- Open access
- Published: 23 January 2019

Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management
- Mahaboob Khan Sulaiman 1
Diabetology & Metabolic Syndrome volume 11 , Article number: 7 ( 2019 ) Cite this article
45k Accesses
124 Citations
1 Altmetric
Metrics details
Diabetic nephropathy (DN) or diabetic kidney disease refers to the deterioration of kidney function seen in chronic type 1 and type 2 diabetes mellitus patients. The progression of the disease is known to occur in a series of stages and is linked to glycemic and blood pressure control. However, despite aggressive blood sugar control the prevalence of chronic kidney disease (CKD) in diabetic patients has not witnessed any decrease in the last two decades; which has lead to identification of additional factors in its progression. The nutritional status of patients is an important and modifiable factor that may influence CKD processes and outcome. It directly stems from the traditional dietary choices that patients make due to poor nutritional awareness. Dietary management of DN patients is challenging, as the twin factors of diet overload on kidney function needs to be balanced with malnutrition. Patient education seems to be the key in avoiding overindulgence of carbohydrate and protein-rich foods while favoring inclusion of essential fats in their diet.
This review will summarize current advances in staging and molecular pathogenesis of DN. It will highlight recent studies focusing on patient-customized dietary interventions that offer new hope as an effective tool in improving quality of life and delaying disease progression in DN patients.
Introduction
In 2015, the International Diabetic Federation estimated that the prevalence of diabetes was 8.8% from ages 20 to 79 years affecting a population of approximately 440 million people [ 1 ]. This is predicted to grow to over 550 million people by the year 2035 [ 2 ]. One of the most important clinical features of diabetes is its association with chronic tissue complications. A short-term increase in hyperglycemia does not result in serious clinical complications. The duration and severity of hyperglycemia is the major causative factor in initiating organ damage. Early morphological signs of renal damage include nephromegaly and a modified Doppler, but the degree of damage is best ascertained from proteinuria and Glomerular filtration rate (GFR) [ 3 ]. The average incidence of diabetic nephropathy is high (3% per year) during the first 10 to 20 years after diabetes onset [ 4 ]. Typically, it takes 15 years for small blood vessels in organs like kidney, eyes and nerves to get affected. It is estimated that more than 20 and up to 40% of diabetic patients will develop chronic kidney disease (CKD) [ 5 , 6 ], depending upon the population, with a significant number that develop end stage kidney disease (ESKD) requiring renal replacement therapies such as kidney transplantation. Incidentally, diabetes with no clinical sign of kidney damage during the initial 20 to 25 years is significantly less likely (1% a year) to cause major renal complication later in life [ 4 ].
Staging of diabetic nephropathy
Until recently, diabetic nephropathy was defined by the evidence of proteinuria ≥ 300 mg/day, in a diabetic patient [ 7 ]. Although urinary albumin is recognized as an early marker of DN, significant glomerular damage has already occurred when albumin appears in urine. Therefore, novel urinary biomarkers are needed to identify patients who are at risk of developing kidney damage. A proteomic study of the condition collectively termed as non-albumin proteinuria (NAP) identified several putative early biomarkers such as α-1 microglobulin, β-1 microglobulin, Nephrin, Cystatin C etc., [ 8 ]. While these markers can serve as sensitive early indicators of tubule damage, currently, they are neither calibrated nor universally available [ 9 ]. Moreover, precipitation of morning urine proteins and subsequent resolution by 2D electrophoresis also identified another putative urinary biomarker kininogen-1. This protein involved in the kallikrein-kinin system also awaits validation in larger cohorts [ 10 ].
Several recent studies have enabled a more robust and comprehensive stratification of DN. In 2010, Tervaert et al. reported a new pathological classification of kidney lesions that involved tubules, interstitium and/or the vessels as shown in Table 1 [ 11 ]. Such a classification was required, as a considerable percentage of patients with diabetes and impaired renal filtration do not exhibit elevated protein excretion. Also, many patients with Type 1 DM show proteinuria without concurrent GFR changes. Since diabetes mellitus studies are often observational and lack biopsy data to prove involvement of lesions, diabetic nephropathy is now classified as diabetic kidney disease (DKD). Interestingly, these classical stages of type 1 DM (T1DM) may not occur in type 2 DM (T2DM) patients as the latter is often diagnosed with concurrent disorders such as hypertension, proteinuria and renal failure [ 11 , 12 ]. Therefore, a new term diabetic chronic kidney disease (DCKD) was proposed to replace diabetic nephropathy to explain the extent of kidney damage. Additionally, in these patients with type 2 DM, it is recommended that screening should be performed at diagnosis and yearly thereafter. More recently, Gheith et al. [ 13 ] have proposed five stages of diabetic nephropathy after a comprehensive review of literature as summarized in Table 1 .
Risk factors for diabetic nephropathy
Many epidemiological studies demonstrate that ethnicity, family history, gestational diabetes, elevated blood pressure, dyslipidaemia, obesity and insulin resistance are the major risk factors of diabetic nephropathy [ 14 ]. Other putative risk factors include elevated glycosylated haemoglobin level (HbA1c), elevated systolic pressure, proteinuria and smoking [ 15 ].
Modifiable vs non-modifiable risk factors: recent advances
Although nephropathy is the strongest predictor of mortality in patients with diabetes, its development involves important inter-individual variations. Genome-wide transcriptome studies [ 16 ] and high-throughput technologies [ 17 ] indicate the activation of inflammatory signaling pathways and oxidative stress highlighting the role of genetic factors. Evidences suggest that epigenetic mechanisms such as DNA methylation, noncoding RNAs and histone modifications can also play a pivotal role in the pathogenesis of diabetic nephropathy. Accordingly, cytokine TNF-alpha, IL-6 and IL-1 beta gene promoter polymorphisms and modulation in expression have been linked to DN susceptibility in subjects.
Dysregulation of local metabolic environment triggered by inflammation and subsequent tissue remodeling may initiate kidney damage [ 18 ]. Excess intracellular glucose have been shown to activate cellular signaling pathways such as diacylglycerol (DAG)-protein kinase C (PKC) pathway, advanced glycation end-products (AGE), polyol pathway, hexosamine pathway and oxidative stress [ 19 ]. Many studies have linked these pathways to key steps in the development of glomerulosclerosis. In addition to these metabolic pathways, Rho-kinase, an effector of small-GTPase binding protein Rho, has been linked to various steps in the ultra structural damage of diabetic nephropathy by inducing endothelial dysfunction, mesangial excessive extracellular matrix (ECM) production, podocyte abnormality, and tubulointerstitial fibrosis. A review on the important pathways that lead to diabetic nephropathy can be found elsewhere [ 20 ].
Type of diabetes and their progression to diabetic nephropathy
Although microalbuminuria is a confirmatory test for diagnosis of diabetic nephropathy, not all patients progress to macroalbuminuria. In fact, some patients may regress to normoalbuminuria [ 21 ]. The progression of kidney disease in type 1 diabetes mellitus is unpredictable and seems to be connected to the intensity of blood sugar and pressure control. Accordingly, while initial studies reported that ~ 80% microalbuminuric patients progress to proteinuria over 6–14 years [ 22 , 23 ], recent studies have reported a regression as a result of better glycemic control. For example, the Joslin type 1 cohort and DCCT/EDIC study reported roughly similar results of 58% patients and 50% patients with microalbuminuria regressed to normoalbuminuria over 6 years and within 10 years, with or without renin–angiotensin–aldosterone system (RAAS) inhibitors respectively, solely with better control of diabetes, hypertension and lipids [ 24 , 25 ]. Improvement in microalbuminuria also resulted in 89% lower risk of developing a decreased GFR in type 1 DM patients.
In contrast, progression and regression of kidney disease in type 2 DM is highly variable as it is usually diagnosed with a secondary disorder, the onset of which is unrecorded. The UKPDS study reported microalbuminuria and reduced GFR in 38% and 29% patients respectively after a median follow-up of 15 years [ 26 ]. In terms of progression, the same study reported a change from microalbuminurea–macroalbuminuria-ESKD at 2.8% and 2.3% per year respectively. In contrast, the Pima Indians study reported that macroalbuminuria was 50% during a median follow-up of 20 years [ 27 ]. Also, a gradual loss of kidney damage with time was noticed as 7.3% patients were diagnosed with microalbuminuria at the onset, 17.3% at 5 years, 24.9% at 10, and 28% at 15 years. Epidemiological studies in Western and Pima Indian populations also suggest that the prevalence of overt nephropathy is about 21% in patients with type 1 DM, and 20–25% in patients with Type 2 DM, depending solely on the duration since onset of disease.
Potential serum biomarkers of diabetic nephropathy: recent advances
Traditionally, biomarkers are evaluated based on their ability to predict the onset or monitor the progression of DN. As albuminuria has certain limitations the quest for more reliable serum and renal biomarkers with higher sensitivity and specificity has led to an explosion of literature in this field. MacIssac et al. [ 28 ] have presented a detailed review of current literature on relevant biomarkers. Recently, Motawi et al. [ 29 ] estimated three new promising biomarkers: neutrophil gelatinase-associated lipocalin (NGAL), beta-trace protein (beta TP) and microRNA-130b (miR-130b) in type 2 DM. They concluded that serum NGAL and betaTP were significantly elevated in T2DM patients and can serve as early biomarkers of tubular and glomerular markers respectively. Other recent reviews on the promise of biomarkers in early detection of DKD can also be seen [ 30 ]. Such advances in biomarker research and metabolic phenotyping offer hope for multiparametric risk assessment of kidney injury and effective interventional strategies in future.
Diet therapy in diabetic nephropathy and its importance
The primary goal of diabetic nephropathy treatment is to prevent microalbuminuria from progressing to macroalbuminuria and an eventual decrease in renal function and associated heart disorders. Consequently, intensive glycaemic control, antihypertensive treatment by blocking RAAS system and lipid-modifying statin therapy are the main cornerstones of treatment. A detailed discussion of the various treatment methods of diabetic nephropathy is beyond the scope of this article, and reviews on the subject are available [ 31 , 32 , 33 ].
The nutritional status of patients is an important and modifiable factor that may influence DN processes and outcome [ 34 ]. Diet is a crucial factor in influencing the nutritional status of an individual. Whereas diabetes advocates a healthy and balanced diet, diet of a CKD or diabetic nephropathy patient is challenging and designed to delay progression of kidney damage and the associated secondary conditions such as hypertension, hyperlipidemia, uremia, etc. It also needs continuous monitoring and must be personalized to the patients’ treatment regimen. As food intake could be a burden on kidney function, a delicate balance between nutrition and sustainable physiological load is essential to maintain quality of life for the patient. A common problem encountered in patients with renal failure and proteinuria is their lack of nutritional knowledge and continued adherence to traditional food choices that are rich in carbohydrate, proteins or minerals. Since a majority of patients are dyslipidemic the only control they exercise is on limiting fat intake. Such a skewed diet places a tremendous burden on kidney function that causes further problems in disease management.
An ideal diet recommended for diabetic nephropathy patients with compromised kidney function includes a proper amount of fat to prevent malnutrition. More so when total calories coming from protein and carbohydrate intake needs to be restricted. A total fat reduction as advised by earlier studies can be a very unhealthy practice. Thus, to achieve these goals nutritionists advice limiting saturated fatty acid consumption while taking vegetable oils and omega-rich fatty acid containing oils in moderation. Many clinical studies have highlighted the renoprotective effects of a low protein diet on DN, although protein restriction alone does not result in a positive outcome for patients [ 35 ]. Moreover, a protein-deficient diet (0.6 to 0.7 g/kg/day) needs to be integrated into the overall care of renal insufficiency with customized dietary interventions to avoid malnutrition [ 36 ]. Interestingly, in animal type 2 DM models a very low protein diet (VLPD) improved tubulo-interstitial damage, inflammation and fibrosis, through restoration of autophagy via reduction of a mammalian target of rapamycin complex 1 (mTORC1) activity [ 37 ]. Although a low protein diet slows progression of renal dysfunction in human subjects with chronic glomerular nephritis, VLPD has not been clinically validated. A low-salt diet that is devoid of salted and pickled foods is highly recommended for DN patients. Restricted sodium intake allows better blood pressure control in such patients. High salt intake and urinary protein excretion were associated with annual creatinine clearance decline in type 2 DKD patients as reported by Kanauchi et al. [ 38 ]. Potassium is an essential electrolyte involved in the contraction and relaxation of muscles. During a deficit in kidney function potassium excretion is reduced leading to an accumulation in body tissues. Therefore, potassium intake specifically from foods such as grains, potatoes, corn, soybean, nuts, tomatoes, banana, melons, kiwi etc. must be restricted. Like potassium, phosphorus excretion is also reduced during chronic kidney damage leading to increased blood phosphorus levels. Since phosphate is in homeostatic equilibrium with the skeletal muscle calcium levels, an imbalance leads to a significant calcium loss and debilitating bone disease. In summary, excessive carbohydrate and protein intake is managed with a target of 1600 kcal of energy per day in which 60 percent comes from carbohydrate and 40 percent from proteins. In a recent study, such a regimen achieved a commendable control in blood lipid and glucose values in a patient with stage 4 chronic kidney disease [ 39 ]. However, patient adherence to the recommended diet seems to be gender-specific. For example, Ahola et al. [ 40 ] assessed frequency of adherence to special diet in a large cohort Finnish DN study and reported that adherents were more frequently women, older, and had longer duration of diabetes. Therefore, effective adherence through patient education may be a crucial factor in the management of DN through diet.
In conclusion, this review summarizes the recent advances in the pathophysiology of diabetic nephropathy and the importance of dietary factors in modifying treatment outcomes for patients. A critical analysis of studies that emphasize the importance of patient-centered dietary intervention in successful management of advanced CKD patients has been presented. Large-scale cohort studies are necessary to evaluate the efficiency of diet as a new therapeutic paradigm. Nevertheless, proactive personalized diet-management plans tailored to the disease stage is likely to be the future trend in diabetic nephropathy therapy as it will have a large impact on the patient’s quality of life and may prolong survival. Notably, in newly diagnosed DN patients these dietary interventions may no longer be regarded as complementary measures but significant factors that delay progression of the disease.
Abbreviations
chronic kidney disease
diabetic nephropathy
glomerular filtration rate
end stage kidney disease
diabetes mellitus
diabetic kidney disease
International Diabetes Federation IDF Diabetes Atlas. International Diabetes Federation. 2015; 7ed, Brussels, Belgium.
Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25:496–501.
Article CAS Google Scholar
Zhang J, Liu J, Qin X. Advances in early biomarkers of diabetic nephropathy. Rev Assoc Med Bras. 2018;64(1):85–92.
Article Google Scholar
Magee C, Grieve DJ, Watson CJ, Brazil DP. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. 2017;31(5–6):579–92.
Papadopoulou-Marketou N, Paschou SA, Marketos N, Adamidi S, Adamidis S, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes. Minerva Med. 2018;109(3):218–28.
PubMed Google Scholar
Nelson RG, Bennett PH, Beck GJ, et al. Diabetic Renal Disease Study Group: development and progression of renal disease in Pima Indians with non-insulin dependent diabetes mellitus. N Engl J Med. 1996;335:1636–42.
American Diabetes Association. Nephropathy in diabetes (Position Statement). Diabetes Care. 2004;27(Suppl. 1):S79–83.
Google Scholar
Ballantyne FC, Gibbons J, O-Reilly DS. Urine albumin should replace total protein for the assessment of glomerular proteinuria. Ann Clin Biochem. 1993;30(1):101–3.
Kim SS, Song SH, Kim IJ, Jeon YK, Kim BH, et al. Nonalbuminuric proteinuria as a biomarker for tubular damage in early development of nephropathy with type 2 diabetic patients. Diabetes Metab Res Rev. 2014;30:736–41.
Vitova L, Tuma Z, Moravec J, Kvapil M, Matejovic M, Mares J. Early urinary biomarkers of diabetic nephropathy in type 1 diabetes mellitus show involvement of kallikrein-kinin system. BMC Nephrol. 2017;18(1):112.
Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2014;21(4):556–63. https://doi.org/10.1681/ASN.2010010010 .
Mogensen CE. The natural history of type 2 diabetic nephropathy. Am J Kidney Dis. 2001;37:S2–6.
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49–56.
Klemens R, Angela G, Sabine H, et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset and sex. Diabetes Care. 2007;30:2523–8.
Eberhard R. Diabetic nephropathy. Saudi J Kidney Dis Transplant. 2006;17:481–90.
Hameed I, Masoodi SR, Malik PA, Mir SA, Ghazanfar K, Ganai BA. Genetic variations in key inflammatory cytokines exacerbates the risk of diabetic nephropathy by influencing the gene expression. Gene. 2018;661:51–9.
Kato M, Natarajan R. Diabetic nephropathy–emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–30.
Zheng Z, Zheng F. Immune cells and inflammation in diabetic nephropathy. J Diabetes Res. 2016;2016:1841690. https://doi.org/10.1155/2016/1841690 .
Article CAS PubMed Google Scholar
Ni WJ, Tang LQ, Wei W. Research progress in signaling pathway in diabetic nephropathy. Diabetes Metab Res Rev. 2015;31(3):221–33.
Kawanami D, Matoba K, Utsunomiya K. Signaling pathways in diabetic nephropathy. Histol Histopathol. 2016;31(10):1059–67.
CAS PubMed Google Scholar
Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408.
Parving HH, Oenboll B, Syendsen PA, Christiansen JS, Andersen AR. Early detection of patients at risk of developing diabetic nephropathy: a longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh). 1982;100:550–5.
Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–2.
de Boer IH, Afkarian M, Rue TC, Cleary PA, Lachin JM, Molitch ME, et al. Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol. 2014;25:2342–50.
Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, et al. Predictors for the developmental of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328(7448):1105–8.
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK prospective diabetes study 74. Diabetes. 2006;55:1832–9.
Pavkov ME, Knowler WC, Bennett PH, Looker HC, Krakoff J, Nelson RG. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int. 2006;70:1840–6.
MacIssac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development of diabetic kidney disease. Am J Kidney Dis. 2014;63(2):S39–62.
Motawi TK, Shehata NI, ElNokeety MM, El-Emady YF. Potential serum biomarkers for early detection of diabetic nephropathy. Diabetes Res Clin Pract. 2018;136:150–8.
Papadopoulou-Marketou N, Kanaka-Gantenbein C, Marketos N, Chrousos GP, Papassotiriou I. Biomarkers of diabetic nephropathy: a 2017 update. Crit Rev Clin Lab Sci. 2017;54(5):326–42.
Oltean S, Coward R, Collino M, Baelde H. Diabetic nephropathy: novel molecular mechanisms and therapeutic avenues. Biomed Res Intl. 2017;2017:3146524.
Montero RM, Covic A, Gnudi L, Goldsmith D. Diabetic nephropathy: what does the future hold? Int Urol Nephrol. 2016;48(1):99–113.
Lytvyn Y, Bjornstad P, Pun N, Cherney DZ. New and old agents in the management of diabetic nephropathy. Curr Opin Nephrol Hypertens. 2016;25(3):232–9.
Meloni C, Tatangelo P, Cipriani S, Rossi V, Suraci C, Tozzo C, et al. Adequate protein dietary restriction in diabetic and nondiabetic patients with chronic renal failure. J Ren Nutr. 2004;14(4):208–13.
Otoda T, Kanasaki K, Koya D. Low-protein diet for diabetic nephropathy. Curr Diab Rep. 2014;14(9):523.
Trimeche A, Selmi Y, Ben Slama F, Ben Amara H, Hazar I, Ben Mami F, et al. Effect of protein restriction on renal function and nutritional status of type 1 diabetes at the stage of renal impairment. Tunis Med. 2013;91(2):121–6.
Kitada M, Ogura Y, Monno I, Koya D. A low-protein diet for diabetic kidney disease: its effect and molecular mechanism, an approach from animal studies. Nutrients. 2018;10(5):544.
Kanauchi N, Ookawara S, Ito K, Mogi S, Yoshida I, Kakei M, et al. Factors affecting the progression of renal dysfunction and the importance of salt restriction in patients with type 2 diabetic kidney disease. Clin Exp Nephrol. 2015;19(6):1120–6.
Kim HY. Nutritional intervention for a patient with diabetic nephropathy. Clin Nutr Res. 2014;3:64–8.
Ahola KAJ, Forsblom C, Groop PH. Adherence to special diets and its association with meeting the nutrient recommendations in individuals with type 1 diabetes. Acta Diabetol. 2018;55(8):843–51.
Download references
Authors’ contributions
The author read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The author declares no competing interests.
Consent for publication
Ethics approval and consent to participate.
No funding was received for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Fatima College of Health Sciences, PO Box 57788, Abu Dhabi, UAE
Mahaboob Khan Sulaiman
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mahaboob Khan Sulaiman .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Sulaiman, M.K. Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetol Metab Syndr 11 , 7 (2019). https://doi.org/10.1186/s13098-019-0403-4
Download citation
Received : 16 October 2018
Accepted : 17 January 2019
Published : 23 January 2019
DOI : https://doi.org/10.1186/s13098-019-0403-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetic nephropathy
- Chronic kidney disease
- Dietary intervention
- Proteinuria
- Malnutrition
Diabetology & Metabolic Syndrome
ISSN: 1758-5996
- Submission enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Diabetic nephropathy – complications and treatment
Andy kh lim.
- Author information
- Article notes
- Copyright and License information
Correspondence: Andy Lim, Department of Nephrology, Monash Medical Center, 246 Clayton Road, Clayton, VIC 3168, Australia, Tel +61 3 9594 6666, Fax +61 3 9594 6730, Email [email protected]
Collection date 2014.
The full terms of the License are available at http://creativecommons.org/licenses/by-nc/3.0/ . Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed.
Diabetic nephropathy is a significant cause of chronic kidney disease and end-stage renal failure globally. Much research has been conducted in both basic science and clinical therapeutics, which has enhanced understanding of the pathophysiology of diabetic nephropathy and expanded the potential therapies available. This review will examine the current concepts of diabetic nephropathy management in the context of some of the basic science and pathophysiology aspects relevant to the approaches taken in novel, investigative treatment strategies.
Keywords: diabetes, diabetic nephropathy, albuminuria, kidney disease, inflammation
Introduction
Diabetic nephropathy (DN) or diabetic kidney disease is a syndrome characterized by the presence of pathological quantities of urine albumin excretion, diabetic glomerular lesions, and loss of glomerular filtration rate (GFR) in diabetics. Diabetes may be classified as type 1 (autoimmune β-cell destruction and absolute insulin deficiency), type 2 (relative insulin deficiency and resistance), and other types (eg, pancreatic disease).
Epidemiology
The prevalence of diabetes is phenomenal and the projections are staggering. When one considers the morbidity, mortality, and cost of health care, the burden of the diabetes epidemic becomes apparent. Worldwide, the prevalence of diabetes was estimated at 171 million in 2000, increasing to 382 million in 2013; and is projected to reach 592 million by 2035. This represents 8%–10% of the global population, resulting in at least 548 billion dollars in health expenditure on diabetes care. Type 2 diabetes constitutes about 85%–95% of all diabetes cases. 1 In the US alone for 2011, 25.8 million children and adults have diabetes with another 79 million having a prediabetic state. 2
The diabetes epidemic has resulted in DN becoming the most frequent cause of end-stage renal disease (ESRD) in most countries. In 2009–2011, diabetes was the primary cause of ESRD in about 60% of patients in Malaysia, Mexico, and Singapore. Countries with an ESRD incidence of 40%–50% include Israel, Korea, Hong Kong, Taiwan, Philippines, Japan, the US, and New Zealand. 2 The incidence of ESRD due to diabetes also rises in the older age group. In 2011, the incident rates of ESRD due to diabetes in the US were 44, 266, and 584 per million for the age groups 20–44, 45–64, and 65–74 years, respectively. A similar finding was noted in the AusDiab study of 11,247 diabetic Australians. 3 Thus, the reason for this boom in diabetes-associated ESRD is the increasing prevalence of diabetes and the aging population.
Risk factors
Not all diabetics develop DN and in those who do, progression is variable. The main modifiable risks are hypertension, glycemic control, and dyslipidemia. Data from the Joslin Diabetes Center, Steno Diabetes Center, and AusDiab studies also strongly implicate smoking as a risk factor for DN. 3 – 5 The main unmodifiable risks are age, race, and genetic profile. DN is more likely to develop in patients with a family history of DN. 6 – 8 Certain racial groups are also at higher risk, such as African Americans, Mexican Americans, and Pima Indians. 9 , 10 One study suggested that males had an increased risk of DN. 11
A meta-analysis of studies identified 24 genetic variants in 16 genes which are associated with DN. These include: ACE , ALR2 , APOC1 , APOE , EPO , eNOS , HSPG2 , VEGF , FRMD3 , CARS , UNC13B , CPVL/CHN2 , and GREM1 . In a subgroup of type 2 diabetic Asians, ELMO1 , CCR5 , and CNDP1 were also relevant. 12 Other meta-analyses implicated polymorphisms of ADIPOQ , PAI-1 , TGFβ1 , and PPARγ in the development of DN. The nature of the polymorphism varies with ethnicity. 13 – 15 The complexity of genetic studies in DN is discussed in a review by Mooyaart. 16
Stages and natural history
Incipient nephropathy is the initial presence of low but abnormal amounts of urine albumin, referred to as microalbuminuria (persistent albuminuria at level 30–299 mg/24 hours). Overt nephropathy or macroalbuminuria (persistent albuminuria at level ≥300 mg/24 hours) develops after many years in type 1 diabetes but may be present at the time of diagnosis of type 2 diabetes. Patients who progress to macroalbuminuria are more likely to develop ESRD. 11 The natural history depends on the type of diabetes.
In untreated type 1 diabetics, approximately 80% of patients with sustained microalbuminuria increase their albumin excretion by 10%–20% per year until overt nephropathy develops, which normally takes 10–15 years. With the development of overt nephropathy, the GFR declines at a rate of 2–20 mL/minute/year and ESRD develops in 50% within 10 years and in 75% by 20 years. 17 Structural changes can precede albuminuria and reduced GFR, with glomerular basement membrane thickening and mesangial expansion, can be detected as early as 2–8 years after onset of diabetes. 18
In type 2 diabetics, more patients have DN at the time of diagnosis of diabetes as type 2 diabetes can go unrecognized for years. The AusDiab study of diabetic Australians showed that albuminuria is common among patients with established diabetes, is present before the onset of diabetes, and becomes more prevalent with worsening glucose tolerance. 3 About 20%–40% of type 2 diabetics with microalbuminuria progress to overt nephropathy; and about 20% will develop ESRD after the development of overt nephropathy. 17 , 19
Screening for DN
Most guidelines recommend screening with a spot urine albumin/creatinine ratio (ACR; normal >30 mg/g creatinine), from either first morning (preferred) or random specimens. An abnormal result is repeated once or twice over a few months for consistency. This is coupled with an assessment of renal function, using the Modification of Diet in Renal Disease or Chronic Kidney Disease Epidemiology Collaboration formulas for estimated GFR (eGFR) in order to stage chronic kidney disease (CKD). 20 , 21 Screening begins at diagnosis of type 2 diabetes and usually 5 years after onset of type 1 diabetes. Timed collections can also be utilized and will average out diurnal variations in albumin excretion (normal >20 μg/minute).
Renal biopsy
The routine use of renal biopsy to confirm DN is much debated. Many nephrologists do not biopsy patients with classic features such as retinopathy, duration of diabetes <10 years, slow decline in GFR, gradual progression of proteinuria, and lack of active urinary sediment. Without standardized criteria, there may be significant variations in epidemiology. An Italian study of 393 type 2 diabetics highlighted this point. In centers with an unrestricted biopsy policy, the rate of finding an underlying glomerulonephritis was lower than those centers with a restricted biopsy policy (33% versus 57%). The unrestricted policy resulted in a greater proportion of patients found to have glomerulonephritis rather than diabetic glomerulosclerosis. 22 The prevalence of specific disease in the population can also affect the biopsy decision. In a Chinese study of 51 type 2 diabetics with >1 g/day proteinuria, one-third of patients had nondiabetic disease, predominantly IgA nephropathy. 23 The largest study to date looked at 620 biopsies from type 1 and 2 diabetics, with a median duration of diabetes of 10 years. Overall, 37% of patients had isolated DN, 36% had isolated nondiabetic disease, and 27% had nondiabetic disease superimposed on DN. The duration of diabetes >12 years was the best predictor for isolated DN. Interestingly, 43% of biopsies with DN demonstrated superimposed acute tubular necrosis. 24 Thus, a renal biopsy is useful to exclude acute tubular injury and diseases amenable to specific therapy.
There are limitations in using albuminuria as a marker of DN as many patients experience GFR loss without deterioration in albuminuria and even normoalbuminuria. 25 In fact, histologically proven advanced diabetic glomerular lesions can develop despite normoalbuminuria. 26 Furthermore, low-grade albuminuria is a lesser predictor of disease progression than macroalbuminuria. 27 Therefore, there is interest in finding biomarkers to detect DN earlier and identify progression risk. There is also interest in urine microRNA profiling but studies are fairly preliminary. 28 , 29 The most promising biomarker currently is serum TNF-α receptor levels, which may predict progression of CKD and ESRD, in type 1 and type 2 diabetics. In type 2 diabetics, the TNF-α receptor level showed prognostic value in addition to albuminuria. 30 , 31 Serum uric acid is another biomarker which may also be pathogenic (discussed later). Studies of tubular biomarkers have been conflicting ( Table 1 ). The larger studies have not shown that these biomarkers offer additional value on top of traditional prediction models. More work is needed to clarify the role of biomarkers in clinical practice.
Tubular biomarkers
| Biomarker | Source | Cohort (size) | Key points | Reference |
|---|---|---|---|---|
| KIM-1 | Blood | Type 1 (124) | Baseline KIM-1 in proteinuric (>500 mg/day) patients predicted rate of eGFR loss and ESRD during 5–15 years of follow-up. | Sabbisetti et al |
| Urine | Type 1 (63) | KIM-1 associated with decline in GFR but not independent of albuminuria. | Nielsen et al | |
| Urine | Type 2 (978) | Urine KIM-1/Cr associated with faster decline in GFR during 4 years follow-up but offered no additional prognostic information to albumin/Cr ratio. | Conway et al | |
| NGAL | Serum/urine | Type 1 (50) | Elevated before microalbuminuria. Serum NGAL correlated with HbA and urine NGAL correlated with albuminuria. | Lacquaniti et al |
| Urine | Type 1 (63) | NGAL associated with decline in GFR but not independent of albuminuria. | Nielsen et al | |
| Serum/urine | Type 2 (140) | No correlation with eGFR. | Chou et al | |
| L-FABP | Urine | Type 1 (1,549) | Patients ranged from normoalbuminuria to macroalbuminuria. | Panduru et al |
| Urine L-FABP/Cr ratio at baseline predicted progression of DN but adding | ||||
| L-FABP to albumin excretion did not improve prediction model. | ||||
| Urine | Type 1 (277) | Urine L-FABP predicted progression of albuminuria or death. | Nielsen et al | |
| Urine | Type 1 (63) | L-FABP not related to decline in GFR. | Nielsen et al | |
| Serum/urine | Type 2 (140) | Serum L-FABP correlated with baseline eGFR but did not predict decline in eGFR. | Chou et al | |
| Urine | Type 2 (618) | Japanese patients without overt proteinuria and serum creatinine ≤1.0 mg/dL followed for median of 12 years. Urine L-FABP in the highest tertile was associated with 50% decline in eGFR or progression to eGFR < 30 mL/minute/m . | Araki et al | |
| Urine | Type 2 (140) | High L-FABP associated with progressive albuminuria, ESRD, or hemodialysis. | Kamijo-Ikemori et al | |
| Cystatin C | Urine | Type 2 (237) | Urine cystatin C/Cr ratio associated with decline in eGFR, with the upper tertile of levels associated with progression to stage 3 CKD or higher after 20 months follow-up. | Kim et al |
Abbreviations: CKD, chronic kidney disease; Cr, creatine; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; GFR, global filtration rate; HbA 1c , hemoglobin A 1c .
Pathogenesis
Pathology and pathophysiology.
DN is characterized by structural and functional changes. In glomeruli, there is mesangial expansion, thickening of the basement membrane, and, characteristically, nodular glomerulosclerosis (Kimmelstiel–Wilson nodules). In early DN, tubular hypertrophy is present but eventually interstitial fibrosis with tubular atrophy develops, along with arteriolar hyalinosis. In advanced cases, there is an infiltrate of macrophages and T-lymphocytes. Ultrastructurally, there is podocyte loss and reduced endothelial cell fenestration. 32 , 33 These characteristic pathological changes are shown in Figure 1 . Functionally, there is early glomerular hyperfiltration and increased albumin excretion; and with advancing nephropathy, increasing proteinuria and declining GFR. A brief description of the functional and cellular pathology is provided below. Although it is conceptually easier to describe these pathways individually, these pathways overlap and interact with one another in vivo, and enhance one another’s biophysiological effects ( Figure 2 ).
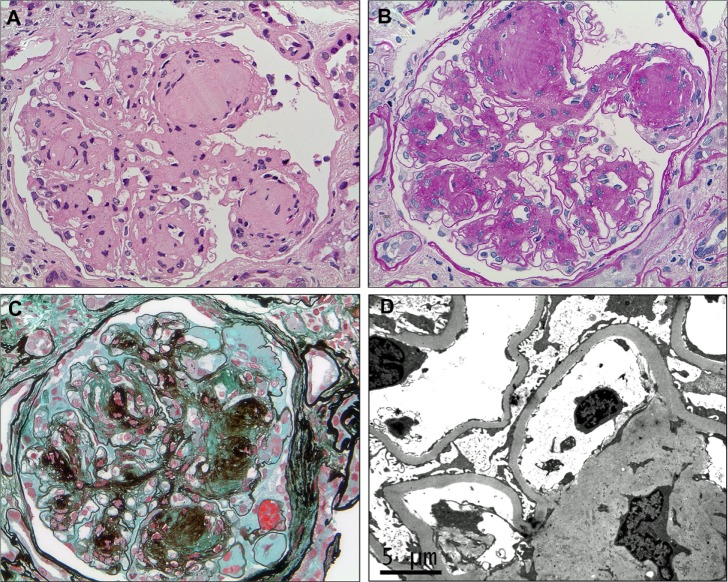
Characteristic histological features of diabetic nephropathy.
Notes: In advanced diabetic nephropathy, there is extensive mesangial expansion due to increased extracellular matrix production, with the formation of spherical, eosinophilic nodules with a central hypocellular or acellular area, known as Kimmelstiel–Wilson nodules ( A ) (hematoxylin–eosin, ×400). These nodules are also typically strongly periodic acid–Schiff-positive and may be seen compressing and narrowing the peripheral capillary loops ( B ) (periodic acid–Schiff, ×400). The increased matrix stains dark with silver and the Kimmelstiel–Wilson nodules may demonstrate a lamellated appearance. Capillary microaneurysms can be seen at the periphery on the right (in the 1–5 o’clock position), in association with mesangiolysis ( C ) (Masson’s trichrome–methenamine silver, ×400). There is diffuse thickening of the glomerular basement membrane, which is apparent on electron microscopy even if it is difficult to discern by light microscopy in early disease, and often accompanied by some degree of podocyte foot process effacement ( D ) (electron microscopy).
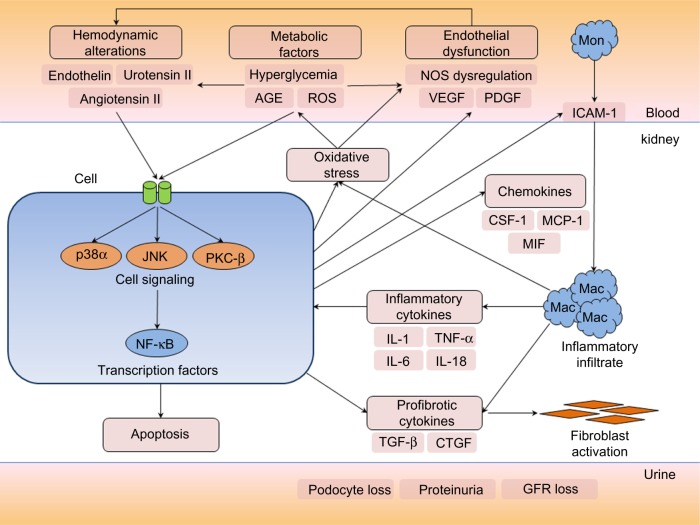
Overview of the pathological pathways in diabetic nephropathy.
Notes: In the diabetic milieu, metabolic derangements and hemodynamic alterations, particularly activation of the renin–angiotensin system, trigger a number of cell signaling cascades, including the MAPKs (p38 and JNK) and PKC-β, which mediate a cellular response through activation of key transcription factors such as NF-κB. In response to such signals, renal cells such as tubular epithelial cells, podocytes, and mesangial cells can produce chemokines, growth factors, and profibrotic cytokines. CSF-1 and MCP-1 function as chemotactic molecules and promote the recruitment of monocytes from the circulation. Upregulation of ICAM-1 on endothelial cells – a key leukocyte adhesion molecule – facilitates infiltration of circulating mononuclear cells into the kidney. CSF-1 also promotes monocyte/macrophage differentiation, proliferation, and activation. MIF functions to retain macrophages at sites of inflammation and has counter-regulatory functions against the anti-inflammatory actions of glucocorticoids. Activated macrophages can produce proinflammatory and profibrotic cytokines, reactive oxygen species, and antiangiogenic factors and contribute to a cycle of inflammation, oxidative stress, cellular injury, progressive fibrosis, and loss of glomerular filtration rate. Podocyte loss, endothelial dysfunction, alterations in the GBM, and tubular injury contribute to increasing proteinuria during the development and progression of diabetic nephropathy.
Abbreviations: AGE, advanced glycation end-products; GBM, glomerular basement membrane; GFR, glomerular filtration rate; Mac, macrophages; Mon, monocyte; NOS, nitric oxide synthase; ROS, reactive oxygen species.
Hemodynamic factors
There is an imbalance in afferent and efferent arteriolar resistance, resulting in increased glomerular hydrostatic pressure and hyperfiltration. Activation of the renin–angiotensin system (RAS) increases angiotensin II levels, leading to efferent arteriolar vasoconstriction and production of proinflammatory and profibrotic molecules through multiple mechanisms. High angiotensin converting enzyme (ACE) levels are associated with greater albuminuria and nephropathy in diabetic mice and humans. 34 , 35 Increased levels of endothelin-1 and urotensin II also contribute to vasoconstriction. Various dysregulation of nitric oxide and nitric oxide synthase has been described in DN. Nitric oxide mediates endothelium-dependent vasodilatation, and is formed from L-arginine by endothelial nitric oxide synthase. Diabetic endothelial nitric oxide synthase knockout mice develop more severe glomerular lesions and proteinuria compared to wild-type mice. 36
Metabolic factors
Oxidative stress and generation of reactive oxygen species (ROS) damage DNA and protein, or function as signaling amplifiers to activate cellular stress pathways such as PKC, MAPK, and NF-κB. 37 , 38 Activation of the polyol pathway, with aldose reductase converting excess glucose to sorbitol, and subsequent conversion to fructose by sorbitol dehydrogenase contributes to oxidative stress by increasing the NADH/NAD+ ratio. 39 , 40 A recently described novel mechanism of injury also involves endogenous fructose production with activation of fructokinase in the proximal tubule. 41 The formation of advanced glycation end-products (AGE) by nonenzymatic binding of glucose to proteins, lipids, and nucleic acids can lead to alteration of protein structure and function, oxidative stress, and expression of proinflammatory cytokines and growth factors. 42
Growth factors/cytokines
Activation of TGF-β and its downstream cytokine, CTGF, induce extracellular matrix formation and fibrosis. In kidney biopsies, glomerular expression of TGF-β1 and CTGF were higher in diabetics compared to controls, and correlated with albuminuria. PDGF expression is also increased in DN, which can modulate chemotaxis, vascular tone, and platelet aggregation. VEGF is crucial in angiogenesis but also mediates vasodilatation and leukocyte trafficking in DN.
Cell signaling and transcription factors
Increased renal gene transcription of PKC-β showed a strong relationship with glycemic control. 43 PKC activation has wide ranging effects, including enhancing angiotensin II actions, nitric oxide dysregulation, endothelial dysfunction, and activation of MAPK and NF-κB. 44 , 45 MAPKs are intracellular kinases which integrate cell signaling into cellular responses. MAPKs activate a number of nuclear transcription factors, including NF-κB, which then regulates the gene expression of various cytokines, chemokines, and adhesion molecules. The activation of p38α isoform of the p38 MAPK pathway is most strongly associated with renal inflammation and DN. 46 , 47 There may also be a role for toll-like receptors (TLR2, TLR4) and B7-1 costimulatory signaling in modulating inflammation and injury in DN. 48 , 49 Finally, transcription factors bind to the promoter regions of genes and modulate transcription of messenger RNA. NF-κB has been the best studied in DN. Activation of NF-κB in both human peripheral blood mononuclear cells and kidney biopsies correlate with severity of proteinuria and glycemic control. 50 , 51 A review of transcription factors in DN is provided by Sanchez and Sharma. 52
Inflammation
In DN, there is recruitment and activation of innate immune cells and elaboration of proinflammatory cytokines. 53 Macrophages and T-lymphocytes are prominent in early diabetic glomeruli while an interstitial infiltrate develops later ( Figure 3 ). Strategies impairing kidney leukocyte recruitment, proliferation, or activation have demonstrated that macrophages mediate DN. 54 , 55 In humans, kidney macrophage accumulation is associated with the severity of glomerulosclerosis. 56 Accumulation of interstitial macrophages correlated strongly with proteinuria, interstitial fibrosis, and GFR decline. 57
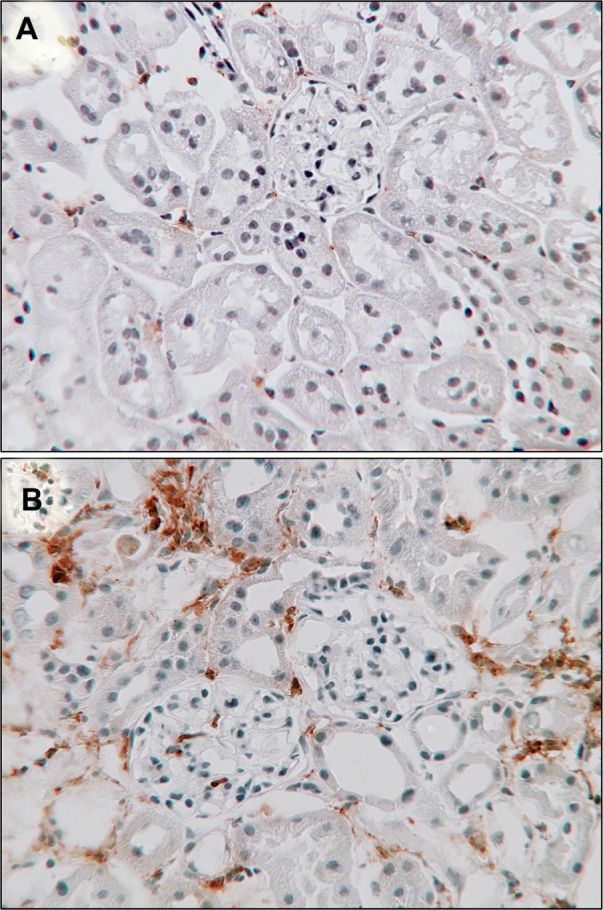
Macrophages in diabetic nephropathy.
Notes: Immunostaining for a macrophage marker (CD68) in kidney sections shows sparse interstitial macrophages in age-matched, nondiabetic control mice ( A ) compared to diabetic mice after 20 weeks of diabetes induced by streptozotocin ( B ) (CD68 brown, counterstained with hematoxylin, ×250).
The role of lymphocytes is less clear. A higher circulating level of activated T-cells is associated with DN. 58 A kidney T-cell influx is common in early type 1 diabetes, and correlates with renal function and albuminuria. 59 However, absence of lymphocytes did not prevent fibrosis and declining renal function in experimental DN. 60 Recent attention has focused on the subset of regulatory T-cells (Treg), which may play a protective role in DN. Treg numbers are increased in diabetic mice. 60 Treg depletion in diabetic mice exacerbated albuminuria and hyperfiltration, while adoptive transfer of Treg improved DN. 61 In type 2 diabetics, the number of Tregs as determined by flow cytometry showed an inverse correlation with albuminuria, particularly in patients with macroalbuminuria. 62 Treg also demonstrated an anti-inflammatory function, which reduces the metabolic abnormalities and insulin resistance in a mouse model of type 2 diabetes. 63 The main proinflammatory cytokines implicated in DN are TNF-α, MCP-1, ICAM-1, IL-1, IL-6, and IL-18. These cytokines are increased in diabetic patients and show correlation with albuminuria and glomerular pathology. 53
Treatment to delay DN progression involves adequate control of metabolic and hemodynamic abnormalities. In practical terms, this means adequate blood glucose lowering and control of hypertension. A description of all glucose lowering agents is beyond the scope of this review but certain agents have theoretical benefits beyond glucose lowering. Certain antihypertensives are also preferred based on studies which have demonstrated reductions in proteinuria or preservation of GFR, or both. The main pharmacological interventions described here are summarized in Table 2 . Nonpharmacological approaches and alternative medicine are briefly discussed. There is also interest in novel agents, gene therapy, and stem cell treatment, which may someday find a place in the treatment armamentarium.
Summary of pharmacological treatment of diabetic nephropathy
| Drug (s) | Antiproteinuric | Preserve GFR | Diabetes type |
|---|---|---|---|
| ACE inhibitor | ++ | ++ | Type 1 and 2 |
| ARB | ++ | ++ | Type 2 |
| ACE inhibitor plus ARB | +++ | − | Type 1 and 2 |
| Aldosterone antagonist | + | ? | Type 2 |
| Aldosterone antagonist plus ACE inhibitor or ARB | +++ | ? | Type 1 and 2 |
| Renin inhibitor | ++ | ? | Type 2 |
| Renin inhibitor plus ACE inhibitor or ARB | +++ | − | Type 2 |
| Non-dihydropyridine CCB | + | ? | Type 2 |
| Non-dihydropyridine CCB plus ACE inhibitor or ARB | ++ | ? | Type 2 |
| Dihydropyridine CCB | − | − | Type 2 |
| Allopurinol | ? | ? | ? |
| Statin | + | ? | Type 2 |
| Vitamin D | + | ? | Type 2 |
Notes: + data exist to indicate benefit; − data exist to indicate lack of benefit or harm; ? insufficient data for conclusion, possible benefit. The number of + indicates a semiquantitative scale of beneficial effect.
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; GFR, glomerular filtration rate.
Glycemic control
Good glycemic control is effective in reducing diabetic microvascular complications. DCCT was a trial involving 1,365 type 1 diabetics and normoalbuminuria. After almost 10 years, patients randomized to intensive glucose control had lower incidences of microalbuminuria and macroalbuminuria. 64 In the UKPDS trial of 3,867 newly diagnosed type 2 diabetics, patients receiving intensive glucose treatment were less likely to develop renal failure. 65 In the ADVANCE trial of 11,140 type 2 diabetics, intensive therapy (mean hemoglobin A 1c [HbA 1c ] ≤6.5%) also reduced the incidence of nephropathy compared to standard control (mean HbA 1c 7.3%). Intensive glucose control reduced the risk of ESRD by 65%. 66 In the VADT study of 1,791 type 2 diabetics, intensive glucose control (median HbA 1c 6.9%) was associated with less worsening of albuminuria and progression to macroalbuminuria but no significant difference in GFR at 6 years. 67 However, intensive glucose control to an HbA 1c of <6% may confer excess mortality, as demonstrated in the ACCORD trial of type 2 diabetics with cardiovascular disease or cardiovascular risk factors. 68 , 69 Thus, an HbA 1c of <6%, particularly if associated with significant hypoglycemic episodes, should be avoided.
Certain drugs may confer beneficial effects independent of glucose lowering. PPAR-γ inhibitors such as pioglitazone and rosiglitazone have demonstrated antifibrotic and anti-inflammatory effects in the kidney of diabetic rats. 70 – 72 In type 2 diabetics, the addition of rosiglitazone to metformin treatment for 32 weeks reduced albuminuria and blood pressure independent of glycemic control. 73 DPP-4 inhibitors (gliptins) have shown anti-inflammatory and antiapoptotic properties in DN models. 74 In type 2 diabetics, sitagliptin treatment for 6 months reduced albuminuria independent of HbA 1c . 75 In a study of alogliptin in type 2 diabetics, researchers showed a reduction in oxidative stress but no change in renal function. 76 Lastly, SGLT-2 inhibitors such as empagliflozin may reduce hyperfiltration by their effect on tubuloglomerular feedback. 77 Further trial evidence is needed to determine if these agents should be preferred agents in patients with DN.
Antihypertensives
Ace inhibitors.
ACE inhibitors have a strong track record in slowing disease progression in type 1 and type 2 diabetics. In the 1990s, captopril demonstrated the ability of ACE inhibitors in reducing the progression of albuminuria and decline in renal function in type 1 diabetics, independent of blood pressure lowering. 78 – 80 In the Collaborative Study Group trial of 409 type 1 diabetics, captopril treatment reduced the risk of doubling of serum creatinine by 48% and reduced the composite outcome of death, dialysis, and transplantation by 50% compared to placebo. 80 This study also demonstrated that a sustained remission of nephrotic-range proteinuria was possible with ACE inhibitors. 81 This was backed up by a study which showed that patients who achieved remission (albuminuria <600 mg/day) for ≥1 year had better outcomes compared to those who did not, including slower decline in GFR and lower risk of dialysis, transplantation, or death. 82 , 83
The perindopril/indapamide combination was studied in the ADVANCE trial of 11,140 type 2 diabetics. After mean follow-up of 4.3 years, perindopril/indapamide treatment reduced new onset microalbuminuria and prevented progression of microalbuminuria to overt nephropathy. However, serum creatinine and ESRD were not affected. It has also been argued that the effect on albuminuria was not independent of blood pressure, given a difference of 5.6/2.2 mmHg between the treatment groups. 84 Finally, the BENEDICT trial also showed that ACE inhibitor treatment could delay onset of microalbuminuria in type 2 diabetics with hypertension and baseline normoalbuminuria. 85
Angiotensin receptor blocker (ARB)
In the IDNT trial, 1,715 hypertensive type 2 diabetics with nephropathy were randomly assigned to receive irbesartan, amlodipine, or placebo. 86 Irbesartan reduced the risk of ESRD or doubling of serum creatinine by 20%–23% compared to amlodipine or placebo. In the RENAAL trial, 1,513 type 2 diabetics with nephropathy were randomly assigned to losartan or placebo, in addition to conventional antihypertensives. Losartan reduced the risk of ESRD or doubling of serum creatinine by 25%–28% compared to placebo. 87 These effects were also independent of blood pressure lowering. Much like the early captopril studies in type 1 diabetics, a lower residual level of albuminuria was associated with lower ESRD risk. 88 The ROADMAP trial of 4,447 type 2 diabetics randomized to olmesartan or placebo demonstrated that olmesartan was more effective in delaying the onset of microalbuminuria. However, the olmesartan group had a slightly lower blood pressure (mean difference 3.1/1.9 mmHg) and there appeared to be a higher rate of fatal cardiovascular events in those with preexisting coronary artery disease. 89
ACE inhibitor versus ARB
In the DETAIL trial, 250 type 2 diabetics with early DN were randomly assigned to enalapril or telmisartan. This trial indicated that telmisartan was not inferior to enalapril in reducing a decline in GFR over 5 years. However, there was only a relatively small proportion of patients with overt nephropathy in this study. 90 Given the paucity of data for ARBs in type 1 diabetics, some clinicians prefer initiating treatment with an ACE inhibitor for type 1 DN.
For primary prevention of DN, a recent meta-analysis of eight studies and 11,906 participants found that ACE inhibitors reduced the risk of new onset microalbuminuria, macroalbuminuria, or both when compared to placebo (relative risk 0.71; 95% confidence interval 0.56–0.89). However, similar benefits could not be demonstrated for ARBs. 91 Thus, there is no proven benefit in starting ARB treatment in normotensive, normoalbuminuric type 1 or type 2 diabetics. Neither ACE inhibitor nor ARB is currently recommended in normotensive, normoalbuminuric diabetics for primary prevention of DN.
ACE inhibitor and ARB
Earlier studies of combination ACE inhibitor and ARB reported superiority of combination therapy for lowering albuminuria and blood pressure versus either alone, in both type 1 and 2 diabetics. 92 – 94 One study also showed a reduction in urinary TGF-β levels as another surrogate marker. 95 Despite the positive effects on these surrogate markers, the impact on preservation of GFR has not been demonstrated. The ONTARGET trial, which combined ramipril and telmisartan in patients with DN, noted no significant difference in the incidence of dialysis or doubling of serum creatinine when compared to single RAS inhibition. 96 In the Veterans Affairs NEPHRON-D study, the addition of lisinopril to losartan treatment did not reduce the composite endpoint of 50% reduction in eGFR, ESRD, or death. 97 Furthermore, combination treatment was associated with higher incidences of acute kidney injury and hyperkalemia in both these trials. Thus, the dual ACE inhibitor/ARB treatment strategy for DN has largely been abandoned.
Aldosterone antagonists
Aldosterone is the final component of the RAS cascade. Aldosterone promotes fibrosis, inflammation, and generation of ROS, along with endothelial dysfunction, cell growth, and proliferation. 98 , 99 Spironolactone appears to reduce proteinuria on its own or in combination with ACE inhibitor or ARB, in both type 1 and type 2 diabetics. 100 , 101 In addition to a blood pressure lowering effect, an anti-inflammatory mechanism is also likely, including reductions in MCP-1, MIF, and macrophage accumulation. 102 In a randomized trial of 268 type 2 diabetics, the addition of eplerenone to an ACE inhibitor reduced albuminuria. 103 However, the combination of aldosterone antagonists and other RAS inhibitors increases the risk of hyperkalemia and there is no long-term data on loss of renal function with combination blockade. Thus, combination of aldosterone antagonists and ACE inhibitor/ARB is unclear but, if used, careful monitoring of blood potassium is recommended along with dietary limitation of potassium intake.
Calcium channel blocker (CCB)
The addition of a non-dihydropyridine CCB to RAS inhibition may also be beneficial. Both verapamil and diltiazem have been shown to lower proteinuria in type 2 diabetics. 104 The effects of adding verapamil to lisinopril or trandolapril treatment were additive in reducing albuminuria and a decline in GFR. 105 , 106 However, the BENEDICT-B study of verapamil in combination with trandolapril did not find an additional benefit in regression of macroalbuminuria in hypertensive type 2 diabetics independent of blood pressure lowering. 107 In the MARVAL study of 332 type 2 diabetics randomized to valsartan or amlodipine (a dihydropyridine CCB) for 24 weeks, valsartan was more effective than amlodipine in reducing albuminuria, including remission to normoalbuminuria. 108 Further evidence from the Nephros and REIN-2 studies in nondiabetic CKD suggests that dihydropyridine CCBs such as felodipine and amlodipine do not have additive value in reducing proteinuria or progression to ESRD when added to ramipril. 109 , 110 Thus, the non-dihydropyridine CCBs may be considered second- or third-line agents after RAS inhibitors.
Similar to dietary sodium restriction, thiazide diuretics (eg, hydrochlorothiazide 50 mg) when combined with an ACE inhibitor (lisinopril 40 mg/day) reduced albuminuria in type 2 diabetics. However, the combination is associated with more frequent orthostatic symptoms. 82 For advanced CKD, a loop diuretic may be more appropriate. Diuretics may increase the effectiveness of ACE inhibitors and ARBs.
Blood pressure target
The current Joint National Committee (JNC 8) guidelines recommend targeting a blood pressure of <140/90 mmHg for diabetic patients, irrespective of CKD. 111 The 2013 European Society of Hypertension/European Society of Cardiology, 112 2014 Kidney Health Australia Caring for Australians with Renal Impairment, 113 and 2012 Kidney Disease: Improving Global Outcomes guidelines advocate a similar target. However, a lower blood pressure target is recommended by some guidelines for better control of proteinuria. The 2014 Kidney Health Australia Caring for Australians with Renal Impairment guidelines recommend a lowering of the blood pressure target from <140/90 mmHg to <130/80 mmHg in the presence of macroalbuminuria. 113 The 2012 Kidney Disease: Improving Global Outcomes guidelines suggest that a target of <130/80 would be more beneficial in those with micro- or macroalbuminuria. The National Kidney Foundation’s (Kidney Disease Outcomes Quality Initiative) 2007 and 2012 updated guidelines advocate blood pressure readings <130/80 mmHg in diabetics with CKD, or even lower in patients with high-level albuminuria (ACR >500 mg/g). 114 The Canadian Society of Nephrology continues to advocate for the lower target of <130/80 mmHg for all diabetics, regardless of CKD or albuminuria. 115 It is probably sufficient to say that low risk diabetics with normoalbuminuria could be treated to a target of <140/90 mmHg, while those at high risk or significant albuminuria should have a lower target of <130/80 mmHg.
Anti-lipid agents
In the Casale Monferrato study of 1,253 type 2 diabetics, apolipoprotein B and high-density lipoprotein cholesterol levels were independent risk factors for progression to overt nephropathy during 7 years follow-up. 19 In a large multinational case–control study of 2,535 type 2 diabetics with good control of low-density lipoprotein cholesterol, triglycerides and high-density lipoprotein cholesterol were associated with a higher risk of DN. 116 Data from the Joslin Diabetes Center from 439 type 1 diabetics also indicated that elevated cholesterol levels (>220 mg/dL) was associated with progression of DN. 117 Experimentally, statins have been shown to reduce NF-κB activation by p38 MAPK in tubular cells, AGE-mediated ROS activation, and tubular apoptosis and suppress RAS activation and aldosterone production. 118 – 120
Despite the epidemiological and experimental data, there is limited data from intervention studies with regards to renal outcomes. In a study of type 2 diabetics, simvastatin reduced albuminuria and improved expression of slit diaphragm proteins compared with cholestyramine despite similar lipid reductions. 121 In an open-label randomized study in 104 type 2 diabetics, rosuvastatin reduced albuminuria and oxidative stress independent of lipid levels. 122 The Heart Protection Study noted that simvastatin treatment was associated with a lesser decline in GFR compared to placebo after an average of 4.6 years, a difference which was bigger in diabetics compared to nondiabetics. 123 The CARDS study of 2,838 type 2 diabetics randomized patients to atorvastatin or placebo, with a median follow-up of 3.9 years. Atorvastatin treatment improved the annual decline in eGFR, particularly in those with albuminuria. 124 Currently, statins are already recommended for diabetics with DN over the age of 40 years, irrespective of their baseline lipid levels. This is primarily for cardiovascular benefit rather than renal disease per se, as albuminuria has been demonstrated to be an independent risk factor for cardiovascular events and mortality. 125
Epidemiological studies demonstrate a strong link between uric acid and DN. The Joslin Diabetes Center study of 355 type 1 diabetics found that higher baseline uric acid levels was associated with early GFR loss over 4–6 years. 126 Data from the Coronary Artery Calcification study, which included 324 type 1 diabetics with normoalbuminuria at baseline who were followed for 6 years, showed that for every 1 mg/dL increase in uric acid levels there was an 80% increased risk of developing micro- or macroalbuminuria. 127 In the Steno Diabetes Center study of 263 type 1 diabetics, baseline serum uric acid at the onset of diabetes predicted development of macroalbuminuria 18 years later. 128
Does lowering uric acid prevent progression of DN? A post hoc analysis of RENAAL noted that uric acid lowering by losartan may have accounted for 20% of the benefit afforded by the intervention. 129 In diabetic mice, allopurinol attenuated albuminuria and tubulointerstitial injury, suggesting that uric acid is not just a potential marker but a therapeutic target. 130 , 131 Allopurinol improves endothelial dysfunction and reduces urinary TGF-β in DN. 132 – 134 The PERL study is currently enrolling type 1 diabetics into a randomized trial of allopurinol versus placebo. 135
A low vitamin D level is common in patients with CKD. Vitamin D deficiency is linked to RAS activation and podocyte injury. 136 , 137 Vitamin D may also play a role in preventing epithelial-to-mesenchymal transformation of tubular epithelial cells. 138 Experimentally, active vitamin D also attenuated oxidative stress by restoring Nrf2 levels, important for cellular protection against oxidative injury. This was associated with reduced NF-κB activation and lower albuminuria. 139
Observational data from the PRONEDI trial of type 2 diabetics with stage 2–3 CKD showed that vitamin D levels <15 ng/mL was independently a risk factor for the composite outcome of >50% increase in serum creatinine, ESRD, or death. 140 In the VITAL study, type 2 diabetics randomized to paricalcitol (a synthetic D 2 agonist) for 24 weeks achieved significantly lower albuminuria than placebo treatment. 141 The upcoming VALIDATE-D study will evaluate the effect of calcitriol supplementation in patients on lisinopril to determine if there is a synergistic effect on RAS activity to lower proteinuria. 142 Future randomized trials will hopefully determine the usefulness of targeting the vitamin D receptor in preserving GFR in DN.
Lifestyle, diet, and alternative medicine
Although moderate-intensity aerobic physical activity is recommended for all diabetics to improve glycemic control and cardiovascular risk, the DCCT study of type 1 diabetics found no evidence that physical activity prevents DN. 143 Exercise may temporarily increase albumin excretion and should be avoided prior to urine collection for albumin excretion. On the other hand, the Look AHEAD study of type 2 diabetics suggested that intensive lifestyle intervention targeting weight loss may reduce progression of CKD, despite no benefit on cardiovascular outcomes. 144
A low protein diet is advocated by the American Diabetes Association. 21 A recent meta-analysis of 13 randomized controlled trials with 779 type 1 and type 2 diabetics found that a low protein diet was associated with significant improvement in GFR. However, adequate compliance was necessary for this effect on GFR. Interestingly, proteinuria was not different between low protein and regular protein patients but HbA 1c decreased slightly with low protein intake (−0.26%; 95% confidence interval −0.35 to −0.18). Low protein intake was defined as 0.6–0.8 g/kg/day and regular protein intake as 1.0–1.6 g/kg/day. 145
Substituting soy protein for animal protein may also be beneficial in diabetics with proteinuria but studies have not been consistent. 146 A number of alternative medicine supplements have also been studied ( Table 3 ). Lastly, sodium restriction to 50–70 mmol daily may enhance the action of RAS inhibitors and result in a greater reduction in albuminuria in type 2 diabetics. 147 , 148 However, this degree of sodium restriction is quite difficult for most and some advocate achieving an intake of <100 mmol/day as adequate restriction.
Diet and alternative medicine
| Product | Diabetes type (patients, n) | Study design | Potential mechanisms | Key findings | Reference |
|---|---|---|---|---|---|
| Silymarin (milk thistle, silybum) | Type 2 (60) | RCT | Antioxidant, anti-inflammatory Antiapoptotic | Silymarin (140 mg three times daily) for 3 months reduced albuminuria, urine TNF-α, urine, and serum malondialdehyde (oxidative stress marker) compared to baseline. | Fallahzadeh et al |
| Zinc | Type 2 (54) | Non-RCT | Antioxidant, improved glycemic control | Zinc supplement (50 mg elemental zinc) for 12 weeks improved glycemic control, lipids, and albuminuria compared to baseline. Effects on albuminuria were not shown to be independent of other metabolic effects. | Khan et al |
| Type 2 (50) | RCT crossover | Zinc supplement (30 mg elemental zinc) for 12 weeks reduced HbA and albuminuria compared to baseline. A 4-week washout was carried out before crossover. | Parham et al | ||
| Curcumin (turmeric) | Type 2 (40) | RCT | Antioxidant | Turmeric capsules 500 mg three times daily for 2 months reduced albuminuria, TGF-β, and IL-18 levels compared to baseline. | Khajehdehi et al |
| Green tea | Recruiting | RCT | Antioxidant | This trial is currently recruiting: Clinical Trials . Diabetic patients randomized to green tea extract, epigallocatechin, or placebo for 3 months. The primary outcome is a change in albuminuria. | None |
| Fish oil | Type 1 (36) | RCT | Anti-inflammatory Immunomodulatory | 1-year fish oil supplementation 4.6 g/day did not affect albuminuria or kidney function. | Rossing et al |
Abbreviation: HbA 1c , hemoglobin A 1c ; RCT, randomized controlled trial.
Multifactorial risk factor reduction
The benefits of intensive multifactorial intervention in type 2 diabetics were shown in the Steno-2 trial of 160 patients with microalbuminuria. Intensive therapy included: reduced dietary fat, light/moderate exercise, smoking cessation, tight glycemic control (<6.5%), tight blood pressure control (<130/80), ACE inhibitors, and anti-lipid medications (cholesterol <4.5 mmol/L). After a mean follow-up of 7.8 years, patients receiving multifactorial intervention had significantly lower risk of overt nephropathy (hazard ratio 0.39; 95% confidence interval 0.17–0.87) than those receiving regular management. 149
Transplantation
Simultaneous pancreas/kidney transplantation is an effective treatment for type 1 diabetics with ESRD, with most achieving insulin independence and preventing recurrence of DN in the allograft. 150 , 151 In patients with CKD after 10 years of pancreas transplantation alone, patients with sustained normoglycemia showed reductions in albuminuria and reversal of DN lesions on serial biopsy, including regression of glomerular basement membrane thickening and mesangial matrix deposition. 152 Some of these benefits may be offset by interstitial fibrosis and arteriolar hyalinosis due to calcineurin inhibitor (eg, cyclosporine) use. However, the same authors note that tubulointerstitial remodeling at 10 years had ameliorated some of the interstitial collagen deposition noted at 5 years, although vascular changes were not affected. 153
Novel agents
The diabetic milieu is a complex environment where a number of interventions may be utilized to target various pathological processes. As no single therapy completely ameliorates DN, novel strategies are needed to complement existing interventions. Some of these novel agents are described below and summarized in Table 4 .
Summary of novel agents
| Category | Mechanism of action | Drug(s) | Human data |
|---|---|---|---|
| Direct renin inhibitors | Blocks conversion of angiotensinogen to angiotensin I. | Aliskiren | RCT |
| Endothelin inhibitors | Predominantly blocks ET receptors on vascular endothelium. | Atrasentan Avosentan | RCT RCT |
| Vasopeptidase inhibitors | Blocks ACE and neutral endopeptidase. Palosuran blocks urotensin II receptor. | Palosuran Omapatrilat Ilepatril | RCT None None |
| PKC inhibitors | Blocks PKC-β intracellular signaling. | Ruboxistaurin | RCT, pooled |
| Aldose reductase | Reduces sorbitol formation by the polyol pathway. | Epalrestat Ponalrestat Tolrestat | Non-RCT Non-RCT None |
| Phosphodiesterase inhibitors | Increases cellular cAMP with broad effects. Cilostazol blocks PDE3, pentoxifylline is nonspecific and also blocks the adenosine receptor. | Cilostazol Pentoxifylline | RCT RCT, MetaAx |
| AGE inhibitors | Blocks AGE formation, enhances breakdown, or breaks crosslinks. | Aminoguanidine Pyridoxamine Alegebrium | RCT RCT None |
| Antioxidative stress | Activation of nuclear transcription factor Nrf2. | Bardoxolone | RCT |
| Glycosaminoglycans | Reduces heparan sulfate degradation in GBM, anti-inflammatory actions. | Sulodexide | RCT |
| Antifibrosis | Reduces TGF-β signaling and TNF-α levels but exact mechanism unclear. | Pirfenidone | RCT |
Abbreviations: AGE, advanced glycation end-products; cAMP, cyclic adenosine monophosphate; RCT, randomized controlled trial; MetaAx, meta-analysis; GBM, glomerular basement membrane.
Renin inhibitors
Renin catalyses the rate-limiting step in the production of angiotensin II. In diabetic rats, aliskiren reduced albuminuria and glomerulosclerosis, and was more effective than perindopril in reducing interstitial fibrosis. 154 In type 2 diabetics after a 4-week washout of previous medications, aliskiren reduced blood pressure and albuminuria, with the effects on albuminuria persisting after withdrawal of medication. 155 In the AVOID trial of 599 type 2 diabetics, the combination of aliskiren 300 mg and losartan 100 mg for 6 months reduced the urine ACR independent of blood pressure. 156 However, the much larger ALTITUDE trial, which randomized 8,561 high-risk type 2 diabetics to aliskiren 300 mg or placebo as adjunctive to RAS inhibition, found no significant difference in renal outcomes. It is noted that the trial was terminated prematurely due to excess hyperkalemia and hypotension in the aliskiren group. 157 Due to the lack of good randomized controlled trial evidence supporting the use of aliskiren in combination with ACE inhibitors or ARBs, and the increased adverse effects, the combination is not recommended. From the US Food and Drug Administration perspective, the combination should be contraindicated in patients with diabetes. However, it could be considered as an alternative RAS blocker for blood pressure lowering and proteinuria reduction. More research is needed to demonstrate that aliskiren is as good as ACE inhibitors or ARBs.
Endothelin inhibitors
In diabetic rats, an ET A receptor blockade with atrasentan or avosentan reduced albuminuria and renal fibrosis. 158 , 159 The ASCEND trial of 1,392 type 2 diabetics with overt nephropathy examined the effect of avosentan on time to doubling of serum creatinine, ESRD, or death. Avosentan halved proteinuria but increased fluid retention, edema, and congestive heart failure, resulting in the trial being stopped early. 160 Since ASCEND, two other randomized controlled trials have noted reduction in albuminuria at the cost of edema and congestive heart failure. 160 , 161 The latter trial involving 1,392 type 2 diabetics was also stopped prematurely after a median follow-up of 4 months. In a randomized trial of 211 type 2 diabetics, atrasentan added to RAS inhibition for 12 weeks reduced albuminuria in association with lowering blood pressure. 162 Fluid overload was reported as manageable, albeit more patients discontinued treatment on the higher dose of atrasentan. The SONAR trial ( NCT01858532 ) with atrasentan is currently in progress to evaluate renal outcomes in type 2 diabetics.
Urotensin and vasopeptidase inhibitors
Vasopeptidase inhibitors can block ACE and neutral endopeptidase. Palosuran is a competitive antagonist of the urotensin II receptor. In diabetic patients with macroalbuminuria, a 2-week course of palosuran in addition to RAS inhibitors reduced albuminuria by 24%. 163 The PROLONG trial is a prospective, randomized controlled crossover trial in hypertensive type 2 diabetics looking at the effects of palosuran on albuminuria and blood pressure. 164 This study found no significant reduction in albuminuria or blood pressure after 4 weeks of treatment. Other vasopeptidase inhibitors such as omapatrilat and ilepatril (AVE7688) have been shown to attenuate albuminuria in diabetic rats but human data are lacking. 165 , 166
PKC inhibitors
Ruboxistaurin is a selective inhibitor of PKC-β. Animal studies with ruboxistaurin showed beneficial effects on reducing mesangial expansion, hyperfiltration, albuminuria, macrophage accumulation, and tubulointerstitial injury. 167 , 168 Small randomized controlled studies have demonstrated that ruboxistaurin reduced urinary TGF-β excretion by >50%, 169 reduced albuminuria, and preserved eGFR at 1 year in type 2 diabetics. 170 However, when pooled data from three large studies of ruboxistaurin from diabetic retinopathy trials were analyzed (n=1,157), ruboxistaurin was no different from placebo after 3 years in reducing the rates of doubling of serum creatinine or stage 4–5 CKD. 171
Aldose reductase inhibitors
These inhibitors suppress sorbitol accumulation in tissues. Epalrestat reduced mesangial expansion and preserved renal function in diabetic rats. 172 Another inhibitor – tolrestat – prevented glomerular hypertrophy and hyperfiltration, mesangial cell hypocontractility, and albuminuria in diabetic rats. 173 A small study of 35 type 2 diabetics showed that epalrestat treatment for 5 years prevented progression of microalbuminuria. 174 A post hoc analysis of the Aldose Reductase Inhibitor–Diabetes Complications Trial concluded that progression of retinopathy/albuminuria was significantly inhibited by epalrestat. 175 This was a re-analysis of the original 3-year, open-label trial using a subset of patients for which data were available. On the other hand, another inhibitor – ponalrestat – did not affect urinary albumin excretion or glomerular filtration in type 1 diabetics. 176
Phosphodiesterase inhibitors
Cilostazol inhibits phosphodiesterase III and reduces thrombospondin-1 and TGF-β expression, attenuating hyperfiltration, albuminuria, and extracellular matrix deposition in diabetic rats. 177 , 178 In humans, one study using cilostazol for 3 months in type 2 diabetics demonstrated a reduction in urinary ACR and renal production of thromboxane B2. 179 A small Chinese study randomized 40 type 2 diabetics to cilostazol or placebo for 6 months. Cilostazol reduced albuminuria, serum ICAM-1, and MCP-1 levels but did not affect kidney function. 180
Pentoxifylline is a methylxanthine-derived phosphodiesterase inhibitor that antagonizes the adenosine receptor and lowers blood viscosity. It also has anti-inflammatory and immunomodulatory properties, and lowers serum and urine TNF-α in diabetic patients with DN. In a Cochrane meta-analysis of 17 randomized trials involving 991 participants, pentoxifylline was better than placebo in reducing albuminuria and preserving serum creatinine but was equivalent to captopril. However, the studies were small and of poor methodology, with no data on ESRD or mortality. 181 Since the meta-analysis, other trials have examined the addition of pentoxifylline to RAS blockers and have consistently found a benefit in reducing proteinuria. Roozbeh et al enrolled 74 patients with type 2 diabetes with overt proteinuria, randomized to pentoxifylline 400 mg daily plus captopril or captopril alone. The reduction in proteinuria from baseline was greater in the pentoxifylline-treated group, associated with a modest reduction in blood pressure. 182 Oliaei et al enrolled 50 type 2 diabetics with proteinuria >500 mg/day despite RAS inhibition, to pentoxifylline 400 mg three times a day versus placebo. The pentoxifylline group had greater reductions in proteinuria but no difference in creatinine clearance. 183 Ghorbani et al enrolled 100 type 2 diabetics with proteinuria randomized to pentoxifylline 400 mg/day or placebo for 6 months. Both groups received losartan and enalapril in combination. After 6 months, pentoxifylline treatment was associated with lower proteinuria and higher creatinine clearance. 184 The results of the PREDIAN study are still expected. 185
AGE inhibitors
AGE inhibitors reduce AGE formation, enhance degradation, or break AGE crosslinks. The prototype AGE inhibitor is aminoguanidine (pimagedine), which acts by scavenging intermediates such as 3-deoxyglucosone and methyglyoxal. 186 Experimentally, aminoguanidine attenuates albuminuria, mesangial expansion, and collagen deposition in diabetic rats. 187 However, the placebo-controlled ACTION trial in 690 type 1 diabetics with overt nephropathy showed no difference in the time taken to double serum creatinine despite a reduction in proteinuria with pimagedine treatment for 2–4 years. 188
Pyridoxamine inhibits AGE formation and scavenges ROS and toxic carbonyls. When data from two 24-week studies were combined, pyridoxamine reduced the change from baseline creatinine in type 1 and type 2 diabetics without affecting albuminuria. 189 However, in a random-ized controlled trial of 317 type 2 diabetics, pyridoxamine treatment for 52 weeks did not significantly affect serum creatinine. 190 GLY-230 is another inhibitor of protein glycation that was studied in 21 diabetic men in a randomized trial for 14 days. GLY-230 reduced glycated albumin and albuminuria compared to baseline but not placebo. 191 AGE crosslink breakers, such as alegebrium, and inhibitors of the AGE receptor have shown benefit in DN models but have not been studied in humans.
Agents targeting oxidative stress
Cellular and mitochondrial ROS formation is an important contributor to the pathophysiology of DN. Targeted inhibitors of ROS generation are emerging but most have not progressed to clinical trials. Oxidative stress and inflammation in DN may also lead to a reduction in Nrf2, a nuclear transcription factor which plays a key role in antioxidant and cytoprotective mechanisms. 192 Bardoxolone is a potent activator of Nrf2. In the BEAM trial of 227 type 2 diabetics with eGFR 20–45 mL/minute/m 2 randomized to bardoxolone (25, 75, or 150 mg daily) or placebo, bardoxolone was associated with an improvement in eGFR at 24 weeks, which was sustained to 52 weeks of treatment. 193 In the much larger BEACON trial of 2,185 type 2 diabetics with stage 4 CKD, patients were randomized to bardoxolone 20 mg daily or placebo. The trial was stopped after a median follow-up of 9 months due to a higher rate of cardiovascular events and increased albuminuria, with no reduction in ESRD or cardiovascular death. 194 Subsequently, one animal study in diabetic rats found unfavorable side effects of bardoxolone analogs, further questioning the safety profile in DN. 195
Glycosaminoglycans
Sulodexide is a mixture of 80% heparan sulfate and 20% dermatan sulfate. Sulodexide may reduce the enhanced heparan sulfate degradation in the glomerular basement membrane that occurs in DN. It has anti-inflammatory properties and inhibits the hyperglycemia-induced production of ROS, MCP-1, and IL-6 in endothelial cells. 196 It may improve endothelial dysfunction, vascular permeability, and renal hemodynamics. It may also attenuate TGF-β gene expression, extracellular matrix expansion, and inhibit HPSE-1, which plays a role in tubular epithelial-to-mesenchymal transition. 197
The DiNAS trial enrolled 223 type 1 and type 2 diabetics with serum creatinine ≤1.7 mg/dL in a randomized trial of sulodexide (50, 100, 200 mg/day) versus placebo for 4 months, with a further 4 months follow-up postintervention. There was a dose-dependent effect, with 200 mg/day the most effective in reducing albuminuria. RAS inhibition was not universal in this trial although post hoc analysis indicated the effect of sulodexide was additive to ACE inhibition. 198 The Sun-MICRO trial enrolled 1,056 type 2 diabetics with microalbuminuria in a randomized trial of sulodexide 200 mg/day versus placebo for 12 months. There was no difference between the groups in normalizing albumin excretion or reducing albuminuria by at least 50%. 199 The Sun-MACRO trial enrolled 1,248 type 2 diabetics with renal impairment and overt nephropathy in a randomized trial of sulodexide 200 mg/day versus placebo. The study was terminated mid-enrollment, with data on 1,029 patient-years analyzed. This showed no significant difference in doubling of serum creatinine, ESRD, or creatinine >6 mg/dL. 200 In both the Sun-MICRO and Sun-MACRO trials, patients were on maximum doses of RAS inhibitors. The latter studies have dampened the enthusiasm for sulodexide in DN.
Antifibrotic agents
Pirfenidone inhibits TGF-β production and TNF-α production in models of DN and non-DN kidney disease. The exact mechanism of action is unclear. In db/db mice with type 2 diabetes, pirfenidone reduced mesangial matrix expansion but did not affect albuminuria. 201 In a small randomized trial of 77 type 1 and 2 diabetics with established DN, pirfenidone at 1,200 mg/day for 1 year improved eGFR from baseline compared to placebo (mean intergroup difference 5.5 mL/minute/1.73 m 2 ). Pirfenidone at the higher dose of 2,400 mg/day did not demonstrate a similar benefit and the dropout rate was high. Pirfenidone did not lower albuminuria. Larger studies are needed to validate the findings.
Gene and cell-based therapy
Gene therapy involves introducing a gene into cells to increase the production of a protein of interest. A carrier or vector such as modified adenovirus is employed to deliver the gene to the nucleus where the protein coded by the gene is produced by the cellular machinery. Gene therapy targeting TGF-β/SMAD signaling has shown promise in reducing kidney injury in diabetic models. Ka et al studied Smad7 gene therapy in the db/db mouse model of type 2 diabetes. Treatment inhibited TGF-β/SMAD and NF-κB activation, resulting in a reduction in proteinuria, macrophage infiltration, inflammation, podocyte injury, and renal fibrosis. 202 A similar finding was noted by Zhang et al by using gene therapy to enhance decorin expression in the streptozotocin model of type 1 diabetes. The beneficial effects were attributed to downregulation of TGF-β/SMAD signaling as decorin is a natural inhibitor of TGF-β1. 203 HGF gene therapy has been shown in db/db mice to enhance renal expression of SDF-1, associated with increased numbers of bone marrow-derived monocyte/macrophages with a higher proportion of M2 markers (anti-inflammatory phenotype). This was associated with a reduction in proinflammatory cytokines, reduced histological injury, and preservation of podocytes. 204 Kosugi et al examined soluble Flt-1 gene therapy in db/db mice. sFlt-1 is an endogenous inhibitor of VEGF and treated animals showed reduced VEGF expression in association with elevated sFlt-1 levels in the kidney. Although sFlt-1 gene therapy reduced podocyte injury and albuminuria, tubulointerstitial injury was enhanced, leading the authors to conclude that this approach would not be beneficial in DN. 205 Thus, there are some potential risks with gene therapy, which may be related to the inserted gene itself or the viral vector utilized but this discussion is beyond the scope of this review.
Progenitor (stem) cells are multipotent cells capable of self-renewal and differentiation into specialized cells, and are broadly categorized into embryonic stem cells and adult stem cells. Adult stem cells can be derived from bone marrow, adipose tissue, or peripheral blood. Stem cells can also be harvested from umbilical cord blood at birth. The potential benefits of stem cell treatment in DN include: 1) replacing or regenerating damaged cells, 2) modulating inflammation, 3) reducing oxidative stress, and 4) improving glycemia. There have been a number of experimental studies of stem cell treatment in DN ( Table 5 ). Most studies have demonstrated a blood glucose lowering effect by improved pancreatic β-cell function and insulin levels, whilst some others have not. This may relate to the nature of the cells utilized or the method of delivery. Some of these studies suggest that a paracrine effect is more important as a renoprotective mechanism, rather than regeneration or replacement of injured cells. This is based on observations of low level engraftment of mesenchymal stem cells in the kidney and the production of beneficial growth factors, antifibrotic factors, and factors which protect from oxidative stress. 206 , 207
Stem cell therapy in experimental diabetic nephropathy
| Source | Model | Main outcomes | Reference |
|---|---|---|---|
| Human B-MSC | T1DM, mice NOD/SCID | ↓ glucose, ↑ insulin and β-cells, ↓ mesangial thickening, ↓ macrophage infiltration | Lee et al |
| Mouse B-MSC | T1DM, mice C57BL/6 | ↓ glucose, ↑ mouse insulin and β-cells, ↓ albuminuria, ↓ glomerular fibrosis and mesangial expansion | Ezquer et al |
| Mouse B-MSC | T1DM, mice C57BL/6 | No effect on glucose, insulin, or β-cells, ↓ albuminuria, ↓ glomerular fibrosis and mesangial expansion, ↓ podocyte loss | Ezquer et al |
| Rat B-MSC | T1DM, rats SD | ↓ glucose, ↓ albuminuria, ↓ renal mass index | Zhou et al |
| Human UC-MSC | T1DM, rats SD | No effect on glucose, ↓ proteinuria, ↓ fibronectin and α-smooth muscle actin, ↑ E-cadherin | Park et al |
| Human UC-MSC | T1DM, rats SD | No effect on glucose, ↓ proteinuria, ↓ mesangial expansion, ↓ α-smooth muscle actin, TGF-β1, and collagen, ↑ E-cadherin | Park et al |
| Rat A-MSC | T1DM, rats SD | ↓ glucose, ↑ insulin, ↓ lipids, ↓ creatinine, ↓ mesangial expansion, ↓ oxidative stress, ↓ proinflammatory cytokines (TNF-α, IL-1β, IL-6), ↓MAPK signaling (p38, ERK, JNK) | Fang et al |
| Rat B-MSC | T1DM, rats SD | No effect on glucose, ↓ albuminuria, ↓ BMP-7, ↓ podocyte injury and loss, ↑ creatinine clearance, ↓ renal mass index | Wang et al |
| Rat B-MSC | T1DM, rats Wistar | ↓ glucose and albuminuria, ↓ glomerulosclerosis, ↓ MCP-1 and macrophages, ↑ HGF, ↓ proinflammatory cytokines (IL-1β, IL-6, TNF-α) | Lv et al |
| Rat B-MSC | T1DM, rats SD | ↓ glucose, ↑ insulin and β-cells, ↓ albuminuria, ↑ synaptopodin, ↓ TGF-β1, ↑ IL-10 | Zhang et al |
| Human A-MSC | T1DM, rats SD | No effect on glucose or β cells, ↓ proteinuria, ↑ creatinine clearance, ↓ cholesterol, ↓ glomerular hypertrophy, ↓ podocyte injury/loss, ↓ interstitial fibrosis | Zhang et al |
| Rat B-MSC | T1DM, rats Albino | ↓ urea and creatinine, ↓ albuminuria, ↓ Bax expression, ↓ TGF-β and TNF-α, ↑ VEGF | Abdel Aziz et al |
Note: a down arrow indicates a reduction or decrease; an up arrow indicates an increase.
Abbreviations: A-MSC, adipose-derived mesenchymal stromal (stem) cells; B-MSC, bone marrow-derived mesenchymal stromal (stem) cells; SD, Sprague Dawley; T1DM, streptozotocin-induced model of type 1 diabetes; UC-MSC, umbilical cord blood-derived mesenchymal stromal (stem) cells.
The main issues facing cell-based therapy include: 1) consistency of manufactured cells (phenotypic change occur with repeat passages), 2) cell delivery method (optimize tissue targeting and minimizing passive entrapment), and 3) engrafting and cell survival. Notwithstanding the limitations mentioned, both gene therapy and stem cell therapy are promising areas of research but there are currently no successful human studies to date. Further studies are also needed to confirm that mesenchymal stem cells ameliorate DN independent of its metabolic benefits.
DN and ESRD remains a significant problem despite best efforts to limit the impact of the disease on such end-organ damage. In such a complex milieu of diabetes where no single treatment can halt DN progression, a multifactorial approach remains the most sensible. This should include optimal glycemic control and single RAS inhibition for hypertension or albuminuria. Based on the evidence, ACE inhibitors are preferred for type 1 diabetics. Second-line antihypertensives include non-dihydropyridine CCBs and diuretics. Lipid management with a statin is prudent for cardiovascular disease even though a direct impact on renal disease has not been conclusively shown other than as part of the multifactorial risk intervention similar to the Steno-2 study (which includes aspirin). No alternative medicines or supplements have been shown to slow GFR decline although effects on albuminuria are reported by some small studies. None can be routinely recommended currently and further studies on vitamin D are awaited. Further data on uric acid management with allopurinol are also awaited. Mild salt and protein restriction may also benefit some patients but strict monitoring and compliance can be problematic.
Understanding the pathophysiology of DN has improved over the years, particularly the molecular biology aspect. Inflammation has emerged as an important theme, while treatment targets and options continue to evolve as knowledge improves. The inflammatory amplification loop mediated by macrophages may be a good candidate for inhibition to reduce DN progression. Leukocyte or monocyte/macrophage culling may not necessarily be the best long-term strategy but manipulation of the macrophage phenotype and the interaction with T-cells should be further investigated. Blocking specific cell signaling pathways involved with inflammation may be useful but can be troubled by off-target effects, which will need to be fully explored before clinical trials can proceed.
A number of potential treatment strategies have shown benefit in improving surrogate markers like albuminuria but the translation to preserving GFR and preventing ESRD has not always followed. Such is the case with dual or triple blockade of the RAS system in DN seen in recent large clinical trials. It is acknowledged that albuminuria as a surrogate marker of disease progression is flawed. Furthermore, experimental interventions which reduce histological injury and inflammation do not always reduce the level of established proteinuria. Novel biomarkers may assist in this area when more data becomes available. Despite these challenges, new strategies to complement existing treatments will nonetheless continue to be looked for.
Acknowledgments
The author thanks Dr Ian Simpson (Renal Pathologist, Department of Anatomical Pathology, Monash Health) for his contribution of the histology images and pathology descriptions. The author also thanks Mr Paul Crammer (Renal Scientist, Department of Anatomical Pathology, Monash Health) for the electron microscopy image.
The author reports no conflicts of interest in this work. This manuscript has not been submitted or published anywhere else.
- 1. International Diabetes Federation . IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [Accessed September 2, 2014]. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf . [ Google Scholar ]
- 2. Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: US Department of Health and Human Services; 2011. [Accessed September 2, 2014]. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf . [ Google Scholar ]
- 3. Tapp RJ, Shaw JE, Zimmet PZ, et al. Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Am J Kidney Dis. 2004;44(5):792–798. [ PubMed ] [ Google Scholar ]
- 4. Scott LJ, Warram JH, Hanna LS, Laffel LM, Ryan L, Krolewski AS. A nonlinear effect of hyperglycemia and current cigarette smoking are major determinants of the onset of microalbuminuria in type 1 diabetes. Diabetes. 2001;50(12):2842–2849. doi: 10.2337/diabetes.50.12.2842. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25(5):859–864. doi: 10.2337/diacare.25.5.859. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Satko SG, Langefeld CD, Daeihagh P, Bowden DW, Rich SS, Freedman BI. Nephropathy in siblings of African Americans with overt type 2 diabetic nephropathy. Am J Kidney Dis. 2002;40(3):489–494. doi: 10.1053/ajkd.2002.34888. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1990;33(7):438–443. doi: 10.1007/BF00404096. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320(18):1161–1165. doi: 10.1056/NEJM198905043201801. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26(8):2392–2399. doi: 10.2337/diacare.26.8.2392. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Smith SR, Svetkey LP, Dennis VW. Racial differences in the incidence and progression of renal diseases. Kidney Int. 1991;40(5):815–822. doi: 10.1038/ki.1991.281. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314(7083):783–788. doi: 10.1136/bmj.314.7083.783. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Mooyaart AL, Valk EJ, van Es LA, et al. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia. 2011;54(3):544–553. doi: 10.1007/s00125-010-1996-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Dellamea BS, Pinto LC, Leitao CB, Santos KG, Canani LH. Endothelial nitric oxide synthase gene polymorphisms and risk of diabetic nephropathy: a systematic review and meta-analysis. BMC Med Genet. 2014;15:9. doi: 10.1186/1471-2350-15-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Lin Z, Huang G, Zhang J, Lin X. Adiponectin gene polymorphisms and susceptibility to diabetic nephropathy: a meta-analysis. Ren Fail. 2014;36(3):478–487. doi: 10.3109/0886022X.2013.868319. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Zhou TB, Guo XF, Yin SS. Association of peroxisome proliferator-activated receptor γ Pro12Ala gene polymorphism with type 2 diabetic nephropathy risk in Caucasian population. J Recept Signal Transduct Res. 2014;34(3):180–184. doi: 10.3109/10799893.2013.868905. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Mooyaart AL. Genetic associations in diabetic nephropathy. Clin Exp Nephrol. 2014;18(2):197–200. doi: 10.1007/s10157-013-0874-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Molitch ME, DeFronzo RA, Franz MJ, et al. American Diabetes Association Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83. doi: 10.2337/diacare.27.2007.s79. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51(5):1580–1587. doi: 10.2337/diabetes.51.5.1580. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Bruno G, Merletti F, Biggeri A, et al. Progression to overt nephropathy in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2003;26(7):2150–2155. doi: 10.2337/diacare.26.7.2150. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Johnson DW, Jones GR, Mathew TH, et al. Chronic kidney disease and measurement of albuminuria or proteinuria: a position statement. Med J Aust. 2012;197(4):224–225. doi: 10.5694/mja11.11468. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. American Diabetes Association Standards of Medical Care in Diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Mazzucco G, Bertani T, Fortunato M, et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis. 2002;39(4):713–720. doi: 10.1053/ajkd.2002.31988. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Mak SK, Gwi E, Chan KW, et al. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12(12):2588–2591. doi: 10.1093/ndt/12.12.2588. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Sharma SG, Bomback AS, Radhakrishnan J, et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8(10):1718–1724. doi: 10.2215/CJN.02510213. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77(1):57–64. doi: 10.1038/ki.2009.399. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52(4):1036–1040. doi: 10.2337/diabetes.52.4.1036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Argyropoulos C, Wang K, McClarty S, et al. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One. 2013;8(1):e54662. doi: 10.1371/journal.pone.0054662. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. DiStefano JK, Taila M, Alvarez ML. Emerging roles for miRNAs in the development, diagnosis, and treatment of diabetic nephropathy. Curr Diab Rep. 2013;13(4):582–591. doi: 10.1007/s11892-013-0386-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23(3):516–524. doi: 10.1681/ASN.2011060628. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507–515. doi: 10.1681/ASN.2011060627. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Weil EJ, Lemley KV, Mason CC, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82(9):1010–1017. doi: 10.1038/ki.2012.234. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56(8):2155–2160. doi: 10.2337/db07-0019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Huang W, Gallois Y, Bouby N, et al. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci U S A. 2001;98(23):13330–13334. doi: 10.1073/pnas.231476798. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Rudberg S, Rasmussen LM, Bangstad HJ, Osterby R. Influence of insertion/deletion polymorphism in the ACE-I gene on the progression of diabetic glomerulopathy in type 1 diabetic patients with microalbuminuria. Diabetes Care. 2000;23(4):544–548. doi: 10.2337/diacare.23.4.544. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Kanetsuna Y, Takahashi K, Nagata M, et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170(5):1473–1484. doi: 10.2353/ajpath.2007.060481. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Haneda M, Araki S, Togawa M, Sugimoto T, Isono M, Kikkawa R. Mitogen-activated protein kinase cascade is activated in glomeruli of diabetic rats and glomerular mesangial cells cultured under high glucose conditions. Diabetes. 1997;46(5):847–853. doi: 10.2337/diab.46.5.847. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int Suppl. 2000;77:S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26(3):380–392. doi: 10.1210/er.2004-0028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Williamson JR, Chang K, Frangos M, et al. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42(6):801–813. doi: 10.2337/diab.42.6.801. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Lanaspa MA, Ishimoto T, Cicerchi C, et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol. 2014 May 29; doi: 10.1681/ASN.2013080901. Epub. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288(20):2579–2588. doi: 10.1001/jama.288.20.2579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Langham RG, Kelly DJ, Gow RM, et al. Increased renal gene transcription of protein kinase C-beta in human diabetic nephropathy: relationship to long-term glycaemic control. Diabetologia. 2008;51(4):668–674. doi: 10.1007/s00125-008-0927-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Noh H, King GL. The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl. 2007;(106):S49–S53. doi: 10.1038/sj.ki.5002386. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43(1):1–8. doi: 10.2337/diab.43.1.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Sakai N, Wada T, Furuichi K, et al. Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis. 2005;45(1):54–65. doi: 10.1053/j.ajkd.2004.08.039. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Adhikary L, Chow F, Nikolic-Paterson DJ, et al. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47(7):1210–1222. doi: 10.1007/s00125-004-1437-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Fiorina P, Vergani A, Bassi R, et al. Role of podocyte B7-1 in diabetic nephropathy. J Am Soc Nephrol. 2014;25(7):1415–1429. doi: 10.1681/ASN.2013050518. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Mudaliar H, Pollock C, Komala MG, Chadban S, Wu H, Panchapakesan U. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol. 2013;305(2):F143–F154. doi: 10.1152/ajprenal.00398.2012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55(11):2993–3003. doi: 10.2337/db06-0477. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Hofmann MA, Schiekofer S, Kanitz M, et al. Insufficient glycemic control increases nuclear factor-kappa B binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care. 1998;21(8):1310–1316. doi: 10.2337/diacare.21.8.1310. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Sanchez AP, Sharma K. Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med. 2009;11:e13. doi: 10.1017/S1462399409001057. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50(2):471–480. doi: 10.1007/s00125-006-0497-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Lim AK, Ma FY, Nikolic-Paterson DJ, Thomas MC, Hurst LA, Tesch GH. Antibody blockade of c-fms suppresses the progression of inflammation and injury in early diabetic nephropathy in obese db/db mice. Diabetologia. 2009;52(8):1669–1679. doi: 10.1007/s00125-009-1399-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Furuta T, Saito T, Ootaka T, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21(5):480–485. doi: 10.1016/s0272-6386(12)80393-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11(3):226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Bending JJ, Lobo-Yeo A, Vergani D, Viberti GC. Proteinuria and activated T-lymphocytes in diabetic nephropathy. Diabetes. 1988;37(5):507–511. doi: 10.2337/diab.37.5.507. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Moriya R, Manivel JC, Mauer M. Juxtaglomerular apparatus T-cell infiltration affects glomerular structure in Type 1 diabetic patients. Diabetologia. 2004;47(1):82–88. doi: 10.1007/s00125-003-1253-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Lim AK, Ma FY, Nikolic-Paterson DJ, Kitching AR, Thomas MC, Tesch GH. Lymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injury. Diabetologia. 2010;53(8):1772–1782. doi: 10.1007/s00125-010-1757-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Eller K, Kirsch A, Wolf AM, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60(11):2954–2962. doi: 10.2337/db11-0358. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Xu J, Su HL, Wang JH, Zhang CH. Role of CD4+CD25+Foxp3+ regulatory T cells in type 2 diabetic nephropathy. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(1):137–139. Chinese. [ PubMed ] [ Google Scholar ]
- 63. Ilan Y, Maron R, Tukpah AM, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107(21):9765–9770. doi: 10.1073/pnas.0908771107. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517–523. doi: 10.1038/ki.2012.401. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Ko GJ, Kang YS, Han SY, et al. Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrol Dial Transplant. 2008;23(9):2750–2760. doi: 10.1093/ndt/gfn157. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Ohga S, Shikata K, Yozai K, et al. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-kappaB activation. Am J Physiol Renal Physiol. 2007;292(4):F1141–F1150. doi: 10.1152/ajprenal.00288.2005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Zhang H, Saha J, Byun J, et al. Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295(4):F1071–F1081. doi: 10.1152/ajprenal.90208.2008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Bakris GL, Ruilope LM, McMorn SO, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens. 2006;24(10):2047–2055. doi: 10.1097/01.hjh.0000244955.39491.88. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Kodera R, Shikata K, Takatsuka T, et al. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014;443(3):828–833. doi: 10.1016/j.bbrc.2013.12.049. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Mori H, Okada Y, Arao T, Tanaka Y. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(3):313–319. doi: 10.1111/jdi.12142. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Fujita H, Taniai H, Murayama H, et al. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1α in type 2 diabetic patients with incipient nephropathy. Endocr J. 2014;61(2):159–166. doi: 10.1507/endocrj.ej13-0305. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Viberti G, Mogensen CE, Groop LC, Pauls JF, European Microalbuminuria Captopril Study Group Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. JAMA. 1994;271(4):275–279. [ PubMed ] [ Google Scholar ]
- 79. The Microalbuminuria Captopril Study Group Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. Diabetologia. 1996;39(5):587–593. doi: 10.1007/BF00403306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Wilmer WA, Hebert LA, Lewis EJ, et al. Remission of nephrotic syndrome in type 1 diabetes: long-term follow-up of patients in the Captopril Study. Am J Kidney Dis. 1999;34(2):308–314. doi: 10.1016/s0272-6386(99)70360-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Hovind P, Rossing P, Tarnow L, Toft H, Parving J, Parving HH. Remission of nephrotic-range albuminuria in type 1 diabetic patients. Diabetes Care. 2001;24(11):1972–1977. doi: 10.2337/diacare.24.11.1972. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Hovind P, Tarnow L, Rossing P, Carstensen B, Parving HH. Improved survival in patients obtaining remission of nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2004;66(3):1180–1186. doi: 10.1111/j.1523-1755.2004.00870.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. de Galan BE, Zoungas S, Chalmers J, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2009;52(11):2328–2336. doi: 10.1007/s00125-009-1484-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–1951. doi: 10.1056/NEJMoa042167. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Eijkelkamp WB, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18(5):1540–1546. doi: 10.1681/ASN.2006050445. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Haller H, Ito S, Izzo JL, Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907–917. doi: 10.1056/NEJMoa1007994. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351(19):1952–1961. doi: 10.1056/NEJMoa042274. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF. Antihypertensive agents for preventing diabetic kidney disease [review] Cochrane Database Syst Rev. 2012;12:CD004136. doi: 10.1002/14651858.CD004136.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin–angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63(5):1874–1880. doi: 10.1046/j.1523-1755.2003.00940.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Jacobsen P, Andersen S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol. 2003;14(4):992–999. doi: 10.1097/01.asn.0000054495.96193.bf. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin–angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440–1444. doi: 10.1136/bmj.321.7274.1440. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Song JH, Cha SH, Lee HJ, et al. Effect of low-dose dual blockade of renin–angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant. 2006;21(3):683–689. doi: 10.1093/ndt/gfi310. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Mann JF, Anderson C, Gao P, et al. Dual inhibition of the renin–angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens. 2013;31(2):414–421. doi: 10.1097/HJH.0b013e32835bf7b0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–1903. doi: 10.1056/NEJMoa1303154. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Wolf G. Renal injury due to renin–angiotensin–aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70(11):1914–1919. doi: 10.1038/sj.ki.5001846. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Huang W, Xu C, Kahng KW, Noble NA, Border WA, Huang Y. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294(6):F1287–F1295. doi: 10.1152/ajprenal.00017.2008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68(6):2829–2836. doi: 10.1111/j.1523-1755.2005.00756.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28(9):2106–2112. doi: 10.2337/diacare.28.9.2106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Han SY, Kim CH, Kim HS, et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17(5):1362–1372. doi: 10.1681/ASN.2005111196. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–951. doi: 10.2215/CJN.00240106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int. 1996;50(5):1641–1650. doi: 10.1038/ki.1996.480. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Bakris GL, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic humans: importance of therapeutic selection. Kidney Int. 1992;41(4):912–919. doi: 10.1038/ki.1992.139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Bakris GL, Weir MR, DeQuattro V, McMahon FG. Effects of an ACE inhibitor/calcium antagonist combination on proteinuria in diabetic nephropathy. Kidney Int. 1998;54(4):1283–1289. doi: 10.1046/j.1523-1755.1998.00083.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Ruggenenti P, Fassi A, Ilieva A, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens. 2011;29(2):207–216. doi: 10.1097/hjh.0b013e32834069bd. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation. 2002;106(6):672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Herlitz H, Harris K, Risler T, et al. The effects of an ACE inhibitor and a calcium antagonist on the progression of renal disease: the Nephros Study. Nephrol Dial Transplant. 2001;16(11):2158–2165. doi: 10.1093/ndt/16.11.2158. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365(9463):939–946. doi: 10.1016/S0140-6736(05)71082-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Pilmore H, Dogra G, Roberts M, et al. Cardiovascular disease in patients with chronic kidney disease. Nephrology (Carlton) 2014;19(1):3–10. doi: 10.1111/nep.12148. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Ruzicka M, Quinn RR, McFarlane P, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for the management of blood pressure in CKD. Am J Kidney Dis. 2014;63(6):869–887. doi: 10.1053/j.ajkd.2014.03.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Sacks FM, Hermans MP, Fioretto P, et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case–control study in 13 countries. Circulation. 2014;129(9):999–1008. doi: 10.1161/CIRCULATIONAHA.113.002529. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Krolewski AS, Warram JH, Christlieb AR. Hypercholesterolemia – a determinant of renal function loss and deaths in IDDM patients with nephropathy. Kidney Int Suppl. 1994;45:S125–131. [ PubMed ] [ Google Scholar ]
- 118. Ishibashi Y, Yamagishi S, Matsui T, et al. Pravastatin inhibits advanced glycation end products (AGEs)-induced proximal tubular cell apoptosis and injury by reducing receptor for AGEs (RAGE) level. Metabolism. 2012;61(8):1067–1072. doi: 10.1016/j.metabol.2012.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Gao P, Wu X, Shui H, Jia R. Fluvastatin inhibits high glucose-induced nuclear factor kappa B activation in renal tubular epithelial cells. J Nephrol. 2013;26(2):289–296. doi: 10.5301/jn.5000128. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Toba H, Mitani T, Takahashi T, et al. Inhibition of the renal renin-angiotensin system and renoprotection by pitavastatin in type1 diabetes. Clin Exp Pharmacol Physiol. 2010;37(11):1064–1070. doi: 10.1111/j.1440-1681.2010.05436.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Tonolo G, Velussi M, Brocco E, et al. Simvastatin maintains steady patterns of GFR and improves AER and expression of slit diaphragm proteins in type II diabetes. Kidney Int. 2006;70(1):177–186. doi: 10.1038/sj.ki.5001515. [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J Atheroscler Thromb. 2011;18(11):1018–1028. doi: 10.5551/jat.9084. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Colhoun HM, Betteridge DJ, Durrington PN, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54(5):810–819. doi: 10.1053/j.ajkd.2009.03.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 125. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343. doi: 10.2337/dc10-0227. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25(6):1865–1869. doi: 10.1093/ndt/gfp740. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58(7):1668–1671. doi: 10.2337/db09-0014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 129. Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58(1):2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [ DOI ] [ PubMed ] [ Google Scholar ]
- 130. Kim SM, Choi YW, Seok HY, et al. Reducing serum uric acid attenuates TGF-β1-induced profibrogenic progression in type 2 diabetic nephropathy. Nephron Exp Nephrol. 2012;121(3–4):e109–e121. doi: 10.1159/000343567. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Kosugi T, Nakayama T, Heinig M, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297(2):F481–F488. doi: 10.1152/ajprenal.00092.2009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Dogan A, Yarlioglues M, Kaya MG, et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20(3):182–187. doi: 10.3109/08037051.2010.538977. [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35(3):746–751. doi: 10.1161/01.hyp.35.3.746. [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27(5):435–440. doi: 10.1159/000105142. [ DOI ] [ PubMed ] [ Google Scholar ]
- 135. Maahs DM, Caramori L, Cherney DZ, et al. Uric acid lowering to prevent kidney function loss in diabetes: the Preventing Early Renal Function Loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–559. doi: 10.1007/s11892-013-0381-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105(41):15896–15901. doi: 10.1073/pnas.0803751105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 137. Wang Y, Deb DK, Zhang Z, et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol. 2012;23(12):1977–1986. doi: 10.1681/ASN.2012040383. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Xiong M, Gong J, Liu Y, Xiang R, Tan X. Loss of vitamin D receptor in chronic kidney disease: a potential mechanism linking inflammation to epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol. 2012;303(7):F1107–F1115. doi: 10.1152/ajprenal.00151.2012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 139. Nakai K, Fujii H, Kono K, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014;27(4):586–595. doi: 10.1093/ajh/hpt160. [ DOI ] [ PubMed ] [ Google Scholar ]
- 140. Fernandez-Juarez G, Luno J, Barrio V, et al. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin–angiotensin system. Clin J Am Soc Nephrol. 2013;8(11):1870–1876. doi: 10.2215/CJN.00910113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 141. de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Brown JM, Secinaro K, Williams JS, Vaidya A. Evaluating hormonal mechanisms of vitamin D receptor agonist therapy in diabetic kidney disease: the VALIDATE-D study. BMC Endocr Disord. 2013;13(1):33. doi: 10.1186/1472-6823-13-33. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 143. Makura CB, Nirantharakumar K, Girling AJ, Saravanan P, Narendran P. Effects of physical activity on the development and progression of microvascular complications in type 1 diabetes: retrospective analysis of the DCCT study. BMC Endocr Disord. 2013;13(1):37. doi: 10.1186/1472-6823-13-37. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Nezu U, Kamiyama H, Kondo Y, Sakuma M, Morimoto T, Ueda S. Effect of low-protein diet on kidney function in diabetic nephropathy: meta-analysis of randomised controlled trials. BMJ Open. 2013;3(5):e002934. doi: 10.1136/bmjopen-2013-002934. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Anderson JW. Beneficial effects of soy protein consumption for renal function. Asia Pac J Clin Nutr. 2008;17(Suppl 1):324–328. [ PubMed ] [ Google Scholar ]
- 147. Kwakernaak AJ, Krikken JA, Binnenmars SH, et al. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(5):385–395. doi: 10.1016/S2213-8587(14)70030-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Houlihan CA, Allen TJ, Baxter AL, et al. A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care. 2002;25(4):663–671. doi: 10.2337/diacare.25.4.663. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Pedersen O, Gaede P. Intensified multifactorial intervention and cardiovascular outcome in type 2 diabetes: the Steno-2 study. Metabolism. 2003;52(8 Suppl 1):19–23. doi: 10.1016/s0026-0495(03)00213-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Bohman SO, Tyden G, Wilczek H, et al. Prevention of kidney graft diabetic nephropathy by pancreas transplantation in man. Diabetes. 1985;34(3):306–308. doi: 10.2337/diab.34.3.306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 151. Bilous RW, Mauer SM, Sutherland DE, Najarian JS, Goetz FC, Steffes MW. The effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med. 1989;321(2):80–85. doi: 10.1056/NEJM198907133210204. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339(2):69–75. doi: 10.1056/NEJM199807093390202. [ DOI ] [ PubMed ] [ Google Scholar ]
- 153. Fioretto P, Sutherland DE, Najafian B, Mauer M. Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int. 2006;69(5):907–912. doi: 10.1038/sj.ki.5000153. [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia. 2007;50(11):2398–2404. doi: 10.1007/s00125-007-0795-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 155. Persson F, Rossing P, Schjoedt KJ, et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73(12):1419–1425. doi: 10.1038/ki.2008.68. [ DOI ] [ PubMed ] [ Google Scholar ]
- 156. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358(23):2433–2446. doi: 10.1056/NEJMoa0708379. [ DOI ] [ PubMed ] [ Google Scholar ]
- 157. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [ DOI ] [ PubMed ] [ Google Scholar ]
- 158. Sasser JM, Sullivan JC, Hobbs JL, et al. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18(1):143–154. doi: 10.1681/ASN.2006030208. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 159. Gagliardini E, Corna D, Zoja C, et al. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009;297(5):F1448–F1456. doi: 10.1152/ajprenal.00340.2009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 160. Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21(3):527–535. doi: 10.1681/ASN.2009060593. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 161. Wenzel RR, Littke T, Kuranoff S, et al. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20(3):655–664. doi: 10.1681/ASN.2008050482. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 162. de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25(5):1083–1093. doi: 10.1681/ASN.2013080830. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Sidharta PN, Wagner FD, Bohnemeier H, et al. Pharmacodynamics and pharmacokinetics of the urotensin II receptor antagonist palosuran in macroalbuminuric, diabetic patients. Clin Pharmacol Ther. 2006;80(3):246–256. doi: 10.1016/j.clpt.2006.05.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. Vogt L, Chiurchiu C, Chadha-Boreham H, et al. Effect of the urotensin receptor antagonist palosuran in hypertensive patients with type 2 diabetic nephropathy. Hypertension. 2010;55(5):1206–1209. doi: 10.1161/HYPERTENSIONAHA.109.149559. [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Schafer S, Linz W, Vollert H, et al. The vasopeptidase inhibitor AVE7688 ameliorates type 2 diabetic nephropathy. Diabetologia. 2004;47(1):98–103. doi: 10.1007/s00125-003-1264-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 166. Davis BJ, Johnston CI, Burrell LM, et al. Renoprotective effects of vasopeptidase inhibition in an experimental model of diabetic nephropathy. Diabetologia. 2003;46(7):961–971. doi: 10.1007/s00125-003-1121-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 167. Koya D, Haneda M, Nakagawa H, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14(3):439–447. doi: 10.1096/fasebj.14.3.439. [ DOI ] [ PubMed ] [ Google Scholar ]
- 168. Wu Y, Wu G, Qi X, et al. Protein kinase C beta inhibitor LY333531 attenuates intercellular adhesion molecule-1 and monocyte chemotactic protein-1 expression in the kidney in diabetic rats. J Pharmacol Sci. 2006;101(4):335–343. doi: 10.1254/jphs.fp0050896. [ DOI ] [ PubMed ] [ Google Scholar ]
- 169. Gilbert RE, Kim SA, Tuttle KR, et al. Effect of ruboxistaurin on urinary transforming growth factor-beta in patients with diabetic nephropathy and type 2 diabetes. Diabetes Care. 2007;30(4):995–996. doi: 10.2337/dc06-2079. [ DOI ] [ PubMed ] [ Google Scholar ]
- 170. Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28(11):2686–2690. doi: 10.2337/diacare.28.11.2686. [ DOI ] [ PubMed ] [ Google Scholar ]
- 171. Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol. 2007;2(4):631–636. doi: 10.2215/CJN.00840207. [ DOI ] [ PubMed ] [ Google Scholar ]
- 172. Itagaki I, Shimizu K, Kamanaka Y, et al. The effect of an aldose reductase inhibitor (epalrestat) on diabetic nephropathy in rats. Diabetes Res Clin Pract. 1994;25(3):147–154. doi: 10.1016/0168-8227(94)90002-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 173. Donnelly SM, Zhou XP, Huang JT, Whiteside CI. Prevention of early glomerulopathy with tolrestat in the streptozotocin-induced diabetic rat. Biochem Cell Biol. 1996;74(3):355–362. doi: 10.1139/o96-038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 174. Iso K, Tada H, Kuboki K, Inokuchi T. Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in type 2 diabetic patients. J Diabetes Complications. 2001;15(5):241–244. doi: 10.1016/s1056-8727(01)00160-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 175. Hotta N, Kawamori R, Fukuda M, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29(12):1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 176. Ranganathan S, Krempf M, Feraille E, Charbonnel B. Short term effect of an aldose reductase inhibitor on urinary albumin excretion rate (UAER) and glomerular filtration rate (GFR) in type 1 diabetic patients with incipient nephropathy. Diabete Metab. 1993;19(2):257–261. [ PubMed ] [ Google Scholar ]
- 177. Wang X, Yan L, Chen W, Xu L, Zhang X. The renal protective effects of cilostazol on suppressing pathogenic thrombospondin-1 and transforming growth factor-beta expression in streptozotocin-induced diabetic rats. J Int Med Res. 2009;37(1):145–153. doi: 10.1177/147323000903700117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 178. Tohma T, Shimabukuro M, Oshiro Y, Yamakawa M, Shimajiri Y, Takasu N. Cilostazol, a phosphodiesterase inhibitor, reduces microalbuminuria in the insulin-resistant Otsuka Long-Evans Tokushima Fatty rat. Metabolism. 2004;53(11):1405–1410. doi: 10.1016/j.metabol.2004.06.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 179. Watanabe J, Sako Y, Umeda F, Nawata H. Effects of cilostazol, a phosphodiesterase inhibitor, on urinary excretion of albumin and prostaglandins in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1993;22(1):53–59. doi: 10.1016/0168-8227(93)90132-o. [ DOI ] [ PubMed ] [ Google Scholar ]
- 180. Jiao XM, Jiao XJ, Zhang XG, et al. Cilostazol reduces microalbuminuria in type 2 diabetic nephropathy. Chin Med J (Engl) 2013;126(22):4395–4396. [ PubMed ] [ Google Scholar ]
- 181. Shan D, Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ. Pentoxifylline for diabetic kidney disease [review] Cochrane Database Syst Rev. 2012;2:CD006800. doi: 10.1002/14651858.CD006800.pub2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 182. Roozbeh J, Banihashemi MA, Ghezlou M, et al. Captopril and combination therapy of captopril and pentoxifylline in reducing proteinuria in diabetic nephropathy. Ren Fail. 2010;32(2):172–178. doi: 10.3109/08860221003602645. [ DOI ] [ PubMed ] [ Google Scholar ]
- 183. Oliaei F, Hushmand S, Khafri S, Baradaran M. Efficacy of pentoxifylline for reduction of proteinuria in type II diabetic patients. Caspian J Intern Med. 2011;2(4):309–313. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 184. Ghorbani A, Omidvar B, Beladi-Mousavi SS, Lak E, Vaziri S. The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: a double blind and randomized clinical trial. Nefrologia. 2012;32(6):790–796. doi: 10.3265/Nefrologia.pre2012.Jun.11242. [ DOI ] [ PubMed ] [ Google Scholar ]
- 185. Navarro-Gonzalez JF, Muros M, Mora-Fernandez C, Herrera H, Meneses B, Garcia J. Pentoxifylline for renoprotection in diabetic nephropathy: the PREDIAN study. Rationale and basal results. J Diabetes Complications. 2011;25(5):314–319. doi: 10.1016/j.jdiacomp.2010.09.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 186. Thornalley PJ. Use of aminoguanidine (pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419(1):31–40. doi: 10.1016/j.abb.2003.08.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 187. Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991;40(10):1328–1334. doi: 10.2337/diab.40.10.1328. [ DOI ] [ PubMed ] [ Google Scholar ]
- 188. Bolton WK, Cattran DC, Williams ME, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24(1):32–40. doi: 10.1159/000075627. [ DOI ] [ PubMed ] [ Google Scholar ]
- 189. Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB. Effects of pyridoxamine in combined Phase II studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol. 2007;27(6):605–614. doi: 10.1159/000108104. [ DOI ] [ PubMed ] [ Google Scholar ]
- 190. Lewis EJ, Greene T, Spitalewiz S, et al. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):131–136. doi: 10.1681/ASN.2011030272. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 191. Kennedy L, Solano MP, Meneghini L, Lo M, Cohen MP. Anti-glycation and anti-albuminuric effects of GLY-230 in human diabetes. Am J Nephrol. 2010;31(2):110–116. doi: 10.1159/000259897. [ DOI ] [ PubMed ] [ Google Scholar ]
- 192. Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. doi: 10.1038/ki.2012.439. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 193. Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [ DOI ] [ PubMed ] [ Google Scholar ]
- 194. de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369(26):2492–2503. doi: 10.1056/NEJMoa1306033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 195. Zoja C, Corna D, Nava V, et al. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol. 2013;304(6):F808–F819. doi: 10.1152/ajprenal.00376.2012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 196. Ciszewicz M, Polubinska A, Antoniewicz A, Suminska-Jasinska K, Breborowicz A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Transl Res. 2009;153(3):118–123. doi: 10.1016/j.trsl.2008.12.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 197. Masola V, Zaza G, Gambaro G. Sulodexide and glycosaminoglycans in the progression of renal disease. Nephrol Dial Transplant. 2014;29(Suppl 1):i74–i79. doi: 10.1093/ndt/gft389. [ DOI ] [ PubMed ] [ Google Scholar ]
- 198. Gambaro G, Kinalska I, Oksa A, et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the DiNAS randomized trial. J Am Soc Nephrol. 2002;13(6):1615–1625. doi: 10.1097/01.asn.0000014254.87188.e5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 199. Lewis EJ, Lewis JB, Greene T, et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am J Kidney Dis. 2011;58(5):729–736. doi: 10.1053/j.ajkd.2011.06.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 200. Packham DK, Wolfe R, Reutens AT, et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):123–130. doi: 10.1681/ASN.2011040378. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 201. RamachandraRao SP, Zhu Y, Ravasi T, et al. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol. 2009;20(8):1765–1775. doi: 10.1681/ASN.2008090931. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 202. Ka SM, Yeh YC, Huang XR, et al. Kidney-targeting Smad7 gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear factor κB (NF-κB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia. 2012;55(2):509–519. doi: 10.1007/s00125-011-2364-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 203. Zhang Z, Wu F, Zheng F, Li H. Adenovirus-mediated decorin gene transfection has therapeutic effects in a streptozocin-induced diabetic rat model. Nephron Exp Nephrol. 2010;116(1):e11–e21. doi: 10.1159/000314669. [ DOI ] [ PubMed ] [ Google Scholar ]
- 204. Flaquer M, Franquesa M, Vidal A, et al. Hepatocyte growth factor gene therapy enhances infiltration of macrophages and may induce kidney repair in db/db mice as a model of diabetes. Diabetologia. 2012;55(7):2059–2068. doi: 10.1007/s00125-012-2535-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 205. Kosugi T, Nakayama T, Li Q, et al. Soluble Flt-1 gene therapy ameliorates albuminuria but accelerates tubulointerstitial injury in diabetic mice. Am J Physiol Renal Physiol. 2010;298(3):F609–F616. doi: 10.1152/ajprenal.00377.2009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 206. Li D, Wang N, Zhang L, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4(5):103. doi: 10.1186/scrt314. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 207. Zhang Y, Yuen DA, Advani A, et al. Early-outgrowth bone marrow cells attenuate renal injury and dysfunction via an antioxidant effect in a mouse model of type 2 diabetes. Diabetes. 2012;61(8):2114–2125. doi: 10.2337/db11-1365. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 208. Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014 Jun 5; doi: 10.1681/ASN.2013070758. Epub. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 209. Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int. 2011;79(10):1113–1118. doi: 10.1038/ki.2010.554. [ DOI ] [ PubMed ] [ Google Scholar ]
- 210. Conway BR, Manoharan D, Jenks S, et al. Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors. Kidney Int. 2012;82(7):812–818. doi: 10.1038/ki.2012.218. [ DOI ] [ PubMed ] [ Google Scholar ]
- 211. Lacquaniti A, Donato V, Pintaudi B, et al. “Normoalbuminuric” diabetic nephropathy: tubular damage and NGAL. Acta Diabetol. 2013;50(6):935–942. doi: 10.1007/s00592-013-0485-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 212. Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP, and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One. 2013;8(1):e54863. doi: 10.1371/journal.pone.0054863. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 213. Panduru NM, Forsblom C, Saraheimo M, et al. Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2013;36(7):2077–2083. doi: 10.2337/dc12-1868. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 214. Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care. 2010;33(6):1320–1324. doi: 10.2337/dc09-2242. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 215. Araki S, Haneda M, Koya D, et al. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36(5):1248–1253. doi: 10.2337/dc12-1298. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 216. Kamijo-Ikemori A, Sugaya T, Yasuda T, et al. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care. 2011;34(3):691–696. doi: 10.2337/dc10-1392. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 217. Kim SS, Song SH, Kim IJ, et al. Urinary cystatin C and tubular proteinuria predict progression of diabetic nephropathy. Diabetes Care. 2013;36(3):656–661. doi: 10.2337/dc12-0849. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 218. Fallahzadeh MK, Dormanesh B, Sagheb MM, et al. Effect of addition of silymarin to renin–angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60(6):896–903. doi: 10.1053/j.ajkd.2012.06.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 219. Khan MI, Siddique KU, Ashfaq F, Ali W, Reddy HD, Mishra A. Effect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patients. J Nat Sci Biol Med. 2013;4(2):336–340. doi: 10.4103/0976-9668.117002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 220. Parham M, Amini M, Aminorroaya A, Heidarian E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud. 2008;5(2):102–109. doi: 10.1900/RDS.2008.5.102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 221. Khajehdehi P, Pakfetrat M, Javidnia K, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45(5):365–370. doi: 10.3109/00365599.2011.585622. [ DOI ] [ PubMed ] [ Google Scholar ]
- 222. Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH. Fish oil in diabetic nephropathy. Diabetes Care. 1996;19(11):1214–1219. doi: 10.2337/diacare.19.11.1214. [ DOI ] [ PubMed ] [ Google Scholar ]
- 223. Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 224. Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14(6):631–640. doi: 10.1016/j.bbmt.2008.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 225. Ezquer F, Ezquer M, Simon V, et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15(11):1354–1365. doi: 10.1016/j.bbmt.2009.07.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 226. Zhou H, Tian HM, Long Y, et al. Mesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in rats. Chin Med J (Engl) 2009;122(21):2573–2579. [ PubMed ] [ Google Scholar ]
- 227. Park JH, Park J, Hwang SH, Han H, Ha H. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury. Transplant Proc. 2012;44(4):1123–1126. doi: 10.1016/j.transproceed.2012.03.044. [ DOI ] [ PubMed ] [ Google Scholar ]
- 228. Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98(3):465–473. doi: 10.1016/j.diabres.2012.09.034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 229. Fang Y, Tian X, Bai S, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines, and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30(1):85–92. doi: 10.3892/ijmm.2012.977. [ DOI ] [ PubMed ] [ Google Scholar ]
- 230. Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013;19(4):538–546. doi: 10.1016/j.bbmt.2013.01.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 231. Lv SS, Liu G, Wang JP, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol. 2013;17(2):275–282. doi: 10.1016/j.intimp.2013.05.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 232. Zhang Y, Ye C, Wang G, et al. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. Biomed Res Int. 2013;2013:526367. doi: 10.1155/2013/526367. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 233. Zhang L, Li K, Liu X, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22(23):3074–3086. doi: 10.1089/scd.2013.0142. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 234. Abdel Aziz MT, Wassef MA, Ahmed HH, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr. 2014;6(1):34. doi: 10.1186/1758-5996-6-34. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (3.0 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- DOI: 10.5867/medwave.2017.01.6839
- Corpus ID: 28658147
[Pathophysiology of diabetic nephropathy: a literature review].
- Carlos Eduardo Meza Letelier , Camilo Alfredo San Martín Ojeda , +1 author Cristobal Jesus Frugone Zaror
- Published in Medwave 2017
41 Citations
Novel biomarkers for the diagnosis of diabetic nephropathy, hirudin in the treatment of chronic kidney disease, new insights into diabetes mellitus and its complications: a narrative review, mechanisms and efficacy of traditional chinese herb monomers in diabetic kidney disease., peroxisome proliferator-activated receptor-alpha agonists in the management of the diabetic acute kidney injury: is the verdict out, does the mediterranean diet reduce the odds of diabetic nephropathy in women a case–control study, effectiveness of novel iron regulators in the treatment of diabetic nephropathy, niclosamide from an anthelmintic drug to a promising adjuvant therapy for diabetic kidney disease: randomized clinical trial, increased levels of circulating igfbp4 and angptl8 with a prospective role in diabetic nephropathy, related papers.
Showing 1 through 3 of 0 Related Papers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 21 October 2024
Association between RBC folate and diabetic nephropathy in Type2 diabetes mellitus patients: a cross-sectional study
- Peixia Yu 1 ,
- Yongjin Ji 2 ,
- Hairu wang 1 &
- Keyu Liu 3
Scientific Reports volume 14 , Article number: 24692 ( 2024 ) Cite this article
Metrics details
- Endocrinology
Folates play a crucial role as cofactors in metabolic pathways, influencing biological methylation and nucleotide synthesis, which has a significant impact on overall health and disease susceptibility. Diabetic nephropathy (DN) is a prevalent and severe complication of diabetes mellitus (DM). The correlation between RBC folate and DN remains unclear currently. This study aims to assess whether RBC folate is associated with DN. Based on data from the NHANES (2011–2018), we conducted a cross-sectional study involving 3070 adults with type 2 DM (T2DM). Demographic factors, levels of folate and vitamin B12, dietary folate intakes, and relevant laboratory data were obtained from all participants. Logistic regression, fitting smooth curves, interaction effects were utilized to support the research objectives. Regression analyses demonstrated a positive relation between RBC folate and DN. (P < 0.001). A positive association between levels of RBC folate and the risk of DN was observed after full adjustment for all the confounding variables (odds ratio: 1.38, 95% confidence interval: 1.27–1.49, P < 0.001). Similar patterns of association were observed for subgroup analysis (all P values for interaction > 0.05). In addition, curve fitting after adjusting for all the confounding variables demonstrated that there was a linear relationship between RBC folate and DN (P for non-linearity = 0.147). Increased RBC folate levels were linked to a higher risk of DN in type 2 diabetes. RBC folate should be considered as a crucial indicator for folate status in DN.
Similar content being viewed by others

Retinol intake is associated with the risk of chronic kidney disease in individuals with type 2 diabetes mellitus: results from NHANES
Non-linear associations of serum and red blood cell folate with risk of cardiovascular and all-cause mortality in hypertensive adults
Folic acid supplementation on inflammation and homocysteine in type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials
Introduction.
The incidence of DM has indeed risen significantly in recent years, coinciding with improvements in living standards and alterations in lifestyle factors, such as dietary choices and physical activity. 1 In recent years, DM has become one of the most common chronic illnesses, leading to significant challenges for both patients and the healthcare system, especially for those suffering from diabetes-related complications like DN. 2 , 3 , 4 DN is a common and serious microvascular complication of DM. Prolonged exposure to high levels of glucose in the blood can indeed cause damage to the small blood vessels in the kidneys, leading to kidney disease, which is a significant cause of end-stage renal disease (ESRD) and can result in the need for dialysis or kidney transplantation. 5 DN can potentially reduce the lifespan of individuals with diabetes, and early identification of DN risks provides an opportunity to delay or stop disease onset. 6 Given the lack of effective pharmacological treatments, it is crucial to explore indicators for preventing the occurrence of DN. Because folate plays a central role in the one-carbon metabolism pathway, deficiencies and insufficiencies of it are associated with a wide range of negative health outcomes, from birth defects to cancer. 7 However, the evidence regarding the adverse effects of high folate level is inconsistent. Many reports suggested that elevated folate levels in the blood were primarily attributed to increased folate intake, without taking into account the interactions of homeostasis, which are mediated by use and excretion of the body. The kidneys are responsible for two crucial roles in folate metabolism. One is the excretion of metabolites, such as folates used as reducing agents and folic acid, as well as waste products, from the bloodstream into the urine. The other is the reabsorbing folates to save and return them to the blood for further use, ensuring that the body maintains an adequate level of folate. RBC folate is a blood biomarker that provides insight into human physiology and health status. 8 Measurement of RBC folate serves as a sensitive gauge of the body’s homeostatic control mechanisms, as it is a dynamic parameter that fluctuates in response to internal and external stimuli. 9 , 10 Based on the current evidence, we postulate that changes in RBC folate concentrations in participants with T2DM are influenced by deteriorating kidney function, and higher concentrations of RBC folate may serve as a predictive factor for the early decline in kidney function in patients with T2DM, indicating a closer link between RBC folate levels and the development of diabetic nephropathy. However, there are limited and conflicting reports, and no substantial evidence from large population studies regarding the association between RBC folate and the risk of DN. In this study, we aim to explore the potential association between RBC folate and risk of DN in 3070 participants with T2DM using data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2011 to 2018.
Materials and methods.
Participants enrollment..
The NHANES is a cross-sectional and nationwide study aimed at assessing the health and nutrition status of adults and children in the US. It has been periodically conducted since the 1960s. The study population is recruited through a complex, multistage, stratified sampling design, making it nationally representative. (Source: https://www.cdc.gov/nchs/nhanes/about_nhanes.Htm ) The data we studied came from four NHANES cycles (2011–2012, 2013–2014, 2015–2016, and 2017–2018) because the data on RBC folate and DN in DM patients was available during these periods. Initially, the study included a total of 39,156 participants. Participants who aged younger than 20 were excluded ( N = 22617). A total of 18,560 participants who were not diagnosed with diabetes were excluded ( N = 4057). The patients who had no data about the urinary albumin to creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) ( N = 438), RBC folate ( N = 516) and serum total folate ( N = 27) were also excluded. 6 patients were excluded due to pregnancy. Finally, 3070 patients with T2DM were involved in the final analysis. The participants were considered to have DN ( N = 1217) if their urinary albumin to creatinine ratio (UACR) was 30 mg/g or higher, or if their estimated glomerular filtration rate (eGFR) was less than 60 mL/min/1.73 m 2 . 11 (Fig. 1 ).
Data Collection.
Professionals collected basic population information and conducted experimental measurements in adherence to the technical standards published on the NHANES website. All data and experimental methods were available for download from the NHANES website. The laboratory work was performed at a facility in Minnesota. Demographics data (sex, age, race, family income, education level, etc.), examination Data (blood pressure, body mass index, etc.), health-related behaviors (smoking and alcohol using, etc.), laboratory data ( fasting blood glucose, glycated hemoglobin, albumin, total protein, alanine aminotransferase, gamma-glutamyltransferase, serum creatinine, cholesterol, high density lipoprotein cholesterol, RBC folate, serum total folate, vitamin B12, RBC count, etc.), and dietary data (dietary folate intake, dietary supplement folate intake)were selected 12 , 13 . Subsequently, all measurements were quantified in accordance with international standard units.
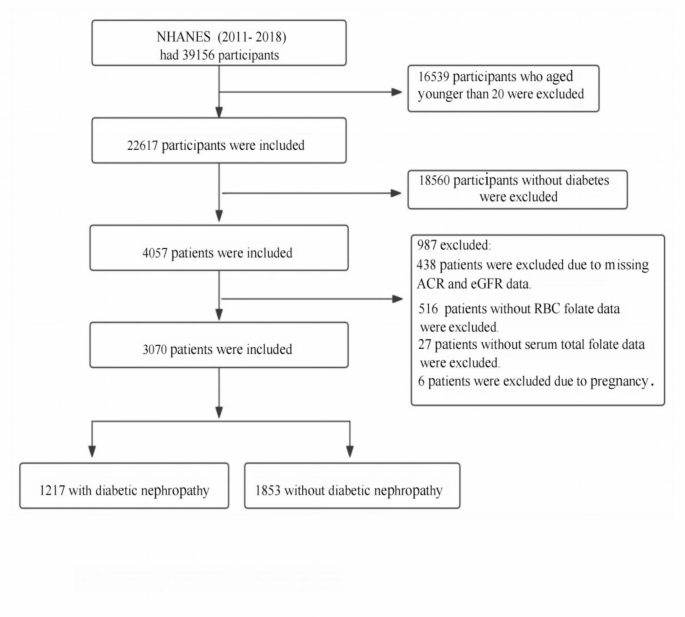
Flowchart of participant selection.

Criteria for evaluation
Diagnosis of dn.
Participants were considered to have diabetic nephropathy (DN) if their urinary albumin to creatinine ratio (UACR) was 30 mg/g or higher, or if their estimated glomerular filtration rate (eGFR) was less than 60 mL/min/1.73 m 2 . 11
Identification of DM
The diagnostic criteria for diabetes were developed based on international and previous research literature. 14 The criteria include: the person who had ever been told that he or she had diabetes, or a fasting blood glucose level of ≥ 7.0 mmol/L, or a glycosylated hemoglobin level of ≥ 6.5 mmol/L.
Defining hypertension.
The diagnostic criteria for hypertension were identified as: individuals who had ever been told that they had hypertension, or individuals with a systolic blood pressure (SBP) of 140mmHg or higher, and/or a diastolic blood pressure (DBP) of 90mmHg or higher. 15 .
Evaluation of BMI
The body mass index (BMI) is an indicator of whether a person is underweight, normal weight, overweight, or obese. The formula is: BMI = weight (kg) / (height (m))^2. According to the standards set by the World Health Organization, if a person’s BMI fell between 18.5 kg/m2 and 24.9 kg/m2, it was considered normal. A BMI of 25.0 to 29.9 kg/m2 was categorized as overweight, and a BMI of 30.0 kg/m2 or higher was classified as obese 16 .
Assessment of of smoking and alcohol using.
Based on the analysis of data and previous research, individuals who have smoked more than 100 cigarettes in their lifetime were defined as smokers, while participants who have not smoked more than 100 cigarettes were considered non-smokers.A non-drinker was defined as a person who had consumed no more than 12 alcoholic drinks in a year, while those who exceeded this limit were categorized as drinkers. 17 , 18 , 19 , 20
Covariable Screening
The covariates were screened based on the following criteria:
Factors that have been identified as potential contributors to the development and advancement of DN in prior research. (11)
In consideration of our clinical experience.
Introduction of variability that may result in a change in the regression coefficient of the base model by over 10%
Avoidance of variables with collinearity.
The collected covariates included demographic data (sex, age, race, family income, education level, etc.), examination data (blood pressure, body mass index, etc.), health-related behaviors (smoking and alcohol use, etc.), laboratory data (albumin, total protein, alanine aminotransferase, gamma-glutamyltransferase, cholesterol, high-density lipoprotein cholesterol, vitamin B12, RBC count, etc.), and dietary data (dietary folate intake, dietary supplement folate intake). A dietary recall interview followed by an interview at the Mobile Examination Center (MEC) was conducted to gather participants’ 24-hour nutritional information, including dietary folate intake, and dietary supplement folate intake, which were determined by questions regarding nutritional consumption. Education level was categorized as less than 9 years, 9–12 years, and more than 12 years. Family income was classified as low (PIR ≤ 1.3), medium (PIR > 1.3–3.5), and high (PIR > 3.5) based on the poverty income ratio (PIR). 20
Statistical Analysis
The baseline characteristics of different RBC folate groups were analyzed using One-Way ANOVA (for normal distribution), Kruskal-Wallis H test (for skewed distribution), and chi-square test (for categorical variables). Histogram distribution, Q-Q plot, or Kolmogorov-Smirnov test was utilized to evaluate whether the variables followed a normal distribution. Continuous variables were presented as mean with standard deviation (SD) or medians with interquartile range (IQR), and categorical variables were presented as number and percentage. The participants were divided into two groups: those with DN and those without DN. Single-factor and multi-factor logistic regressions were used to explore the associations between RBC folate and DN. In the context of multi-factor analyses, RBC folate was treated as both continuous and categorical variables, in which RBC folate levels were transformed into their logarithmic base 2 values for analysis or categorized by quartiles with Q1 as the reference group, respectively. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to assess the relationship between RBC folate and the risk of DN.
Model 1 represented the basic model without accounting for any variables. Model 2 was controlled for age, gender, race/ethnicity, PIR, and education level. Model 3 was further adjusted from Model 2 to include BMI, smoking, alcohol use, and hypertension. Model 4 was adjusted as for Model 3, additionally adjusted for ALT, ALB, GGT, TP, cholesterol, HDL, dietary folate intake, dietary folate supplement intake, vitamin B12, and RBC count. A smooth curve fitting graph between RBC folate and DN using the restricted cubic spline approach was created and fine-tuned based on the covariates in model 4. Subgroup analysis was performed on all subgroups to assess the stability of the results. Interaction tests were conducted, followed by effect adjustment tests on subgroup measures, and likelihood ratio tests were carried out subsequently.
The analysis was performed using R 4.2.1 ( http://www.Rproject.org ; The R Foundation, Vienna, Austria) and the Free Statistics software (version 1.9.2; Beijing FreeClinical Medical Technology Co., Ltd, Beijing, China). A two-sided p-value less than 0.05 was deemed statistically significant.
Basic information feature when collecting the data of participant.
This study involved 39,156 participants from the NHANES dataset over a duration of eight years across four cycles. Following the strict screening criteria outlined above, the final analysis included 3,070 participants with T2DM, among whom 1,217 had DN and 1,853 had no DN (refer to Fig. 1 ). Table 1 presented the baseline data for diabetic nephropathy and non-diabetic nephropathy. DN was detected in 1,217 participants (39.6%). Significant differences in age, race, serum folate, RBC folate, ALB, RBC count, hypertension, smoking, and ALT were observed between the DN and non-DN groups (all P-value < 0.05). The DN group showed higher levels of serum folate and RBC folate ( P < 0.001) and lower levels of ALB, RBC count, and ALT ( P < 0.001).
The univariate logistic regression analysis showed that age, race, RBC folate, serum folate, ALT, ALB, RBC count, hypertension, smoking, and family income were related factors for DN. Non-Hispanic White individuals had an increased risk of DN compared to other races, and higher family income was associated with a decreased likelihood of developing DN. Serum folate, RBC folate, age, hypertension, and smoking were positively correlated with the occurrence of DN. Conversely, ALT, ALB, and RBC count were negatively correlated with the occurrence of DN (refer to Table 2 ).
Multivariate analysis of RBC folate and DN related factors
After multiple imputations were performed to account for missing covariates, we conducted a logistic multiple factor regression analysis to assess the relationship between RBC folate and DN. RBC folate was considered both as continuous and categorical variables. The RBC folate levels were transformed into their logarithmic base 2 values for analysis or categorized by quartiles, with Q1 serving as the reference group. We created four logistic regression models to explore the correlation between RBC folate and DN, and the results pointed to a positive correlation between RBC folate and DN. Table 3 provided a detailed analysis of the relationship between RBC folate and DN, with the effect value expressed as odds ratio (OR) and 95% confidence interval (CI). The effect value can be interpreted as the proportional increase in the risk of DN for each additional unit of RBC folate. For instance, in the unadjusted model 1, where RBC folate was transformed into logarithmic base 2 values, the effect size of 1.57 with a confidence interval of 1.47 to 1.68 suggested a 57% rise in the risk of DN for every additional 2 units of RBC folate. In the slightly adjusted model 2, the effect value of 1.38 (95% CI 1.28 ~ 1.48) implied a 38% increase in the risk of DN for every additional 2 units of RBC folate. In the further adjusted model 3, the effect size of 1.34 (95% CI 1.25 ~ 1.45) indicated a 34% increase in the risk of DN for every additional 2 units of RBC folate. In the fully adjusted model 4, the effect value was 1.38 (95% CI 1.27 ~ 1.49), suggesting that every additional 2 units of RBC folate increased the risk of DN by 38%. These results were statistically significant with a P value of less than 0.001. In the unadjusted model, participants in the highest quartile (Q4) group of RBC folate exhibited a higher risk of DN, with an effect value of 2.11 (95% CI 1.87 ~ 2.38) compared to the Q1 group. Moreover, as RBC folate levels increased, the risk of DN gradually increased. These associations remained statistically significant in all multivariate logistic regression models, even after adjusting for various potential influencing factors. In model 4, compared with Q1, participants in the Q4 group had a higher risk of DN (OR = 1.69, 95% CI 1.47–1.94). The Q2 and Q3 groups also showed significantly higher risk of DN ( P for trend < 0.001). In all models, the rise in RBC folate was consistently associated with an increased risk of DN, as indicated by a consistent trend test, with P for trend < 0.001 (Table 3 ). To ensure the stability of the results, subgroup analyses were conducted. Additionally, smooth fitting curves of RBC folate and DN were plotted to verify the relationship between the two variables. After adjusting for potential confounding factors based on clinical consensus, if the influencing factors changed by more than 10%, RBC folate can be considered to have a strong positive correlation with the incidence of DN.
Model1:Non-adjusted.
Model2: Adjusted for age, sex, race. family poverty income ratio, and education level.
Model3: Adjusted for Model2 + BMI, hypertension, alcohol using, smoking.
Model4: Adjusted for Model3 + ALT, ALB, GGT, TP, Cholesterol, HDL, dietary folate intake, dietary folate supplement intake, VitaminB12, RBC count.
Subgroup Analysis and Curve Fitting
We investigated whether there were variations in age, BMI, race, gender, education level, and PIR in relation to RBC folate and DN. The findings revealed that the association between RBC folate and DN remained consistent in all subgroups (Fig. 2 ), and there was no significant interaction ( P > 0.05). This suggests that the relationship between RBC folate and DN was not influenced by demographic or clinical characteristics, such as age, BMI, race, gender, education level, and PIR. These results support the idea that RBC folate may serve as a reliable biomarker for identifying individuals at risk of DN, regardless of these factors. Furthermore, to better understand the link between RBC folate and DN, fitting curves of RBC folate and DN were plotted after adjusting according to model 4 (Fig. 3 ). The results showed a linear relationship between RBC folate and DN (P for non-linearity = 0.147). This indicates that RBC folate was positively correlated with DN.
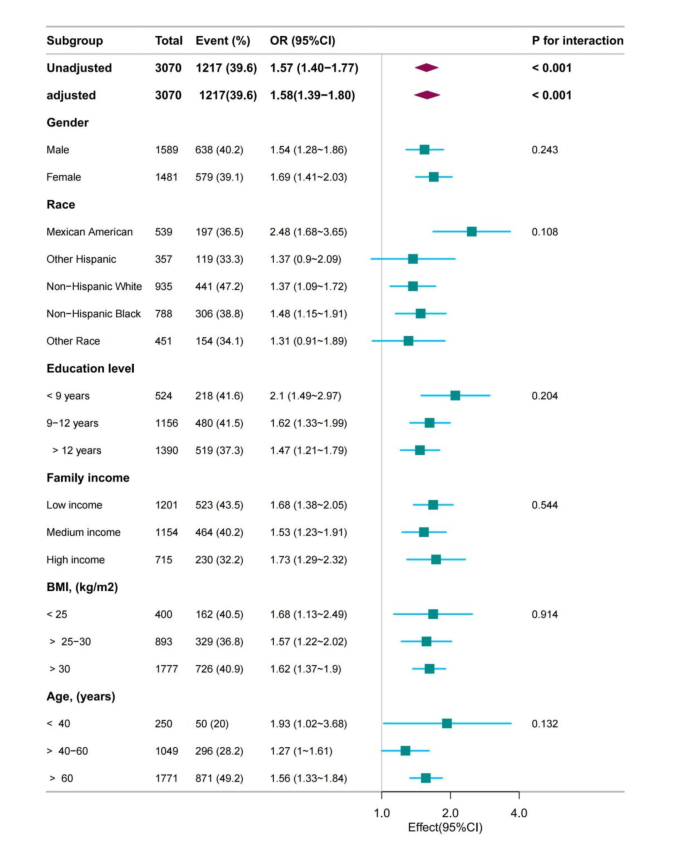
Forest plot of RBC folate and DN.
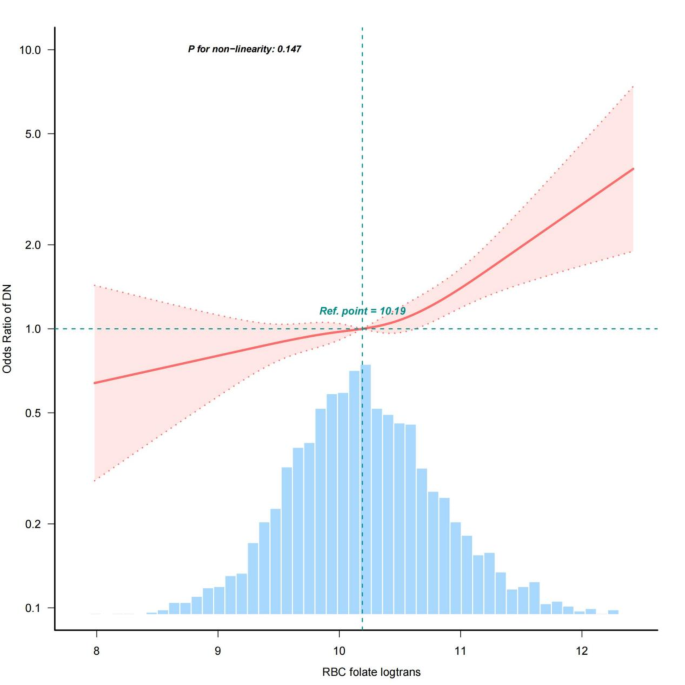
Fitting curves of RBC folate and DN after adjustment according to model 4.
This extensive cross-sectional study of Type 2 Diabetes Mellitus utilized the NHANES 2011–2018 database to demonstrate a correlation between RBC folate concentrations and Diabetic Nephropathy (DN) in a nationally representative sample of the US population. The investigation aimed to explore the association between RBC folate levels and the occurrence of DN in a large, diverse population. The study findings suggested that RBC folate levels were positively correlated with the risk of DN, indicating that homeostatic changes in kidney function among patients with diabetes mellitus have a significant impact on RBC folate concentrations. Even after adjusting for demographic variables and other relevant factors, such as dietary folate intake, dietary supplement folate intake, levels of serum vitamin B12, and RBC count, which could potentially influence the results, the relationship between the variables remained robust and consistent. Furthermore, the relationship between RBC folate and DN was found to be stable across all subgroups ( P for interaction > 0.05). These observations have significant implications for the current management strategies of Diabetic Nephropathy in Type 2 Diabetes Mellitus. The study suggested that there is a positive relationship between RBC folate and DN ( P < 0.001), and that RBC folate concentrations in DN risk were independent of dietary folate intake, dietary supplement folate intake, levels of serum vitamin B12, and RBC count. The study also shed light on the potential oversight in studies that investigate the effects of elevated levels of RBC folates, which often assume that high folate concentrations indicate high folic acid intake, overlooking the potential impacts of other homeostatic and biological processes, including kidney function. These findings underscore the need to consider the complex interplay of factors influencing RBC folate levels and the risk of DN in individuals with Type 2 Diabetes Mellitus. This differs from the limited reports that have been conducted in the past. Wang A et al. reported that when examining the relationships between kidney function (measured CKD risk) and levels of RBC and serum folate, the levels of RBC and serum folate increased as kidney function declined without increased folic acid intake. 8 Additionally, elevated levels of RBC folate were independently linked to a higher risk of all-cause and cardiovascular disease (CVD) mortality in individuals with diabetes. Conversely, high levels of RBC folate appeared to have a mild protective effect in non-diabetic individuals. 9 However, there are very few studies that have investigated the relation to RBC folate. 21 , 22 , 23 , 24 , 25 Folates are essential as cofactors in metabolic pathways, influencing biological methylation and nucleotide synthesis, significantly impacting overall health and susceptibility to diseases. 26 , 27 , 28 Serum total folate concentration is generally regarded as an indicator of recent folate intake, while RBC folate concentration is viewed as an indicator of long-term folate status. 29 , 30 , 31 , 32 There is a lack of extensive literature explaining the biological mechanism that associates high RBC folate with diabetic nephropathy (DN) risk among patients with type 2 diabetes.
The precise mechanism linking the effect of higher RBC folate and the risk of Diabetic Nephropathy (DN) is still unclear. One potential explanation is the adverse effects of unmetabolized folic acid (UMFA) in the bloodstream, which may disrupt cellular folate uptake and intracellular folate metabolism. UMFA in the blood has been associated with decreased natural killer (NK) cell cytotoxicity, which plays a role in inflammation and insulin resistance, and is hence involved in the development of diabetes mellitus (DM). Additionally, metabolic and homeostatic changes resulting from alterations in kidney function can lead to an increase in RBC folate concentration. 8 , 33 , 34 , 35 , 36 The studies mentioned above suggested a potential link between RBC folate and DN in Type 2 Diabetes Mellitus. Due to the current very limited reports, we emphasize the importance of conducting additional research to explore the potential link between kidney function, RBC folate concentrations, and the effects of unmetabolized folic acid on cellular processes. This could provide valuable insights into the underlying mechanisms and potential implications for conditions such as DM and DN.
It is important to note that previous research was limited by small sample sizes and a restricted number of covariates, which hindered the ability to make broad generalizations about the relationship between the variables under investigation. Future studies should aim to overcome these limitations by using larger and more diverse samples, as well as considering a wider range of potential confounding variables. The NHANES provided us with a unique opportunity to investigate the potential association between RBC folate and DN, as well as to comprehensively assess the level-risk connection between RBC folate and DN, fully adjusted for a large number of covariates and a range of stratified analyses.
The strength of the present study lies in its status as the first large-scale and ethnically diverse population-based investigation to uncover the link between RBC folate and DN in T2DM patients among US adults. Our findings have the potential to contribute to the existing limited literature on the association between RBC folate and DN.
However, our study was limited by its use of data from a cross-sectional survey, which prevented us from establishing a cause-and-effect relationship between RBC folate and DN. Therefore, additional longitudinal studies are necessary to verify and support our findings. Moreover, relying on self-reported data may have resulted in recall bias and potential misclassification of DN. Finally, the study’s narrow focus on a limited sample of US adults raises questions about the generalizability of the findings to other populations. Despite these limitations, our study offered valuable insights into the potential link between RBC folate and DN in individuals with T2DM. Future research should focus on addressing these limitations and delving deeper into this relationship.
Conclusions
In conclusion, our study suggested that a high level of RBC folate was independently associated with the presence of DN in diabetic patients, indicating RBC folate as a potential biomarker for early detection, prevention, and intervention planning for this serious complication. RBC folate might predict the possible existence of DN in patients with T2DM. Furthermore, when examining associations between disease outcomes and factors such as folic acid intake and folate biomarkers, it is crucial to take into account the potential mediating role of kidney function. It is essential for researchers to carefully assess and control for the potential influence of kidney function when investigating these relationships.
Data availability
All data and experimental methods were available for download from the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm .
Demir S, Nawroth PP, Herzig S, et al. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv Sci (Weinh). 2021;8(18):e2100275.
Dixon D, Edmonds M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs. 2021;81(1):29–56.
Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337 − 47
Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018;71(6):884 − 95.
Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124(3):139 − 52.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045.
Wang W, Yang A, Zhang H, et al. Associations of RBC and Serum Folate Concentrations with Cervical Intraepithelial Neoplasia and High-Risk Human Papillomavirus Genotypes in Female Chinese Adults. J Nutr. 2022 ;8;152(2):466 − 74.
Wang, A., Yeung, L. F., Ríos Burrows, N., et al. Reduced Kidney Function Is Associated with Increasing Red Blood Cell Folate Concentration and Changes in Folate Form Distributions (NHANES 2011–2018). Nutrients, 2022,14(5), 1054.
Xiong H, Li X, Cheng S, et al. Folate Status and Mortality in US Adults With Diabetes: A Nationally Representative Cohort Study. Front Cardiovasc Med. 2022;25;9:802247
Wang J, Liu F, Kong R, et al. Association Between Globulin and Diabetic Nephropathy in Type2 Diabetes Mellitus Patients: A Cross-Sectional Study. Front Endocrinol (Lausanne). 2022;8;13:890273.
Yang H, Lin H, Liu X, Liu H, Chen T, Jin Z. Association between dietary fiber intake and diabetic nephropathy among adult diabetes mellitus in the United States: A cross-sectional study. Heliyon. 2024 Apr 23;10(9):e30036.
Yeung, L.F.; Cogswell, M.E.; Carriquiry, A.L, et al. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children:National Health and Nutrition Examination Survey (NHANES), 2003–2006. Am. J. Clin. Nutr. 2011, 93, 172 − 85.
Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; et al. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2012. Br. J. Nutr. 2015, 113, 1965-77.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011 Jan;34 Suppl 1(Suppl 1):S62-9.
Elliott WJ. Systemic Hypertension. Curr probl Cardiol (2007) 32(4):201 − 59.
White GE, Mair C, Richardson GA, Courcoulas AP, King WC. Alcohol Use Among U.S. Adults by Weight Status and Weight Loss Attempt: NHANES, 2011–2016. Am J Prev Med. 2019;57(2):220 − 30.
Ricci C, Schutte AE, Schutte R, et al. Trends in Alcohol Consumption in Relation to Cause-Specific and All-Cause Mortality in the United States: A Report From the NHANES Linked to the US Mortality Registry. Am J Clin Nutr (2020) 111(3):580–9.
Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA (2016) 315(21):2284–91.
Mortada I. Hyperuricemia, Type 2 Diabetes Mellitus, and Hypertension: An Emerging Association. Curr hypertens Rep (2017) 19(9):69.
Liu, H., Wang, L., Chen, C., et al. Association between Dietary Niacin Intake and Migraine among American Adults: National Health and Nutrition Examination Survey. Nutrients, 2022,14(15), 3052.
Shen M, Chaudhry SH, MacFarlane AJ, et al. Serum and red-blood-cell folate demonstrate differential associations with BMI in pregnant women. Public Health Nutr. 2016 Oct;19(14):2572-9.
Colapinto CK, O’Connor DL, Sampson M, et al. Systematic review of adverse health outcomes associated with high serum or red blood cell folate concentrations. J Public Health (Oxf). 2016;38(2):e84-97
Bird JK, Ronnenberg AG, Choi SW, et al. Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J Nutr. 2015 Jan;145(1):79–86.
Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr. 2011 Jul;94(1):303S-312S.
Liwinski, T., & Lang, U. E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients, 2023;15(17), 3859.
Xie K, Xu P, Fu Z, et al. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci Nutr. 2019 ;18;7(11):3759-65.
Hung J, Beilby JP, Knuiman MW, et al. Folate and vitamin B-12 and risk of fatal cardiovascular disease: cohort study from Busselton, Western Australia. BMJ. 2003;326:131.
Kyte B, Ifebi E, Shrestha S, et al. High red blood cell folate is associated with an increased risk of death among adults with diabetes, a 15-year follow-up of a national cohort. Nutr Metab Cardiovasc Dis. 2015;25:997–1006.
Cho CE, Sánchez-Hernández D, Reza-López SA, et al. Anderson GH. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics. 2013;8(7):710-9.
Yajnik, C. S., Deshpande, S. S., Jackson, A. A., et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune maternal nutrition study. Diabetologia, 2008, 51(1), 29–38.
Marchetta CM, Hamner HC. Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001–2010. Matern Child Nutr. 2016 Jan;12(1):39–50
Mahabir S, Ettinger S, Johnson L, et al. Measures of adiposity and body fat distribution in relation to serum folate levels in postmenopausal women in a feeding study. Eur J Clin Nutr 2008;62:644–50
Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol 2004;19:1029–36.
Twum F, Morte N, Wei Y, et al. Red blood cell folate and cardiovascular deaths among hypertensive adults, an 18-year follow-up of a national cohort. Hypertens Res. (2020) 43:938–12.
Peng Y, Wang Z. Red blood cell folate concentrations and coronary heart disease prevalence: a cross-sectional study based on 1999–2012 National Health and Nutrition Examination Survey. Nutr Metab Cardiovasc Dis. 2017;27:1015–20.
Chen MY, Rose CE, Qi YP, et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019,1;109(5):1452-61.
Download references
Author information
Authors and affiliations.
Department of Laboratory Medicine, Tongji Shanxi Hospital, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Third Hospital of Shanxi Medical University, Taiyuan, 030032, China
Peixia Yu & Hairu wang
Department of Otolaryngology Head and Neck Surgery, Shanxi Medical University Second Affiliated Hospital, Taiyuan, 030001, China
Department of Clinical Laboratory, Affiliated Hospital of Hebei Engineering University, Handan, 056002, China
You can also search for this author in PubMed Google Scholar
Contributions
Peixia Yu contributed to the design of our study, and wrote the manuscript. Keyu Liu contributed to the design of our study, data analysis and interpretation. Yongjin Ji, and Hairu wang were responsible for the management and retrieval of data. All authors reviewed the manuscript and met the authorship of ICMJE criteria.
Corresponding author
Correspondence to Keyu Liu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Informed consent
The NCHS Ethics Review Board has given approval for the NHANES research protocol, and all participants have given their written informed consent.
Study or trial registration: The study was registered in a registry with the registration number #2011-17, and data collection began in January 2011.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, P., Ji, Y., wang, H. et al. Association between RBC folate and diabetic nephropathy in Type2 diabetes mellitus patients: a cross-sectional study. Sci Rep 14 , 24692 (2024). https://doi.org/10.1038/s41598-024-76456-0
Download citation
Received : 02 June 2024
Accepted : 14 October 2024
Published : 21 October 2024
DOI : https://doi.org/10.1038/s41598-024-76456-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetic nephropathy
- Type2 diabetes mellitus
- Serum folate
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Therapeutic Renin Inhibition in Diabetic Nephropathy—A Review of the Physiological Evidence
Bianca domingues massolini, stephanie san gregorio contieri, giulia severini lazarini, paula antoun bellacosa, mirela dobre, georg petroianu, andrei brateanu, luciana aparecida campos, ovidiu constantin baltatu.
- Author information
- Article notes
- Copyright and License information
Edited by: Joaquin Garcia-Estañ, University of Murcia, Spain
Reviewed by: Carlos P. Vio, Pontifical Catholic University of Chile, Chile; Bruno Vogt, University of Bern, Switzerland
*Correspondence: Ovidiu Constantin Baltatu, [email protected] ; [email protected]
† These authors have contributed equally to this work
This article was submitted to Integrative Physiology, a section of the journal Frontiers in Physiology
Received 2019 Nov 18; Accepted 2020 Feb 19; Collection date 2020.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
The purpose of this systematic review was to investigate the scientific evidence to support the use of direct renin inhibitors (DRIs) in diabetic nephropathy (DN). MEDLINE was searched for articles reported until 2018. A standardized dataset was extracted from articles describing the effects of DRIs on plasma renin activity (PRA) in DN. A total of three clinical articles studying PRA as an outcome measure for DRIs use in DN were identified. These clinical studies were randomized controlled trials (RCTs): one double-blind crossover, one post hoc of a double-blind and placebo-controlled study, and one open-label and parallel-controlled study. Two studies reported a significant decrease of albuminuria associated with PRA reduction. One study had a DRI as monotherapy compared with placebo, and two studies had DRI as add-in to an angiotensin II (Ang II) receptor blocker (ARB). Of 10,393 patients with DN enrolled in five studies with DRI, 370 (3.6%) patients had PRA measured. Only one preclinical study was identified that determined PRA when investigating the effects of aliskiren in DN. Moreover, most of observational preclinical and clinical studies identified report on a low PRA or hyporeninemic hypoaldosteronism in DM. Renin inhibition has been suggested for DN, but proof-of-concept studies for this are scant. A small number of clinical and preclinical studies assessed the PRA effects of DRIs in DN. For a more successful translational research for DRIs, specific patient population responsive to the treatment should be identified, and PRA may remain a biomarker of choice for patient stratification.
Keywords: diabetes mellitus, diabetic nephropathy, renin inhibitor, plasma renin activity, renin- angiotensin system
Introduction
Diabetic nephropathy (DN) is the primary cause of chronic kidney disease. Despite therapeutic advances, DN remains the principal cause of mortality in diabetic patients ( Dugbartey, 2017 ).
Renin–angiotensin system (RAS) has been classically involved in the progression of diabetic cardiovascular disease. A chronically activated endocrine or paracrine RAS is considered as a principal contributor to the pathophysiology of end-organ damage in diabetes mellitus (DM), including the DN ( Urushihara and Kagami, 2017 ). As a result, therapeutic drugs for DN are targeting mostly the renin–angiotensin–aldosterone system ( Yacoub and Campbell, 2015 ).
Although debate remains, the therapeutic drugs for DN currently consist mainly of angiotensin II (Ang II) receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) used for their antihypertensive and antiproteinuric measures ( Bhattacharjee et al., 2016 ). Direct renin inhibitors (DRIs) acting on rate-limiting enzyme of the RAS offered probability of a greater inhibition of the system so as to have better therapeutic outcomes in patients with DN ( Parving et al., 2008 ). The rationale for developing renin inhibitors was as follows: renin is the first and rate-limiting step in RAS cascade (low renin concentration in the pM range), renin has high specificity for angiotensinogen (little side effects anticipated), ACEIs and ARBs result in incomplete RAS suppression [reactive rise in plasma renin activity (PRA), “escape” mechanism, and other products of RAS (e.g., Ang1–7, AIII, and AIV)] ( Wood and Close, 1996 ; Nussberger et al., 2002 ; Stanton, 2003 ). However, larger trials of the DRI aliskiren in combination with an ACE inhibitor or ARB in patients with DN did not reduce cardiovascular or renal outcomes ( Parving et al., 2012 ).
Plasma renin activity played a central role as a pharmacological biomarker for drug development, safety, and dosing in the research and development (R&D) of DRIs such as remikiren, enalkiren, zankiren, and aliskiren. Generally, DRIs induced rapid reductions of 65–95% PRA ( Lambers Heerspink et al., 2009 ). PRA has been used in the estimation of the extracellular volume, because this correlates inversely with PRA ( Juncos, 2013 ). Hence, Brunner et al. (1972) categorized hypertensive patients by their volume status using PRA levels. Augmented PRA levels represent a risk factor of cardiovascular disease ( Alderman et al., 1991 ; Parving et al., 2009 ). Whereas several pathologies are associated with an augmented PRA, DM and associated DN apparently are not.
The therapeutic effects of RAS inhibitors may be important depending on the pathological activation of endocrine and/or tissue RAS ( Gasparo et al., 2013 ; de Alencar Franco Costa et al., 2015 ). For instance, disease conditions with low baseline PRA levels reduced the treatment efficacy of DRIs ( Stanton et al., 2009 ). Few reviews on DRI for DN as therapeutic target discussed PRA as an outcome measure ( Abassi et al., 2009 ; Rafiq et al., 2011 ; Jagadeesh et al., 2012 ). These reviews document that PRA is reduced in DM with or without DN.
Early studies described hyporeninemia or low-renin state as a characteristic state of circulatory RAS in DM patients with or without DN ( Sousa et al., 2016 ). Our and other studies ( de Alencar Franco Costa et al., 2015 ; Sousa et al., 2016 ) evidencing a diabetes-induced low-renin status may indicate that a DRI is not always effective in treating DN. Therefore, the purpose of this study was to examine and synthesize the existing literature on DRI effects on PRA in DN. Literature search included studies that investigated DRIs such as remikiren, enalkiren, zankiren, or aliskiren in DN.
Literature Search Strategy
We conducted a systematic review of investigative studies in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) consensus guidelines ( Moher et al., 2016 ). A literature search of the MEDLINE database via PubMed was performed using a structured approach to identify relevant studies. A manual search was also conducted through searching the reference lists of relevant articles to expand the included studies. Eligibility assessment of identified articles was performed independently by two reviewers for preclinical studies and two reviewers for clinical studies, and inconsistencies were settled by one of the senior reviewers.
Inclusion Criteria
To identify relevant articles on original research, we associated terms referring to the use of PRA and/or renin inhibitors in DN. All experimental studies on humans and animals were eligible. Document types included were those produced as original and review papers written in English and published until 2018. The following Medical Subject Headings (MeSH) were used in the search: DN OR “diabetic kidney disease” AND “renin inhibitor” OR “aliskiren” OR “remikiren” OR “enalkiren” OR “zankiren.” We used filters to select the type of study, and we gathered data from clinical trials and from case–control and cohort studies and reviews, designed to assess the effects of DRIs on PRA in DN.
Exclusion Criteria
Articles were excluded if they were clinical case reports, clinical case series, letters, editorials, opinions, points of views, or anecdotes. Also, articles that were written in languages other than English were discarded.
Data Extraction and Quality Assessment
Four investigators evaluated independently titles and abstracts and selected the articles for further full-text evaluation. Disagreements were resolved by consensus or by consultation with one of the senior investigators. When data were not found in the published article, authors were contacted to provide the missing information. The following data were collected: title, author and study group, publication year, DN, DRI, and PRA.
Search Results and Study Selection
Figure 1 details the search and selection process of articles that determined (or discussed in case of reviews) PRA when investigating DRI effects in DN. Of 920 potentially relevant papers initially identified through the PubMed search, after de-duplication, we reviewed 918 titles and abstracts; from these, we included 878 in a full-text review. A further 873 articles were excluded after full review, and five were included in the present study: one preclinical study and four clinical studies.
PRISMA Flow Diagram.
Clinical Studies With Direct Renin Inhibitor in Diabetic Nephropathy That Determined Plasma Renin Activity
Three clinical studies have been identified to have reported effects of DRI (aliskiren) on PRA in DN ( Table 1 ). Two studies reported a significant decrease of albuminuria associated with PRA reduction ( Persson et al., 2009 ; Abe et al., 2012 ). The post hoc analysis of ALTITUDE study ( Parving et al., 2012 ) in a subset of 133 patients reported a non-significant reduction of urinary albumin creatinine of 22 and 9% in the aliskiren and placebo groups, respectively ( Persson et al., 2012a ).
Clinical Studies with DRI in diabetic nephropathy that determined plasma renin activity.
| Double-blind, randomized, crossover trial | Aliskiren, irbesartan, and aliskiren/irbesartan, 2-month treatment | 26 patients with T2DM, HTN, and albuminuria (>100 mg/day) | Significant reduction in urinary albumin, glomerular filtration rate, and 24-h blood pressure from placebo | 72%↓ as monotherapy compared with placebo | |
| AVOID analysis | Add-in: aliskiren or placebo in addition to losartan, 6-month treatment | Patients with HTN and T2DM with nephropathy: a prespecified subset of 133 (22%) patients from a total of 599 patients | Not significant reduction in urinary albumin–creatinine ratio | 71%↓ compared with placebo (90%↓ compared with baseline; placebo: 19%↓) | |
| Open-label, randomized, parallel-controlled study | Add-in: aliskiren or placebo in addition to telmisartan and amlodipine, 6-month treatment | 64 patients with T2DM, DN, and HTN | Significant reduction in urinary albumin–creatinine ratio | 70–77%↓ compared with baseline; 89%↓ compared with calcium channel blocker (CCB) group |
T2DM, type 2 diabetes mellitus; DN, diabetic nephropathy; HTN, hypertension; PRA, plasma renin activity; DRI, direct renin inhibitor.
One double-blind, randomized study that investigated the effect of aliskiren as monotherapy in patients with DM and hypertension (HTN) reported a decrease of 72% in PRA ( Persson et al., 2009 ). Two other studies that investigated aliskiren or placebo in addition to an ARB [one a post hoc analysis ( Persson et al., 2012a ) and the other an open-label, randomized study ( Abe et al., 2012 )] reported a PRA decrease of 71–77%. The data from the studies were heterogeneous and not sufficient to carry out a quantitative analysis. There were not enough data in two studies ( Abe et al., 2012 ; Persson et al., 2012a ), the reported PRA data had a skewed distribution in one study ( Persson et al., 2009 ), and there was no blindness in one study ( Abe et al., 2012 ). In addition, in one study, PRA was determined only in a subset of patients from the total investigated in the aliskiren group: 22% (133 of 599) patients in the ( Persson et al., 2012a ) study.
Of 10,393 patients with DN enrolled in five studies [599 in Parving et al. (2008) ; 26 in Persson et al. (2009) ; 8,561 in the ALTITUDE study ( Parving et al., 2012 ); 64 in Abe et al. (2012) ; 1,143 in the VIvID study ( Bakris et al., 2013 )], 370 (3.6%) patients had PRA measured ( Persson et al., 2009 , 2012a ; Abe et al., 2012 ).
Preclinical Studies With Direct Renin Inhibitor in Diabetic Nephropathy That Determined Plasma Renin Activity
One preclinical proof-of-concept study testing the effects of aliskiren in DN determined PRA ( Table 2 ). This study used as model for DM the streptozotocin (STZ)-induced DM in C57BL/6J mice fed on a high-fat diet, determined PRA, and found higher levels in DN when compared with the control non-DN ( Kidokoro et al., 2016 ). In Table 2 are included articles that reported renal renin outcome measures, including plasma renin concentration and renin mRNA expression.
Preclinical studies with DRI in diabetic nephropathy that determined plasma renin or renal RAS.
| db/db mice, with obesity and T2DM | Aliskiren, 6 weeks’ treatment | Protects against cardiovascular complications and pancreatic injury | Renal renin mRNA not different than that of control db/m mice | Increased renal renin mRNA expression | |
| db/db mice with obesity and T2DM | Aliskiren, 6 weeks’ treatment | Protects against DN | Renal renin mRNA higher than that in control db/m mice | Increased renal renin mRNA expression | |
| db/db mice with obesity and T2DM | Aliskiren, 3 months’ treatment | Decreased albuminuria, glomerulosclerosis, interstitial fibrosis, improved insulin resistance | Lower plasma renin concentration (PRC) in db/db mice than in db/m mice (control non-DM) | Increased PRC | |
| STZ-DBA/2J mice fed on a high-fat diet | Aliskiren, 6 weeks’ treatment | Protects against DN | Renal renin mRNA in DN higher than that in control non-DN | Increased renal renin mRNA expression | |
| db/db mice, with obesity and T2DM + uninephrectomy | Aliskiren, 4 weeks’ treatment | Protects against DN | PRC normal, renal renin mRNA higher than that in control non-DN | Increased PRC and renal renin mRNA expression | |
| STZ-C57BL/6J mice fed on a high-fat diet | Aliskiren, 4 weeks’ treatment | Protects against DN | PRA and imaging of renal renin activity higher than that in control non-DN | Decreased PRA and imaging of renal renin activity |
T2DM, type 2 diabetes mellitus; DRI, direct renin inhibitor; RAS, renin–angiotensin system; DN, diabetic nephropathy.
The present study shows that a low number of preclinical and clinical studies with DRIs as monotherapy or add-in therapy in DN assessed PRA. Only two randomized controlled studies reported renoprotective effects in DN associated with a significant reduction in PRA.
Of eight publications identified to report DRI effects on urinary albumin in DN ( Parving et al., 2008 , 2012 ; Persson et al., 2009 , 2010 , 2011 , 2012a , b ; Abe et al., 2012 ), only three clinical studies presented data on PRA. All three involved patients with both DM and HTN. As aliskiren does not lower blood pressure in hypertensive patients with low PRA ( Sealey and Laragh, 2009 ), Jagadeesh et al. (2012) suggested that “it may be useful to dichotomize RAAS-related pathologic syndromes into ones associated with high renin (some HTN, any HTN after diuretic treatment), where aliskiren appears to be quite effective, and low-renin” (like diabetes), where aliskiren is of uncertain value. Indeed, in the AVOID study ( Parving et al., 2008 ), a prespecified subset of 133 (22%) patients from a total of 599 patients was identified with a significant decrease in PRA by aliskiren ( Persson et al., 2012a ). The study of Uresin et al. (2007) that reported a significant reduction in 24-h ambulatory blood pressure presented PRA data on 32% of the total patients recruited in the DRI study group. As such, important information that could lead to patient stratification could be learned from disclosing the PRA data from the ALTITUDE study ( Parving et al., 2012 ).
Dual therapy of the DRI aliskiren with ACEI or ARBs was commonly investigated in patients with HTN, heart failure, and diabetes with or without proteinuria. It is conceptualized that the antihypertensive efficacy of aliskiren is increased when adding ACEIs, ARBs, or diuretics, which produce a reactive increase in PRA. Indeed, aliskiren in combination with ACEIs or ARBs showed significant blood pressure and proteinuria reductions than monotherapy alone in phase II trials with hypertensive patients with or without DM ( Persson et al., 2009 ), reviewed by Şen et al. (2013) . Clinical trials that studied the combination of aliskiren and ACEI or ARBs and involved patients with DM include Pool 2007, ALOFT 2008, ALLAY 2009, AVANTE GARDE 2010, VANTAGE 2010, and ASPIRE 2011. These studies were systematically reviewed by Harel et al. (2012) . They were not designed to investigate outcomes in DM patients. A meta-analysis of 13,395 patients with diabetes showed no benefit from the addition of aliskiren to standard medical therapy ( Zheng et al., 2017 ).
Finding a preclinical experimental model for DM and DN was challenged by the high selectivity of aliskiren for human renin compared with renin from other species (IC50 values [50% inhibitory concentrations]: human 0.6, marmoset 2, rat 80, dog 7) ( Wood et al., 2003 ). The first studies of DRI on an experimental DM model used the STZ-induced DM in high-renin hypertensive (mRen-2)27 rats ( Kelly et al., 2007 ; Feldman et al., 2008 ). However, this DM model cannot consider the phenotype alterations as primarily induced by DM because these rats genetically activated renin production. Four studies reported DRI effects on DN of db/db mice ( Table 2 ). Another study on db/db mice showed no significant differences in their PRA compared to control db/m mice ( Gallo et al., 2016 ).
Experimental models investigated for proof-of-concept efficacy of aliskiren in DN were db/db mice for type 2 DM (T2DM) and STZ mice (DBA/2J and C57BL/6J strains) for type 1 DM (T1DM). The db/db mice are characterized by T2DM, elevated systolic blood pressure, obesity, and hyperlipidemia. They develop T2DM with high plasma levels of insulin and glucose at weeks 9–10 of age ( Forbes and Cooper, 2013 ). The main outcomes studied for DN, including increased albumin excretion and glomerular pathology, are very similar between mouse lines with T1DM or T2DM and humans with DM ( Azushima et al., 2017 ).
Locally activated synthesis and activity of renin have been identified in kidney and other organs in different pathologies ( Bader et al., 2001 ). Such organs where a local tissue RAS has been postulated include the heart, blood vessels, kidney, brain, adipose tissue, adrenal gland, pancreas, liver, reproductive system, lymphatic tissue, placenta, and eyes ( Nehme et al., 2019 ). Diseases where chronically activated local tissue RAS has been identified include HTN, atherosclerosis, heart failure, cardiac hypertrophy and fibrosis, chronic kidney disease, and glaucoma ( Ames et al., 2019 ; Nehme et al., 2019 ). An increase in local production of active angiotensins could be through the classical renin–ACE pathway or through alternative pathways ( Ferrario et al., 2014 ). Translational proof-of-concept studies shall distinguish the enzymes involved in these RAS pathways in order to identify therapeutic targets. For instance, we have demonstrated in a proof-of-concept study that renin might be a therapeutic target in glaucoma ( Wang et al., 2012 ). This does not seem to be the case in DN where both preclinical and clinical proof-of-concept studies indicate that DN is associated with a low-renin state. In Table 3B , we summarized the clinical studies that described hyporeninemia in DM, with the first report dated year 1973. These studies indicate that hyporeninemic hypoaldosteronism is underdiagnosed in DM ( Sousa et al., 2016 ). Several mechanisms have been suggested as responsible for the reduction in renin release in patients with DM, including juxtaglomerular injury, autonomic dysfunction, and primary increase in renal salt retention with volume expansion ( Phelps et al., 1980 ; Sousa et al., 2016 ). The first experimental studies of RAS in DM used alloxan-DM or STZ-DM rat models ( Table 3A ). Preclinical evidence for an activated renal RAS in DM is suggested by our and others studies on increased synthesis and urinary secretion of renal angiotensinogen ( Zimpelmann et al., 2000 ; Saito et al., 2009 ; de Alencar Franco Costa et al., 2015 ; Lee et al., 2017 ) ( Figure 2 shows urinary angiotensinogen as a potential biomarker). STZ-induced DM in rats caused a 69% increase of Ang II in the renal interstitial fluid, which was decreased 27% by aliskiren (6 weeks’ treatment) ( Matavelli et al., 2012 ). As aliskiren did not normalize the DM-increased renal interstitial fluid Ang II, alternative Ang II-forming pathways might have been activated. One candidate enzyme that may take over the renin activity in kidney to activate the local Ang II production is cathepsin L. Cathepsin L was identified as a potential sex-specific biomarker for renal damage by the Actelion group ( Bauer et al., 2011 ). Cathepsin L appears to be importantly involved in the development of albuminuria and renal damage in early experimental DN ( Garsen et al., 2016 ) ( Figure 2 ). Angiotensinogen may be degraded by cathepsins including cathepsin L, which may degrade angiotensinogen ( Watanabe et al., 1989 ). As cathepsin L can be involved in the pathogenesis of DN through several mechanisms, targeting with suitable antagonists may hold promises for therapeutic interventions ( Kumar and Anders, 2016 ). PRA is not a good indicator of local RAS activity as measure circulating production of angiotensin I, where ACE, alternative Ang II-forming enzymes, and Ang II might be increased in the local tissue. Although PRA is a pharmacological efficacy biomarker for aliskiren and has been considered as an outcome measure in DM, the effects of renin inhibitors on local tissue RAS are not easily demonstrable in clinical studies because there are no available biomarkers for local RAS activation. Studies on urinary peptidome might lead to the characterization of biomarkers for local renal RAS activation, such as cathepsin L or D ( Krochmal et al., 2017 ).
Clinical studies on low PRA in DN.
| One patient with DM | Hyporeninemic hypoaldosteronism | |
| Two patients with DM | Hypoaldosteronism due to low PRA | |
| 31 Patients with T1DM | Intrarenal RAS activated without PRA | |
| 80 Patients with T1DM | Low PRA and high plasma ANP | |
| One patient with DM | Hyporeninemic, hypoaldosteronism, and autonomic neuropathy | |
| One patient with DM | Hyporeninemic hypoaldosteronism | |
| 118 Patients with DM | Hyporeninemic selective hypoaldosteronism may be associated with DM nephropathy or DM neuropathy | |
| 100 Teenage patients with T1DM | Decline of PRA over 5 years | |
| 13 Patients with DM and chronic renal failure | Hyporeninemic hypoaldosteronism associated with type IV renal tubular acidosis | |
| 12 Patients with DM | Low PRA and active renin (AR) | |
| 16 Normotensive diabetics with long-term disease | Hyporeninemia | |
| Four patients with DM | Diabetic hyporeninemic hypoaldosteronism | |
| Five patients with DM with mild renal insufficiency | Hyporeninemic hypoaldosteronism associated with DM and neuropathy may be due to decreased sympathetic nervous system activity | |
| Three patients with DM | Hyporeninemic hypoaldosteronism | |
| 44 Patients with DM | Hyporeninemic hypoaldosteronism is frequent in diabetics with nephropathy | |
| 48 Patients with DM | (1) PRA is normal in normotensive diabetics (2) Patients with diabetes, hypertension, and nephropathy have “low renin hypertension” | |
| Eight patients with DM | Low PRA | |
| 60 Patients with DM | Low PRA | |
| 12 Patients with DM | Hyporeninemia and hypoaldosteronism | |
| Four patients with DM | Low PRA |
PRA, plasma renin activity; DN, diabetic nephropathy; DM, diabetes mellitus; RAS, renin–angiotensin system; ANP, atrial natriuretic peptide; T1DM, type 1 diabetes mellitus.
Preclinical studies that determined PRA in experimental DM.
| Alloxan-DM rat, acute DM (alloxan is nephrotoxic) | Low PRA | |
| Alloxan-DM rat, 3 months | PRA decreased progressively | |
| STZ-DM rat, 1 month | PRC values in untreated DM rats were lower than those of insulin-treated rats or controls | |
| Alloxan-DM rat, 7 weeks | Low PRA | |
| STZ-DM rat, 1.5 months | Hyporeninemic hypoaldosteronism | |
| STZ-DM rat, 2 months | Hyporeninemic hypoaldosteronism | |
| Alloxan-DM rat, 1 month | Low PRA, glucose overload did not significantly affect these values | |
| STZ-DM rat, 3 months | Low PRA, PRC, and renal renin mRNA |
PRA, plasma renin activity; DN, diabetic nephropathy; DM, diabetes mellitus; STZ, streptozotocin.
Renal renin-angiotensin system in diabetes mellitus. AOGEN, angiotensinogen; ACE, angiotensin-converting enzyme; Ang, angiotensin; AT1, angiotensin type 1 receptor.
Very few studies addressed the PRA as the outcome measure of DRI treatment effect in DN. Therefore, for a more successful translational research, specific patient population where DRI treatment is effective in DN should be identified. Additional well-designed randomized controlled trials (RCTs) using PRA as a marker for patient stratification and randomization may be warranted.
Author Contributions
OB and LC contributed to the conception and design of the study. MD, AB, GP, OB, and LC analyzed and interpreted the data. MD, AB, GP, OB, and LC drafted the manuscript. All authors provided critical revision of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. BM, SC, GL, and PB received institutional fellowship for this study. OB is supported by the National Council for Scientific and Technological Development (CNPq, 307760/2018-9). This publication is based upon work supported by the Khalifa University of Science and Technology under Award No. FSU-2020-33 to OB.
- Abassi Z., Winaver J., Feuerstein G. Z. (2009). The biochemical pharmacology of renin inhibitors: implications for translational medicine in hypertension, diabetic nephropathy and heart failure: expectations and reality. Biochem. Pharmacol. 78 933–940. 10.1016/j.bcp.2009.05.018 [ DOI ] [ PubMed ] [ Google Scholar ]
- Abe M., Maruyama N., Suzuki H., Fujii Y., Ito M., Yoshida Y., et al. (2012). Additive renoprotective effects of aliskiren on angiotensin receptor blocker and calcium channel blocker treatments for type 2 diabetic patients with albuminuria. Hypertens. Res. 35 874–881. 10.1038/hr.2012.45 [ DOI ] [ PubMed ] [ Google Scholar ]
- Alderman M. H., Madhavan S., Ooi W. L., Cohen H., Sealey J. E., Laragh J. H. (1991). Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N. Engl. J. Med. 324 1098–1104. 10.1056/NEJM199104183241605 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ames M. K., Atkins C. E., Pitt B. (2019). The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 33 363–382. 10.1111/jvim.15454 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Antonipillai I., Tan S. Y., Suzuki S., Franco-Saenz R., Mulrow P. J. (1981). Active and inactive renin in low renin states: studies in human plasma. J. Clin. Endocrinol. Metab. 53 694–697. 10.1210/jcem-53-4-694 [ DOI ] [ PubMed ] [ Google Scholar ]
- Azushima K., Gurley S. B., Coffman T. M. (2017). Modelling diabetic nephropathy in mice. Nat. Rev. Nephrol. 14 48–56. 10.1038/nrneph.2017.142 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bader M., Peters J., Baltatu O., Müller D. N., Luft F. C., Ganten D. (2001). Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J. Mol. Med. 79 76–102. 10.1007/s001090100210 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bakris G. L., Oparil S., Purkayastha D., Yadao A. M., Alessi T., Sowers J. R. (2013). Randomized study of antihypertensive efficacy and safety of combination aliskiren/valsartan vs valsartan monotherapy in hypertensive participants with type 2 diabetes mellitus. J. Clin. Hypertens. 15 92–100. 10.1111/jch.12032 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ballermann B. J., Skorecki K. L., Brenner B. M. (1984). Reduced glomerular angiotensin II receptor density in early untreated diabetes mellitus in the rat. Am. J. Physiol. 247 F110–F116. 10.1152/ajprenal.1984.247.1.F110 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bauer Y., Hess P., Qiu C., Klenk A., Renault B., Wanner D., et al. (2011). Identification of Cathepsin L as a Potential Sex-Specific Biomarker for Renal Damage. Hypertension 57 795–801. 10.1161/HYPERTENSIONAHA.110.157206 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bhattacharjee N., Barma S., Konwar N., Dewanjee S., Manna P. (2016). Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur. J. Pharmacol. 791 8–24. 10.1016/j.ejphar.2016.08.022 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bojestig M., Nystrom F. H., Arnqvist H. J., Ludvigsson J., Karlberg B. E. (2000). The renin-angiotensin-aldosterone system is suppressed in adults with Type 1 diabetes. JRAAS J. Renin Angiotensin Aldoster. Syst. 1 353–356. 10.3317/jraas.2000.065 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bonnet F., Thivolet C. H. (1996). Reversible hyperkalemia at the initiation of ACE inhibitors in a young diabetic patient with latent hyporeninemic hyporeninemic hypoaldosteronism. Diabetes Care 19 781. 10.2337/diacare.19.7.781a [ DOI ] [ PubMed ] [ Google Scholar ]
- Brunner H. R., Laragh J. H., Baer L., Newton M. A., Goodwin F. T., Krakoff L. R., et al. (1972). Essential hypertension: renin and aldosterone, heart attack and stroke. N. Engl. J. Med. 286 441–449. 10.1056/NEJM197203022860901 [ DOI ] [ PubMed ] [ Google Scholar ]
- Chelaghma N., Oyibo S. O. (2018). Hyporeninemic hypoaldosteronism in a patient with diabetes mellitus: an unforgettable case report. Int. Med. Case Rep. J. 11 69–72. 10.2147/IMCRJ.S158628 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Christlieb A. R. (1974). Renin, angiotensin, and norepinephrine in alloxan diabetes. Diabetes 23 962–970. 10.2337/diab.23.12.962 [ DOI ] [ PubMed ] [ Google Scholar ]
- Christlieb A. R., Kaldany A., D’Elia J. A. (1976). Plasma renin activity and hypertension in diabetes mellitus. Diabetes 25 969–974. 10.2337/diab.25.10.969 [ DOI ] [ PubMed ] [ Google Scholar ]
- Christlieb A. R., Kaldany A., D’Elia J. A., Williams G. H. (1978). Aldosterone responsiveness in patients with diabetes mellitus. Diabetes 27 732–737. 10.2337/diab.27.7.732 [ DOI ] [ PubMed ] [ Google Scholar ]
- Christlieb A. R., Long R., Underwood R. H. (1979). Renin-angiotensin-aldosterone system, electrolyte homeostasis and blood pressure in alloxan diabetes. Am. J. Med. Sci. 277 295–303. 10.1097/00000441-197905000-00008 [ DOI ] [ PubMed ] [ Google Scholar ]
- Christlieb A. R., Munichoodappa C., Braaten J. T. (1974). Decreased response of plasma renin activity to orthostasis in diabetic patients with orthostatic hypotension. Diabetes 23 835–840. 10.2337/diab.23.10.835 [ DOI ] [ PubMed ] [ Google Scholar ]
- de Alencar Franco Costa D., Todiras M., Campos L. A. A., Cipolla-Neto J., Bader M., Baltatu O. C. C. (2015). Sex-dependent differences in renal angiotensinogen as an early marker of diabetic nephropathy. Acta Physiol. 213 740–746. 10.1111/apha.12441 [ DOI ] [ PubMed ] [ Google Scholar ]
- de Chatel R., Weidmann P., Flammer J., Ziegler W. H., Beretta-Piccoli C., Vetter W., et al. (1977). Sodium, renin, aldosterone, catecholamines, and blood pressure in diabetes mellitus. Kidney Int. 12 412–421. 10.1038/ki.1977.132 [ DOI ] [ PubMed ] [ Google Scholar ]
- deLeiva A., Zager P. G., Melby J. C., Christlieb A. R., Graham C. A., Luetscher J. A., et al. (2010). Big renin and biosynthetic defect of aldosterone in diabetes mellitus. N. Engl. J. Med. 295 639–643. 10.1056/nejm197609162951203 [ DOI ] [ PubMed ] [ Google Scholar ]
- Di Loreto V., Moreno H. S., Puche R. C., Locatto M. E. (2004). Severe hyperglycemia: a determinant factor for hypofiltration in alloxan diabetic rats. Acta Diabetol. 41 56–62. 10.1007/s00592-004-0145-z [ DOI ] [ PubMed ] [ Google Scholar ]
- Dong Y. F., Liu L., Kataoka K., Nakamura T., Fukuda M., Tokutomi Y., et al. (2010a). Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes. Diabetologia 53 180–191. 10.1007/s00125-009-1575-1575 [ DOI ] [ PubMed ] [ Google Scholar ]
- Dong Y. F., Liu L., Lai Z. F., Yamamoto E., Kataoka K., Nakamura T., et al. (2010b). Aliskiren enhances protective effects of valsartan against type 2 diabetic nephropathy in mice. J. Hypertens. 28 1554–1565. 10.1097/HJH.0b013e328338bb11 [ DOI ] [ PubMed ] [ Google Scholar ]
- Dugbartey G. J. (2017). Diabetic nephropathy: a potential savior with ‘rotten-egg’ smell. Pharmacol Rep. 69 331–339. 10.1016/j.pharep.2016.11.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- Elisaf M. S., Tomos P. P., Milionis H. J., Siamopoulos K. C. (1999). Prerenal azotemia in a diabetic patient with hyporeninemic hypoaldosteronism and autonomic neuropathy. Diabetes Metab. 25 344–346. [ PubMed ] [ Google Scholar ]
- Farese R. V., Rodriguez-Colomé M., O’Malley B. C. (1980). Urinary prostaglandins following frusemide treatment and salt depletion in normal subjects and subjects with diabetic hyporeninaemic hypoaldosteronism. Clin. Endocrinol. 13 447–453. 10.1111/j.1365-2265.1980.tb03410.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Feldman D. L., Jin L., Xuan H., Contrepas A., Zhou Y., Webb R. L., et al. (2008). Effects of aliskiren on blood pressure, albuminuria, and (Pro)renin receptor expression in diabetic TG(mRen-2)27 Rats. Hypertension 52 130–136. 10.1161/HYPERTENSIONAHA.107.108845 [ DOI ] [ PubMed ] [ Google Scholar ]
- Fernandez-Cruz A., Noth R. H., Lassman M. N., Hollis J. B., Mulrow P. J. (1979). Low plasma renin activity in normotensive patients with diabetes mellitus: relationship to neuropathy. Hypertens 3 87–92. 10.1161/01.hyp.3.1.87 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ferrario C. M., Ahmad S., Nagata S., Simington S. W., Varagic J., Kon N., et al. (2014). An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin. Sci. 126 461–469. 10.1042/CS20130400 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Forbes J. M., Cooper M. E. (2013). Mechanisms of diabetic complications. Physiol. Rev. 93 137–188. 10.1152/physrev.00045.2011 [ DOI ] [ PubMed ] [ Google Scholar ]
- Fukuchi S. (1991). Studies on the cause and the treatment of hyporeninemic selected hypoaldosteronism in diabetic nephropathy. Jpn. J. Med. 30 617–618. [ PubMed ] [ Google Scholar ]
- Gallo L. A., Ward M. S., Fotheringham A. K., Zhuang A., Borg D. J., Flemming N. B., et al. (2016). Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 6:26428. 10.1038/srep26428 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Garsen M., Rops A. L., Dijkman H., Willemsen B., van Kuppevelt T. H., Russel F. G., et al. (2016). Cathepsin L is crucial for the development of early experimental diabetic nephropathy. Kidney Int. 90 1012–1022. 10.1016/j.kint.2016.06.035 [ DOI ] [ PubMed ] [ Google Scholar ]
- Gasparo M. D., Speth R. C., Baltatu O. C., Vanderheyden P. (2013). Brain RAS: hypertension and beyond. Int. J. Hypertens. 2013:157180. 10.1155/2013/157180 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Harel Z., Gilbert C., Wald R., Bell C., Perl J., Juurlink D., et al. (2012). The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ 344:e42. 10.1136/bmj.e42 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hollenberg N. K., Price D. A., Fisher N. D. L., Lansang M. C., Perkins B., Gordon M. S., et al. (2003). Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 63 172–178. 10.1046/j.1523-1755.2003.00701.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Jagadeesh G., Balakumar P., Stockbridge N. (2012). How well do aliskiren’s purported mechanisms track its effects on cardiovascular and renal disorders? Cell. Signal. 24 1583–1591. 10.1016/j.cellsig.2012.04.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- Juncos L. (2013). Direct renin inhibition: extricating facts from façades. Ther. Adv. Cardiovasc. Dis. 7 153–167. 10.1177/1753944713479995 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kang Y. S., Lee M. H., Song H. K., Hyun Y. Y., Cha J. J., Ko G. J., et al. (2011). Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrol. Dial. Transplant. 26 1194–1204. 10.1093/ndt/gfq579 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kelly D. J., Zhang Y., Moe G., Naik G., Gilbert R. E. (2007). Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia 50 2398–2404. 10.1007/s00125-007-0795-799 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kidokoro K., Satoh M., Itano S., Kuwabara A., Sasaki T., Kashihara N. (2016). Feasibility of fluorescence energy transfer system for imaging the renoprotective effects of aliskiren in diabetic mice. JRAAS J. Renin Angiotensin Aldoster. Syst. 17 1–8. 10.1177/1470320315625704 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kigoshi T., Imaizumi N., Azukizawa S., Yamamoto I., Uchida K., Konishi F., et al. (1986). Effects of angiotensin II, adrenocorticotropin, and potassium on aldosterone production in adrenal zona glomerulosa cells from streptozotocin-induced diabetic rats. Endocrinology 118 183–188. 10.1210/endo-118-1-183 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kim H. J. (1994). Mechanisms of hyperkalemia associated with hyporeninemic hypoaldosteronism in streptozotocin-induced diabetic rats. J. Korean Med. Sci. 9 107–115. 10.3346/jkms.1994.9.2.107 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Krochmal M., Kontostathi G., Magalhães P., Makridakis M., Klein J., Husi H., et al. (2017). Urinary peptidomics analysis reveals proteases involved in diabetic nephropathy. Sci. Rep. 7:15160. 10.1038/s41598-017-15359-15359 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kuhlmann U., Vetter W., Fischer E., Siegenthaler W. (1978). Control of plasma aldosterone in diabetic patients with hyporeninemic hypoaldosteronism. Klin. Wochenschr. 56 229–234. 10.1007/BF01477829 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kumar V. R. S., Anders H.-J. (2016). Cathepsins are potential therapeutic targets in kidney disease. Kidney Int. 90 933–935. 10.1016/j.kint.2016.07.034 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lambers Heerspink H. J., Perkovic V., De Zeeuw D. (2009). Renal and cardio-protective effects of direct renin inhibition: a systematic literature review. J. Hypertens. 27 2321–2331. 10.1097/HJH.0b013e3283310f92 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lee M. J., Kim S. S., Kim I. J., Song S. H., Kim E. H., Seo J. Y., et al. (2017). Changes in urinary angiotensinogen associated with deterioration of kidney function in patients with type 2 diabetes Mellitus. J. Korean Med. Sci. 32 782. 10.3346/jkms.2017.32.5.782 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Matavelli L. C., Huang J., Siragy H. M. (2012). Combined aliskiren and amlodipine reduce albuminuria via reduction in renal inflammation in diabetic rats. J. Cardiovasc. Pharmacol. 59 281–287. 10.1097/FJC.0b013e31823fc3f5 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. (2016). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev. Esp. Nutr. Humana Diet. 4:1. 10.1186/2046-4053-4-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nehme A., Zouein F. A., Zayeri Z. D., Zibara K. (2019). An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 6 1–17. 10.3390/jcdd6020014 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nussberger J., Wuerzner G., Jensen C., Brunner H. R. (2002). Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension 39 E1–E8. 10.1161/hy0102.102293 [ DOI ] [ PubMed ] [ Google Scholar ]
- Parving H.-H., Brenner B. M., McMurray J. J. V., de Zeeuw D., Haffner S. M., Solomon S. D., et al. (2009). Aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE): rationale and study design. Nephrol. Dial. Transplant. 24 1663–1671. 10.1093/ndt/gfn721 [ DOI ] [ PubMed ] [ Google Scholar ]
- Parving H.-H., Brenner B. M., McMurray J. J. V., de Zeeuw D., Haffner S. M., Solomon S. D., et al. (2012). Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367 2204–2213. 10.1056/NEJMoa1208799 [ DOI ] [ PubMed ] [ Google Scholar ]
- Parving H.-H., Persson F., Lewis J. B., Lewis E. J., Hollenberg N. K. (2008). Aliskiren Combined with losartan in Type 2 diabetes and nephropathy. N. Engl. J. Med. 358 2433–2446. 10.1056/NEJMoa0708379 [ DOI ] [ PubMed ] [ Google Scholar ]
- Paulsen E. P., Seip R. L., Ayers C. R., Croft B. Y., Kaiser D. L. (1989). Plasma renin activity and albumin excretion in teenage type I diabetic subjects a prospective study. Hypertension 13 781–788. 10.1161/01.HYP.13.6.781 [ DOI ] [ PubMed ] [ Google Scholar ]
- Perez G. O., Lespier L., Jacobi J., Oster J. R., Katz F. H., Vaamonde C. A., et al. (1977). Hyporeninemia and hypoaldosteronism in diabetes mellitus. Arch. Intern. Med. 137 852–855. 10.1001/archinte.137.7.852 [ DOI ] [ PubMed ] [ Google Scholar ]
- Persson F., Lewis J. B., Lewis E. J., Rossing P., Hollenberg N. K., Hans-Henrik P. (2012a). Impact of aliskiren treatment on urinary aldosterone levels in patients with type 2 diabetes and nephropathy: an AVOID substudy. JRAAS J. Renin Angiotensin Aldoster. Syst. 13 118–121. 10.1177/1470320311417272 [ DOI ] [ PubMed ] [ Google Scholar ]
- Persson F., Lewis J. B., Lewis E. J., Rossing P., Hollenberg N. K., Parving H. H. (2012b). Impact of glycaemic control on the effect of direct renin inhibition in the AVOID study. JRAAS J. Renin Angiotensin Aldoster. Syst. 13 250–253. 10.1177/1470320312437068 [ DOI ] [ PubMed ] [ Google Scholar ]
- Persson F., Lewis J. B., Lewis E. J., Rossing P., Hollenberg N. K., Parving H.-H. (2010). Impact of baseline renal function on the efficacy and safety of aliskiren added to losartan in patients with type 2 diabetes and nephropathy. Diabetes Care 33 2304–2309. 10.2337/dc10-0833 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Persson F., Lewis J. B., Lewis E. J., Rossing P., Hollenberg N. K., Parving H. H. (2011). Aliskiren in combination with losartan reduces albuminuria independent of baseline blood pressure in patients with type 2 diabetes and nephropathy. Clin. J. Am. Soc. Nephrol. 6 1025–1031. 10.2215/CJN.07590810 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Persson F., Rossing P., Reinhard H., Juhl T., Stehouwer C. D. A., Schalkwijk C., et al. (2009). Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care 32 1873–1879. 10.2337/dc09-0168 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Phelps K. R., Lieberman R. L., Oh M. S., Carroll H. J. (1980). Pathophysiology of the syndrome of hyporeninemic hypoaldosteronism. Metabolism 29 186–189. 10.1016/0026-0495(80)90145-90146 [ DOI ] [ PubMed ] [ Google Scholar ]
- Pratt J. H., Parkinson C. A., Weinberger M. H., Duckworth W. C. (1985). Decreases in renin and aldosterone secretion in alloxan diabetes: an effect of insulin deficiency. Endocrinology 116 1712–1716. 10.1210/endo-116-5-1712 [ DOI ] [ PubMed ] [ Google Scholar ]
- Rafiq K., Hitomi H., Nakano D., Ichihara A., Nishiyama A. (2011). Possible involvement of the (pro)renin receptor-dependent system in the development of insulin resistance. Front. Biosci. 3 1478–1485. 10.2741/238 [ DOI ] [ PubMed ] [ Google Scholar ]
- Saito T., Kobori H., Urushihara M., Kotani Y., Kagami S. (2009). Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am. J. Med. Sci. 338 478–480. 10.1097/MAJ.0b013e3181b90c25 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sealey J. E., Laragh J. H. (2009). Aliskiren fails to lower blood pressure in patients who have either low PRA levels or whose PRA falls insufficiently or reactively rises. Am. J. Hypertens. 22 112–121. 10.1038/ajh.2008.275 [ DOI ] [ PubMed ] [ Google Scholar ]
- Şen S., Sabırlı S., Özyiğit T., Üresin Y. (2013). Aliskiren: review of efficacy and safety data with focus on past and recent clinical trials. Ther. Adv. Chronic Dis. 4 232–241. 10.1177/2040622313495288 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sousa L. S., de Sousa A. G. P., Nunes A. B., Cabral J. V., de S., El-Feghaly W. B. (2016). Hyporeninemic hypoaldosteronism and diabetes mellitus: pathophysiology assumptions, clinical aspects and implications for management. World J. Diabetes 7 101–111. 10.4239/wjd.v7.i5.101 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stanton A. (2003). Potential of renin inhibition in cardiovascular disease. JRAAS J. Renin Angiotensin Aldoster. Syst. 4 6–10. 10.3317/jraas.2003.008 [ DOI ] [ PubMed ] [ Google Scholar ]
- Stanton A. V., Dicker P., O’Brien E. T. (2009). Aliskiren monotherapy results in the greatest and the least blood pressure lowering in patients with high- and low-baseline PRA levels, respectively. Am. J. Hypertens. 22 954–957. 10.1038/ajh.2009.114 [ DOI ] [ PubMed ] [ Google Scholar ]
- Tuck M. L., Sambhi M. P., Levin L. (1979). Hyporeninemic hypoaldosteronism in diabetes mellitus. Studies of the autonomic nervous system’s control of renin release. Diabetes 28 237–241. 10.2337/diab.28.3.237 [ DOI ] [ PubMed ] [ Google Scholar ]
- Uresin Y., Taylor A. A., Kilo C., Tschöpe D., Santonastaso M., Ibram G., et al. (2007). Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. JRAAS J. Renin Angiotensin Aldoster. Syst. 8 190–198. 10.3317/jraas.2007.028 [ DOI ] [ PubMed ] [ Google Scholar ]
- Urushihara M., Kagami S. (2017). Role of the intrarenal renin–angiotensin system in the progression of renal disease. Pediatr. Nephrol. 32 1471–1479. 10.1007/s00467-016-3449-3447 [ DOI ] [ PubMed ] [ Google Scholar ]
- Villoria G., Nunez M., Miralles J. M., del Pozo D. C., Romo T. (1988). Hyporeninemic hypoaldosteronism in diabetic patients with chronic renal failure. Am. J. Nephrol. 8 127–137. 10.1159/000167571 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wang R.-F., Podos S. M., Serle J. B., Baltatu O. C. (2012). Effect of SPP 635, a renin inhibitor, on intraocular pressure in glaucomatous monkey eyes. Exp. Eye Res. 94 146–149. 10.1016/j.exer.2011.11.019 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wang W., Qiu L., Howard A., Solis N., Li C., Wang X., et al. (2014). Protective effects of aliskiren and valsartan in mice with diabetic nephropathy. JRAAS J. Renin Angiotensin Aldoster. Syst. 15 384–395. 10.1177/1470320313507123 [ DOI ] [ PubMed ] [ Google Scholar ]
- Watanabe T., Waguri S., Watanabe M., Ishii Y., Kominami E., Uchiyama Y. (1989). Immunocytochemical localization of angiotensinogen and cathepsins B, H, and L in rat hepatocytes, with special reference to degradation of angiotensinogen in lysosomes after colchicine. J. Histochem. Cytochem. 37 1899–1911. 10.1177/37.12.2685113 [ DOI ] [ PubMed ] [ Google Scholar ]
- Weidmann P., Reinhart R., Maxwell M. H., Rowe P., Coburn J. W., Massry S. G. (1973). Syndrome of hyporeninemic hypoaldosteronism and hyperkalemia in renal disease. J. Clin. Endocrinol. Metab. 36 965–977. 10.1210/jcem-36-5-965 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wood J. M., Close P. (1996). Renin inhibitors: cardiovascular drugs of the future? Cardiovasc. Drugs Ther. 10 309–312. 10.1007/bf02627954 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wood J. M., Maibaum J., Rahuel J., Grütter M. G., Cohen N. C., Rasetti V., et al. (2003). Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem. Biophys. Res. Commun. 308 698–705. 10.1016/S0006-291X(03)01451-1457 [ DOI ] [ PubMed ] [ Google Scholar ]
- Yacoub R., Campbell K. N. (2015). Inhibition of RAS in diabetic nephropathy. Int. J. Nephrol. Renovasc. Dis. 8 29–40. 10.2147/IJNRD.S37893 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zheng S. L., Roddick A. J., Ayis S. (2017). Effects of aliskiren on mortality, cardiovascular outcomes and adverse events in patients with diabetes and cardiovascular disease or risk: a systematic review and meta-analysis of 13,395 patients. Diabetes Vasc. Dis. Res. 14 400–406. 10.1177/1479164117715854 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhou G., Liu X., Cheung A. K., Huang Y. (2015). Efficacy of aliskiren, compared with angiotensin ii blockade, in slowing the progression of diabetic nephropathy in db/db mice: should the combination therapy be a focus? Am. J. Transl. Res. 7 825–840. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zimpelmann J., Kumar D., Levine D. Z., Wehbi G., Imig J. D., Navar L. G., et al. (2000). Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int. 58 2320–2330. 10.1046/j.1523-1755.2000.00416.x [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Open access
- Published: 19 October 2024
Mechanism of the cardioprotective effect of empagliflozin on diabetic nephropathy mice based on the basis of proteomics
- Zongchao Yu 1 na1 ,
- Yongping Lu 2 na1 ,
- Mengxian Zhang 3 na1 ,
- Yanshan Lin 1 ,
- Tak-sui Wong 1 ,
- Baozhang Guan 1 ,
- Yu Meng 1 ,
- Fan-na Liu 1 ,
- Lianghong Yin 1 ,
- Yankun Li 4 ,
- Han Zhang 4 ,
- Donge Tang 5 &
- Yong Dai 6
Proteome Science volume 22 , Article number: 9 ( 2024 ) Cite this article
66 Accesses
Metrics details
Diabetic nephropathy affects a significant proportion of individuals with diabetes, and its progression often leads to cardiovascular disease and infections before the need for renal replacement therapy arises. Empagliflozin has been shown to have various protective effects in cardiovascular disease studies, such as improving diabetic myocardial structure and function, and reducing myocardial oxidative stress. However, the impact of empagliflozin on cardiac protein expression and signaling pathways has not been comprehensively analyzed. To address this gap, we conducted proteome analysis to identify specific protein markers in cardiac tissue from the diabetes model group, including Myh7, Wdr37, Eif3k, Acot1, Acot2, Cat, and Scp2, in cardiac tissue from the diabetes model group. In our drug model, empagliflozin primarily modulates the fat-related metabolic signaling pathway within the heart. Empagliflozin downregulated the protein expression levels of ACOX1, ACADVL and CPT1A in the model group. Overall, our findings demonstrate that empagliflozin provides cardiac protection by targeting metabolic signaling pathways, particularly those related to fat metabolism. Moreover, the identification of cardiac biomarkers in a mouse model of diabetic nephropathy lays the foundation for further exploration of disease biomarkers in cardiac tissue.
The number of people living with diabetes worldwide is projected to increase to 693 million by 2045 [ 1 ]. Diabetic nephropathy affects approximately 40% of people with diabetes and has emerged as a leading cause of chronic kidney disease (CKD) worldwide [ 2 ]; however, most patients actually die from cardiovascular disease and infections before they need renal replacement therapy [ 3 ].
Empagliflozin (Jardiance ® ) is a potent and highly selective inhibitor of sodium glucose cotransporter 2 ( SGLT2 ), which inhibits the reabsorption of glucose in the proximal tubule of the kidney by inhibiting SGLT2 . Empagliflozin provides a novel insulin-independent mechanism for lowering blood sugar has been developed [ 4 ]. In addition to lowering blood sugar, empagliflozin had a favorable effect on several nonglycemic outcomes, including modest reductions in body weight, blood pressure, and major adverse cardiovascular events (55% relative risk reduction in the risk empagliflozin group); Empagliflozin was approved by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) in 2014.
Research has shown that empagliflozin has multiple protective functions in cardiovascular disease research [ 5 , 6 , 7 ]. Empagliflozin improves diabetic myocardial structure and function, reduces myocardial oxidative stress, and improves myocardial fibrosis. Further studies have shown that empagliflozin inhibits oxidative stress and fibrosis by transforming growth factor beta→TGF-β, adenosine triphosphate→ATP, and less reactive oxygen species→ROS [ 8 , 9 ].
Treatment with empagliflozin inhibits the production of inflammatory factors and reverses changes in redox parameters (e.g., glutathione and lipid peroxides), attenuates the levels of the protein kinase GIα, improves myofilament function, reduces cardiomyocyte stiffness and increases the diastolic capacity of the heart [ 10 ]. In a mouse model of ventricular fibrillation-induced cardiac arrest [ 11 ], treatment with ipagliflozin improved left ventricular function and increased survival time. GLT2 inhibitors affect lipid metabolism at several different levels, reducing lipid oxidation and shifting the utilization of substrates to the use of ketone bodies, which are more efficient for myocardial metabolism, and that less reactive oxygen species pass through these ketone bodies, affecting β-oxidation and the transport of lipid molecules in cells [ 12 ]. Despite these promising findings, there has been a lack of systematic analysis of the effects of empagliflozin on cardiac protein expression and signaling pathways.
In this study, we conducted proteomic analysis of heart tissue from the healthy group, disease group, and medication group to investigate the regulatory mechanism of empagliflozin at the cardiac proteome level.
Quality control of samples
We evaluated cardiac collagen levels in a total of 17 mice as illustrated in Fig. 1 A, which were categorized into three groups: 4 mice in the healthy group (db/m), 6 mice in the disease model group (db/db), and 7 mice in the EMPA-treated group (EMPA-treated) (Supplementary Excel Table 1). Protein integrity was assessed via SDS‒PAGE combined with Coomassie brilliant blue staining, as demonstrated in Supplementary Fig. 1. The observed sample were clearly defined, and the distribution of protein molecular weights was uniform, as depicted in Supplementary Fig. 1. LC‒MS-based quantitative proteomics was conducted on each sample individually, and the resulting data were analyzed via computer software. Supplementary Figure Figs. 2 and 3 present the chromatograms of all the samples. As shown in the figure, the chromatographic separation peaks of all the samples had relatively stable retention times, uniform peptide peak time distributions, and chromatographic peaks. Narrow, sharp and symmetrical without obvious trailing. By counting the number of peptide missed cleavage sites and the proportion of the corresponding total ion current intensity, the enzyme cleavage efficiency of the experiment was approximately evaluated. The total number of identified peptides was 32,701, the number of missed cleavage sites > = 2 was 158, and the missed cleavage ratio was 0.48. %, and the enzymatic hydrolysis efficiency was 99.52%.

Proteomic analysis of differences in cardiac tissue before and after drug treatment. ( A ) Experimental grouping design. ( B ) PCA clustering diagrams for three sample groups. ( C ) Heatmap of differentially expressed proteins. ( D ) Expression levels of ACOX1, ACADVL and CPT1A proteins in the three sample groups

KOG analysis of differentially expressed proteins between the db/m and db/db groups ( A ) and between the db/db and EMPA-treated groups ( B )

GO (BP, biological process) enrichment analysis of differentially expressed proteins in each group. ( A ) db/m vs. db/db; ( B ) db/db vs. EMPA-treated
Differential protein analysis
Through PCA analysis, we found that the sample group was within the 95% confidence interval (Hotelling’s T-squared ellipse), which met the statistical requirements (Supplementary Fig. 4). In addition, we also used PCA analysis to select the top 200 highly variable proteins, that were significantly different among the three groups (Fig. 1 C). The comparison strategy is as follows: A total of 2 groups are compared, namely, the Empa (EMPA-treated) group versus the db/db (model) group and the db/db group versus the db/m (healthy) group. We used statistical methods to screen for differentially expressed proteins, and the screening criteria for differentially expressed proteins were Student’s t-test P -value < 0.05 and FOLD CHANGE < 0.83 or FOLD CHANGE > 1.2. The original results of the difference analysis are shown in the Supplementary Table 1. Compared those in with the normal group, 213 proteins were downregulated and 228 proteins were highly expressed in the model group. The top highly expressed proteins in the model group were Myh7, Wdr37, Eif3k, Acot1, Acot2, Cat and Scp2 in the model group ( Fig. 1 D). With drug treatment, the increase in protein expression in the model group was reversed.
Signaling pathways of the differentially expressed proteins
To determine the changes in the signaling pathways of heart proteins in mice before and after taking empagliflozin treatment, we conducted a multidimensional signal dimension analysis, which included clusters of orthologous groups for eukaryotic complete genomes (KOG), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Ontology (GO) analyses. The details are as follows.
We performed KOG analysis on the differentially expressed proteins ( Fig. 2 ). The proteins exhibiting differential expression between the disease and healthy groups were predominantly predominantly enriched in several key categories: posttranslational modification and chaperone protein turnover, lipid transport and metabolism, and signal transduction mechanisms. Specifically, 47, 59, and 58 differentially expressed proteins were identified and enriched in these respective categories. ( Fig. 2 A). After taking the drug, the differentially expressed proteins between the disease group and the drug group were mainly enriched in mainly signal transduction mechanisms and posttranslational modification, protein turnover, and chaperones, which were enriched to 50 and 36, respectively. In the lipid transport and metabolism pathway, only 14 differentially expressed proteins were enriched ( Fig. 2 B). These findings indicated that the lipid transport and metabolism pathway-related proteins in cardiac tissue were significantly downregulated under the action of drugs.
Analysis of the GO (BP, biological process) pathway, revealed that the enriched upregulated ptoteins in the disease group were associated mainly withfatty acid derivative metabolism and fatty acid catabolic processes (Fig. 3 A). After taking the empagliflozin treatment, the differentially expressed proteins were enriched mainly in signaling pathways such as fatty acid metabolic process, negative regulation of membrane permeability cellular calcium ion homeostasis, and RNA splicing, via transesterification reactions (Fig. 3 B).
The GO analysis also revealed that the fatty acid metabolism-related proteins enriched in differential proteins in the disease group were downregulated after treatment with empagliflozin ( Fig. 3 ). Analysis of the KEGG signaling pathways revealed that, the upregulated ptoteins in the disease group are mainly metabolic pathways and peroxisome, Protein processing in endoplasmic reticulum (Supplementary Fig. 5A). After the empagliflozin treatment, the differentially expressed proteins were mainly enriched in signaling pathways such as amyotrophic lateral sclerosis, and the enriched signaling pathways in the disease model group were altered (Supplementary Fig. 5B).
Through KOG, GO and KEGG analyses, the signaling pathways that reflect the possible effects of empagliflozin are mainly fatty acid metabolic-related signaling pathways.
Network interaction analysis of differential proteins
When proteins exercise their biological functions, they form a PPI network to maintain temporal and spatial coordination, and construct an interaction network of differentially expressed proteins, which can reveal the changing trends of differentially expressed proteins at the proteome level, and further help us identify the differences in differentially expressed proteins. A visual analytics platform for comprehensive gene expression profiling was used with NetworkAnalyst 3.0 ( https://www.networkanalyst.ca ) [ 13 ]. In the disease model group, the upregulated signaling pathways ( p < 0.05) in heart tissue included mainly fatty acid degradation, fatty acid metabolism and peroxisome (Supplementary Excel Table 2 A) and the downregulated signaling pathways involved mainly protein processing in the endoplasmic reticulum, metabolic pathways and arginine and proline metabolism (Supplementary Excel Table 2B). In addition, the significantly enriched involved were Acox1 , Acadvl and Cpt1a . The transcription factors involved are mainly: Chd1 , Jund , Rcor1 , Tbp , Gata1 , Ubtf , Sin3a , Ep300 , Mef2 and Hcfc1 (Fig. 4 A and B). After EMPA treatment, the enriched pathways were mainly the proteasome, cell cycle and TGF-beta signaling pathways (Supplementary Excel Table 2 C). The main transcription factors involved are: Zmiz1 , Mxi1 , Nrf1 , Chd2 and Irf4 ( Fig. 4 C). In other words, the empagliflozin protects mouse heart tissue primarily by modulating signaling pathways associated with fatty acids, thereby potentially reducing oxidative stress and inflammation, and promoting overall cardiac health (Fig. 5 ).

Gene regulatory network analysis of differentially expressed proteins in each group. ( A ) Upregulated differential proteins between the db/m and db/db groups; ( B ) downregulated differential proteins between the db/m and db/db groups; (3) upregulated differential proteins between the db/db and EMPA-treated groups

Summary of the potential mechanism of action of empagliflozin
Conclusions
On the basis of our observations in mouse models, we have identified specific protein markers associated with the diabetes model group, namely Myh7, Wdr37, Eif3k, Acot1, Acot2, Cat and Scp2. Furthermore, our findings suggest that empagliflozin primarily modulates the fat-related metabolic signaling pathway in the heart. According to the results of the bioinformatics analysis, Acox1 , Acadvl and Cpt1a may be involved in regulating the transcription factors associated with the reversal of the metabolic pathway. Collectively, our evidence from a mouse model indicates that empagliflozin affects heart tissue by targeting molecular metabolism and the fatty acid signaling pathway, thereby influencing the molecular function of the heart. However, further research is warranted to fully understand the underlying mechanisms and potential therapeutic implications.
The underlying mechanism behind the beneficial effects of SGLT2 inhibitors in patients at high risk of cardiovascular disease remains poorly understood. To investigate and compare the impact of hypoglycemic agents on ventricular electrophysiological substrates in metabolic mice, the relationship between adipocytokines present in pericardial fat and arrhythmia has been explored through electrophysiological techniques. These adipocytokines can contribute to an increase in the area of fibrosis area within the heart and ventricular tissues of patients diagnosed with metabolic syndrome [ 14 , 15 ] and SGLT2 inhibitors can reduce the amount of pericardium fat [ 16 , 17 , 18 ], and can alter autonomic nervous function [ 19 , 20 ]. Previous studies have also shown that SGLT2 inhibitors improve mitochondrial function in diabetic rats [ 21 , 22 ]. Empagliflozin regulates the effect of adipocytokines in adipose tissue on the ion current of cardiomyocytes and reduces arrhythmia [ 23 ], which may provide a possible mechanism for antiarrhythmic effects [ 24 ]. Metabolic syndrome-induced cardiac fibrosis [ 25 , 26 ] may be because adipose tissue in visceral fat resides in macrophages, producing more proinflammatory cytokines [ 26 , 27 , 28 ], and causing arrhythmia [ 29 , 30 ]. However, the effects of SGLT2 inhibitors, especially empagliflozin, on ventricular electrophysiological substrates have not been fully elucidated.
In this study, we utilized animal models to assess the impact of drugs on the hearts of mice and identified specific cardiac protein markers associated with diabetes models in mice. Additionally, through the utilization of mass spectrometry technology, we discovered that the protective effects of empagliflozin on the hearts of mice were mediated through metabolic signaling pathways. Overall, the drugs investigated in this study were found to intervene in cardiac tissue metabolism and peroxiproteasome signaling, with a close involvement of genes related to fat metabolism. Specifically, we focused on three genes associated with fat metabolism. First, peroxisomal ACOX1 (Acyl-CoA oxidase 1) was identified as the initial and rate-limiting enzyme in fatty acid β-oxidation. ACOX1 serves as a significant source of H2O2 production, and dysfunction of ACOX1 has been associated with peroxide enzyme dioxin dyads. The protein in the CPT1 family, CPT1A-mediated lipid oxidation, also has potential as a therapeutic target [ 31 , 32 , 33 ]. In the context of cancer, CPT1A can mediate the fatty acid oxidation pathway [ 31 ] and can also inhibit colorectal cancer cell metastasis by inhibiting inactivation; O-GlcNAcylation regulates long-chain fatty acid metabolism by inhibiting ACOX1 ubiquitination-dependent degradation [ 34 ]. ACOX1 complexes are also new targets for liver lipid metabolism disorders induced by perfluoroalkyl and perfluoroalkyl substances [ 35 ]. High methylation of ACADVL is associated with reduced cardiac fibrosis in patients with high-intensity interval training-related heart failure [ 35 ]. These three genes are closely correlated with fatty acid metabolism from the perspectives of cytology and tissue organs and are expected to become targets.
The expression of the CPT1A gene is tightly regulated by hormones and diet in tissues that rely heavily on fatty acid utilization, such as the heart. The regulation of CPT1A is a complex process and has been implicated in various diseases, including genetic mutations, metabolic disorders, and cancer. However, the majority of the existing data on CPT1A regulation have been derived from animal studies [ 32 ]. Furthermore, reduced expression of CPT1A has been shown to impair the cells’ capacity of cells to produce aspartate, which is an essential nucleotide precursor for DNA synthesis [ 36 ].
Mutations in the ACADVL gene result in very long-chain acyl-CoA dehydrogenase deficiency, a severe and potentially life-threatening disorder affecting mitochondrial fatty acid oxidative metabolism [ 37 ]. Further research is necessary to explore the potential of targeting three specific genes in the drug signaling pathway for intervention purposes.
Sample information
In accordance with previous studies [ 38 ], male db/db mice (C57BLKS/J-leprdb/leprdb) and matched littermate db/m mice (C57BLKS/J-leprdb/leprm) were obtained from the Nanjing University Experimental Animal Center. The db/db mice were 6 weeks old and numbered 30, whereas the db/m mice served as healthy controls and numbered 15. After a 2-week adaptation period, the db/db mice were randomly divided into two groups: the db/db (disease model) group and the empagliflozin (EMPA-treated) group, with each group consisting of 15 mice. The mice in the EMPA-treated group received a daily oral gavage of 10 mg/kg EMPA daily via oral gavage for 12 weeks. Similarly, the mice in the model and control groups were administered 10 mL/kg of sterile water via the same method. After the 12-week treatment period, heart tissue samples were collected from the mice. All experimental procedures in this study were conducted in compliance with the National Law for Laboratory Animal Experimentation and were approved by the Commission on Experimental Animal Ethics of Jinan University (No. 202069-04). A total of 17 heart tissue samples from mice were included in the proteomic analysis, comprising the db/db group (6 samples), db/m group (4 samples), and EMPA-treated group (7 samples).
Sample preparation
(1) Sample preparation: An appropriate amount of sample and was placed on dry ice. The sample was transferred to a 2 mL EP tube and add 300 µL of RIPA working solutionwas added. The samples were sonicated in an ice–water bath via a cell sonicator for 2 min.The tube was centrifuged at 4 °C and 12,000 rpm for 10 min. The clear supernatant was carefully removed. (2) Protein concentration determination: Perform a BCA assay was performed to calculate the protein concentration of each sample. (3) Acetone precipitation: 100 µg of total protein was taken from each sample and diluted with H2O to a concentration of approximately 1 mg/mL. The acetone was precooled to 20 °C, and 5 times the volume of acetone was added to the sample. The mixture was mixed well, and the sample was precipitated at 20 °C overnight. The tube was centrifuged at 4 °C and 12,000 rpm for 10 min. The supernatant was carefully removed, and the pellet was rinsed twice with 200 µL of precooled 80% acetone. The mixture was centrifuged again at 12,000 rpm, and the supernatant was carefully removed. (4) Protein reconstitution, reduction, and alkylation: 100 µL of reconstituted protein mixture was added to the precipitated protein. The samples were sonicated in a water bath for 5 min to dissolve the protein precipitate. DTT was added to a final concentration of 5 mM, and the sample was incubated at 55 °C with shaking for 10 min to reduce disulfide bonds. The samples were cooled to room temperature, and IAA was added to a final concentration of 10 mM. The sample was reacted in the dark for 15 min to alkylate the reduced disulfide bonds. (5) Protein digestion: Trypsin was dissolved to a concentration of 0.5 µg/µL with resuspension buffer. The trypsin solution was incubated at room temperature for 5 min. Trypsin was mixed with each sample thoroughly at a ratio of trypsin to protein of 1:50. The mixture was incubated overnight at 37 °C with shaking at 1000 rpm after brief centrifugation. (6) SDC cleanup: TFA was added to the mixed sample to a final concentration of 2%, ensuring a pH below 2. The mixture was mixed well to precipitate SDC. The tube was centrifuged at high speed for 10 min, and the supernatant was transferred to a new EP tube. Next, 100 µL of 2% TFA was added, and the mixture was mixed thoroughly. The mixture was centrifuged at 13,000 rpm for 10 min to extract the coprecipitated polypeptides. This extraction process was repeated twice. The three supernatant fractions were combined and centrifuged at high speed for 10 min. The supernatant was transferred to a new EP tube to obtain labeled peptide samples. (7) Peptide desalting: A C18 cartridge was used following the provided instructions. The eluate was dried under vacuum at 4 °C overnight.
nanoLCMS/MS analysis
Each sample was subjected to separation and analysis via a nano-UPLC (EASYnLC1200) coupled to a Q Exactive HFX Orbitrap instrument (Thermo Fisher Scientific) with a nanoelectrospray ion source. The separation was carried out via a reversed-phase column (100 ID ×15 cm, ReprosilPur 120 C18AQ, 1.9, Dr. Math). The mobile phases consisted of H 2 O with 0.1% formic acid and 2% acetonitrile (phase A) and 80% acetonitrile with 0.1% formic acid (phase B). Sample separation was performed via a 120-minute gradient at a flow rate of 300 nL/min. The gradient for phase B was as follows: 2–5% for 2 min, 5–22% for 88 min, 22–45% for 26 min, 45–95% for 2 min, and 95% for 2 min. Data-dependent acquisition (DDA) was carried out in profile and positive mode using the Orbitrap analyzer at a resolution of 120,000 (@200 m/z) and a m/z range of 350–1600 for MS1. For MS2, the resolution was set to 15,000 with a dynamic first mass. The automatic gain control (AGC) target for MS1 was set to 3E6 with a maximum injection time of 50 ms, whereas for MS2, it was set to 1E5 with a maximum injection time of 110 ms. The top 20 most intense ions were fragmented by higher-energy collisional dissociation (HCD) with a normalized collision energy (NCE) of 27% and an isolation window of 1.2 m/z. A dynamic exclusion time window of 45 s was applied, and single charged peaks and peaks with a charge exceeding 6 were excluded from the DDA procedure.
Proteome Discoverer database search
The raw MS files acquired from the vendor were processed with Proteome Discoverer (PD) software, version 2.4.0.305, employing the Sequest HT search engine. MS spectra lists were queried against species-specific UniProt FASTA databases (uniport-Mus + musculus-10090-2020-10.fasta) at the species level. The following modifications were considered during the search: carbamidomethyl [C] as a fixed modification, Oxidation (M), and acetyl (protein N-term) as variable modifications. Trypsin was selected as the protease. The peptide tolerance was set to 10 ppm, and the MS/MS tolerance was set to 0.02 Da. Up to 2 missed cleavages were allowed during the search. The false discovery rate (FDR) was set to 0.01 for both peptide-spectrum matches (PSMs) and peptide levels. For protein quantification, unique peptides and Razor peptides were used, while the total peptide amount was used for normalization. All other parameters were set to the default settings.
Using R (version 3.6.3) or SIMCA software (V16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden), the data were logarithmically and centered, and then modeled for analysis [ 39 ].
Screening of differentially expressed proteins
We used statistical methods to screen differentially expressed proteins, where the screening criteria for differentially expressed proteins were Student’s t-test P -VALUE < 0.05 and FOLD CHANGE < 0.83 or FOLD CHANGE > 1.2 [ 40 ].
Cluster of orthologous groups (COG) analysis is a method used to classify gene products based on their homology and functional classification. In this study, we conducted a differential protein analysis using the KOG (euKaryotic Orthologous Groups) database, which is a variant of COG analysis specifically designed for eukaryotes. This analysis was performed based on basis of the methodology described in the authors’ previous literature [ 41 ]. The results of the KOG analysis for differentially expressed proteins were visualized via a histogram or bar plot [ 42 ].
GO and KEGG
For functional enrichment analysis, we utilized the Gene Ontology (GO) database, available at geneontology.org. In this project, we mapped the genes to the corresponding nodes in the Gene Ontology database and performed functional enrichment analysis using the GO database [ 43 ]. This allowed us to classify the differentially expressed proteins into their respective functional categories.
Additionally, we utilized the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database ( https://www.kegg.jp/kegg/pathway.html .) to analyze the metabolic pathways associated with the differentially expressed proteins. The KEGG database provides functional information on genes and genomes, including cellular biochemical processes such as metabolism, membrane transport, signaling, cell cycle, and conserved sub-pathways. By identifying the metabolic pathways that are significantly enriched with differentially expressed proteins, we can gain insights into the systematic alterations occurring under different experimental conditions.
Analysis of gene regulatory networks build
For gene regulatory network analysis, we employed NetworkAnalyst ( https://www.networkanalyst.ca ), a web-based tool that allows researchers to perform various meta-analyses on gene expression data [ 13 ]. In our project, we used the differentially expressed proteins for the analysis and selected specific parameters. The organism was specified as Mus musculus , and the gene regulatory network (GRN) database utilized was the TF‒gene interaction database from ENCODE. The ENCODE database provides transcription factor and gene target data derived from ChIP-seq data. Only peak intensity signals less than 500 and predicted regulatory potential scores less than 1 were used, employing the BETA Minus algorithm.
Availability of data and materials
The original chromatograms of the proteome are attached, and the original proteome file can be obtained from the author. The author's email address is [email protected].
Data availability
No datasets were generated or analysed during the current study.
Khan MZ, Hussain M, Khan AA, Hassan U, Akhter N, Hameed M, Mushtaq S, Awan UA. Frequency of non-diabetic renal disease in type 2 diabetes mellitus patients undergoing renal biopsy. J Ayub Med Coll Abbottabad. 2021;33(Suppl 1):S757–62.
Google Scholar
Obrador GT, Levin A. CKD hotspots: challenges and areas of opportunity. Semin Nephrol. 2019;39(3):308–14. https://doi.org/10.1016/j.semnephrol.2019.02.009 .
Article PubMed Google Scholar
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45. https://doi.org/10.2215/CJN.11491116 .
Article PubMed PubMed Central CAS Google Scholar
Frampton JE, Empagliflozin. A review in type 2 diabetes. Drugs 78 (10), 1037–48. https://doi.org/10.1007/s40265-018-0937-z .
Cooper S, Teoh H, Campeau MA, Verma S, Leask RL. Empagliflozin restores the integrity of the endothelial glycocalyx in vitro. Mol Cell Biochem. 2019;459(1–2):121–30. https://doi.org/10.1007/s11010-019-03555-2 .
Article PubMed CAS Google Scholar
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney D. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–58. https://doi.org/10.1161/CIRCULATIONAHA.117.030012 .
Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019a;18(1):165. https://doi.org/10.1186/s12933-019-0964-4 .
Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. https://doi.org/10.1186/s12933-019-0816-2 .
Article PubMed PubMed Central Google Scholar
Zou R, Shi W, Qiu J, Zhou N, Du N, Zhou H, Chen X, Ma L. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol. 2022;21(1):106. https://doi.org/10.1186/s12933-022-01532-6 .
Kolijn D, Pabel S, Tian Y, Lodi M, Herwig M, Carrizzo A, Zhazykbayeva S, Kovacs A, Fulop GA, Falcao-Pires I, Reusch PH, Linthout SV, Papp Z, van Heerebeek L, Vecchione C, Maier LS, Ciccarelli M, Tschope C, Mugge A, Bagi Z, Sossalla S, Hamdani N. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase galpha oxidation. Cardiovasc Res. 2021;117(2):495–507. https://doi.org/10.1093/cvr/cvaa123 .
Tan Y, Yu K, Liang L, Liu Y, Song F, Ge Q, Fang X, Yu T, Huang Z, Jiang L, Wang P. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function after cardiac arrest in rats by enhancing mitochondrial energy metabolism. Front Pharmacol. 2021;12:758080. https://doi.org/10.3389/fphar.2021.758080 .
Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33(5). https://doi.org/10.1002/dmrr.2886 .
Zhou G, Soufan O, Ewald J, Hancock R, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47(W1):W234–41. https://doi.org/10.1093/nar/gkz240 .
Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017;38(17):1294–302. https://doi.org/10.1093/eurheartj/ehw045 .
Sanchez J, Gomez JF, Martinez-Mateu L, Romero L, Saiz J, Trenor B. Heterogeneous effects of fibroblast-myocyte coupling in different regions of the human atria under conditions of atrial fibrillation. Front Physiol. 2019;10:847. https://doi.org/10.3389/fphys.2019.00847 .
Tam WC, Lin YK, Chan WP, Huang JH, Hsieh MH, Chen SA, Chen YJ. Pericardial fat is associated with the risk of ventricular arrhythmia in Asian patients. Circ J. 2016;80(8):1726–33. https://doi.org/10.1253/circj.CJ-16-0047 .
Chang D, Zhang S, Yang D, Gao L, Lin Y, Chu Z, Jiang X, Yin X, Zheng Z, Wei X, You D, Xiao X, Cong P, Bian X, Xia Y, Yang Y. Effect of epicardial fat pad ablation on acute atrial electrical remodeling and inducibility of atrial fibrillation. Circ J. 2010;74(5):885–94. https://doi.org/10.1253/circj.cj-09-0967 .
Saxon DR, Rasouli N, Eckel RH. Pharmacological prevention of cardiovascular outcomes in diabetes mellitus: established and emerging agents. Drugs. 2018;78. https://doi.org/10.1007/s40265-017-0857-3 .
Jhuo SJ, Liu IH, Tasi WC, Chou TW, Lin YH, Wu BN, Lee KT, Lai WT. Characteristics of ventricular electrophysiological substrates in metabolic mice treated with empagliflozin. Int J Mol Sci. 2021;22(11). https://doi.org/10.3390/ijms22116105 .
Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90–100. https://doi.org/10.1177/1479164114559852 .
Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019b;18(1):165. https://doi.org/10.1186/s12933-019-0964-4 .
Ring A, Brand T, Macha S, Breithaupt-Groegler K, Simons G, Walter B, Woerle HJ, Broedl UC. The sodium glucose cotransporter 2 inhibitor empagliflozin does not prolong QT interval in a thorough QT (TQT) study. Cardiovasc Diabetol. 2013;12:70. https://doi.org/10.1186/1475-2840-12-70 .
Lee KT, Tang PW, Tsai WC, Liu IH, Yen HW, Voon WC, Wu BN, Sheu SH, Lai WT. Differential effects of central and peripheral fat tissues on the delayed rectifier K(+) outward currents in cardiac myocytes. Cardiology. 2013;125(2):118–24. https://doi.org/10.1159/000350360 .
Jhuo SJ, Liu IH, Tsai WC, Chou TW, Lin YH, Wu BN, Lee KT, Lai WT. Effects of Secretome from Fat Tissues on Ion currents of Cardiomyocyte modulated by sodium-glucose transporter 2 inhibitor. Molecules. 2020;25(16). https://doi.org/10.3390/molecules25163606 .
Lee HC, Chen CC, Tsai WC, Lin HT, Shiao YL, Sheu SH, Wu BN, Chen CH, Lai WT. Very-low-density lipoprotein of metabolic syndrome modulates gap junctions and slows cardiac conduction. Sci Rep. 2017;7(1):12050. https://doi.org/10.1038/s41598-017-11416-5 .
Ladeiras-Lopes R, Moreira HT, Bettencourt N, Fontes-Carvalho R, Sampaio F, Ambale-Venkatesh B, Wu C, Liu K, Bertoni AG, Ouyang P, Bluemke DA, Lima JA. Metabolic syndrome is associated with impaired diastolic function independently of MRI-Derived myocardial extracellular volume: the MESA study. Diabetes. 2018;67(5):1007–12. https://doi.org/10.2337/db17-1496 .
Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2(4):367–73. https://doi.org/10.2174/1573399810602040367 .
Pellman J, Zhang J, Sheikh F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: mechanisms and model systems. J Mol Cell Cardiol. 2016;94:22–31. https://doi.org/10.1016/j.yjmcc.2016.03.005 .
Adamsson ES, Smith JG, Melander O, Hedblad B, Engstrom G. Inflammation-sensitive proteins and risk of atrial fibrillation: a population-based cohort study. Eur J Epidemiol. 2011;26(6):449–55. https://doi.org/10.1007/s10654-011-9565-6 .
Article CAS Google Scholar
Hu YF, Yeh HI, Tsao HM, Tai CT, Lin YJ, Chang SL, Lo LW, Tuan TC, Tzeng CH, Huang SH, Lin YK, Chen SA. Impact of circulating monocyte CD36 level on atrial fibrillation and subsequent catheter ablation. Heart Rhythm. 2011;8(5):650–6. https://doi.org/10.1016/j.hrthm.2010.12.036 .
Liu Z, Liu W, Wang W, Ma Y, Wang Y, Drum DL, Cai J, Blevins H, Lee E, Shah S, Fisher PB, Wang X, Fang X, Guo C, Wang XY. CPT1A-mediated fatty acid oxidation confers cancer cell resistance to immune-mediated cytolytic killing. Proc Natl Acad Sci U S A. 2023;120(39):e1992089176. https://doi.org/10.1073/pnas.2302878120 .
Schlaepfer IR, Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020;161(2). https://doi.org/10.1210/endocr/bqz046 .
Wang YN, Zeng ZL, Lu J, Wang Y, Liu ZX, He MM, Zhao Q, Wang ZX, Li T, Lu YX, Wu QN, Yu K, Wang F, Pu HY, Li B, Jia WH, Shi M, Xie D, Kang TB, Huang P, Ju HQ, Xu RH. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018;37(46):6025–40. https://doi.org/10.1038/s41388-018-0384-z .
Zhang M, Zhou W, Cao Y, Kou L, Liu C, Li X, Zhang B, Guo W, Xu B, Li S. O-GlcNAcylation regulates long-chain fatty acid metabolism by inhibiting ACOX1 ubiquitination-dependent degradation. Int J Biol Macromol. 2024;266(Pt 2):131151. https://doi.org/10.1016/j.ijbiomac.2024.131151 .
Yang W, Ling X, He S, Cui H, Yang Z, An H, Wang L, Zou P, Chen Q, Liu J, Ao L, Cao J. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: an integrated approach. Environ Int. 2023;178:108138. https://doi.org/10.1016/j.envint.2023.108138 .
Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Godde L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquiere B, Lunt SY, Fendt SM, Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520(7546):192–7. https://doi.org/10.1038/nature14362 .
Arunath V, Liyanarachchi MS, Gajealan S, Jasinge E, Weerasekara K, Moheb LA. A novel mutation in ACADVL causing very long-chain acyl-coenzyme-A dehydrogenase deficiency in a south Asian pediatric patient: a case report and review of the literature. J Med Case Rep. 2021;15(1):441. https://doi.org/10.1186/s13256-021-03013-y .
Lu YP, Zhang ZY, Wu HW, Fang LJ, Hu B, Tang C, Zhang YQ, Yin L, Tang DE, Zheng ZH, Zhu T, Dai Y. SGLT2 inhibitors improve kidney function and morphology by regulating renal metabolic reprogramming in mice with diabetic kidney disease. J Transl Med. 2022;20(1):420. https://doi.org/10.1186/s12967-022-03629-8 .
Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem. 2008;80(1):115–22. https://doi.org/10.1021/ac0713510 .
Liu X, Wang J, Gao L, Liu H, Liu C. ITRAQ-Based proteomic analysis of neonatal kidney from offspring of protein restricted rats reveals abnormalities in intraflagellar transport proteins. Cell Physiol Biochem. 2017;44(1):185–99. https://doi.org/10.1159/000484626 .
Zhang H, Fu Y, Wang L, Liang A, Chen S, Xu A. Identifying novel conopepetides from the venom ducts of Conus Litteratus through integrating transcriptomics and proteomics. J Proteom. 2019;192:346–57. https://doi.org/10.1016/j.jprot.2018.09.015 .
Huerta-Cepas J, Szklarczyk D, Heller D, Hernandez-Plaza A, Forslund SK, Cook H, Mende DR, Letunic I, Rattei T, Jensen LJ, von Mering C, Bork P. EggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47(D1):D309–14. https://doi.org/10.1093/nar/gky1085 .
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. https://doi.org/10.1038/nprot.2008.211 .
Download references
This work was supported by the Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (No. 20232025).
Author information
Zongchao Yu, Yongping Lu and Mengxian Zhang contributed equally to this work.
Authors and Affiliations
Department of Nephrology, the First Affiliated Hospital of Jinan University, Guangzhou, China
Zongchao Yu, Yanshan Lin, Tak-sui Wong, Baozhang Guan, Yu Meng, Bo Hu, Fan-na Liu & Lianghong Yin
Department of Nephrology, Center of Kidney and Urology, Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
Yongping Lu
Department of Internal Medicine, Humen Hospital, Dongguan City, Guangdong Province, China
Mengxian Zhang
Dongguan Maternal and Child Health Care Hospital, Postdoctoral Innovation Practice Base of Southern Medical University, Dongguan, China
Yankun Li & Han Zhang
Clinical Medical Research Center, Shenzhen People’s Hospital, The Second Clinical Medical College of Jinan University, Shenzhen, 518020, China
The First Affiliated Hospital, School of Medicine, Anhui University of Science and Technology, Huainan, Anhui, China
You can also search for this author in PubMed Google Scholar
Contributions
All the authors discussed the results and contributed to the fifinal manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Han Zhang , Donge Tang or Yong Dai .
Ethics declarations
Ethics approval and consent to participate.
All experimental procedures in this study were conducted in compliance with the National Law for Laboratory Animal Experimentation and were approved by the Commission on Experimental Animal Ethics of Jinan University (No. 202069-04).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12953_2024_232_moesm1_esm.xlsx.
Additional file 1: Supplementary Excel Table 1: Analysis of protein expression levels and differences in each sample; Supplementary Excel Table 2 :KEGG enrichment results obtained from network interaction analysis of differential proteins; Supplementary table1 :Sample information.
Additional file 2: Supplementary table2: KOG catalog.
12953_2024_232_moesm3_esm.docx.
Additional file 3: Supplementary figure 1: SDS-PAGE and Coomassie brilliant blue staining of 17 samples; Supplementary figure 2: Total ion chromatograms for each sample grouping; Supplementary figure 3: Total Ion Chromatography and Base Peak Chromatogram of 17 samples; Supplementary figure 4: Score scatter plot for PCA model; Supplementary figure 5: KEGG enrichment analysis of differential proteins in each group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, Z., Lu, Y., Zhang, M. et al. Mechanism of the cardioprotective effect of empagliflozin on diabetic nephropathy mice based on the basis of proteomics. Proteome Sci 22 , 9 (2024). https://doi.org/10.1186/s12953-024-00232-1
Download citation
Received : 03 January 2024
Accepted : 23 September 2024
Published : 19 October 2024
DOI : https://doi.org/10.1186/s12953-024-00232-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetic nephropathy
- Empagliflozin
Proteome Science
ISSN: 1477-5956
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Recent status and trends of innate immunity and the gut-kidney aixs in IgAN: A systematic review and bibliometric analysis
Affiliations.
- 1 School of Clinical Medicine, Chengdu Medical College, Chengdu 610500, China; Department of Nephrology, The First Affiliated Hospital of Chengdu Medical College, Chengdu 610500, China.
- 2 School of Clinical Medicine, Chengdu Medical College, Chengdu 610500, China; Department of Nephrology, The First Affiliated Hospital of Chengdu Medical College, Chengdu 610500, China. Electronic address: [email protected].
- 3 School of Clinical Medicine, Chengdu Medical College, Chengdu 610500, China; Department of Nephrology, The First Affiliated Hospital of Chengdu Medical College, Chengdu 610500, China. Electronic address: [email protected].
- PMID: 39423662
- DOI: 10.1016/j.intimp.2024.113335
Background: There is a significant global demand for precise diagnosis and effective treatment of IgA nephropathy (IgAN), with innate immunity, particularly the complement system, exerting a profound influence on its pathogenesis. Additionally, the gut-kidney axis pathway is vital in the emergence and development of IgAN.
Methods: We conducted a comprehensive search in the Web of Science database, spanning from January 1, 2000 to December 18, 2023. The gathered literature underwent a visual examination through CiteSpace, VOSviewer, and Scimago Graphica to delve into authors, nations, organizations, key terms, and other pertinent elements.
Result: Between 2000 and 2023, a total of 720 publications were identified, out of which 436 publications underwent screening for highly relevant literature analysis. The average annual number of articles focusing on IgAN, innate immunity, and the gut-kidney axis is approximately 31, with an upward trend observed. In terms of research impact encompassing publication count and authorship, the United States emerged as the leading contributor. Prominent keywords included "complement", "activation", "microbe", "gut-kidney axis", "C4d deposition", "alternative pathway" and "B cells" along with other prospective hot topics.
Conclusion: The correlation between IgAN and innate immunity is a focal point in current scientific research. Recent literature underscores the significance of the gut-kidney axis, where intestinal microorganisms and metabolites may influence IgAN. The complement system, a key component of innate immunity, also has a crucial function.Advancements in prevention, diagnosis, and treatment hinge on unraveling this intricate relationship.
Keywords: Bibliometrics; Complement system; Gut-kidney aixs; IgAN; Innate immunity.
Copyright © 2024 Elsevier B.V. All rights reserved.
PubMed Disclaimer
Similar articles
- A bibliometric analysis of complement in IgA nephropathy from 1991 to 2022. Guo Y, Zhang H, Yu X. Guo Y, et al. Front Pharmacol. 2023 Jul 27;14:1200193. doi: 10.3389/fphar.2023.1200193. eCollection 2023. Front Pharmacol. 2023. PMID: 37576817 Free PMC article.
- Bibliometric analysis of mucosal immunity in IgA nephropathy from 1990 to 2022. Chen X, Yan Z, Pan Q, Zhang C, Chen Y, Liang X, Li S, Wu G. Chen X, et al. Immun Inflamm Dis. 2024 Jan;12(1):e1156. doi: 10.1002/iid3.1156. Immun Inflamm Dis. 2024. PMID: 38270317 Free PMC article.
- Bibliometric Analysis of Research Relating to IgA Nephropathy from 2010 to 2021. Xi M, Gao X. Xi M, et al. Med Sci Monit. 2022 Nov 23;28:e937976. doi: 10.12659/MSM.937976. Med Sci Monit. 2022. PMID: 36415119 Free PMC article. Review.
- Global research trends on innate lymphoid cells in the brain, gut and lung field: a bibliometric and visualized analysis. Huang J, Deng K, Liu Y, Xia M, Lei M, Wu M. Huang J, et al. Front Immunol. 2024 Feb 7;15:1336666. doi: 10.3389/fimmu.2024.1336666. eCollection 2024. Front Immunol. 2024. PMID: 38384457 Free PMC article.
- Research trends in the field of the gut-brain interaction: Functional dyspepsia in the spotlight - An integrated bibliometric and science mapping approach. Zhang T, Zhang B, Ma X, Zhang J, Wei Y, Wang F, Tang X. Zhang T, et al. Front Neurosci. 2023 Mar 8;17:1109510. doi: 10.3389/fnins.2023.1109510. eCollection 2023. Front Neurosci. 2023. PMID: 36968499 Free PMC article. Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
VIDEO
COMMENTS
Diabetic nephropathy (DN) is a major disorder of diabetes mellitus (DM) which ends up in chronic renal failure (Schrijvers et al., 2004; Sulaiman, 2019). People with DM are ten times more prone to end-stage kidney failure. The International Diabetes Federation (IDF) reports that 40% of diabetic people might develop final stage renal failure.
Diabetic nephropathy (DN) is a major healthcare challenge. It occurs in up to 50% of those living with diabetes, is a major cause of end-stage kidney disease (ESKD) that requires treatment with dialysis or renal transplantation, and is associated with significantly increased cardiovascular morbidity and mortality.
Background Diabetic nephropathy (DN) or diabetic kidney disease refers to the deterioration of kidney function seen in chronic type 1 and type 2 diabetes mellitus patients. The progression of the disease is known to occur in a series of stages and is linked to glycemic and blood pressure control. However, despite aggressive blood sugar control the prevalence of chronic kidney disease (CKD) in ...
Abstract. Diabetic nephropathy (DN) is a type of nephropathy that is caused by a diabetic condition. Diabetic nephropathy is seen in type 1 and type 2 diabetes. End-stage renal disorders are brought on by DN. Diabetic nephropathy is thought to be linked to metabolic changes in the body. Proteinuria and glomerular filtration rate are the two ...
Abstract. Type 2 diabetes mellitus (T2DM) leads to many health problems like diabetic nephropathy (DN). One of the key factors for chronic kidney disease and end-stage renal disease (ESRD) is T2DM. Extensive work is being done to delineate the pathogenesis of DN and to extend possible remedies. This review is intended to understand the nature ...
The development of this disease pathophysiology is being studied and it is known that a series of complex molecular pathways determining a microvascular disease are involved. This review addresses the known pathways in the development of diabetic nephropathy aiming to improve the understanding of potential therapeutic targets that could be ...
Serum inflammatory factors, and coagulation and endothelial functions are good predictors of diabetic nephropathy. This literature review was conducted with access to scholarly databases and Google Scholar through Qassim University, and it analyzes studies from early 2010 until November 2020. Many studies have inferred that diabetes severely ...
Multiple pathophysiological disturbances contribute to the onset and progression of diabetic kidney disease (DKD). This Review describes these pathogenic processes and discusses the ability of ...
Diabetic nephropathy (DN) is the predominant secondary nephropathy resulting in global end-stage renal disease. It is attracting significant attention in both domestic and international research due to its widespread occurrence, fast advancement, and limited choices for prevention and treatment.
Diabetic nephropathy is a significant cause of chronic kidney disease and end-stage renal failure globally. ... NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health. ... Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ ...
Diabetic kidney disease (DKD) is a serious microvascular complication that affects approximately 40% of individuals with diabetes ().Presently the leading cause of end-stage kidney disease (ESKD) worldwide, DKD affects 700 million people, and it disproportionately affects those who are socially disadvantaged ().The global percentage of prevalent ESKD patients with diabetes increased from 19.0% ...
Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature Diab Vasc Dis Res. 2021 Nov-Dec;18(6):14791641211058856. doi: 10.1177/14791641211058856. ... Several factors and mechanisms contribute to the development and outcome of diabetic nephropathy. Microalbuminuria is an early marker of DN and ...
Diabetic nephropathy is more prevalent among African Americans, Asians, and Native Americans than Caucasians (1,12).Among patients starting renal replacement therapy, the incidence of diabetic nephropathy doubled from the years 1991-2001 ().Fortunately, the rate of increase has slowed down, probably because of the adoption in clinical practice of several measures that contribute to the early ...
INTRODUCTION. Diabetes is the leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) in the United States and worldwide. While the gold standard for diagnosis of diabetic nephropathy is defined by histology of the kidney, the majority of patients do not undergo kidney biopsy, as they are presumed to have diabetic ...
Even in the Diabetes Control and Complications Trial (DCCT), of the 11% of patients with type 1 diabetes who developed an eGFR of <60 ml/min/1.73 m 2 40% had never experienced overt nephropathy 12.
The microvascular complications of diabetes induce to renal damage known as diabetic nephropathy (DN), the most common complication of type 2 diabetes mellitus, 5 and it is the leading cause of end-stage renal disease worldwide, which is associated with high morbidity and mortality. 6 It develops in approximately 40% of patients with diabetes, 7 after 10 years of type 2 diabetes mellitus were ...
Methods: A literature review was done from databases like Google Scholar, PUBMEDMEDLINE, and Scopus using standard keywords "Diabetic Nephropathy," "Biomarkers," "Pathophysiology," "Cellular Mechanism," "Cell Therapy," "Treatment Therapies" from 2010- 2023. It has been studied that metabolic as well as hemodynamic pathways resulting from ...
This review addresses the known pathways in the development of diabetic nephropathy aiming to improve the understanding of potential therapeutic targets that could be developed in the future. Chronic kidney disease is a common complication of diabetes. Its importance lies in its high prevalence and future projection. It is associated with high health costs and global cardiovascular ...
29,30,31,32 There is a lack of extensive literature explaining the biological mechanism that associates high RBC folate with diabetic nephropathy (DN) risk among patients with type 2 diabetes.
Abstract. The purpose of this systematic review was to investigate the scientific evidence to support the use of direct renin inhibitors (DRIs) in diabetic nephropathy (DN). MEDLINE was searched for articles reported until 2018. A standardized dataset was extracted from articles describing the effects of DRIs on plasma renin activity (PRA) in DN.
Diabetic nephropathy (DN) is a common and severe complication of diabetes mellitus and is the leading cause of chronic kidney disease (CKD) worldwide. ... Comprehensive advancements in the prevention and treatment of diabetic nephropathy: A narrative review Medicine (Baltimore). 2023 Oct 6;102 ... We comprehensively reviewed the literature on ...
Diabetic nephropathy affects a significant proportion of individuals with diabetes, and its progression often leads to cardiovascular disease and infections before the need for renal replacement therapy arises. Empagliflozin has been shown to have various protective effects in cardiovascular disease studies, such as improving diabetic myocardial structure and function, and reducing myocardial ...
Background: There is a significant global demand for precise diagnosis and effective treatment of IgA nephropathy (IgAN), with innate immunity, particularly the complement system, exerting a profound influence on its pathogenesis. Additionally, the gut-kidney axis pathway is vital in the emergence and development of IgAN. Methods: We conducted a comprehensive search in the Web of Science ...