using active microwave imaging
Microwave imaging is an upcoming technique that offers therapeutic applications. The interactions with the tissues are studied. The malignant cancer tissues show visibly more scattering than the normal tissues. This can help in the detection of the tissues in the early stages where cancer could be cured. In this technique, malignant tissues are estimated to have higher water content than normal ones.
Methods in Active Microwave Imaging are sated below:
Confocal microwave technique: It is a non-ionizing technique. This method takes advantage of the fact of the see-through properties of the breast to create a dielectric contrast of tissues based on water content. Time shifting and the summing of the signals are used for the detection of cancer tissues. Acquiring data: There is an antenna that is used as a trans-receiver. The antenna is set around the sample to observe it. An observation is taken every 10 degrees. At last, signals are recorded at every step added to get the final signal. The data is validated using time-domain analysis based on the finite difference method.
2D-microwave tomographic imaging: This is the traditional method used in breast cancer diagnosis. Acquiring data: The breast sample is brought into view using the bow-tie antenna. A frequency of 3000MHz is used for the sample. The antenna behavior is the same as the confocal microwave technique. The sample readings are taken in steps of 10 degrees. With the experimentation on active microwave imaging, following conclusions are made:
Active microwave imaging is very useful in the detection of cancer in its early stages.
Studies show that microwave tomographic imaging could effectively distinguish and image the tissues showing the significant difference in dielectric-permittivity.
Tumor location can be determined using Confocal Imaging. This is achieved by variations seen in signal strength due to water content (Bindu et al. 2006 ).
Table 1 represents the observations among AI-enabled Breast Cancer detection techniques.
3 AI enabled lung cancer detection techniques
The AI techniques share similarities in their application to breast cancer as well as the lung cancer. Each cancer type mentioned here presents unique challenges and considerations. Therefore, AI solutions are tailored to address the specific characteristics and complexities of each cancer type, as well as the clinical context in which they are deployed. The safety of AI-based approaches in cancer diagnosis and treatment for breast cancer and the lung cancer is measure with the similar set of tools.
AI-based lung cancer detection techniques use advanced machine learning algorithms to analyze medical data including chest X-rays, CT scans, and PET scans for the early detection and diagnosis of lung cancer. These algorithms are trained on large datasets of annotated images to recognize subtle patterns and abnormalities indicative of lung tumors, often achieving high levels of accuracy in classification tasks. AI systems can assist radiologists by flagging suspicious areas for further examination, reducing interpretation time, and potentially improving diagnostic accuracy. The most popular ways of detecting lung cancer are lung radiography and computed tomography (CT) scans (Mustafa and Azizi 2016 ).
3.1 CT lung cancer detection
CT lung cancer detection is one such AI-backed solution that is used to aid physicians, lowering their workload, improving hospital operational processes and giving them more time to create a high-quality doctor-patient connection. Computer-aided detection (CAD) employing CT scans relieves clinicians’ workload and increases efficiency by discovering previously undetected lung cancer nodules (Sathykumar and Munoz 2020 ).
Small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC) are the two common types of lung cancer used for therapeutic reasons. Lung cancer staging is a method of determining the extent to which cancer has progressed from its initial site and it is one of the elements that affect lung cancer diagnosis and possible treatments (Mustafa and Azizi 2016 ).
Radiologists employ X-ray imaging of the lungs to diagnose early lung cancer. When existent tissue is to be separated from the absence of an early tumor, radiology physician monitoring becomes more challenging. This accurate detection result may aid radiologists in more properly assessing and diagnosing patients’ ailments. The study’s purpose is to develop smart application software or intelligent machines that can identify and categorize early tumor types by utilizing an artificial neural network. This precise detection result may assist radiologists to analyze and diagnose patients’ diseases more effectively so that early lung tumor test findings might benefit patients in treatment by avoiding danger or no risk (Pandiangan et al. 2019 ).
The authors compared and assessed the results of four separate research experiments. Sensitivity, accuracy, the area under the curve (AUC), specificity, and the receiver operation characteristics (ROC) curve are some of the metrics that the models examined. High specificity number suggests a low rate of lung cancer misdiagnosis whereas a low specificity value indicates a high probability of false positives. The model’s accuracy is the percentage of data that was properly categorized. Finally, both the ROC and AUC curves are employed in the various research group model performance measurements.
In a research experiment done by Toğaçar et al. the authors used lung CT images for lung nodule cancer detection. The use of image augmentation, Minimum Redundancy Maximum Relevance (MRMR), principal component analysis (PCA), and suitable feature selection resulted in the model performing very accurately. Out of the various iterations, the use of deep features with KNN and MRMR gives the best result with an accuracy of 99.51%. Three other research studies considered got a less accurate result in comparison due to the lack of image augmentation and feature selection techniques (Sathykumar and Munoz 2020 ).
3.2 AI based lung segmentation techniques
The researchers devised a lung segmentation technique to improve segmentation accuracy and separate and remove the trachea from the lungs. Digital image processing techniques have been utilized to improve quality and accuracy, as well as to demonstrate the evolution of the field.
The approach used in research is focused on meeting the goals of the study. Traditional lung X-ray image processing and ANN procedures are used to identify and classify early cancers. These findings are utilized to help enhance visual observation of the target item. The image processing system includes pre-processing, image noise reduction,image enhancement,lung organ segmentation, object edge detection, and tumor boundary identification. In X-ray pictures, low contrast is used to distinguish between malignant and non-malignant tumors.
The back-propagation technique is used in this study. The benefit of this technique is that it properly reduces mistakes caused by the discrepancy between the actual output and the predicted outcomes. The performance of artificial neural networks with graphics is demonstrated.
Image processing techniques are used on 25 lung nodule samples and 25 non-lung nodule samples from each of the 50 standard lung X-ray samples of parents. An artificial intelligence (AI) machine has been developed that can identify 10 samples correctly. The machine works by combining two-dimensional (2D) X-ray images with previously studied tumor characteristics. After training with a large dataset, it should be able to achieve close to 100% detection performance (Pandiangan et al. 2019 ).
Recently, Cengil and Cinar proposed a CNN-based model for the prediction of lung cancer. This technique makes use of the TensorFlow library for the detection mechanism. The authors used SPIE-AAPM-LungX dataset which contains images of 60 patients. The model supports an accuracy of 70% which is less than the standards required.
Another CNN-based approach is presented by Sasikala et al. This approach is applied to Lung Images Dataset Consortium and Image Dataset Resource Initiative (LIDC-IDRI) dataset. The pre-processing layers consisted of median filters which removed any unwanted features from the images. The accuracy achieved was 96%. A comparison study is presented by Gunaydin et al. They compared models like KNN, SVM, and decision trees to detect lung cancer. Data set used in this study is the Standard Digital Image Dataset from the Japanese Society of Radiology Technology. All techniques shows good results with the accuracy of 95.05%. Asuntha and Srinivasan proposed a different model with new approaches using deep learning techniques. They have used highly efficient feature extraction techniques such as wavelet transform, HoG nodes, Zernike moment, SIFT, and LBP. After the primary feature has been extracted, a fuzzy particle swarm optimization is used to select the most evident features. The model is tested on several data sets and has shown the accuracy of 97% (Patel 2022 ).
Chemotherapy, radiation, and surgery are the methods for treating lung cancer, depending on the kind of cancer. One of the most commonly used surgical treatments for the initial stage of lung cancer is the elimination of a lung lobe. The chemotherapy treatment is determined by the kind of tumor. In advanced instances, chemotherapy increases overall survival as well as the quality of life when compared to supportive treatment alone. For patients who are unable to have surgery, radiotherapy is frequently combined with chemotherapy. Smoking cessation and prevention are both effective methods for avoiding the development of lung cancer. Long-term vitamin A, D, or E supplementation has not been shown to lessen the incidence of lung cancer. The larger consumption of fruits and vegetables appears to reduce the risk, though the fact is that there is no established link between food and lung cancer (Mustafa and Azizi 2016 ).
Despite the many diverse types of cancer, lung cancer, with its unique development and spreading processes, can influence normal cells and disturb the cell signaling process, which modifies the function of cell division. To date, an enormous number of studies have been conducted in several aspects of diagnosis of cancer or pre-cancer stages by using artificial intelligent systems-based algorithms. Some algorithms are supervised and some set of algorithms are un-supervised along with different features extracted from pathological images. To the best of the author’s knowledge, there have been no systematic review and meta-analysis studies to evaluate the performance as well as to estimate the current status of existing approaches to lung cancer. The well-known databases are explored in this systematic review and meta-analysis based solely on a Boolean query for lung cancer and the accompanying artificial intelligent systems. Preferred Reporting Items for Systematic Reviews and Meta-Analyses Diagnostic Test Accuracy (PRISMA-DTA) is used to conduct the systematic review, The English-language papers that use various sorts of prediction models to distinguish between healthy and malignant cell pictures are taken from the databases. The relevant publications that had the necessary data, such as the true positive, true negative, total sample size, false positive, and false negative values, are then chosen from the search results. The studies with insufficient data are excluded from further examination.
The subsequent research employed a variety of artificial intelligent systems, including support vector machines (SVMs), artificial neural networks (ANNs) with diverse training strategies, and statistical techniques, despite the different retrieved features from lung cancer images. Artificially intelligent systems are used in each of them to make decisions similar to those of a clinical practitioner when diagnosing lung cancer. I-squared parameters generated by Meta-DiSc software enable a detailed assessment of the heterogeneity in a meta-analysis study and the potential impact of the included studies on it. I-squared indices are classified as low, moderate, or high depending on whether they fall within the range of 0 to 25%, 25 to 50%, or above 75%. Additionally, the SROC curve and the estimated values for the combined diagnostic odds ratio, AUC, sensitivity, and specificity are 0.77, 0.74, 17.22, and 0.872, respectively. Since the AUC value is higher, it is concluded that artificial intelligence systems are effective in differentiating between types of lung cancer. The findings for the development trends in the quantity of success in the performance of the artificially intelligent systems have been presented, taking lung cancer diagnosis and conducting a meta-analysis on the papers into consideration. Eventually, two publication bias tests have shown that the possibility of publication bias exists. Additionally, while sensitivity and specificity trends are moderate, the diagnostic odds ratio and AUC values shows enormously high trends (Sokouti et al. 2019 ).
Table 2 represents the critical observations of AI techniques for lung cancer detection.
4 AI techniques for colorectal cancer
The lung cancer detection techniques discussed in the previous section share some commonalities with colorectal cancer in terms of diagnostic and treatment challenges. These two cancer types are distinct in terms of anatomical notation. Clinical decision support systems are used in both the cases of lung cancer as well as colorectal cancer. Artificial intelligence has seen significant growth in tumor evaluation for CRC over the past years. Various techniques and steps involved in tumor recognition are the evaluation of some categorical as well as numeric data. Obtaining a correct diagnosis from clinical data during the initial steps of the medical assessment might reduce the chance of human error while also saving time. Colonoscopy, one of the most widely used computer-aided diagnosis techniques, is the most commonly used method for detecting and screening colorectal cancer (Lorenzovici and Dulf 2021 ).
4.1 Prediction of colorectal cancer using AI
The high occurrence and fatality rate of CRC presents the question of artificial intelligence’s usage in colorectal cancer epidemiology. As broad approaches to understanding research and data have started, several stages of using deep learning in epidemiological studies have started. However, they do experience some difficulty because of factors like data access, classification and accuracy. Various facets of artificial intelligence (AI) utilized for colorectal cancer prediction include:
Geo AI: It is a subset of AI that was initially used in environmental healthcare and now being used by healthcare professionals. It functions by using information from a given region to collect more specialized data using artificial intelligence. Information is readily available on a wide range of topics, including geographic (terrain) information, food consumption information, healthcare information etc. The idea behind GeoAI is that particular regions that have a higher rate of colorectal cancer can gain the most from this kind of data collection.
Digital epidemiology: It is a promising but possibly divisive area of AI. This technique focuses on obtaining data from digital sources including social media and digital devices, and this data is utilized for the early identification of SARS and real-time surveillance of Covid-19. This provides access to a vast body of knowledge that was previously unavailable to doctors and can help with early illness identification and public health surveillance. The predicted information might not, however, be what the doctors require.
Data mining: It is another application that serves as a practical tool. It analyzes massive amounts of data gathered from several sources and extracts facts, patterns, and data from them. When used to diagnose colorectal cancer, data mining tools helps in finding connections, linkages, and a variety of other potentially hidden characteristics that are never explored and may contain a significant piece of the information for what causes colorectal cancer (Yu and Helwig 2022 ).
4.2 AI techniques in colorectal cancer screening
Colonoscopy: It is a highly rated method due to its attributes like high sensitivity as well as decision-making power on lesions. However, small-size or flat polyps have a high chance of being missed by the naked eye (Yu and Helwig 2022 ). A flexible tube (endoscope) with a small video camera at the tip is used to do colonoscopy exploration. When polyps bigger than 10 mm are detected, traditional colonoscopy is effective. Although DCE (dye-based chromoendoscopy) may identify microscopic or flat polyps, it has significant limitations, such as interobserver and intraobserver variability. White light alone is used in an AI-based method to determine whether polyps are present in a video frame. Polyp categorization is the process of cataloging the type of polyp found after it has been discovered. Using three well-defined methods, depending on whether the polyp seems to be benign, pre-malignant, or malignant, the doctor can make a better judgment about whether or not to remove it. Colorectal polyps are characterized as adenomatous, hyperplastic, or serrated and inflammatory based on their anatomical pathogenesis. AI might help medics distinguish polyps in the future, which is a promising development. There is a form of serrated polyp known as a sessile serrated adenoma that is not generally neoplastic (Viscaino and Bustos 2021 ). An experiment study of colonoscopy with 1058 patients was conducted, which resulted in the conclusion that colonoscopy reduces the mortality rate.
Blood tests: Studies have found that certain blood test data and some features could be used to identify patients carrying the risk of CRC. One such test is the Complete Blood Count. Slow bleeding from cancer in CRC causes iron deficiency anemia, which is accounted for by this test. A machine learning model, MeScore, is developed and tested rigorously for calculating the risk factors of CRC.
CT colonography: A non-invasive imaging test for CRC is CT colonography (CTC). The ability of CTC to diagnose and distinguish between various lesions can be reduced, and the capacity for detection can be increased by CAD.
Colon capsule endoscopy: It is an invasive procedure. It is to be noted that the invasion is minimal. It required more laxatives. It removes the capsule from the GI tract by flushing it out. It required manual reading, which increases the error rate. AI techniques are applied to these images to interpret the results.
Polyp characterization: This is a critical step. The characterization of polyps carry a lot of weight. AI comes into play here for predicting malignant or benign lesions. Most colorectal polyps are hyperplastic. Even in that case, it is absolutely important to have a high-precision diagnosis. A retrospective study was carried out at three hospitals, using methods like conventional white light endoscopy, etc., to construct and evaluate deep learning models for the automatic classification of colorectal lesions histologically in white-light colonoscopy images. This study contained a total of 3828 pictures from 1339 participants. The results showed a promising future for deep learning models.
Magnifying narrow-band imaging: It is a less bandwidth-intensive form of endoscopy that uses image enhancement. It is an optical filter. These filters are used for green and blue light illumination in a sequence. It comes in handy for polyp characterization. A computer-based method is tested for classification. All 214 study participants underwent a zoomed NBI (narrow band imaging) colonoscopy and had a total of 434 polyps measuring 10 mm or less in size. The diagnostic agreement between two specialists and computer-aided classifier systems is 98.7%, with no discernible difference.
Magnifying chromoendoscopy: It is a technique to amp up the visualization of pit patterns on the surfaces of polyps to distinctly identify benign and neoplastic polyps. An analysis is done on an automated computer system named HuPAS, which could outline the boundaries of the pits. The aided system is in 100% compliance with the expert diagnosis for type I and II pits. For types IIIL and IV, it shows values of 96.6 and 96.7, respectively.
Endocytoscopy: This technique involves magnifying real-time images by 380–500 folds. This allows for clear visuals. In esophageal cancer (EC), a contact light microscopy system is added to the colonoscope’s distal tip, which permits instantaneous evaluation of nuclei and cytological structures. CAD-EC is developed to automate export-dependent diagnosis. The database consists of images from 242 patients. CAD-EC shows a sensitivity of 89.4%, a specificity of 98.9%, an accuracy of 94.1%, a PPV of 98.8%, and an NPV of 90.1% in differentiating invasive cancer from adenoma.
Confocal endomicroscopy/confocal laser endomicroscopy: It allows for real-time \(\times 1000\) image magnification. This technique requires a lot of training and is hence performed only by experts. To distinguish between neoplastic and non-neoplastic polyps, a study of the diagnosis capacity of computer-based automated pCLE classification was conducted, and it was contrasted with experienced endoscopists who made a diagnosis based solely on pCLE recordings. For computer-based automated pCLE classification and expert performance in differentiating neoplastic and non-neoplastic lesions, the results showed a sensitivity, specificity, and accuracy of 92.5 vs. 91.4%, 83.3 vs. 84.7%, and 89.6 vs. 89.6%, respectively. These differences were not statistically significant.
Laser-induced fluorescence spectroscopy: This technique gives us a real-time automated distinction between benign and neoplastic polyps. It includes an optical fiber device called WavSTAT. This component emits laser waves onto targeted tissue and then releases the light to give the results. A new version of LIFS is studied which makes use of WavSTAT4. The accuracy of LIFS using WavSTAT4 in predicting polyp histology was 84.7%. For distal colorectal diminutive polyps only, the NPV for excluding adenomatous histology increased to 100%. The dataset used was a combination of electronic medical record data from two unrelated patient populations in Israel and the UK to develop an ML-based prediction model for identifying individuals at high risk of colorectal cancer based on attributes such as complete blood count (CBC), age, and sex (Goyal and Mann 2020 ).
4.3 AI for colorectal cancer diagnosis
One of the numerous sectors in which machine learning techniques are gaining prominence is the development of computer-aided diagnosis systems. The most widely used traditional machine-learning algorithms in medical applications for data analysis are decision trees. Important factor when working with CAD (computer-aided diagnosis) in tumor recognition is confidence analysis (Lorenzovici and Dulf 2021 ). CAD tries to locate aberrant or suspicious areas in order to improve detection rates while lowering false-negative rates (FNR). (Viscaino and Bustos 2021 )
Endoscopy and MRI/CT imaging: One of the most effective methods for diagnosing colorectal cancer makes use of convolution neural networks (CNN), an ANN type, and computer vision, endoscopy, and MRI/CT imaging. It analyses a vast number of images to find patterns and objects. The first steps in improving the endoscope have been made possible by segmentation technology, which derives from computer vision’s capacity to distinguish between things. The important thing to remember is that optimal segmentation would allow for the separation of anatomy and malignant and normal masses. Pattern recognition is used to distinguish between normal and abnormal conditions. The detection, segmentation and classification of pictures are currently the most significant areas where AI has been able to influence this kind of imaging. Additionally, general initiatives to enhance picture quality for better reading and advancements in segmentation technology have been observed. Along with the obvious advantages of improved diagnostic accuracy, using deep learning in colonoscopies also has the added benefit of reducing any variability in detection rates. One of the major challenges was dealing with bigger artifacts, which might lead to misunderstanding of data or false-positive results. However, the lack of relevant datasets is the main issue. When attempting to employ CNNs for image or object identification, there are generally certain restrictions or problems. Pixel appearance anomalies in images can cause misinterpretations owing to missing or irregular pixelation. Because anatomy is not always the same in all people, it might be mistakenly perceived. Anatomical variations or patient posture practices can affect how an image is taken, causing positional alterations that could result in misunderstanding. Additionally, it might be difficult to distinguish between useful information and information that is only an artifact when segmenting data. The limitations of endoscopes and CT/MRI imaging serve as a reminder that AI is still being improved and is not yet ready for full application and they also highlight the continued need for technicians in the interpretation of imaging.
Genetic and pathological diagnosis: Some progress has been made in training AI to categorize cancers based on histology. When inflammatory tissue are present in data sets, the systems, however, has identification problems. The inability of AI to detect pictures may not be its fault; instead, CNNs requires higher-quality training data, and stain normalization in hematoxylin and eosin pictures are able to aid AI by increasing its accuracy. By paying more attention to characteristics like textures, spatial connections, and morphology, the accuracy may be increased even further. Fuzzy systems have also proven effective since they allow for more thorough information interpretation. Any type of pathology can use this scope to find abnormalities or forecast the possibility of malignancy based on predetermined criteria. (Yu and Helwig 2022 )
4.4 AI for colorectal cancer treatment
Therapeutic assessment: Rectal cancer treatment using an AI model enables the early-onset differentiation between therapeutic full response (CR) and not-respond to therapy (NR). AI makes it possible to comprehend some metabolic processes and drug-induced changes that are directly related to the development of colorectal cancer. AI has significantly enhanced the processing of complicated networks of biological information. Additionally, the accuracy of AI’s colorectal cancer ANN algorithm prediction is rising in importance. ANN is distinguished by nonlinear models that are adaptable to medical and clinical research. The following are a few advantages observed with this technique:
It has the potential to improve the optimization process, resulting in adaptable nonlinear models that are cost-effective, particularly in huge data scenarios.
It predicts clinical outcomes with high accuracy and reliability.
Improves academic dialogue and knowledge dissemination.
IBM Watson for oncology: Clinical decision-support systems (CDSSs), a new AI trend for therapeutic suggestions, shows enormous potential for therapeutic management in cancer given the increasing growth of clinical information. By imitating human thinking, it makes it possible to acquire and analyze knowledge in a way that is superior to the traditional human touch. The selection of cancer therapies has been expedited by AI with a cognitive computing system called IBM Watson for Oncology (WFO). WFO is gaining recognition in the field of cancer therapy. It formulates recommendations using natural language processing and clinical data from many sources (treatment guidelines, professional opinions, works of literature, and medical records). The datasets used in the study included 10,000 to 40,000 CT images for studies on CT imaging and AI. These images were gathered by the research parties themselves. AI training data is frequently labeled and supervised. The known input and output values have been connected clearly. However, doctors’ notes and other unstructured, person-specific information make up the majority of a case record, making it challenging to feed WFO (Yu and Helwig 2022 ).
Colorectal surgery is another promising area in which AI has been used. According to research, AI greatly decreased the need for extra surgery after T1 CRC endoscopic resection. In particular, a support vector machine for supervised machine learning was developed to identify patients who required extra surgical intervention. As a result of surgery’s difficult, time-consuming, and non-scalable nature, the use of AI in surgery is still limited. The only purpose of AI’s presentable results is to expedite the decision-making process (Yu and Helwig 2022 ).
An experiment was performed by Noémi Lorenzovici et al. to maximize the effectiveness of the colorectal cancer detection system by using an innovative dataset. For every patient, the dataset contains 33 blood and urine samples. Also, when determining the diagnosis, the patient’s living environment was taken into consideration. Various machine learning approaches, such as classification and shallow and deep neural networks are used to create the intelligent computer-aided colorectal cancer diagnosis system. The problem is solved using two approaches, traditional machine learning algorithms, and the regression problem solved using artificial neural networks. The initial step in the first approach is pre-processing and it is done by classifying each record from the dataset as unhealthy (denoted by 1) or healthy (denoted by 0). If there is the presence of other diseases or risk factors, then it is denoted as 1, otherwise 0. The response variable is obtained using two approaches; the first, absolute deviation; and the second, by finding the weight of each predictor. In the first approach, the weights are calculated on the assumption that each variable had roughly the same effect on the diagnosis. The second method for determining the proper weights is to expand individual variables’ healthy and unhealthy ranges. But even though the second technique appeared to be more rational than the first, the results did not support these assumptions in the theory.
The first goal of the experiment is to solve the binary classification problem. Therefore, for the training of the model, a labeled response variable is needed to represent the answer of the model to the input data. After obtaining the predictors and the response variable, various models are trained using the Classification Learner toolbox in MATLAB. This toolbox allows to train models, and each model is trained using two separate validation strategies, each with three alternative approaches. The first validation technique used is k-fold cross-validation and it had various approaches such as 5-Fold Cross-Validation, 25-Fold Cross-Validation, and 50-Fold Cross-Validation. Regarding the percentage of data held out, three different methods are tested, and those are 5% of data held out for validation, 15% of data held out for validation, and 25% of data held out for validation.
In the second approach, taking into consideration the fact that deep neural networks perform better on large datasets than basic machine learning algorithms, solving a regression problem was not performed using the regression learner but rather with the Neural Network Toolbox in MATLAB. The first three types of neural networks are shallow neural networks, having only one hidden layer and a varying number of neurons on that hidden layer. In the first case, when the hidden layer has only three neurons, an MSE(mean squared error) of 1.94 is obtained after training the network. In the second case, a network with 10 neurons on one hidden layer is constructed, and in the third case, a shallow neural network having 20 neurons was made. The next three networks are built on a 5-hidden layer architecture and, therefore are called deep neural networks. Evaluating the results obtained after training the first network with five hidden layers, it is concluded that this network has the worst performance. After training the classification algorithms, the resulting models’ performances are compared. Traditional machine learning techniques achieves a maximum accuracy of 77.8% while solving the binary classification problem. In terms of mean squared error minimization, however, the regression issue addressed with deep neural networks performed substantially better, with a value of 0.0000529. In conclusion, deep neural networks are both more reliable and more efficient in colorectal cancer detection than traditional machine learning algorithms (Lorenzovici and Dulf 2021 ).
Although evaluation of slides by pathologists is the standard for cancer detection, with the increase in workload the authors have seen the development of computerized screening. This is done by whole slide images (WSI), which are a digital form of glass slides. computer-aided diagnosis (CAD) is possible through the application of medical image analysis, CNN, and AI. With increasing cases of CRC, the number needed to diagnose increases as a study shows only 7.4% of cancers are detected as positive.
Unique AI based deep learning model is trained for screening colonic malignancies in colorectal specimens for better detection and classification. This unique algorithm developed by Qritive consists of two models, first to detect the high-risk regions in Whole Slide Images (WSIs) by gland segmentation and second to classify the WSIs into “low risk” (benign, inflammation) and “high risk” (dysplasia, malignancy) categories. The WSIs of 294 colorectal specimens are collected from the archives of Singapore General Hospital’s pathology and The Cancer Genome Atlas (TCGA). The attributes selected from the data included the histological features present in the WSIs, such as glandular structures, dysplastic regions, blood vessels, necrosis, mucin, and inflammation, which were used to train the AI models for accurate detection and classification of high-risk regions indicative of malignancy. Slides containing poor-quality images and other malignancies are excluded. The gland segmentation model is a deep learning model that uses Faster Region-Based Convolutional Neural Network (Faster-RCNN) and Resnet-101 for feature extraction. The second model is a gradient-boosted decision tree classifier, which classifies WSIs as high risk and low risk. The model is initially trained based on 39 WSIs annotated by pathologists. The gland segmentation model is trained by dividing the WSIs into tiles. These tiles are separated into categories if tissue is found and others are discarded. The model is thoroughly trained by using iterative training to reduce the loss function. Data augmentation by rotation, mirroring, contrasting, changing brightness, and adding Gaussian noise and Gaussian blur is done to increase the training set and make the model robust.
The trained segmentation model was applied to the 105 WSIs from the Singapore General Hospital to number them according to the following features:
Area classified as dysplasia or adenocarcinoma with a minimum of 70% certainty.
Area-weighted average prediction confidence for adenocarcinoma or dysplasia objects.
The boolean flag is set to 1 if the slide has a prediction certainty of more than 85%.
Predictions for cancer or dysplasia are in the bottom 1 percentile.
After the numeric classification, a fivefold cross-validation is carried out to train, tune, and compare several models. The best-performing model is selected. This model is tested on 150 WSIs and the results are compared to those of pathologists. It is found that the model could classify 119 out of 150 slides correctly, thus resulting in a high sensitivity of 97.4%, a specificity of 60.3%, and an accuracy of 91.7%.
The confusion matrix generated from the predicted results of biopsies is depicted in table 3 below.
On evaluating the data from the testing dataset, it is observed that its high accuracy of 91.7 shows uniformity between the AI model and the pathologist. With a sensitivity of 97.4%, the model assures the minimization of false negatives (Goyal and Mann 2020 ). This model guarantees the identification of high-risk CRCs. These identified specimens are then be sent to a pathologist for final verification. Thus, the AI model is used as a screening tool to help reduce the diagnostic burden of pathologists by identifying significant cases (Ho and Zhao 2022 ; Viscaino and Bustos 2021 ).
Table 4 lists the observations in literature for colorectal cancer.
5 Prostate cancer
Though the risk factors, diagnostic methods, and treatment modalities are different for prostate cancer with respect to breast cancer, lung cancer, and colorectal cancer, there are common AI methods to deal with all four types of cancers. Medical Image analysis is the common technique used among all the mentioned types of cancers. AI techniques for colorectal cancer involve analyzing medical imaging data like CT scans. Similarly, AI techniques for skin cancer, especially melanoma, use deep learning to analyze dermatoscopic images, identifying malignant lesions by visual patterns. By utilizing imaging data and machine learning, AI enhances early detection and treatment strategies, highlighting its transformative potential in cancer care. The second most prevalent cancer to cause death in males is prostate cancer, which is also the most frequently diagnosed non-skin malignancy in men. The volume of prostate biopsies has increased, and there is a dearth of urological pathologists, which strains the ability to diagnose prostate cancers. These difficulties are encountered during prostate therapeutic interventions (Booven and Kuchakulla 2021 ). Prostate cancer should be found early and located while it is still curable since excellent cancer-specific survival is anticipated for the majority of locally contained illnesses. Radiation therapy and radical prostatectomy are frequent therapies for individuals with locally advanced, high-risk illnesses. The Gleason grading system’s pathologic grade is the main factor in clinical risk evaluation. Generally speaking, cancers with just Gleason pattern 3 are regarded as low risk. The risk of developing metastatic illness and dying from cancer is increased in tumors with dominant Gleason patterns 4 and 5 (Harmon and Tuncer 2019 ).
It is anticipated that the development and acceptance of digital pathology technology will increase the precision of anatomic pathology diagnosis. Machine-learning algorithms have shown some accuracy in recognizing disease phenotypes and characteristics when applied to digital images (Perincheri and Levi 2021 ).
There is widespread knowledge of the value of multiparametric magnetic resonance imaging (mpMRI) as a method for detecting prostate cancer. The prostate and pelvic areas can be seen in high-contrast, high-resolution anatomical pictures thanks to mpMRI. Radiologists must possess a high degree of competence in order to assess and interpret all mpMRI sequences. Literature has been developed to describe the complicated heterogeneity of localized prostate cancer using the functional imaging properties of mpMRI. In the past ten years, there has been a significant rise in the usage of AI applications in both radiology and pathology (Harmon and Tuncer 2019 ).
5.1 AI in localized prostate cancer
Contributions of AI to prostate mpMRI are particularly expected to increase the sensitivity of prostate cancer diagnosis and lower inter-reader variability. The TZ shows the largest advantage, enabling readers with average competence to reach 83.8% sensitivity with automatic detection as opposed to 66.9% with mpMRI alone. Litjens et al. are able to show that integrating AI-based prediction with the Prostate Imaging Reporting and Data System (PI-RADS) enhances both cancer detection and clinically relevant (aggressive) disease classification. While there haven’t been as many studies on the automated identification of prostate cancer using mpMRI using deep learning. Early research suggests increased detection rates compared to earlier studies. Here, PI-RADS v2 classification in conjunction with automated detection enhanced the diagnosis of clinically relevant malignancy over radiologist-only detection. Biopsy intra-lesion spatial targeting of aggressive spots on mpMRI is not best suited for AI algorithms. The algorithm should be educated using voxel-based classifiers against a system that classifies each tumor “patch” according to its regional Gleason score in order to enable more precise spatial learning (Harmon and Tuncer 2019 ).
Machine learning algorithms used in cancer therapy often include semantic segmentation of stromal, epithelial, and lumen components. Due to how time-consuming it is to geographically annotate at the full resolution of digital pathology pictures, this degree of spatial annotation is not viable. Theoretically, more thorough pathologic details of each lesion would be necessary as input to enhance spatial labeling at the mpMRI level. The use of AI in pre-processing stages to enable more intricate histopathologic spatial analysis with radiologic imaging has shown potential. According to research by Kwak et al., the tissue component density is substantially correlated with the MRI signal characteristics. The differences between tumors with Gleason patterns 3, 4, and 5 were shown by Chatterjee et al. to be bigger for the gland component volumes than for cellularity measures. The detection and classification of intermediate prostate tumors are now constrained by pathology and imaging. Most models are built using weakly labeled data, and there aren’t many high-quality annotations available. The foundation for high-resolution radio-logic labeling can be improved through pathological classifier performance (Harmon and Tuncer 2019 ).
Digital pathological evaluation using automated Gleason grading has been the main focus of prostate cancer research. Building classifiers from manually created characteristics obtained in relatively uniform environments has been the foundation of traditional machine-learning techniques. Prostate cancer grading systems may now perform better thanks to more recent developments in deep learning applications. Deep learning and machine learning were used to create a novel network for prostate cancer diagnosis and grading. According to the results, the accuracy of epithelial identification was 99%, while the accuracy of low- and high-grade illness was between 71 and 79%. New possibilities for image-based jobs at full-scale resolution will open up as technology advances in processing power (Harmon and Tuncer 2019 ).
5.2 AI methods in prostate cancer diagnosis
ANN and Histopathologic Diagnosis of Prostate Cancer: A prostate cancer diagnosis frequently depends on the histological recognition of prostatic adenocarcinoma. Numerous investigations were undertaken, and it was shown that many of them demonstrate the potential of AI ANNs to provide more precise patient counseling and circumvent histopathologic variabilities.
ANN and Magnetic Resonance Imaging (MRI) diagnosis of prostate cancer: Detection and aggressiveness assessment using magnetic resonance imaging (MRI) have both been studied as treatment options. Although MR spectroscopy, T2-weighted MR imaging, and apparent diffusion coefficient (ADC) have been useful methods to evaluate prostate cancer, there is still disagreement about how to best apply them. These AI classifier techniques have been proposed as potential methods for non-invasive tumor progression detection and decision support for active monitoring programs.
Artificial Neural Network in Biomarker Diagnosis and Risk Stratification: PSA level testing has helped to inform prostate cancer diagnosis and prognosis. A deluge of biomarkers has been discovered and incorporated into clinical assays over the past ten years. Analyzing and verifying the biomarkers can be greatly aided by ANNs. In several investigations, clinical prediction models were created using machine learning techniques. The findings demonstrate that novel non-invasive biomarkers can be found using computationally directed proteomics.
ANN for patient interaction and patient centered care Many prostate cancer patients are still uncertain about their potential treatment options after receiving a diagnosis. Understanding how specific therapies are implemented might therefore help patients feel more at ease and enjoy their treatment more. Numerous research shows the potential of AI ANNs to enable the creation of useful patient-centric tools to aid in informing patients about their treatment options and illness progress monitoring.
Classification system for prostate cancer risk stratification using ANN: Numerous criteria work in conjunction with ANN to classify patients into risk groups that are classified into 7 categories ranging from extremely low to very high risk. Although these risk categories provide a solid framework for stratification, none of them takes the possibility of repetition into account. Therefore, a deeper understanding of the mechanisms underlying recurrence could equip us with the knowledge necessary to modify risk variables and, consequently, select the most appropriate therapies (Booven and Kuchakulla 2021 ).
5.3 Study experiments
A study experiment is presented in this section to evaluate Paige Prostate’s performance on a prostate core biopsy. In this study, Sudhir Perincheri et al. evaluated Paige Prostate’s performance on a prostate core biopsy using the Whole Slide Images dataset from Yale Medicine, which the algorithm has never seen before. Paige Prostate is a machine-learning algorithm that classifies a whole-slide image as “suspicious” if any prostatic lesions are found or “not suspicious” if none are found. It is developed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York using data from its digital slide collection.
The primary objective of the study is to investigate two possible use case scenarios. The first is its use as a pre-screening tool to spot negative cores that don’t need a pathologist to manually evaluate them, and the second is its use as a second read tool to spot cancer foci the pathologist missed. 1876 prostate core samples from 118 consecutive individuals are included in the analysis. The primary diagnostic is a board-certified pathologist’s clinically recorded unique diagnosis. The Paige Prostate algorithm is run on scanned, identifier-free stained samples.
Except for High-Grade Prostatic Intraepithelial Neoplasia (HG-PIN), the algorithm classified each core biopsy as suspicious if it discovered abnormalities.
Additionally, if the distribution of the core-needle biopsy slides differed noticeably from the thumbnail, it flagged the slides as being out of distribution. Digital images and/or glass slides were inspected to confirm the presence of diagnostic lesion tissue at the scanned level. Without being aware of the final diagnosis or algorithm classification, two genitourinary pathologists manually inspected the entire slides. Thirty randomly chosen core biopsy photos are combined with erratic biopsy images. When one of the two reviewers observed atypia in a core biopsy, the algorithm classified the sample as “suspicious,” and when neither of the two reviewers did, the sample is classified as “discrepant.” The same pathologists manually evaluated the pictures of any core biopsies that had discrepancies following blinded assessment while emphasizing the area of concern.
5.3.1 Results of the study experiment
Of the 118 patients represented by the 1876 core biopsies in the research, 86 are found to have prostatic adenocarcinoma, and 32 are found to be cancer-free. According to Paige Prostate’s analysis, at least one core is deemed questionable in 84 of the 86 individuals with adenocarcinoma, whereas none is in 26 of the 32 patients who did not have carcinoma or glandular atypia.
Among the non-suspicious labels, 16 of the 46 discrepant cores are not interpretable manually, resulting in the final diagnosis of adenocarcinoma. Diagnostic lesional tissue is not found in the scanned picture of 19 additional discrepant cores. Taking away the 16 faulty scans and considering the 19 cores with no diagnostic tissue in the scanned picture as concordant, leaves 11 discrepant cores.
Thirty-four (34) of the 477 core biopsies classified as “suspicious” by Paige Prostate resulted in a benign prostatic tissue diagnosis. In 16 instances, at least one of the reviewers identified unusual glands during the manual review. The system classified two cores as out of distribution because they lacked prostatic glandular tissue. With the exception of one core that developed granulomatous prostatitis, the remaining cores were completely benign.
In suspected circumstances, Paige Prostate includes a tool that shows regions of concern. At least one of the two manual reviewers classified six more cores as odd after re-examining suspected discrepant cores with Paige Prostate annotation. To avoid bias, 30 randomly chosen core biopsy images were combined with discrepant cores for manual reviewers, which included 22 benign cores. The manual readings and the final results are identical, with the exception of one core having adenocarcinoma. In the data set, Paige Prostate shows a positive predictive value of 97.9%, a negative predictive value of 99.2%, sensitivity of 97.7%, and specificity of 99.3%.
The study by Sudhir Perincheri et al. carefully studies the model’s performance on unseen data and identifies areas where the algorithm might be improved. Through their findings, the false negative rate might be decreased, for instance, by better out-of-focus scan detection. On the other hand, disregarding photos with a single focus smaller than 0.25 mm would reduce the algorithm’s false positive rate. Paige Prostate has the potential to be a powerful tool with a wide range of use-case applications in anatomic pathology clinical settings (Perincheri and Levi 2021 ).
In a study experiment conducted by Derek J Van Booven, et al. the authors analyzed the advancements in AI-based artificial neural networks (ANN) and their current role in prostate cancer diagnosis and management. For this systematic review, the authors explored PubMed, Embase, and the Web of Science to find and evaluate publications about the use of active surveillance and machine learning in the detection, staging, and management of prostate cancer. To make decisions during the diagnosis and treatment of prostate cancer, evidence from several modalities must be interpreted. MRI-guided biopsies, genomic biomarkers, Gleason, and PSA levels grading are just a few examples of the tools that are utilized to diagnose, risk stratify, and subsequently monitor patients during corresponding follow-ups. A process that is extremely challenging and time-consuming for humans can be made possible by artificial intelligence (AI), allowing therapists to recognize complex correlations and manage vast data volumes. Utilizing artificial intelligence-based ANN tools that could be seamlessly integrated with some of the crucial instruments frequently used in prostate cancer detection and lowering the degree of subjectivity, it is possible to use limited resources while enhancing the overall efficiency and accuracy in prostate cancer diagnosis and treatment (Booven and Kuchakulla 2021 ). An exceptional possibility for AI applications to enhance detection, classification and overall prognostication arises from the close relationship between histology and functional imaging parameters. Improvement in systematic assessment and characterization of pathological and radiologic interpretation is clinically necessary for prostate cancer. To continue making progress in this area, mature data sets with top-notch annotations are required (Harmon and Tuncer 2019 ).
Observations in literature for prostate cancer have been shown in the table 5 .
6 AI techniques for skin cancer detection
In most of the available literature, Gray Level Co-occurrence Matrix1 (GLCM) is a go-to technique for feature extraction and GoogleNet for classification. Such models had an average accuracy of around 85%. Out of all the literature’s, Esteva et al. achieved a notable accuracy of 96% using GoogleNet.
Stanley et al. uses fuzzy-based histogram analysis for differentiating benign skin lesions from melanomas in clinical images. The model is trained upon 129 melanoma and 129 benign clinical images. Results shows that the model is able to distinguish between both of them with true positive rates between 89.17 and 93.30% and true negative rates between 84.33 and 86.04%.
Al Nazi and Abir proposes a model where augmentation techniques are used to overcome the issue of having fewer images in the dataset. ISIC 2018 and \(PH^{2}\) data set is used for this purpose. The model achieves an impressive 92% accuracy despite having fewer images in the dataset.
Han et al. proposes a Region-based CNN model for SC prediction. 11,06,886 images are used to train the region-based CNN. The model is trained with two approaches, one with 80% sensitivity to malignant modules with an F1 score of 72.1% and the second with 90% sensitivity to malignant nodules with an F1 score of 76.1%. The model provides better accuracy than dermatologists (Patel 2022 ).
The computer-aided systems can easily extract features like color variation, asymmetry, texture, etc. There have been many different methods, like the 7-point checklist, ABCD rule and Menzies method to improve the diagnosis of skin cancer. The major steps in the computer-aided diagnosis of melanoma are the acquisition of skin lesions, segmentation of lesions from the skin region (to detect areas of interest), extraction of geometric features (accountable for increasing accuracy) and feature classification. The feature extraction in CAD depends majorly on the ABCD rule i.e. asymmetry, border irregularity, color, and diameter (Kanimozhi and Murthi 2016 ). In treating suspicious cases, physicians have been assisted by system-aided technology. They can also be used by less experienced doctors as an extra tool to obtain a preliminary estimate and enhance patient follow-up processes. These techniques are often divided into two categories: the key category is related to attribute mining from skin images, and the second category uses picture properties like texture and color to recognize patterns.
6.1 AI-based melanoma detection
The technique that is used for melanoma detection is computerized detection using Artificial Neural Networks, and this technique proves to be more helpful and powerful than dermoscopy. MATLAB has been extensively used for image processing and developing diagnostic algorithms. The methodology for the detection of melanoma using image processing is depicted below:
Image pre-processing: An image of the lesion is captured by any lens and in any lighting condition. This image thus needs to be pre-processed.
Image scaling: The size of the image is enlarged or reduced to make the lesion more clearly visible.
RGB to grayscale: The colored image is then converted into a grayscale image using the rgb2gray function.
Grayscale to binary image: This grayscale image is then converted into a binary image using the im2bw function. The output image performs thresholding and converts all the pixels having a value above the threshold to 1(white) and all other pixels to 0 (black).
Segmentation: This is used to divide the image into several parts, identifying the different objects in the image.
Background subtraction: This technique helps in extracting the foreground of the image (objects of interest) for further processing. It is also known as blob detection.
Edge detection: It segregates the objects in an image by boundaries by detecting discontinuities in brightness.
Masking: It sets the pixel values of the background to 0 or some other value and highlights only the lesion.
Feature extraction: Melanoma skin lesions are mainly identified by their geometric features. The features that are most significant for melanoma detection are:
Major Axis Length
Minor Axis Length
Circularity Index
Irregularity Indices (A, B, C, D)
Classification: ANN is used in the classification stage according to the ABCD rule. The neural networks are constructed in three layers with a feed-forward architecture.
The input layer contains information from the features extracted in the previous stage.
These input units are integrated with the hidden units in the hidden layer. These hidden units are then linked to the output layer
The output layer gives a result that indicates if cancer is present or not.
Once the layers are developed, the neural network is then trained with data having the results already present. The backpropagation algorithm is used to train the ANN.
Results: The values received from the network are input into a confusion matrix. Once the algorithm is completely trained, the unknown images are given to the algorithm for classification as cancerous or non-cancerous. The trained algorithm gives an accuracy of more than 96%. This tool is made more feasible and robust since it can detect cancer in an image taken in any condition and hence can be used in rural areas or where an expert diagnosis is not possible (Kanimozhi and Murthi 2016 ).
6.2 Skin lesion analysis on gray level profile
Some of the visible features of melanoma in its early stages are skin dryness, fungus, and allergic symptoms. Currently, the captured skin images are analyzed in the frequency domain, but this doesn’t prove to be an efficient way as the skin color in these images does not vary much. Hence, this study aims at analyzing images in the gray level profile. This serves as the input parameter for our system. This method analyzes the variations of the RGB spectrum of the skin images using novel six-sigma threshold and region connectivity concepts to detect the border of a lesion and identify the incidence and propagation. An automated method for the diagnosis of skin cancer based on dermoscopic images has been developed. The retrieved features are based on a gray level co-occurrence matrix (GLCM) and a multilayer perceptron classifier (MLP) to differentiate Melanocytic Nevi from malignant melanoma.
It is important to recognize the texture in an image by its contrast, correlation, homogeneity, and energy properties. In gray images, texture can be identified using 2-D histograms and co-occurrence matrices.
The images captured by the dermatoscope are converted from RGB to grayscale images. The following steps are followed for extracting the gray level co-occurrence matrix:
The image data is quantized into a given number of gray levels.
An NxN GLCM matrix is created by considering a window of pixels surrounding the given pixel. Then the number of times the given intensities occur in the given matrix is calculated. The total number of times the given spatial relationship occurs in W will equal the sum of all the GLCM elements.
The GLCM is then made symmetric.
Then the probability of each pixel is obtained by dividing it by the sum of all intensities in W.
Then, characteristics like homogeneity, entropy, energy, contrast, and correlation can be quantified.
Then the area and perimeter of the region of interest can be calculated by using segmentation(using K-means clustering) and boundary extraction.
Convert RGB image to a L*a*b* image which helps in segmentation using k-means clustering.
Convert L*a*b* image to a grayscale image.
Convert gray image to a binary image by using the Otsu algorithm.
Based on the features extracted, the researchers trained a model of Back-Propagation network. The algorithm uses 50 images, 10 input neurons, and 4 training classes. Researchers have used around 20 images for testing purposes.
The results shows that the developed classifier has 100% accuracy for classifying non-cancerous images. The accuracy for the classification of skin cancer of type 1 is 95%, while that of skin cancer of types 2 and 3 is 95.8% and 95%, respectively. Thus, the authors have developed a model with high accuracy for the classification of cancerous images into 3 types and non-cancerous images (Goel and Singh 2015 ).
6.3 Skin cancer classification with convolutional neural network and transfer learning
With present AI systems, in the detection of skin cancer, the following challenges are observed. A freely accessible dataset called HAM10000 containing 10,015 dermoscopy images is acquired from patients belonging to Australia and Austria. 6705 images are benign, 1113 are malignant, and 2197 belong to unknown lesions:
The high variances in the shape and size of images make it difficult to give an accurate prediction.
Pre-processing is essential for good accuracy.
The contrast from neighboring skin cells plays a part in the analysis. So if contrast is low, detection becomes tricky.
Colour illumination hinders detection capabilities due to properties like texture, light rays, and reflections.
The presence of moles on the human body, which is unrelated to skin cancer, can throw the system off track.
In this classification technique, transfer learning is used. It is a type of machine learning where a pre-existing model is applied to related problems. Training a DNN model from scratch requires a lot of images. These types of datasets with sufficient quality are sparse. The proposed model is a Deep Convolutional Neural Network (DCNN) model with the Adam optimizer as a gradient descent algorithm. An augmentation step is applied in the end to reduce the drawbacks of images. The dataset is divided into two ways as 70% training, 20% validation, 10% testing set, and 80% training and 20% validation. The results are put up against existing DNN models like AlexNet, DenseNet, and ResNet which are implemented using Python due to support of powerful libraries like Keras, Tensorflow and PyTorch. The best results are with 100 epochs and an accuracy value of 0.847 (Ali and Miah 2021 ). Performance comparison with AlexNet, ResNet, VGG-16, DenseNet and MobileNet is presented below:
Precision: The proposed DCNN model has a precision of 94.63, which is only lower than AlexNet, which stands above it with an accuracy of 96.89.
Recall: The proposed model has a recall value of 93.91, with BesNet, VGG-16, and MobileNet besting it with recall values of 97.50, 95.18, and 94.57, respectively.
F1 score: The proposed model has the highest F1 score with a value of 94.27.
Training accuracy: The proposed model had a training accuracy of 92.69, just behind BesNet and MobileNet, which has higher values of 92.78 and 92.93, respectively.
Testing accuracy: The proposed model has the highest training accuracy of 90.16 among the models.
In the following section the performance of the CNN model is presented with 80% training set against the above models.
Precision: The proposed DCNN model has a high precision of 96.57, which is only slightly lower than AlexNet. Alexnet has a precision of 97.88.
Recall: The suggested model has a recall value of 93.66, with DenseNet and MobileNet besting it with recall values of 94.83 and 94.15, respectively. BesNet has a very close value of 93.63.
F1 score: The proposed model has the highest F1 score with a value of 95.09.
Training accuracy: Training accuracy for the suggested model is 93.16, just behind AlexNet’s higher value of 93.82.
Testing accuracy: With a training accuracy of 91.43, the presented model outperformed the others.
The comparison of computation time is as given below:
Time per epoch: Among all the models, the proposed model required the least amount of computation. It shows a tie per epoch value of 9–10 s which is the lowest when compared to others. The only close model performance is by MobileNet, with 11 to 12 secs. The rest of the models take an average time of 14–17 secs for time per epoch value.
Total time: The total time taken to compute images is 16 min, which is 3 min lower than the next highest performance of MobileNet. This is a great achievement. The rest of the models averaged around 24 min.
The proposed model is most suitable for CAD systems which will classify lesions at an early stage. The proposed model has a better classification rate than existing models and transfer learning models. The other models were trained on the same data set while comparing them (Ali and Miah 2021 ).
6.4 Improved skin cancer classification using a combination of human and artificial intelligence
With the advancement of artificial intelligence in healthcare, various studies conducted in the field have often highlighted the comparison of computer-aided diagnosis with physicians to evaluate which is the more efficient of the two. However, the researchers combined both diagnostic methods in an effort to improve skin cancer detection. The authors fused the systems by first obtaining an independent classification of the lesion and then merging the procured results to form an overall classifier, taking both systems into account. The task is to correctly classify the most common skin cancer lesions. The three systems compared includes dermatologists from German University Hospitals, a convolution neural network(CNN), and a combination of both.
The study utilizes the International Skin Imaging Collaboration (ISIC) archive, which contains dermoscopic images. The majority of the images used in the study came from the HAM10000 Dataset, which is a subset of the ISIC archive. The dataset was supplemented with an additional 4291 images from the ISIC archive, resulting in a total of 11,444 images used in the study out of which 6390 were verified for biopsy. To avoid bias for the 300 test images, a random generator is programmed using Python. An online questionnaire is circulated with the test images to 13 skin cancer experts at 13 university hospitals in Germany.
As a concentrated evaluation of 300 samples is impractical, the test set is splited into 6 questionnaires with 50 images each and randomly assigned to each clinic. The dermatologists verifies the skin lesions from the 50 test samples and answered on the identified type of lesion along with the certainty of their response on a scale of 10 with 0 being least certain, 10 being very certain and 5 being most uncertain. To avoid careless responses, the authors have established statistical criteria for the detection of outliers.
The CNN Model is trained on the same training set which included 585 images of class a, 910 of class b, 3101 of class k, 4219 of class n, and 3521 images of class m accounting for a total of 12,336 samples of different classes of lesions. Considering the reliable classification of the ResNet50 architecture, the authors opted for the same model for this study.
To obtain the best results, the classification from dermatologists have to be fused with the CNN Model. The specification of confidence obtained from the dermatologist is also used as an input to the fusion algorithm as a probability metric of the selected class. XGBoost, an efficient tree algorithm with gradient boosting, is used iteratively to avoid bias in the results. RandomizeSearchCV is used for hyperparameter tuning to obtain the largest possible test set.
The results from the three classifiers are evaluated, considering the differential diagnosis task and how well benign lesions are distinguished from malignant ones. From the results obtained, the mean accuracy of physicians’ classification is noted as 42.94%, while the CNN model accounts for 81.59% accuracy, and the fusion method shows the best results by displaying accuracy of 82.95%. The sensitivity and specificity of the three systems are also evaluated, and the results obtained are as follows.
The classification by dermatologists displayes a mean sensitivity of 66% and a mean specificity of 62%. Those of CNN are 86.1% and 89.2%, and those of the fusion method are 89% and 84%, respectively.
From the evaluation, it is observed that the combination of man and machine increases the average sensitivity from 86.1% solely obtained through AI to greater than 89% by including human evaluation. Thus, the study concludes that the combination of human decisions with those of artificial intelligence achieves higher accuracy and better results and could be further utilized not only for skin cancer but also for prospective assessment of oncological transformations (Hekler 2019 ).
Artificial intelligence is gaining ground swiftly in the field of dermatology. It has the potential to revolutionize patient care, particularly by improving the sensitivity and precision of screening for skin lesions, including cancer. However, clinical and photographic data of all skin types are needed for AI research, and this can only be acquired through increased global skin imaging collaboration. It is necessary to record the sensitivities, specificities, and performance in future research and in actual environments. AI is not a danger to dermatologists’ knowledge; rather, in the next few years, it may be employed to enhance clinical practice. If practicing dermatologists had a greater understanding of AI concepts, they would be able to deliver better skin care. The review of the techniques displays an ever-increasing scope of AI and Computer-aided diagnosis in skin cancer detection (Das 2021 ).
6.5 Deep learning techniques for skin lesion analysis and melanoma cancer detection
This section describes segmentation Techniques for skin lesions analysis.
U-Net architecture: Fully Convolutional Network (FCN) was the one to develop this. It is made up of a convolutional layer-based encoder-decoder network. Decoder layers are made up of upsampling layers, while encoder levels are made up of pooling layers. Due to the loss of image features caused by this approach, some important information may be lost.
FCN: It is a band of the pool and convolutional layers. When tested at the International Symposium on Biomedical Imaging (ISBI), a multi-stage FCN that was created with parallel integration produced a system with a state-of-the-art performance of 95.51% segmentation accuracy and 91.18% dice coefficient score.
Deep residual network: It is an ANN subtype. It uses skip connections to skip across convolutional layers as it rises in a pyramidal pattern. To extract features, a system with more than 50 layers was used. Because it consumes a lot of computational resources, this can limit its practical application.
Convolutional DE-Convolutional Neural Networks (CDCNN): Both convolutional and deconvolutional networks make up this architecture. Deconvolutional networks are CNNs that function in the opposite direction. For feature extraction, both networks are applied. These systems demand a lot of computing power. Due to the intensive adjusting of several factors, this method is not particularly practical (Adegun and Viriri 2021 ).
Skin lesion segmentation algorithms and techniques in ISIC 2018 database are given below. Major metrics for performance evaluation of the segmentation and classification deep learning models include:
Dice coefficient: This gauges the degree to which the actual result and the projected result are comparable and overlap.
Sensitivity: This is the proportion of positive outcomes(prediction) among those who are positive.
Specificity: This is the proportion of negative outcomes(prediction) among those who tested negative.
Accuracy: In relation to the total number of cases investigated, it calculates the percentage of true results (including true positives and true negatives).
Some of the best-performing cutting-edge methods used in ISIC 2018 have been investigated, and their performance has been documented.
A model developed using an encoder-decoder technique with Deeplab and PSPNET shows an accuracy of 94.2%, specificity of 96.3%, and sensitivity of 90.6%.
The model developed using Ensemble VGG16, U-net, DenseNet and Inception v3 with Conditional Random Field (CRF) post-processing shows an accuracy of 94.5%, specificity of 95.2%, and sensitivity of 93.4%.
A model developed using a Fully convolutional encoder-decoder network with CRF shows an accuracy of 94.3%, specificity of 96.4%, and sensitivity of 91.8%.
The model developed using a Fully convolutional encoder-decoder network with CRF shows 94.5%, 94.2%, and 94.0% accuracy, specificity, and sensitivity respectively.
A model developed using UNet with DeepLabV3 shows 94.7%, 94.5%, and 94.5% accuracy, specificity, and sensitivity respectively.
The model developed using FCN with a region-proposal network (RPN) and CRF shows 93.7%, 91.8%, and 92.1% accuracy, specificity, and sensitivity respectively.
A model developed using Mask-RCNN shows 93.9%, 96.8%, and 90.4% accuracy, specificity, and sensitivity respectively.
The model developed using the Mask R-CNN model with ResNet50 shows 93.7%, 94.3%, and 93.6% accuracy, specificity, and sensitivity respectively.
Model developed using U-Net variants shows 93.8%, 93.9%, and 94.5% accuracy, specificity, and sensitivity respectively.
Model developed using U-Net shows 93.7%, 97.9%, and 87.9% accuracy, specificity and sensitivity respectively.
Similarly, models are also prepared for ISIC 2019 but results shows ISIC 2018 models performed better. The reason for better performance is found to be pre-processing images before the application of learning algorithms. Generally, ensemble models with state-of-the-art models such as Resnext, NASNet, SENet, DenseNet121, InceptionV3, InceptionResNetV2, Xception, EfficientNetB1, EfficientNetB2, and EfficientNetB3 seem to be more successful and perform at a high level. However, developing this model takes a significant amount of time and resources. Performance-wise deep learning algorithms are recommended for usage (Adegun and Viriri 2021 ).
Table 6 represents the observations of AI techniques in the literature for skin cancer.
7 AI based techniques for digestive cancer detection
White Light Imaging (WLI) and Narrow Band Imaging (NBI) are widely used methods for gastric cancer detection. It has been discovered that narrow-band imaging (NBI) magnification endoscopy is more effective than white light imaging (WLI) for the early identification of gastric cancer.
AI methods are proposed for gastric cancer and stomach diseases. A high-performance computer, deep learning and a significant amount of digital data have been determined to be three components that make artificial intelligence (AI) valuable to doctors in the field of picture identification in recent times. The very first CNN-based AI system for identifying gastric and esophageal malignancies has been developed by Japanese endoscopists in the field of gastrointestinal endoscopy. Numerous researchers have noted the value of AI in separating cancerous from non-cancerous tissue using magnified NBIs. The system is created to forecast the depth of invasion of stomach cancer using artificial intelligence, and its specificity, sensitivity, and accuracy are noticeably better than those of experienced endoscopists. By utilizing its great sensitivity and speed, AI may be employed for picture verification following endoscopy to ensure that malignancies are not missed by accident. The decision to assist AI in the treatment of gastric cancer still needs a more thorough categorization than stage classification. Although AI proved successful in identifying cancerous lesions, the diagnostic precision was insufficient since it was unable to distinguish between malignant and benign lesions with appropriate accuracy. In order to address this problem, AI is utilized to identify gastric ulcers, a frequent condition that serves as a non-cancerous gastrointestinal lesion that must be separated from gastric cancer. Furthermore, the stomach’s anatomical components can be recognized by AI. An artificial intelligence (AI) system is created to anatomically categorize the upper gastrointestinal tract, locate the stomach in real-time, and assess how comprehensively the stomach is covered (Suzuki and Yoshitaka 2021 ).
AI plays active role in Pharyngeal And Esophageal Cancer detection. High-precision AI systems have been proven in reports to employ both still and moving pictures. To detect early esophageal cancer in a clinical scenario, AI systems still need to be improved, especially during screening endoscopies, in which the endoscope is swiftly moved through the esophagus. In terms of anatomy, it can be very difficult for non-experts to see the cervical esophagus and the esophageal mucosa close to the esophagogastric junction. Additionally, many reports don’t include validation films with images that are of low quality due to defocus, halation, mucus, blur, and inadequate air insufflation. So, rather than using the actual speed in clinical practice, the researchers believe that high-speed videos should be used to test the AI system. It would also be useful if AI could predict the cancer risk with normal background esophagus in NBI or WLI, as the presence of several unstained areas after iodine stain indicates the possibility of esophageal squamous cell carcinomas (ESCCs). Such a prediction would help to detect cancers in the daily practice of endoscopic examination. With these developments, AI systems will be able to help endoscopists more successfully identify early esophageal and pharyngeal tumors, increasing survival rates. (Suzuki and Yoshitaka 2021 )
Methods exists in literature for endoscopic detection of early Esophageal Cancer. Current CAD systems are incapable of detecting Barrett’s esophagus-related early neoplastic lesions as well as selecting a biopsy site. To solve this, a CAD system was developed using deep learning. The sensitivity and specificity were higher than those of the existing ones. Ebigbo et al. developed a model using CNN with modalities of High Definition- White Light Endoscopy (HD-WLE) and NBI. The authors used a dataset of 296 images, with 148 in each training and test. The results showed that HD-WLE had a sensitivity of 97% and a specificity of 88%, while NBI had a sensitivity of 94% and a specificity of 80%. De Groof et al. developed an HD-WLE model using SVM. He used 120 images, with 60 for training and testing datasets. It resulted in 95% sensitivity and 85% specificity (Niu and Zhao 2020 ).
AI based Endoscopic Optical Coherence Tomography and Confocal Laser Endomicroscopy identifies lesions using the esophageal, mucosal, and submucosal structures. The primary challenges are complex imaging technology and high time consumption while reading images. After the application of Volumetric Laser Endomicroscopy(VLE) by Swager et al., the system becomes superior to VLE experts. Ebigbo et al. performed real-time diagnosis on 62 images captured with a CAD system, yielding a sensitivity of 83.7% and a specificity of 100%. Veronese et al. developed a model using CLE images and support vector machine (SVM). This system could distinguish between gastric metaplasia (GM), intestinal metaplasia (IM), and neoplasia. It achieved a sensitivity of 96%, 95%, and 100% for GM, IM, and neoplasia, respectively (Niu and Zhao 2020 ). Following are three important methods of AI in digestive cancer (Niu and Zhao 2020 ):
Artificial Intelligence based on White Light Endoscopy and Narrow Band Imaging: To increase the accuracy of WLE, Cai et al. developed a CAD system using DL. This system is tested with WLE images. There is no significant difference between senior endoscopists. Several models are developed, but none of them gave any significant results. A few models managed to get better results than senior endoscopists, but the specificity dropped down.
Artificial Intelligence in the pathological diagnosis of early Esophageal Cancer: When it comes to EC detection, pathological diagnosis is the way to go. Thus, many have tried to use AI in combination with pathological diagnosis. The results did not outclass the experts. The performance of the system as a whole was not far from an expert. A slight inferiority was observed.
Models developed using Deep Convolutional Neural Network for Gastric Cancer Detection: Using a backpropagation algorithm, deep learning allows us to break down images and extract relevant clinical features from them. The model trains itself on the accumulated images and then diagnoses new images. Currently, neural networks developed using CNN are best for image recognition(Hirasawa 2018 ).
Sabo et al. developed two models to distinguish low-grade and high-grade dysplasia. He made use of HE-stained (hematoxylin and eosin) image features. This model performed well in borderline lesions, which were not distinguishable before. Overall, HE-stained pathological image features may pave the way toward AI pathological diagnosis (Niu and Zhao 2020 ). Model developed by Dehua Tanga, et al. is white light imaging and is one method for detecting EGC, but its sensitivity rate is only 40–60%. A system has been developed by the authors for the detection of EGC under WLI using AI based on deep convolutional neural networks (DCNN).
A study is conducted on 1568 patients belonging to 4 institutions in China. Around 45,000 images were gathered after examination by expert endoscopists, and they are used for temporal validation of the DCNN system. Another dataset containing 26 oesophago gastroduo denoscopy (OGD) videos are used for assessing the performance of the DCNN system. The external validation dataset contained around 1514 images. The testing dataset contained around 300 cancerous images and 300 control images (non- malignant). The boundaries of the cancerous lesion are marked by 4 expert endoscopists, and these boundaries are then circumscribed as annotation boxes by the computer.
The parameters of the neurons of the DCNN system are set to random values and then trained according to the images, with every image undergoing the same process multiple times. The architecture of deep networks has 2 parts: backbone structure for image feature extraction and detection and decision layers for detection of the location of the lesion. The backbone structure chosen for this system is the Darknet-53 model, which has 53 layers of neurons. The four coordinates of the surrounding box for the decision of location are predicted by the decision layer. These boxes are then classified into three categories: small, medium, or large. The average accuracy of the system for the four institutions was found to be 88.2%. The sensitivity and negative predictive value was found to be greater than 85%. The specificity of this system was found to be between 81.7 to 90.3%. The positive predictive value ranged from 80.5 to 90.5%. The AUC values were found to be quite high between 0.887 and 0.940, resulting in excellent performance of the system. The accuracy of the developed system(95.3%) was found to be higher than expert endoscopists (87.3%)and trainees (73.6%). The results clearly indicate that this DCNN system has much better results compared to expert endoscopists. The system can identify only EGC and pre-cancerous lesions under WLI but not NBI (Tang 2020 ).
The model developed by Toshiaki Hirasawa, et al. is presented in this section.
Preparation and data set of images: The dataset for this study comprises 13,584 images of gastric cancer lesions used for training and 2296 stomach images designated for independent testing. These images were obtained from two hospitals and two clinics in Japan during routine clinical procedures through standard endoscopic methods using conventional equipment. Exclusion criteria involved filtering out images of inferior quality and those acquired through specialized techniques like chromoendoscopy or NBI. An expert manually delineated cancerous areas in the training images, excluding magnified and subpar-quality ones.
Construction of CNN: To construct the model deep neural network architecture known as Single Multishot box Detector is used (SSD, https://arxiv.org/abs/1512.02325 ). No alterations to the algorithm are made. The SSD consists of 16 layers or more. Every CNN layer is fine tuned using stochastic learning gradients. It has a global learning rate of 0.0001. The images are resized to appropriate size along with the bounding box. For evaluation of accuracy an independent dataset is considered. This dataset consist of 69 patients. This dataset includes images which has 77 gastric cancer lesions. 62 patients has a single lesion, 6 patients has a couple lesions and a single patient has 3 lesions. The final dataset consist of 2296 images in total. Each case has 18 to 69 images taken.
Results: The model takes 47 s to analyse the 2296 submitted test images. It gives a sensitivity(detected number of correct gastric cancer lesions/actual number of gastric cancer lesions) of 92.2% and PPV (detected number of correct gastric cancer lesions/number of lesions that are diagnosed as gastric cancer by the CNN) of 30.6%. There are two prime reasons for misdiagnosis, with the first one being the presence of gastritis, which induced a change in color tone, and the second being normal anatomical structures of the cardia, angulus, and pylorus. The missed six lesions are even difficult to diagnose even for experts.
Study limitations
Use of high quality images only.
Large training data set
Use of only gastric cancer images. Endoscopic surveys shows low cases of gastric cancer.
Occult cancer could be present in false positives.
A single person marked the training and set images.
Accuracy of CNN is not compared with that of endoscopists.
This CNN model detects early stage cancer. Despite all of its limitations, it achieves an incredible processing speed and have good accuracy. CNNs have an aptitude for diagnostics, and this could be leveraged further (Hirasawa 2018 ).
It would be possible to detect cancer early by using AI-based endoscopes. When it comes to early gastrointestinal malignancies, endoscopists’ diagnostic skills and accuracy might be inconsistent, so AI technology’s improved diagnostic capabilities may be helpful. Endoscopic diagnosis would be more accurate if AI and endoscopists’ knowledge were combined (Suzuki and Yoshitaka 2021 ).
Table 7 shows the observations on digestive cancer detection techniques.
8 Discussions
After thoroughly evaluating the AI techniques for different cancer types, the following discussions are made and presented with the critical evaluation. The visual representations employed contribute to the effectiveness of conveying the comparative analyses. With a detailed review of each category of cancer, the authors present the following discussions. These discussions lead to the future development of AI in cancer detection and prediction:
For breast cancer, the evaluated techniques as shown in Fig. 3 encompassed Convolutional Auto-Encoder, Artificial Neural Network (ANN), AI-CAD, and ANN with extreme learning. Notably, the findings underscored ANN’s outstanding performance, securing an accuracy rate of 98.24%. Subsequently, ANN with Extreme Learning demonstrated the next-best accuracy at 96.4%. AI-CAD and Convolutional Auto-Encoder yielded an accuracy of 95% and 93%, respectively. Domain-specific libraries such as Mahotas and sci-kit-image cater to the unique requirements of medical image analysis, contributing to the accuracy and reliability of breast cancer detection models. Based on these results, the study finds the utilization of ANN as the optimal technique for breast cancer detection.

Performance evaluation of breast cancer detection techniques
Performance evaluation of lung cancer detection techniques
In case of lung cancer detection, prominent techniques such as Artificial Neural Network (ANN), k-Nearest Neighbors (KNN), Convolutional Neural Network (CNN), and back propagation-based Artificial Neural Network (bp-ANN) have been evaluated. ANN achieved a remarkable accuracy of 100%, an achievement tempered by the limitation of a relatively small dataset used for testing. Following closely as it can be seen in Fig. 4 , KNN emerged as the top performer, boasting an accuracy of 99.51%. CNN demonstrated a commendable accuracy of 97%, leveraging its inherent flexibility. Concluding the set of techniques, bp-ANN exhibited a noteworthy accuracy of 96.6%. These results collectively underscore the effectiveness of these methodologies in lung cancer detection, with KNN standing out as the most accurate performer among the assessed techniques.
Artificial Neural Networks (ANNs) are frequently developed using Python, and alternative implementations can be pursued in Java or R. The implementation of ANNs is prominently facilitated through widely adopted frameworks such as TensorFlow, PyTorch, and Keras. It is crucial to highlight that these frameworks offer a sophisticated high-level interface, streamlining the process of constructing intricate neural network architectures.
Convolutional Neural Networks (CNNs) are conventionally constructed using programming languages such as Python, with a focus on leveraging the robust ecosystems of deep learning frameworks. Commonly utilized frameworks, such as TensorFlow and PyTorch, form the foundation for CNN development. The high-level abstraction provided by Keras, often integrated with TensorFlow, facilitates streamlined design and implementation of convolutional operations crucial for image-based data processing.
The k-Nearest Neighbors (KNN) algorithm is typically instantiated using languages suitable for numerical computations, with Python frequently chosen for its versatility. Implementations commonly employ specialized libraries, such as scikit-learn, renowned for its concise interface and effectiveness in the construction and training of KNN models.
The reviewed model was implemented using Matlab NNTOOL.However,Back Propagation-based Artificial Neural Networks (bp-ANN) share an implementation approach with general Artificial Neural Networks. They are often developed using languages like Python, with a focus on leveraging deep learning frameworks, including TensorFlow, PyTorch, and Keras. These frameworks provide built-in support for the backpropagation algorithm, optimizing neural network weights during the training process.

Performance evaluation of colorectal cancer detection techniques
In order to detect and classify colorectal polyps, artificial intelligence (AI) and machine learning algorithms are used. These include support vector machines (SVM) for medical imaging and surgical performance classification, random forests, decision trees, CNN, and deep neural networks. The CNN and deep neural networks are implemented using MATLAB’s machine learning and neural network toolboxes to support the algorithms for cancer detection. Utilizing scientific computer libraries and programming languages like MATLAB and Python, biophysics-inspired models were employed for numerical simulations and modeling.
In the context of colorectal cancer, the available range of techniques presented a diverse array of options, as illustrated above in Fig. 5 . The best performing models are Random Forest CNN Bayesnet and SVM, KNN, Decision Tree and Random Forest with accuracy of 97% each. Despite exhibiting equal performance Random Forest CNN BayesNet is preferred due to its neural network nature rather than being a conventional algorithm. This characteristic affords Random Forest CNN BayesNet greater potential for growth and adaptability.

Performance evaluation of prostate cancer detection techniques
In the evaluation of Prostate Cancer detection techniques, the examined methodologies comprised the Paige Prostate Algorithm, Multi-parametric Magnetic Resonance Imaging using AI, and Artificial Neural Network (ANN). Notably, Multi-parametric Magnetic Resonance Imaging using AI exhibited the highest performance, boasting a 99% accuracy, closely trailed by the Paige Prostate Algorithm at 98%, while ANN demonstrated an accuracy of 93% as illustrated in Fig. 6 .
The Paige Prostate algorithm, a notable tool in prostate-related analyses, is typically implemented using Python, although Java or R can be considered as alternative languages. The preference for Python stems from its widespread use and robust support within the medical image processing and machine learning domains.
Multiparametric magnetic resonance imaging (mp-MRI) is implemented using dedicated libraries and frameworks tailored for medical image analysis. Python, with its versatile ecosystem, is frequently chosen, with libraries like SimpleITK or Pydicom playing crucial roles. Further integration with specialized image processing libraries such as scikit-image ensures comprehensive analysis of the multiparametric data.
The findings of this study unequivocally designate Multi-parametric Magnetic Resonance Imaging using AI as the superior technique. For scenarios necessitating the segregation of high-risk and low-risk cancer tissues, the Paige Prostate Algorithm is a more favorable choice due to its high accuracy.

Performance evaluation of skin cancer detection techniques
In Fig. 7 of Skin cancer detection the top models evaluated are GoogleNet, Transfer Learning, CNN, Image Processing with ANN and Back Propagation Neural Network using gray-level co-occurrence matrix.
The models like GoogleNet, Transfer Learning and CNN are implemented using Python with the availability of powerful libraries like Keras, Tensorflow and PyTorch. For implementing Image Processing and GLCM, we can use libraries like OpenCV or scikit-image in Python, or functions provided by MATLAB’s Image Processing Toolbox.
BPNN can be implemented using neural network libraries like TensorFlow, PyTorch, or Keras in Python, or MATLAB’s Neural Network Toolbox.
A notable observation is the reliance of models on CNN as their base. The illustrative findings unequivocally highlight the capabilities inherent in neural networks, particularly exemplified by two standout performers, Image processing with an impressive accuracy of 96.9% and GoogleNet closely following with a commendable accuracy of 96%. This clearly points out that neural networks have a high utility ceiling for cancer detection.
We find usage of ANN with image processing here as the all over best technique here but GoogleNet is a close second choice for skin cancer detection.

Performance evaluation of digestive cancer detection techniques
In Fig. 8 evaluation of digestive cancer detection techniques such as Convolutional Neural Network (CNN), Deep Convolutional Neural Network (DCNN), and Deep Neural Network (DNN) revealed that DCNN emerged as the leading performer, achieving an accuracy of 95.3%. These networks are commonly deployed in Python, utilizing libraries like Tensorflow, Keras, PyTorch, among others. This outcome not only signifies the notable utility of DCNN in the context of digestive cancer detection but also highlights potential of neural networks. The current accuracy of Deep Convolutional Neural Network (DCNN) holds promise for substantial improvement over time by training it on new samples. This indicates a high growth potential for DCNN, leading us to find this technique as particularly well-suited for digestive cancer detection. Specialized CNN implementation such as the Single Shot MultiBox Detector (SSD), Hirasawa ( 2018 ), further supports the efficacy of tailored approaches in this domain.
Following a thorough comparison of various AI techniques, it is evident that deep learning techniques such as Convolutional Neural Network (CNN) with further fine-tuning shows the best performance in detecting malignancies across a variety of cancers and can be a suitable choice for further research for detection.
A noticeable point is that CNN by itself wasn’t up to par with the task. But the custom version of CNN performed a lot better than traditional CNN. CNN by itself is versatile in giving decent accuracies across any type of cancer but to bring out its full potential it needs to be tailored. The pairing of CNN with professional cancer specialists has the potential to significantly enhance the early diagnosis of various cancer types.
A series of prediction techniques were also studied in review, and following a thorough evaluation, it was found that an algorithm created by Qritive, an AI-powered platform had an accuracy of 91.7% in predicting Colorectal Cancer, whereas the accuracy of AI’s Colorectal Cancer ANN algorithm prediction is rising in importance. Although, ANN prediction approaches for Breast Cancer showed an accuracy of 98.24%. CNN was used to predict Lung Cancer with a 70% accuracy rate and Skin Cancer using a region-based CNN. Paige Prostate an AI software has been created to identify significant area in prostate biopsy images, highlighting regions with highest probability of having cancerous tissue helping in the prediction of prostate cancer at an early stage. ANN produced the best outcome with the highest accuracy out of all the prediction approaches covered in this review.
8.1 Challenges with AI in cancer diagnosis
The following are the major challenges associated with using AI techniques for the detection as well as the prediction of cancer:
Bias in results caused by bias in training data or design decisions is a significant and well-observed issue for machine learning systems.
Transferability is a significant concern for AI; successful algorithms frequently fail when applied to various contexts.
Because of the dependency of AI on computational requirements for information management, AI approaches becomes expensive.
Sensitizing people to the ethical dilemma of using patient data without their agreement in some circumstances is crucial.
Confidentiality of Data- Large amounts of high-quality data are needed for training and validating artificial intelligence (AI) systems. Critical problems include who owns the data, who permits its usage, and how it is protected. Despite the concerns, implementation is now moving forward with significant enthusiasm. Strong motivations for the quick adoption of AI are created by the social, cultural, and economic systems in healthcare. It is very challenging to adopt traditional opt-in or opt-out consent methods. A few AI developers are working on novel methods of data security. Any health system that wants to use deep learning applications must first deal with data privacy as a fundamental first step.
AI prevents emotional problems, social and ethical norms, and tiredness. However, radiotherapists and other medical professionals will not be replaced by AI in the medical field. AI is not completely self-dependent and cannot overrule human participation.
9 Conclusion
The majority of developments have been in the field of “narrow AI”, which are now capable of performing a wide range of specialized tasks, such as playing board games, translating between languages, listening to and acting on human commands, or spotting specific patterns in visual data (such as recognizing faces in CCTV images or suspicious areas). However, lately AI has made a significant impact on the healthcare industry, particularly in the field of cancer prediction. An in-depth study of the machine and deep learning models used in cancer early detection utilizing medical imaging is provided, which offers a critical and analytical assessment of the most cutting-edge cancer diagnostic and detection methodologies now available.
In the clinical and translational fields of oncology, the power of AI regarding therapeutic advice in cancer shows great potential, which translates to better and more individualized therapies for people in need. AI-based computer-assisted systems can help physicians with essential aspects like detecting and classifying various types of cancers. To overcome AI’s shortcomings, physicians and technicians must work together to create clinically relevant solutions.
Deep learning technologies significantly improves the way we acquire data, make diagnoses, and treat cancer. Though methods for obtaining medical information may be disputed, there is little doubt that cancer early detection will have a major impact as soon as acceptable data collection methods are discovered. In the numerous fields of study that AI-based cancer prediction algorithms are applied to using machine and deep learning approaches for extracting and categorizing disease characteristics, AI techniques play a vital role in early cancer prediction and diagnosis. With more active efforts toward developing novel biomarkers, creating tailored treatments, and accumulating big datasets, the prospective advantages and reach of AI in cancer detection are only going to improve. It further draws information from digital health tools and help physicians make accurate medical decisions.
The outcomes of AI-based models are promising and provide a competitive edge over traditional techniques. Future research will have to overcome several restrictions to develop clinically relevant techniques. Overcoming real-time limits, as well as the capacity to identify additional components like folds and blood veins, and the capacity to get uncertainty estimates from machine learning and artificial intelligence model predictions. In this instance, the promise of AI-powered technologies is that they will revolutionize the delivery and consumption of healthcare, significantly improve cancer prevention, and open up enormous financial opportunities.
10 Future scope
This review promotes future research opportunities. Hyperparameter tuning techniques may be used to further improve the performance of current models. The use of ensemble models, which may combine machine learning classifiers might improve performance and robustness. Identifying cancer may benefit from the inclusion of additional deep learning models and architectures, such as the combination of CNN-RNN models. Improved feature extraction from digital images may be used as a reliable input to models, enhancing their performance even further.
AI implementation may alter the options available to patients in more or less predictable ways. By using personal health and non-health data in addition to research information, for instance, the range of therapy options may be modified and restricted, giving the patient fewer alternatives than are now available. AI certainly has the ability to produce both good and bad outcomes. However, every algorithm will encode values, either overtly or, increasingly frequently in the ‘new AI’ age, implicitly.
The authors also present here the future research direction of AI in Colorectal Cancer. It is observed that deep learning technologies significantly improve data acquisition, diagnosis, and treatment of colorectal cancer. Though methods for obtaining medical information may be disputed, there is little question that colorectal cancer early detection will have a major impact as soon as acceptable data collection methods are discovered. Modes for related patterns also increase significantly. In the clinical and translational fields of oncology, the power of AI regarding therapeutic advice in colorectal cancer shows great potential, which translates to better and more individualized therapies for people in need. The outcomes of AI-based models are promising and provide a competitive edge over traditional techniques. Future research will have to overcome several restrictions to develop clinically relevant techniques. Overcoming real-time limits, as well as the capacity to identify additional components like folds and blood veins, and the capacity to get uncertainty estimates from machine learning/artificial intelligence model predictions.
AI-based computer-assisted systems can help physicians with essential aspects like detecting and classifying colorectal polyps. To overcome AI’s shortcomings, physicians and technicians must work together to create clinically relevant solutions.
Adegun A, Viriri S (2021) Deep learning techniques for skin lesion analysis and melanoma cancer detection: a survey of state-of-the-art. Artif Intell Rev 54:811–841
Article Google Scholar
Ali MS, Miah MS et al (2021) An enhanced technique of skin cancer classification using deep convolutional neural network with transfer learning models. Mach Learn Appl 5:2666–8270
Google Scholar
Bindu G, Lonappan A, Thomas V et al (2006) Active microwave imaging for breast cancer detection. Progr Electromagn Res 58:149–169
Booven H, Kuchakulla M et al (2021) A systematic review of artificial intelligence in prostate cancer. Media Neliti Com 13:31–39
Das K et al (2021) Machine learning and its application in skin cancer. Int J Environ Res Public Health 18(24):13409
Dong J, Geng Y, Lu D, Li B, Tian L, Lin D, Zhang Y (2020) Clinical trials for artificial intelligence in cancer diagnosis: a cross-sectional study of registered trials in clinicaltrials.gov. Front Oncol 10(September):1–6
Goel R, Singh S (2015) Skin cancer detection using GLCM matrix analysis and back propagation neural network classifier. Int J Comput Appl 112(9):975–8887
Goyal H, Mann R et al (2020) Scope of artificial intelligence in screening and diagnosis of colorectal cancer. J Clin Med 9(10):3313
Harmon S, Tuncer S et al (2019) Artificial intelligence at intersection of pathology and radiology in prostate cancer. Diagn Interv Radiol 25(3):183–188
Hekler A et al (2019) Superior skin cancer classification by the combination of human and artificial intelligence. Eur J Cancer 120:114–121
Hirasawa T et al (2018) Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 21(4):653–660
Ho C, Zhao Z et al (2022) A promising deep learning-assistive algorithm for histopathological screening of colorectal cancer. Artif Intell Rev 12(1):1–9
Injadat M, Moubayed A, Nassif A, Shami A (2021) Machine learning towards intelligent systems: applications, challenges, and opportunities. Artif Intell Rev 54(5):3299–3348
Kanimozhi T, Murthi A (2016) Computer aided melanoma skin cancer detection using artificial neural network classifier. Singaporean Journal of Scientific Research (SJSR). J Selected Areas Microelectron (JSAM) 8(2):35–42
Kim H et al (2020) Changes in cancer detection and false-positive recall in mammography using artificial intelligence: a retrospective, multireader study. Lancet Digital Health 2(3):e138–e148
Lorenzovici N, Dulf E et al (2021) Artificial intelligence in colorectal cancer diagnosis using clinical data: non-invasive approach. Diagnostics 11(3):514
Mustafa M, Azizi A et al (2016) Lung cancer: risk factors, management, and prognosis. IOSR J Dental Med Sci 15(10):94–101
Naderan M (2021) Review methods for breast cancer detection using artificial intelligence and deep learning methods. Syst Res Inf Technol 2021(1):98–102
Niu PH, Zhao L-L et al (2020) Artificial intelligence in gastric cancer: application and future perspectives. World J Gastroenterol 26(36):5408–5419
Pandiangan T, Bali I, Silalahi A (2019) Early lung cancer detection using artificial neural network. Atom Indones 45(1):9–15
Patel K et al (2022) A survey on artificial intelligence techniques for chronic diseases: open issues and challenges. Artif Intell Rev 55(5):3747–3800
Perincheri S, Levi A et al (2021) An independent assessment of an artificial intelligence system for prostate cancer detection shows strong diagnostic accuracy. Mod Pathol 34:1588–1595
Prasetyo C, Kardiana A, Yuliwulandari R (2014) Breast cancer diagnosis using artificial neural networks with extreme learning techniques. Int J Adv Res Artif Intell 3(7):10–14
Rodriguez-Ruiz A, Lång K, Gubern-Merida A, Broeders M, Gennaro G, Clauser P, Helbich TH, Chevalier M, Tan T, Mertelmeier T et al (2019) Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. JNCI J Natl Cancer Inst 111(9):916–922
Saba T (2020) Recent advancement in cancer detection using machine learning: systematic survey of decades, comparisons and challenges. J Infect Public Health 13(9):1274–1289
Sathykumar K, Munoz M et al (2020) Automated lung cancer detection using artificial intelligence (AI) deep convolutional neural networks: a narrative literature review. Cureus 12(8):1–9
Sharif HU (2021) Breast cancer detection using artificial neural networks. Int J Res Appl Sci Eng Technol 09(01):1121–1126
Shastry KA, Sanjay H (2022) Cancer diagnosis using artificial intelligence: a review. Artif Intell Rev 55(4):2641–2673
Sitarz R, Skierucha M et al (2018) Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 10:239–248
Sokouti M, Sokouti M, Sokouti B (2019) A systematic review and meta-analysis on performance of intelligent systems in lung cancer: where are we? Artif Intell Rev 53:3287–3298
Suzuki H, Yoshitaka T et al (2021) Artificial intelligence for cancer detection of the upper gastrointestinal tract. Dig Endosc 33(2):254–262
Tang D et al (2020) Development and validation of a real-time artificial intelligence-assisted system for detecting early gastric cancer: a multicentre retrospective diagnostic study. EBioMedicine 62:103146
Viscaino M, Bustos J et al (2021) Artificial intelligence for the early detection of colorectal cancer: a comprehensive review of its advantages and misconceptions. World J Gastroenterol 27(38):6399–6414
Watanabe A, Lim V, Vu H et al (2019) Improved cancer detection using artificial intelligence: a retrospective evaluation of missed cancers on mammography. J Digit Imaging 32(4):625–637
Yu C, Helwig E (2022) The role of AI technology in prediction, diagnosis and treatment of colorectal cancer. Artif Intell Rev 55(1):323–343
Download references
Author information
Authors and affiliations.
Information Technology, Ramrao Adik Institute of Technology, D. Y. Patil Deemed to be University, Sector-7, Nerul Navi Mumbai, Maharashtra, 400706, India
Gaurav Singh, Anushka Kamalja, Rohit Patil, Ashutosh Karwa, Akansha Tripathi & Pallavi Chavan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Pallavi Chavan .
Ethics declarations
Conflict of interest.
All the authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Research involving human participants and/or animals
No human or animal was harmed while performing this review.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Singh, G., Kamalja, A., Patil, R. et al. A comprehensive assessment of artificial intelligence applications for cancer diagnosis. Artif Intell Rev 57 , 179 (2024). https://doi.org/10.1007/s10462-024-10783-6
Download citation
Accepted : 04 May 2024
Published : 20 June 2024
DOI : https://doi.org/10.1007/s10462-024-10783-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Artificial intelligence
- Cancer detection
- Cancer diagnosis
- Deep learning
- Machine learning
- Image Processing
- Find a journal
- Publish with us
- Track your research
- DOI: 10.1117/12.3004212
- Corpus ID: 268787633
Synchronization and analysis of multimodal bronchoscopic airway exams for early lung cancer detection
- Qi Chang , Vahid Daneshpajooh , +4 authors W. Higgins
- Published in Medical Imaging 29 March 2024
- Medicine, Engineering
One Citation
Self-supervised monocular depth and ego-motion estimation for ct-bronchoscopy fusion, 17 references, bronchoscopic video synchronization for interactive multimodal inspection of bronchial lesions, interactive analysis system for narrow-band imaging bronchoscopy, optimal procedure planning and guidance system for peripheral bronchoscopy, video analysis framework for lesion detection in narrow band imaging bronchoscopy, interactive ct-video registration for the continuous guidance of bronchoscopy, esfpnet: efficient deep learning architecture for real-time lesion segmentation in autofluorescence bronchoscopic video, efficient bronchoscopic video summarization, application of quantitative autofluorescence bronchoscopy image analysis method in identifying bronchopulmonary cancer, hands-free system for bronchoscopy planning and guidance, the diagnostic value of narrow-band imaging for early and invasive lung cancer: a meta-analysis, related papers.
Showing 1 through 3 of 0 Related Papers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Biomed Res Int

This article has been retracted.
Lung cancer classification and prediction using machine learning and image processing, sharmila nageswaran.
1 Department of Sensor and Biomedical Technology, School of Electronics Engineering, Vellore Institute of Technology, Tamil Nadu, India
G. Arunkumar
2 Department of Computer Science and Engineering, Madanapalle Institute of Technology & Science, Madanapalle, Andhra Pradesh, India
Anil Kumar Bisht
3 Department of CS&IT, MJP Rohilkhand University, Bareilly, U. P., India
Shivlal Mewada
4 Department of Computer Science, Govt. College, Makdone (Vikram University), Ujjain, India
J. N. V. R. Swarup Kumar
5 Department of CSE, SR Gudlavalleru Engineering College, Gudlavalleru, India
Malik Jawarneh
6 Faculty of Computing Sciences, Gulf College, Oman
Evans Asenso
7 Department of Agricultural Engineering, University of Ghana, Ghana
Associated Data
The data shall be made available on request.
Lung cancer is a potentially lethal illness. Cancer detection continues to be a challenge for medical professionals. The true cause of cancer and its complete treatment have still not been discovered. Cancer that is caught early enough can be treated. Image processing methods such as noise reduction, feature extraction, identification of damaged regions, and maybe a comparison with data on the medical history of lung cancer are used to locate portions of the lung that have been impacted by cancer. This research shows an accurate classification and prediction of lung cancer using technology that is enabled by machine learning and image processing. To begin, photos need to be gathered. In the experimental investigation, 83 CT scans from 70 distinct patients were utilized as the dataset. The geometric mean filter is used during picture preprocessing. As a consequence, image quality is enhanced. The K -means technique is then used to segment the images. The part of the image may be found using this segmentation. Then, classification methods using machine learning are used. For the classification, ANN, KNN, and RF are some of the machine learning techniques that were used. It is found that the ANN model is producing more accurate results for predicting lung cancer.
1. Introduction
One of the most lethal types of the disease, lung cancer, is responsible for the passing away of about one million people every year. The current state of affairs in the world of medicine makes it absolutely essential to perform lung nodule identification on chest CT scans. This is due to the fact that lung nodules are becoming increasingly common. As a direct result of this, the deployment of CAD systems is required in order to accomplish the objective of early lung cancer identification [ 1 ].
When doing a CT scan, sophisticated X-ray equipment is utilized in order to capture images of the human body from a number of different angles. Following this, the images are fed into a computer, which processes them in such a way as to produce a cross-sectional view of the internal organs and tissues of the body [ 2 ].
A CAD approach was trained and assessed in two separate experiments. One research used a computer simulation using ground truth that was generated by computers. In this work, the cardiac-torso (XCAT) digital phantom was used to replicate 300 CT scans. The second research made use of patient-based ground truth using human subjects and implanted spherical nodules of varied sizes (i.e., 3-10 mm in diameter) at random inside the lung area of the simulated pictures. CT images from the LIDC-IDRI dataset were used to create the CAD technique. 888 CT pictures left for processing after CT scans with a wall thickness of more than 2.5 mm were disregarded. In all investigations, a 10-fold cross-validation approach was used to assess network hyperparameterization and generalization. The detection sensitivities were measured in response to the average false positives (FPs) per picture to assess the overall accuracy of the CAD approach. Using the free-receiver response operating characteristic (FROC) curve, the detection accuracy in the patient research was further evaluated in 9 previously published CAD investigations. The mean and standard error between the anticipated value and ground truth were used to measure the localization and diameter estimate accuracies. In all investigations, the average outcomes throughout the 10 cross-validation folds showed that the CAD approach had a high level of detection accuracy. In the patient trial, the corresponding sensitivities were 90.0 percent and 95.4 percent, showing superiority in the FROC curve analysis over many traditional and CNN-based lung nodule CAD approaches. In both investigations, the nodule localization and diameter estimation errors were fewer than 1 mm. The CAD approach that was created was highly efficient in terms of computing [ 3 ].
It is likely that intravenous injection of contrast (X-ray dye) may considerably improve the quality of CT imaging, which can reveal a wide variety of organs and tissues. This is one of the potential benefits of contrast injection. In addition, CT scans can reliably detect kidney or gallstones, as well as abnormal fluid buildup or enlarged lymph nodes in the abdominal region or pelvis. This is in addition to the capacity to detect gallstones and kidney stones. Because the CT scan is unable to provide a precise diagnosis of certain organs, such as the stomach, it can, however, be used to reveal abnormalities in the soft tissues that are positioned nearby, offering an indirect diagnosis of these organs [ 4 , 5 ].
If lung cancer is detected at an early stage, the American Cancer Society estimates that a patient has a 47 percent chance of surviving the disease. It is quite unlikely that X-ray pictures may accidentally reveal lung cancer in its earlier stages [ 6 ]. It is famously difficult to detect lesions that are round and have a diameter of 510 millimeters or less. A CT scan of a patient diagnosed with lung cancer is shown in Figure 1 .

CT scan image for lung cancer.
The processing of images is an essential activity for a diverse variety of business sectors. It is utilized in X-ray imaging of the lungs in order to find regions that contain cancerous growths. In order to detect areas of the lung that have been affected by cancer, image processing techniques such as noise reduction, feature extraction, identification of damaged regions, and maybe a comparison with data on the medical history of lung cancer are utilized. The majority of the time, digital image processing makes use of a diverse set of methods to merge a number of distinct aspects of a picture into a single coherent entity. This research takes an innovative technique in order to zero down on a particular aspect of the overall lung image. The split region may be seen in a variety of ways, including from different viewpoints and when illuminated in different ways. When utilizing this method, one of the key benefits is the ability to differentiate between portions of a picture that have been impacted by cancer and sections that have not been affected by cancer by comparing the intensity of the two sets of photos [ 6 , 7 ].
As a result of the fact that the majority of patients are diagnosed at a more advanced stage, lung cancer is the primary cause of death resulting from cancer. There is currently no chance of a successful treatment being developed. Lung cancer is consistently ranked as one of the most lethal forms of the disease, regardless of whether a country is industrialized or developing. The incidence of lung cancer in developing countries is on the rise as a result of a longer life expectancy, more urbanization, and the adoption of Western lifestyles. The early detection of cancer and the survival of people with the disease are both essential to the control of lung disease [ 8 , 9 ].
The literature survey section contains a review of various techniques for the classification and detection of cancer using image processing and classification. The methodology section presents accurate classification and prediction of lung cancer using machine learning and image processing-enabled technology. First, images are acquired. Then, images are preprocessed using the geometric mean filter. This results in improving image quality. Then, images are segmented using the K -means algorithm. This segmentation helps in the identification of the region of interest. Then, machine learning classification techniques are applied. The result section contains details related to the dataset and results achieved by various techniques.
To reduce the amount of data that has to be broken down, this study illustrates a method to separate the lung tissue from a chest CT. We will likely have a fully automated computation for cutting the lung tissue into sections and for separating the two sides of the lung as well. The threshold shown in the image separates fat from low-thickness tissue (the lungs). Cleaning is done to get rid of the commotion, air, and flight routes. Finally, a combination of morphological operations is used to tame the unexpected limit. The database used for the evaluation was obtained from a book that instructs radiologists. The current analysis shows that the linked division computation attempts to handle a wide range of different circumstances. The portioned lungs' textural accents were taken off, and it was provided. The neurological system is used to differentiate between the various lung diseases [ 10 ].
1.1. Literature Survey
Palani and Venkatalakshmi [ 11 ] have given predictive modeling of lung cancer illness by continuous monitoring. They did this by using fuzzy cluster-linked augmentation with a categorization. The fuzzy clustering approach is essential to the production of accurate picture segmentation. We instead utilized the fuzzy C -means clustering approach in order to accomplish our goal of further disentangling the characteristics of the transitional area from those of the lung cancer image. In this particular investigation, the Otsu thresholding method was applied in order to distinguish the transition area from the lung cancer representation. In addition to this, the right edge picture is utilized in conjunction with the morphological, thinning procedure in order to improve the presentation of the segmentation. The current Association Rule Mining (ARM), the conventional decision tree (DT), and the CNN are combined with a novel incremental classification technique in order to accomplish classification in an incremental fashion. In order to carry out the operations, standard images from the database were utilized, as well as the most recent data on the patient's health collected from IoT devices that were attached to the patient. The culmination of the research indicates that the predictive modeling system has become more accurate.
Deep residual learning was utilized by Bhatia et al. in order to develop a method for determining whether or not a CT picture contains lung cancer. The researchers have devised a preprocessing pipeline by making use of the UNet and ResNet models. This pipeline is intended to highlight and extract features from sections of the lung that are cancerous. An ensemble of XGBoost and random forest classifiers is used to gather predictions about the likelihood that a CT scan is malignant. The results of each classifier's predictions are then pooled, and the final result is used to determine the likelihood that a CT scan is malignant. The LIDC-IRDI has an accuracy that is 84 percent higher than that of typical techniques [ 12 ].
Joon et al. [ 13 ] segmented lung cancer using an active spline model as their method of analysis. With X-ray photos, through the use of this technique, X-ray images of the lung have been obtained. To begin, it is recommended that a median filter be used for noise detection while the preprocessing stage is being carried out. During the phase devoted to segmentation, further K -means and fuzzy C -means clustering are utilized for the purpose of feature capture. In this research, the ultimate feature retrieval outcome is reached after the X-ray picture has been segmented. The recommended model was developed by the application of the SVM approach for classification. In order to simulate the findings of the cancer detection system, MATLAB is utilized. The purpose of this study was to detect and categorize lung cancer by making use of images that were both normal and malignant.
Nithila and Kumar [ 14 ] have developed an active contouring model, and this model has been deployed. An application of a variation level set function was used for the segmentation of the lungs. It is essential to properly segment the parenchyma in order to arrive at an appropriate diagnosis of lung illness. CT, which stands for computerized tomography, was the first imaging modality to make use of image analysis in this manner. A significant advancement in CT lung image segmentation has been made by the development of the SBGF-new SPF function, which stands for selective binary and Gaussian filtering-new signed pressure force. By taking this strategy, external lung limitations have been identified, and inefficient expansion at the margins has been prevented. Comparisons are being made between the currently under consideration algorithm and four distinct active contour models. The results of the tests demonstrate that the strategy that was provided is reliable and can be computed very quickly [ 13 ].
Lakshmanaprabu et al. [ 15 ] created OODN (Optimal Deep Neural Network) by lowering the number of characteristics in lung CT scans and comparing it to other classification algorithms. This allowed them to design a more accurate method. The adoption of an automated classification method for lung cancer has cut down on the amount of time needed for human labeling and removed the possibility of mistakes being made by the individual doing the labeling. According to the findings of the researchers, the performance of the machine learning algorithms in terms of accuracy and precision in the detection of normal and abnormal lung photos has significantly increased. According to the findings, the research was successful in classifying lung pictures with a peer specificity of 94.56 percent, a level of accuracy of 96.2 percent, and a level of sensitivity of 94.2 percent. It has been shown that it is feasible to increase the performance of cancer detection in CAT scans [ 14 ]. The research has shown that this is the case.
Talukdar and Sarma have placed a strong emphasis on the use of image processing methods for the diagnosis of lung cancer (2018). Deep learning methodologies are being applied to the study of lung cancer. The most prevalent kind of cancer, lung cancer, is taking the lives of an alarmingly high number of individuals. The likelihood of an individual acquiring lung cancer was evaluated with a computed tomography (CT) scan. The growth of precancerous tissue is referred to as “nodules,” and their presence is utilized as a general indication of cancer. Educated radiologists are able to detect nodules and often predict their relationship with cancer. However, these radiologists are also capable of producing false positive and false negative findings. Because the patient is under continual stress, a tremendous quantity of data is evaluated, and a decision that is suitable for the patient is made in a timely manner. As a consequence of this, developing a computer-aided detection system that is capable of rapidly detecting features based on the input of radiologists is most likely to be the answer [ 15 ].
Yu et al. have obtained histopathology whole-slide slides of lung cancer and squamous cell carcinoma that have been stained with hematoxylin and eosin (2016). Patients' photographs were taken from TCGA (The Cancer Genome Atlas) and the Stanford TMA (Tissue Microarray Database), plus an additional 294 photos. Even when conducted with the greatest of intentions, an assessment of human pathology cannot properly predict the patient's prognosis. A total of 9,879 quantitative elements of an image were retrieved, and machine learning algorithms were used to select the most important aspects and differentiate between patients who survived for a short period of time and those who survived for a long period of time after being diagnosed with stage I adenocarcinoma or squamous cell carcinoma. The researchers used the TMA cohort to validate the survival rate of the recommended framework (P0.036 for tumor type). According to the findings of this study, the characteristics that are created automatically may be able to forecast the prognosis of a lung cancer patient and, as a consequence, may help in the development of personalized medication. The methodologies that were outlined can be utilized in the analysis of histopathology images of various organs [ 16 ].
Pol Cirueda and his colleagues used an aggregation of textures that kept the spatial covariances across features consistent. Mixing the local responses of texture operator pairs is done using traditional aggregation functions like the average; nonetheless, doing so is a vital step in avoiding the problems of traditional aggregation. Pretreatment computed tomography (CT) scans were utilized in order to assist in the prediction of NSCLC nodule recurrence prior to the administration of medication. After that, the recommended methods were put to use in order to compute the kind of NSCLC nodule recurrence according to the manifold regularized sparse classifier. These discoveries, which offer up new study possibilities on how to use morphological, tissue traits to evaluate cancer invasion, need to be confirmed and investigated further. However, this will not be possible without more research. When modeling orthogonal information, the author focused on the textural characteristics of nodular tissue and coupled those characteristics with other variables such as the size and shape of the tumor [ 17 ].
The creation of a method for the early detection and accurate diagnosis of lung cancer that makes use of CT, PET, and X-ray images by Manasee Kurkure and Anuradha Thakare in 2016 has garnered a significant amount of attention and enthusiasm. The utilization of a genetic algorithm that permits the early identification of lung cancer nodules by diagnostics allows for the optimization of the findings to be accomplished. It was necessary to employ both Naive Bayes and a genetic algorithm in order to properly and swiftly classify the various stages of cancer images. This was done in order to circumvent the intricacy of the generation process. The categorization has an accuracy rate of up to eighty percent [ 18 ].
Sangamithraa and Govindaraju [ 19 ] have used a preprocessing strategy in order to eliminate the unwanted unaffected by the use of median and Wiener filters. This was done in order to improve the quality of the data. The K -means method is used to do the segmentation of the CT images. EK-mean clustering is the method that is used to achieve clustering. To extract contrast, homogeneity, area, corelation, and entropy features from images, fuzzy EK-mean segmentation is utilized. A back propagation neural network is utilized in order to accomplish the classification [ 20 ].
According to Ashwini Kumar Saini et al. (2016), a summary of the types of noise that might cause lung cancer and the strategies for removing them has been provided. Due to the fact that lung cancer is considered to be one of the most life-threatening kinds of cancer, it is essential that it be detected in its earlier stages. If the cancer has a high incidence and mortality rate, this is another indication that it is a particularly dangerous form of the disease. The quality of the digital dental X-ray image analysis must be significantly improved for the study to be successful. A pathology diagnosis in a clinic continues to be the gold standard for detecting lung cancer, despite the fact that one of the primary focuses of research right now is on finding ways to reduce the amount of image noise. X-rays of the chest, cytological examinations of sputum samples, optical fiber investigations of the bronchial airways, and final CT and MRI scans are the diagnostic tools that are utilized most frequently in the detection of lung malignancies (MRI). Despite the availability of screening methods like CT and MRI that are more sensitive and accurate in many parts of the world, chest radiography continues to be the primary and most prevalent kind of surgical treatment. It is routine practice to test for lung cancer in its early stages using chest X-rays and CT scans; however, there are problems associated with the scans' weak sensitivities and specificities [ 19 ].
Neural ensemble-based detection is the name given to the automated method of illness diagnosis that was suggested in Kureshi et al.'s research [ 21 ] (NED). The approach that was suggested utilized feature extraction, classification, and diagnosis as its three main components. In this experiment, the X-ray chest films that were taken at Bayi Hospital were utilized. This method is recommended because it has a high identification rate for needle biopsies in addition to a decreased number of false negative identifications. As a result, the accuracy is improved automatically, and lives are saved [ 22 ].
Kulkarni and Panditrao [ 23 ] have created a novel algorithm for early-stage cancer identification that is more accurate than previous methods. The program makes use of a technology that processes images. The amount of time that passes is one of the factors that is considered while looking for anomalies in the target photographs. The position of the tumor can be seen quite clearly in the original photo. In order to get improved outcomes, the techniques of watershed segmentation and Gabor filtering are utilized at the preprocessing stage. The extracted interest zone produces three phases that are helpful in recognizing the various stages of lung cancer: eccentricity, area, and perimeter. These phases may be found in the extracted interest zone. It has been revealed that the tumors come in a variety of dimensions. The proposed method is capable of providing precise measurements of the size of the tumor at an early stage [ 21 ].
Westaway et al. [ 24 ] used a radiomic approach to identify three-dimensional properties from photos of lung cancer in order to provide prediction information. As is well known, classifiers are devised to estimate the length of time an organism will be able to continue existing. The Moffitt Cancer Center in Tampa, Florida, served as the location from where these photographs for the experiment's CT scans were obtained. Based on the properties of the pictures produced by CT scans, which may suggest phenotypes, human analysis may be able to generate more accurate predictions. When a decision tree was used to make the survival predictions, it was possible to accurately forecast seventy-five percent [ 23 ] of the outcomes.
CT (computed tomography) images of lung cancer have been categorized with the use of a lung cancer detection method that makes use of image processing. This method was described by Chaudhary and Singh [ 25 ]. Several other approaches, including segmentation, preprocessing, and the extraction of features, have been investigated thus far. The authors have distinguished segmentation, augmentation, and feature extraction, each in its own unique section. In Stages I, II, and III, the cancer is contained inside the chest and manifests as larger, more invasive tumors. By Stage IV, however, cancer has spread to other parts of the body [ 24 ], at which point it is said to be in Stage IV.
2. Methodology
This section shows an accurate classification and prediction of lung cancer using technology that is enabled by machine learning and image processing. To begin, photos need to be gathered. After that, a geometric mean filter is used to perform preprocessing on the images. This ultimately leads to an improvement in image quality. After that, the K -means method is used to segment the images. The identification of the region of interest is facilitated by this segmentation. After that, categorization strategies based on machine learning are utilized. Figure 2 illustrates the classification and prediction of lung cancer utilizing technology that enables machine learning and image processing.

Classification and prediction of lung cancer using machine learning and image processing-enabled technology.
The preprocessing of images plays a significant role in the proper classification of photographs of illnesses. CT scans provide images with a broad variety of artefacts, including noise, which may be seen in these scans. These artefacts may be removed by using image filtering methods. A geometric mean filter is applied to the input pictures in an effort to decrease the amount of noise [ 25 ].
This is accomplished by using a method known as linear discriminant analysis (LDA), which cuts down on the amount of space required for the initial data matrix. The PCA and LDA are two examples of parallel transformation algorithms. In contrast to the supervised LDA method, the PCA is an unsupervised analysis method. In contrast to principal component analysis (PCA), latent dynamic analysis (LDA) seeks to identify a feature subspace that maximizes the possibility of class restoration. It is possible to avoid overfitting by placing more importance on the class-reparability of the data rather than the processing costs [ 26 ].
The method of segmentation is used in the process of medical image processing. The basic role of a picture is to differentiate between components that are beneficial and those that are harmful. As a consequence of this, it separates a picture into distinct pieces based on the degree to which each component is similar to its surrounding components. This effect may be achieved by manipulating the intensity as well as the texture. An area of interest that has been segmented may be utilized as a diagnostic tool to quickly get information that is pertinent to the issue at hand. When it comes to the process of segmenting medical pictures, the technique known as K -means clustering is the one that is used most often. During the clustering process, the picture is divided into a number of different groups, also known as clusters, which do not overlap with one another. These clusters are not connected to one another in any way. In this picture, there are a few distinct clusters that can be noticed. Every one of them has its own one-of-a-kind collection of reference points to which each pixel is assigned. To divide the available data into k separate groups, the K -means clustering algorithm divides the available information based on k reference points [ 27 ].
Artificial neural networks, also known as ANNs, are used often in the medical industry for the purpose of classifying medical images for the goal of diagnosing illness. In terms of the way it performs its tasks, the ANN is fairly comparable to the human brain. It is feasible to get the knowledge required to make an informed guess about the category that a photograph belongs to by looking at a collection of images that have already been categorized. This may be accomplished by looking at a collection of pictures that have been categorized. A category has already been selected for each of the pictures included in this gallery. An artificial neural network (ANN) is constructed up of artificial neurons, which are programmed to behave in a manner that is analogous to that of their biological counterparts in the human brain. Neurons are able to communicate with one another outside of their bodies through connections. It is possible to assign weights to neurons and edges, and those weights may be changed at any time throughout the process of learning. The standard structure of an artificial neural network has three layers: an input layer, a hidden layer, and an output layer that is in charge of creating the signal. This is the architecture that is used the most often. The most popular topologies for artificial neural networks include an input layer, a hidden layer, and a final layer; however, there are other possible configurations as well. It is conceivable that there is just one hidden layer, that there are several hidden levels, or that there are no hidden layers at all. Each and every one of these options is not completely out of the question. The weights that need to be adjusted until the desired output is reached are tucked away in a layer that is below the active layer [ 28 ]. The iterations are closely related to computing efficiency during the training of the ANN model. Precision will suffer by having too few hidden layer neurons, while too many neurons would lengthen training time.
The KNN approach, which is the method that is used in ML the most commonly, makes it easy to learn about the algorithms that are employed in ML. It is a technique of supervised learning that does not need the use of any parameters. The phase that the k -training NN goes through is thus significantly quicker than the phase that other classifiers go through. The testing stage, on the other hand, takes longer and uses more memory as it goes on. In order to use k -nearest neighbors to categorize new kinds of data points, one needs first to have data that is already organized into many different categories. Because training observations are included in each labeled dataset, the algorithm is able to establish a connection between x and y in each training dataset ( x , y ). The typical practice at this location is delaying the processing in order to locate the KNN function. The contributions of neighbors may be weighted in classification models as well as regression models, which can result in a higher average score for those who live in close proximity to one another in comparison to those who live farther away. As the distance between two neighbors increases, an additional weighting of 1/ d is applied to each neighbor [ 29 ]. Despite producing good precision on the test dataset, KNN is still slower and more expensive to run in terms of both time and memory. To store the whole training dataset for prediction, it needs a lot of memory. Additionally, as Euclidean distance is very reactive to orders of magnitude, features in the dataset with high magnitudes always have a higher weight than those with low magnitudes. Last but not least, we must remember that KNN is not appropriate for large-dimensional datasets.
It is possible to construct predictive models by using the random forest approach, which is used by a lot of people. Only two of the many applications that may be accomplished using RF are regression and classification [ 30 ]. It is possible to develop machine learning algorithms that are capable of making predictions with a high degree of accuracy so long as datasets are changed appropriately [ 31 ]. This approach is highly user-friendly in comparison to other algorithms, and it has a lot of support from members of the general public. For the purposes of this model, RF is an abbreviation for “random forest,” and true to its moniker, the model creates random forests. With the help of this technique, one may generate an entire grove of decision trees, each of which is trained in a distinct way. This method was used to build the current thicket of trees representing the many possible multiple-choice responses. As a direct consequence of this, they were integrated in order to provide even more accurate projections [ 22 ].
3. Result Analysis
A dataset of 83 CT images from 70 different patients was used in the experimental study [ x ]. Images are preprocessed using the geometric mean filter. This results in improving image quality. Then, images are segmented using the K -means algorithm. This segmentation helps in the identification of the region of interest. Then, machine learning classification techniques are applied.
For performance comparison, three parameters, accuracy, sensitivity, and specificity, are used:
where TP is true positive, TN is true negative, FP is false positive, and FN is false negative.
Results of different machine learning predictors are shown in Figures Figures3 3 3 – 5 . The accuracy of ANN is better.

Accuracy of machine learning techniques for lung cancer detection.

Sensitivity of machine learning techniques for lung cancer detection.

Specificity of machine learning techniques for lung cancer detection.
4. Conclusion
Lung cancer is one of the deadliest types of the disease, claiming the lives of approximately one million people each year. Given the current state of affairs in medicine, it is critical that lung nodule identification be performed on chest CT scans. As a result, the use of CAD systems is crucial for the early detection of lung cancer. Image processing is a necessary activity that is employed in a wide range of economic domains. It is used in X-ray imaging of the lungs to find areas of the body that have developed malignant growths. Image processing techniques such as noise reduction, feature extraction, identification of damaged regions, and maybe comparison with data on the medical history of lung cancer are used to locate sections of the lung that have been affected by cancer. This study demonstrates accurate lung cancer classification and prediction using technologies enabled by machine learning and image processing. To begin, photographs must be collected. Following that, the images are preprocessed using a geometric mean filter. This eventually leads to an increase in image quality. The K -means approach is then used to segment the images. This segmentation makes it easier to identify the region of interest. Following that, machine learning-based categorization algorithms are used. ANN predicts lung cancer with more accuracy. This research will help to increase the accuracy of lung cancer detection systems that use strong classification and prediction techniques. This study brings cutting-edge images based on machine learning techniques for implementation purposes.
Data Availability
Conflicts of interest.
The authors declare that they have no conflict of interest.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Patient Navigation for Lung Cancer Screening at a Health Care for the Homeless Program : A Randomized Clinical Trial
- 1 Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Boston
- 2 Mongan Institute, Massachusetts General Hospital, Boston
- 3 Harvard Medical School, Boston, Massachusetts
- 4 Institute for Research, Quality & Policy in Homeless Health Care, Boston Health Care for the Homeless Program, Boston, Massachusetts
- 5 New York University Grossman School of Medicine, New York
- 6 University of Massachusetts Chan Medical School, Worcester
- Invited Commentary Progress in Lung Cancer Screening Adoption Ilana B. Richman, MD, MHS; Cary P. Gross, MD JAMA Internal Medicine
- Original Investigation Lung Cancer Screening in the US, 2022 Priti Bandi, PhD; Jessica Star, MA, MPH; Kilan Ashad-Bishop, PhD; Tyler Kratzer, MPH; Robert Smith, PhD; Ahmedin Jemal, DVM, PhD JAMA Internal Medicine
Question Does providing patient navigation improve lung cancer screening completion in a homeless health care setting?
Findings In this randomized clinical trial that included 260 patients in a large Health Care for the Homeless program from 2020 to 2023, patient navigation in addition to usual care produced a statistically significant 4.7-fold increase in lung cancer screening receipt at 6 months compared with usual care alone (43.4% vs 9.2%).
Meaning Study findings suggest that patient navigation may be an effective tool for promoting lung cancer screening engagement among people experiencing homelessness.
Importance People experiencing homelessness die of lung cancer at rates more than double those in the general population. Lung cancer screening (LCS) with low-dose computed tomography (LDCT) reduces lung cancer mortality, but the circumstances of homelessness create barriers to LCS participation.
Objective To determine whether patient navigation, added to usual care, improved LCS LDCT receipt at a large Health Care for the Homeless (HCH) program.
Design, Setting, and Participants This parallel group, pragmatic, mixed-methods randomized clinical trial was conducted at Boston Health Care for the Homeless Program (BHCHP), a federally qualified HCH program that provides tailored, multidisciplinary care to nearly 10 000 homeless-experienced patients annually. Eligible individuals had a lifetime history of homelessness, had a BHCHP primary care practitioner (PCP), were proficient in English, and met the pre-2022 Medicare coverage criteria for LCS (aged 55-77 years, ≥30 pack-year history of smoking, and smoking within the past 15 years). The study was conducted between November 20, 2020, and March 29, 2023.
Intervention Participants were randomized 2:1 to usual BHCHP care either with or without patient navigation. Following a theory-based, patient-centered protocol, the navigator provided lung cancer education, facilitated LCS shared decision-making visits with PCPs, assisted participants in making and attending LCS LDCT appointments, arranged follow-up when needed, and offered tobacco cessation support for current smokers.
Main Outcomes and Measures The primary outcome was receipt of a 1-time LCS LDCT within 6 months after randomization, with between-group differences assessed by χ 2 analysis. Qualitative interviews assessed the perceptions of participants and PCPs about the navigation intervention.
Results In all, 260 participants (mean [SD] age, 60.5 [4.7] years; 184 males [70.8%]; 96 non-Hispanic Black participants [36.9%] and 96 non-Hispanic White participants [36.9%]) were randomly assigned to usual care with (n = 173) or without (n = 87) patient navigation. At 6 months after randomization, 75 participants in the patient navigation arm (43.4%) and 8 of those in the usual care–only arm (9.2%) had completed LCS LDCT ( P < .001), representing a 4.7-fold difference. Interviews with participants in the patient navigation arm and PCPs identified key elements of the intervention: multidimensional social support provision, care coordination activities, and interpersonal skills of the navigator.
Conclusions and Relevance In this randomized clinical trial, patient navigation support produced a 4.7-fold increase in 1-time LCS LDCT completion among HCH patients in Boston. Future work should focus on longer-term screening participation and outcomes.
Trial Registration ClinicalTrials.gov Identifier: NCT04308226
- Invited Commentary Progress in Lung Cancer Screening Adoption JAMA Internal Medicine
Read More About
Baggett TP , Sporn N , Barbosa Teixeira J, et al. Patient Navigation for Lung Cancer Screening at a Health Care for the Homeless Program : A Randomized Clinical Trial . JAMA Intern Med. Published online June 10, 2024. doi:10.1001/jamainternmed.2024.1662
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

An organoid co-culture model for probing systemic anti-tumor immunity in lung cancer
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected] [email protected] [email protected]
- Info/History
- Supplementary material
- Preview PDF
Deciphering the interactions between tumor micro- and systemic immune macro-environment is essential for developing more effective cancer diagnosis and treatment strategies. Here, we established a gel-liquid interface (GLI) co-culture of lung cancer organoids (LCOs) and paired peripheral blood mononuclear cells (PBMCs), featuring with enhanced interactions of immune cells and tumor organoids, to mimic the in vivo systemic anti-tumor immunity induced by immune checkpoint inhibitors (ICI). The co-culture model recapitulates the in vivo ICI-induced T cell recruitment and subsequent tumor regression, predicting the clinical results precisely. We demonstrated that circulating tumor-reactive T cells, which are effector memory-like with high expression levels of GNLY, CD44 and CD9, can serve as an indicator of the immunotherapy efficacy. Interestingly, enhanced inflammatory signaling in blood T cells is accompanied with prompted exhaustion and compromised anti-tumor function, when encountering with organoids. Our findings suggest that the GLI co-culture can be used for developing diagnostic strategies for precision immunotherapies as well as understanding the underlying mechanisms.
- Download figure
- Open in new tab
Competing Interest Statement
The authors have declared no competing interest.
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about bioRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Cancer Biology
- Animal Behavior and Cognition (5387)
- Biochemistry (12153)
- Bioengineering (9100)
- Bioinformatics (30041)
- Biophysics (15421)
- Cancer Biology (12542)
- Cell Biology (17990)
- Clinical Trials (138)
- Developmental Biology (9718)
- Ecology (14560)
- Epidemiology (2067)
- Evolutionary Biology (18722)
- Genetics (12521)
- Genomics (17169)
- Immunology (12265)
- Microbiology (28920)
- Molecular Biology (11990)
- Neuroscience (62960)
- Paleontology (462)
- Pathology (1929)
- Pharmacology and Toxicology (3354)
- Physiology (5166)
- Plant Biology (10763)
- Scientific Communication and Education (1704)
- Synthetic Biology (2989)
- Systems Biology (7516)
- Zoology (1689)
Worldwide research landscape of artificial intelligence in lung disease: A scientometric study
- Wang, XianQi
To perform a comprehensive bibliometric analysis of the application of artificial intelligence (AI) in lung disease to understand the current status and emerging trends of this field. AI-based lung disease research publications were selected from the Web of Science Core Collection. Citespace, VOS viewer and Excel were used to analyze and visualize co-authorship, co-citation, and co-occurrence analysis of authors, keywords, countries/regions, references and institutions in this field. Our study included a total of 5210 papers. The number of publications on AI in lung disease showed explosive growth since 2017. China and the United States lead in publication numbers. The most productive author were Li, Weimin and Qian Wei, with Shanghai Jiaotong University as the most productive institution. Radiology was the most co-cited journal. Lung cancer and COVID-19 emerged as the most studied diseases. Deep learning, convolutional neural network, lung cancer, radiomics will be the focus of future research. AI-based diagnosis and treatment of lung disease has become a research hotspot in recent years, yielding significant results. Future work should focus on establishing multimodal AI models that incorporate clinical, imaging and laboratory information. Enhanced visualization of deep learning, AI-driven differential diagnosis model for lung disease and the creation of international large-scale lung disease databases should also be considered.
- Bibliometric analysis;
- Artificial intelligence (AI);
- Lung disease;
- artificial intelligence;
- chronic obstructive pulmonary disease;
- tuberculosis;
- Web of Science Core Collection;
- Total link Strength;
- betweenness centrality value;
- completely portal robot lobectomy with 4 arms;
- stereotactic body radiotherapy;
- computed tomography;
- support vector machine
Minimally Invasive and Sublobar Resections for Lung Cancer.
No abstract available.
- Lambright ES

IMAGES
VIDEO
COMMENTS
Lung cancer is the primary cause of cancer death worldwide, with 2.09 million new cases and 1.76 million people dying from lung cancer in 2018 1.Four case-controlled studies from Japan reported in ...
This paper presents recent achievements in lung cancer segmentation, detection, and classification using deep learning methods. This study highlights current state-of-the-art deep learning-based lung cancer detection methods. This paper also highlights recent achievements, relevant research challenges, and future research directions.
Lung cancer is the main reason for cancer-related deaths, according to the American Cancer Society. Following to the statistics for cancer in 2022, there were almost 1.9 million reported cases and ...
A report from the International Agency for Research on Cancer (IARC) states that 27 million new cases of cancer are expected before 2030. 1 in 18 men and 1 in 46 women are estimated to develop lung cancer over a lifetime. This paper discusses an overview of lung cancer, along with publicly available benchmark data sets for research purposes.
This paper compares three of the most popular ML techniques commonly used for breast cancer detection and diagnosis, namely Support Vector Machine (SVM), Random Forest (RF) and Bayesian Networks (BN).
The proposed system is used to detect the cancerous nodule from the lung CT scan image using watershed segmentation for detection and SVM for classification of nodule as Malignant or benign. Proposed model detects the cancer with 92% accuracy which is higher than current model and classifier has accuracy of 86.6%.
Evidence from the US and Europe . In 2011, results from the US-based National Lung Screening Trial (NLST) indicated a 20% decrease in lung cancer-related mortality after a median follow-up of 6.5 years in patients undergoing annual LDCT screening compared with scanning by radiography at the same frequency for 3 years 19.Notably, a relative decrease of 6.7% (95% CI 1.2-13.6, P = 0.02) in all ...
Lung Cancer has different types: small cell lung cancer and non-small cell Lung Cancer 8. Figure 1 explains the CT Scan images used to detect the presence of a Lung Nodule, a cancer tumor.
The following set of keywords was used in the search and selection process to find the papers that made up this review paper: Set 1. lung cancer detection OR diagnosis, machine learning OR deep learning, classification. Set 2. Medical imaging, machine learning, lung cancer. Set 3. automated system, lung cancer, classification. Set 4
Purpose To determine whether deep learning algorithms developed in a public competition could identify lung cancer on low-dose CT scans with a performance similar to that of radiologists. Materials and Methods In this retrospective study, a dataset consisting of 300 patient scans was used for model assessment; 150 patient scans were from the competition set and 150 were from an independent ...
Lung cancer is one of the key causes of death amongst humans globally, with a mortality rate of approximately five million cases annually. The mortality rate is even higher than breast cancer and prostate cancer combination. However, early detection and diagnosis can improve the survival rate. Different modalities are used for lung cancer detection and diagnosis, while Computed Tomography (CT ...
Introduction. Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer deaths worldwide. About 2.20 million new patients are diagnosed with lung cancer each year [1], and 75% of them die within five years of diagnosis [2].High intra-tumor heterogeneity (ITH) and complexity of cancer cells giving rise to drug resistance make cancer treatment more challenging [3].
The detection of lung cancer has previously been considered using techniques of image. processing as the work implemented by (Abdillah et al., 2017) in initiation with deep learning. and neural ...
Numerous systems have been created, and research into the detection of lung cancer is still ongoing. However, some systems still need to be improved in order to obtain the best detection accuracy possible, which is going towards 100%. ... The review paper examines the body of knowledge on lung cancer diagnosis and presents several ...
Purpose: To compare the diagnostic performance of standalone deep learning (DL) algorithms and human experts in lung cancer detection on chest computed tomography (CT) scans. Materials and methods: This study searched for studies on PubMed, Embase, and Web of Science from their inception until November 2023. We focused on adult lung cancer patients and compared the efficacy of DL algorithms ...
Lung cancer remains a dangerous global health challenge, which desires for innovation for early detection and personalized treatment. This research paper introduces an integration of deep learning framework that uses the power of large language models (LLMs) on medical and image data for increasing the accuracy in lung cancer detection. Our proposed system focuses on integrating patient-report ...
Popular datasets used in lung cancer research using machine learning include the Lung ... This paper provided a literature review of how cancer has been detected using various machine learning methods. ... Oh S, Hong S, Kang M, Kang D, Ji Y-G, Choi BH, Kang K-W, Jeong H, Park Y, et al. Early-stage lung cancer diagnosis by deep learning-based ...
Lung cancer is considered one of the most threatening carcinoma diseases. If detected early and treated it can save the lives of thousands of people each year. Due to the increasing popularity of image processing techniques in medical fields, it has been possible to improve the detection and treatment of various cancer tumors, such as breast cancer and lung cancer. Processing techniques can ...
This survey consist of 36 research and review-based papers detailing AI applications and their comparisons across the diagnosis of major cancers. This review is created by thoroughly examining the relevant prior research and compiling the data in an organized manner. ... CT lung cancer detection is one such AI-backed solution that is used to ...
In this paper, the author prop oses a method of detecting lung cancer in a CT scan using a 2D-UNet. model on a web application. The author cropp ed 2D cancer masks on its reference image using the ...
A framework for video synchronization and fusion tailored to multimodal bronchoscopic airway examination is proposed, built into an interactive graphical system and results with patient multimodal bronchoscopic airway exams show the promise of the methods. Because lung cancer is the leading cause of cancer-related deaths globally, early disease detection is vital.
An algorithm has been proposed for Automatic detection of Lung cancer using Deep Learning Techniques. The Base network has chosen from VGG-16 Architecture. In this work, classification of lung cancer such as Adenocarcinoma, Large Cell Carcinoma, Squamous Cell Carcinoma has been differentiated from the normal lung images through deep learning ...
"Lung tissue samples can now be analyzed in minutes by our computer program to provide fairly accurate predictions of whether their cancer will return, predictions that are better than current ...
IC2: all papers related to the lung cancer detection process: IC3: publications in academic journals, book chapters, conference/workshop proceedings, and thesis dissertations: ... Challenges and Research Direction. Lung cancer detection techniques are improving day by day. Currently, available lung cancer detection techniques are quite good in ...
The creation of a method for the early detection and accurate diagnosis of lung cancer that makes use of CT, PET, and X-ray images by Manasee Kurkure and Anuradha Thakare in 2016 has garnered a significant amount of attention and enthusiasm. ... ANN predicts lung cancer with more accuracy. This research will help to increase the accuracy of ...
Key Points. Question Does providing patient navigation improve lung cancer screening completion in a homeless health care setting?. Findings In this randomized clinical trial that included 260 patients in a large Health Care for the Homeless program from 2020 to 2023, patient navigation in addition to usual care produced a statistically significant 4.7-fold increase in lung cancer screening ...
Deciphering the interactions between tumor micro- and systemic immune macro-environment is essential for developing more effective cancer diagnosis and treatment strategies. Here, we established a gel-liquid interface (GLI) co-culture of lung cancer organoids (LCOs) and paired peripheral blood mononuclear cells (PBMCs), featuring with enhanced interactions of immune cells and tumor organoids ...
Deep Convolutional Neural Network CNNs is used to identify or label a medical image in some research papers. Diagnosed lung cancer in 2015 with a multiscal two-layer CNN ... the predictive models using the machine learning algorithms reported in the literal works are less for lung cancer detection with IoT integration. There is a high scope to ...
Radiology was the most co-cited journal. Lung cancer and COVID-19 emerged as the most studied diseases. Deep learning, convolutional neural network, lung cancer, radiomics will be the focus of future research. AI-based diagnosis and treatment of lung disease has become a research hotspot in recent years, yielding significant results.
This is a pbulication by a member of the Early Detection Research Network. This is a pbulication by a member of the Early Detection Research Network. Log in; Home. Data and Resources ... Minimally Invasive and Sublobar Resections for Lung Cancer. Minimally Invasive and Sublobar Resections for Lung Cancer. Abstact. No abstract available. Authors ...